Abstract
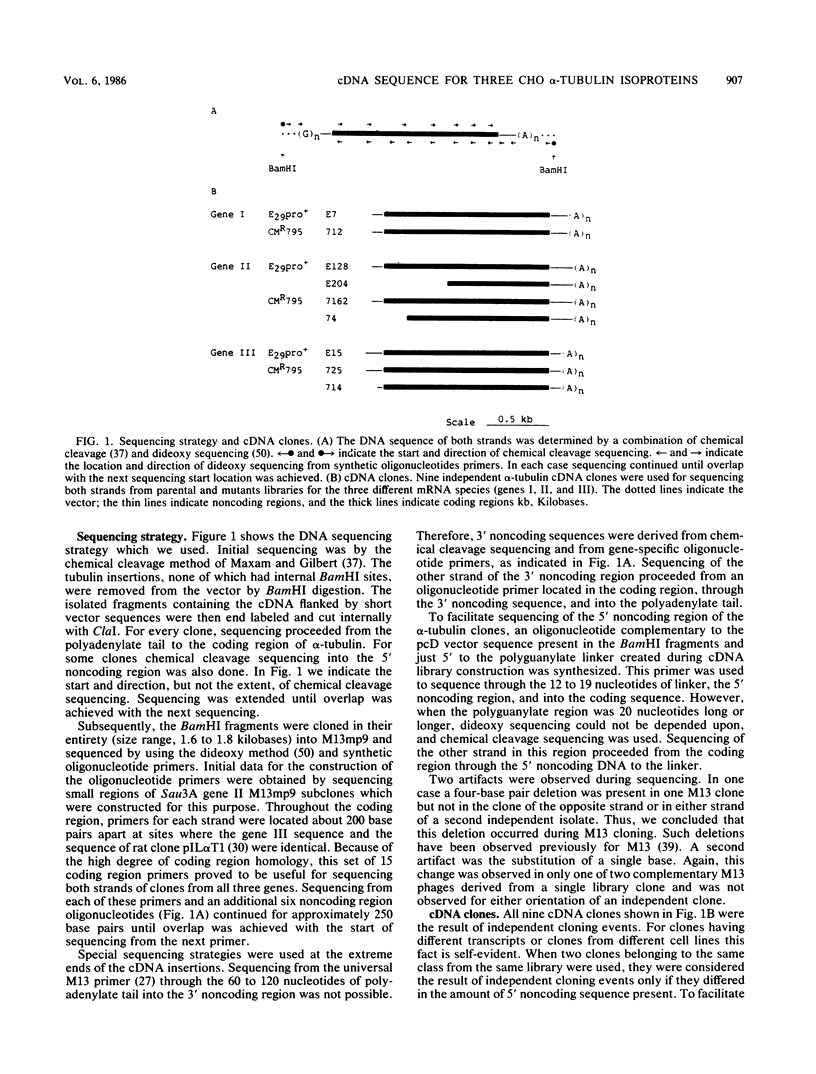
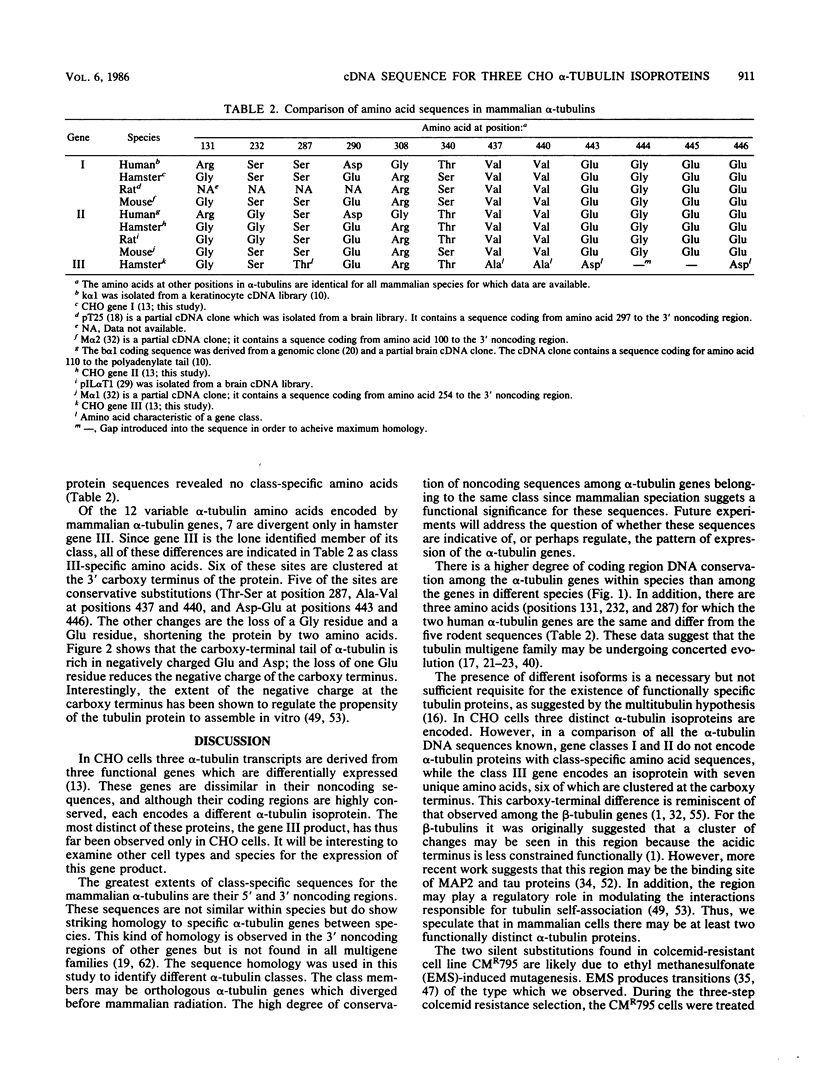
The genome of Chinese hamster ovary (CHO) cells contains a complex family of approximately 16 alpha-tubulin genes, many of which may be pseudogenes. We present here the complete cDNA sequences of three expressed alpha-tubulin genes; one of these genes has been identified only in CHO cells. The noncoding regions of these three CHO alpha-tubulin genes differed significantly, but their coding regions were highly conserved. Nevertheless, we observed differences in the predicted amino acid sequences for the three genes. A comparison of the CHO alpha-tubulin sequences with all of the sequences available for mammals allowed assignment of the alpha-tubulin genes to three classes. The proteins encoded by the members of two of these classes showed no class-specific amino acids among the mammalian species examined. The gene belonging to the third class encoded an isoprotein which was clearly distinct, and members of this class may play a unique role in vivo. Sequencing of the three alpha-tubulin genes was also undertaken in CMR795, a colcemid-resistant clonal CHO cell line which has previously been shown to have structural and functional alterations in its tubulin proteins. We found differences in the tubulin nucleotide sequence compared with the parental line; however, no differences in the alpha-tubulin proteins encoded in the two cell lines were observed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandraki D., Ruderman J. V. Evolution of alpha q- and beta-tubulin genes as inferred by the nucleotide sequences of sea urchin cDNA clones. J Mol Evol. 1983;19(6):397–410. doi: 10.1007/BF02102315. [DOI] [PubMed] [Google Scholar]
- Aubin J. E., Tolson N., Ling V. The redistribution of fluoresceinated concanavalin A in Chinese hamster ovary cells and in their colcemid-resistant mutants. Exp Cell Res. 1980 Mar;126(1):75–85. doi: 10.1016/0014-4827(80)90472-3. [DOI] [PubMed] [Google Scholar]
- Baum H. J., Livneh Y., Wensink P. C. Homology maps of the Drosophila alpha-tubulin gene family: one of the four genes is different. Nucleic Acids Res. 1983 Aug 25;11(16):5569–5587. doi: 10.1093/nar/11.16.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Burland T. G., Schedl T., Gull K., Dove W. F. Genetic analysis of resistance to benzimidazoles in Physarum: differential expression of beta-tubulin genes. Genetics. 1984 Sep;108(1):123–141. doi: 10.1093/genetics/108.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Ling V. Microtubules in colcemid-resistant mutants of CHO cells. Exp Cell Res. 1981 Mar;132(1):147–155. doi: 10.1016/0014-4827(81)90091-4. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. J., Wilde C. D., Chow L. T., Wefald F. C. Structural variation among human beta-tubulin genes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4877–4881. doi: 10.1073/pnas.78.8.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E. M., Ling V. Selection and characterization of Chinese hamster ovary cell mutants resistant to melphalan (L-phenylalanine mustard). Cancer Res. 1981 Feb;41(2):393–400. [PubMed] [Google Scholar]
- Elliott E. M., Okayama H., Sarangi F., Henderson G., Ling V. Differential expression of three alpha-tubulin genes in Chinese hamster ovary cells. Mol Cell Biol. 1985 Jan;5(1):236–241. doi: 10.1128/mcb.5.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E. M., Sarangi F., Henderson G., Ling V. Cloning of 11 alpha-tubulin gene sequences from the genome of Chinese hamster ovary cells. Can J Biochem Cell Biol. 1985 Jun;63(6):511–518. doi: 10.1139/o85-070. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Carter W. Concerted evolution of human interferon alpha genes. J Interferon Res. 1983;3(1):83–88. doi: 10.1089/jir.1983.3.83. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Behar L., Givol D., Littauer U. Z. The nucleotide sequence of rat alpha-tubulin: 3'-end characteristics, and evolutionary conservation. Nucleic Acids Res. 1981 Jun 25;9(12):2691–2697. doi: 10.1093/nar/9.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Mohun T., Ng S. Y., Ponte P., Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3' untranslated regions of the mRNAs. J Mol Evol. 1984;20(3-4):202–214. doi: 10.1007/BF02104727. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Cowan N. J. Structural features and restricted expression of a human alpha-tubulin gene. Nucleic Acids Res. 1985 Jan 11;13(1):207–223. doi: 10.1093/nar/13.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida H., Miyata T., Yamawaki-Kataoka Y., Honjo T., Wels J., Blattner F. Concerted evolution of the mouse immunoglobulin gamma chain genes. EMBO J. 1984 Sep;3(9):2047–2053. doi: 10.1002/j.1460-2075.1984.tb02090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs D. R., Hill A. V., Bowden D. K., Weatherall D. J., Clegg J. B. Independent recombination events between the duplicated human alpha globin genes; implications for their concerted evolution. Nucleic Acids Res. 1984 Sep 25;12(18):6965–6977. doi: 10.1093/nar/12.18.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. A., Childs G. A new family of tandem repetitive early histone genes in the sea urchin Lytechinus pictus: evidence for concerted evolution within tandem arrays. Nucleic Acids Res. 1984 Aug 24;12(16):6455–6471. doi: 10.1093/nar/12.16.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C. C., Lugg D. K., Overton G. C. Post-transcriptional regulation of the abundance of mRNAs encoding alpha-tubulin and a 94,000-dalton protein in teratocarcinoma-derived stem cells versus differentiated cells. Mol Cell Biol. 1984 Nov;4(11):2428–2436. doi: 10.1128/mcb.4.11.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keates R. A., Sarangi F., Ling V. Structural and functional alterations in microtubule protein from Chinese hamster ovary cell mutants. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5638–5642. doi: 10.1073/pnas.78.9.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Gilmartin M. E., Hall J. L., Cowan N. J. Three expressed sequences within the human beta-tubulin multigene family each define a distinct isotype. J Mol Biol. 1985 Mar 5;182(1):11–20. doi: 10.1016/0022-2836(85)90023-3. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Lee M. G., Cowan N. J. Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol. 1985 Sep;101(3):852–861. doi: 10.1083/jcb.101.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Aubin J. E., Chase A., Sarangi F. Mutants of Chinese hamster ovary (CHO) cells with altered colcemid-binding affinity. Cell. 1979 Oct;18(2):423–430. doi: 10.1016/0092-8674(79)90061-8. [DOI] [PubMed] [Google Scholar]
- Malling H. V., de Serres F. J. Identification of genetic alterations induced by ethyl methanesulfonate in Neurospora crassa. Mutat Res. 1968 Sep-Oct;6(2):181–193. doi: 10.1016/0027-5107(68)90033-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. Boundaries of gene conversion within the duplicated human alpha-globin genes. Concerted evolution by segmental recombination. J Biol Chem. 1983 Dec 25;258(24):15245–15254. [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Chang A. C., Cohen S. N., Schimke R. T. Structure and genomic organization of the mouse dihydrofolate reductase gene. Cell. 1980 Feb;19(2):355–364. doi: 10.1016/0092-8674(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Sherman F. Mutagenic specificity: reversion of iso-1-cytochrome c mutants of yeast. J Mol Biol. 1973 Sep 5;79(1):65–82. doi: 10.1016/0022-2836(73)90270-2. [DOI] [PubMed] [Google Scholar]
- Raff E. C. Genetics of microtubule systems. J Cell Biol. 1984 Jul;99(1 Pt 1):1–10. doi: 10.1083/jcb.99.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett D. L., Bhattacharyya B., Wolff J. Tubulin subunit carboxyl termini determine polymerization efficiency. J Biol Chem. 1985 Jan 10;260(1):43–45. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Avila J., Maccioni R. B. Controlled proteolysis of tubulin by subtilisin: localization of the site for MAP2 interaction. Biochemistry. 1984 Sep 25;23(20):4675–4681. doi: 10.1021/bi00315a024. [DOI] [PubMed] [Google Scholar]
- Serrano L., de la Torre J., Maccioni R. B., Avila J. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5989–5993. doi: 10.1073/pnas.81.19.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano M. J., Adair G. M., Atkinson E. N., Humphrey R. M. Induced somatic cell mutations detected in cultured cells by electrophoresis. Isozymes Curr Top Biol Med Res. 1983;10:41–49. [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Sequence of a highly divergent beta tubulin gene reveals regional heterogeneity in the beta tubulin polypeptide. J Cell Biol. 1984 Nov;99(5):1754–1760. doi: 10.1083/jcb.99.5.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Chow L. T., Wefald F. C., Cowan N. J. Structure of two human alpha-tubulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(1):96–100. doi: 10.1073/pnas.79.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cowan N. J. Diverse mechanisms in the generation of human beta-tubulin pseudogenes. Science. 1982 Aug 6;217(4559):549–549. doi: 10.1126/science.6178164. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Nudel U., Mayer Y., Neuman S. Highly conserved sequences in the 3' untranslated region of mRNAs coding for homologous proteins in distantly related species. Nucleic Acids Res. 1985 May 24;13(10):3723–3737. doi: 10.1093/nar/13.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]



