Abstract
Bacillus cereus, aseptically isolated from potato tubers, were screened for cereulide production and for toxicity on human and other mammalian cells. The cereulide-producing isolates grew slowly, the colonies remained small (∼1 mm), tested negative for starch hydrolysis, and varied in productivity from 1 to 100 ng of cereulide mg (wet weight)−1 (∼0.01 to 1 ng per 105 CFU). By DNA-fingerprint analysis, the isolates matched B. cereus F5881/94, connected to human food-borne illness, but were distinct from cereulide-producing endophytes of spruce tree (Picea abies). Exposure to cell extracts (1 to 10 μg of bacterial biomass ml−1) and to purified cereulide (0.4 to 7 ng ml−1) from the potato isolates caused mitochondrial depolarization (loss of ΔΨm) in human peripheral blood mononuclear cells (PBMC) and keratinocytes (HaCaT), porcine spermatozoa and kidney tubular epithelial cells (PK-15), murine fibroblasts (L-929), and pancreatic insulin-producing cells (MIN-6). Cereulide (10 to 20 ng ml−1) exposed pancreatic islets (MIN-6) disintegrated into small pyknotic cells, followed by necrotic death. Necrotic death in other test cells was observed only after a 2-log-higher exposure. Exposure to 30 to 60 ng of cereulide ml−1 induced K+ translocation in intact, live PBMC, keratinocytes, and sperm cells within seconds of exposure, depleting 2 to 10% of the cellular K+ stores within 10 min. The ability of cereulide to transfer K+ ions across biological membranes may benefit the producer bacterium in K+-deficient environments such as extracellular spaces inside plant tissue but is a pathogenic trait when in contact with mammalian cells.
INTRODUCTION
Cereulide, the emetic toxin of Bacillus cereus, is most likely responsible for the severe cases of illness connected to the consumption of food contaminated with B. cereus (1–9). Cereulide-producing B. cereus isolates are frequently reported in processed foods, implicated (3–16) or not implicated (17–21) with food-borne illness, but appear infrequently in farming or natural outdoor environments (22–26).
B. cereus is known to occur in the rhizomicrobiota and endophytic community of plants, as well as in root vegetables, including potato (27), but these habitats have not been searched for cereulide producers (28). The extracellular spaces of plants, as well as natural waters, including soil water, contain <1 mM K+ ions, whereas the concentration of K+ in the interior of the cytoplasmic space of plant cells, as well as in bacteria, is >100 mM (29, 30). Bacteria living in the extracellular spaces of the tuber of a crop plant must thus compete for K+ ions with its plant host and with other bacteria inside the crop plant.
Cereulide is known to be a heat-stable cyclic depsipeptide (6, 31, 32) with high affinity and selectivity for sequestering K+ ions from a low-potassium environment (33–35). We recently found (36) that an endophytic, cereulide-producing Bacillus cereus strain (37) from Picea abies (Norway spruce), had a competitive advantage against nonproducers in potassium-deficient (<1 mM K+) but not in potassium-rich (30 mM K+) media. We hypothesized that the ability to produce cereulide might give a similar advantage to B. cereus in the intercellular spaces of root crops. Furthermore, we also sought to determine whether B. cereus could use its produced cereulide to withdraw K+ ions from intact mammalian cells, with pathological consequences. We describe here the isolation of cereulide-producing endophytic B. cereus from potato tubers and show the mitochondrial toxicity and the potassium-translocating effects of the produced cereulide on human, porcine, and murine primary cells and cell lines.
MATERIALS AND METHODS
Isolation of cereulide-producing potato endophytic B. cereus.
Healthy potatoes (n = 5, Solanum tuberosum) with no visible rot or scab lesions purchased from a local supermarket were washed with water, peeled, surface sterilized with 96% ethanol, and flamed. An aseptically cut piece from the tuber interior was streaked onto nonselective medium (tryptic soy agar [TSA]). After 7 to 10 days at 22°C, colonies with a B. cereus-like appearance (a few colonies per plate) were picked for pure culturing. The cultures were initially screened for heat-stable toxin production as follows. Biomass (∼10 mg [wet weight]) looped from the culture plate was suspended in methanol (0.2 ml) in a screw-cap glass vial with Teflon lining and placed in boiling water for 15 min. After cooling, the vials were vortexed (2 min), and the obtained lysates were tested for sperm motility inhibition (100%) by a rapid assay as described previously (38). The methanol lysates that inhibited motility within 15 min in the rapid assay were serially diluted in methanol (10-fold dilution series), and no more than 20 μl of the dilutions was applied to boar spermatozoa (2 ml, 27 × 106 cells ml−1) for 1 and 4 days at 22 ± 2°C. Corresponding volumes of methanol only served as a control. For reading the 100% effective concentration (EC100), the exposed sperm suspension was prewarmed to 37°C (Thermoblock), and the motility loss (taken as 100% of the immotile cells) was determined by phase-contrast microscopy (×40 objective lens) using a heated (37°C) stage.
Characterization of the potato isolates.
DNA fingerprinting (ribopatterns) was performed using automated equipment (Riboprinter microbial characterization system; DuPont Qualicon, Wilmington, DE) with whole-cell lysates using EcoRI and Pvu2 for the cleavage. A commercial library (DuPont version 2.1.4216.0) amended with an in-house library (containing 120 well-characterized strains of B. cereus) was used for species identification as described previously (20). Starch hydrolysis was tested according to established methods (39) using B. cereus DSM31T as the positive and strain F4810/72 as the negative reference.
Purification, identification, and quantitation of cereulide from the potato endophytes.
Cereulide was purified from the lysates (isolate AB1A, strain NS-58) as described previously (33, 36, 40). The purified compound was identified with mass spectrometry (MS) and tandem MS (MS/MS) analyses. The concentration of cereulide was measured by liquid chromatography-electrospray ionization ion trap MS using an isocratic elution with 0.1% formic acid (solvent A) 6% and methanol (solvent B) 94% at a flow rate of 1 ml min−1 with an Atlantis C18 column T3 (4.6 by 150 mm, 3 μm; Waters, Milford, MA) and valinomycin for calibration.
Target cells used for in vitro toxicity assessments.
Porcine spermatozoa were retrieved from boars (ejaculates of eight individuals), delivered by a commercial supplier (Figen, Ltd., Tuomikylä, Finland) as suspensions of 27 × 106 cells ml−1 in a commercial extender (MR-A), and stored at 18 to 20°C until used (within 48 h) for assays (2). PK-15 is a porcine kidney tubular epithelial cell line (41), cultured as described earlier (40). It was used because cereulide is known to cause renal dysfunction of Na+/K+ homeostasis. Human monocyte-enriched peripheral blood mononuclear cells (PBMC) were purified from buffy coats of healthy individual donors (five separate batches) obtained (with ethical permission) from the Finnish Red Cross Blood Service (Helsinki, Finland) by centrifugation using the Ficoll gradient method of Bøyum (42) and Valmu et al. (43), washed four times in phosphate-buffered saline (PBS; Mg2+- and Ca2+-free) at 22°C, resuspended in RPMI 1640 (complete medium) to 5 × 106 cells ml−1, and used within 36 h. The viability of each batch of PBMC was measured by trypan blue exclusion (Countess cell counter; Invitrogen) as described previously (44).The PBMC were used to model blood cells that become exposed when cereulide is sorbed from the gut into the arteries. The non-tumor-derived epithelial cell line, HaCaT, originates from adult human skin and exhibits normal differentiation (45). MIN-6 cells, kindly donated by J. Miyazaki (46), is a murine pancreatic beta cell line that grows as islets and retains glucose-inducible insulin secretion. The murine fibroblast cell line L929 was obtained from the American Type Culture Collection (ATCC) and used to show responses of a rodent cell line other than the beta MIN-6 cells.
All cells, except for the boar spermatozoa, were maintained in an atmosphere of 95% air, 5% CO2, 37°C, and 95% relative humidity in a cell culture cabinet (Heracell 150i; Thermo Fisher Scientific, Vantaa, Finland). The HaCaT, PK-15, and L-929 cell lines were grown in RPMI 1640 (complete medium), and the MIN-6 cells were grown in Dulbecco modified Eagle medium (DMEM; with supplements [see below]). HaCaT, PK-15, and L-929 cells were adherently cultured. For passaging HaCaT and PK-15, the monolayers of ca. 70% confluence were incubated with 0.02% EDTA for 5 to 10 min, followed by 0.05% (wt/vol) trypsin in 0.02% (wt/vol) EDTA for 3 to 5 min. MIN-6 cells (grown as attached islets) were washed three times with PBS and treated with trypsin for 20 s; the flask was then turned upside down and allowed to drip for 50 to 70 s at 37°C, and the trypsin activity was stopped by rapidly adding 20 to 30 ml of freshly supplemented DMEM.
Toxicity assays.
The boar spermatozoa in commercial extender (MR-A, containing 2 to 5 mM K+ and 150 to 200 mM Na+, was exposed as a suspension as described by Hoornstra et al. (47). PBMC (5 × 106 cells ml−1) in suspension were exposed in RPMI 1640 medium. The HaCaT, L-929, PK-15, and MIN-6 cells were grown in 8-well flat-bottom chamber glass slides, seeded to a density of 4 × 104 cells ml−1, in the respective medium for 48 h. MIN-6 cells were exposed as islet-like agglomerates, attached to the chamber slide bottom, while the other cells were exposed as monolayers. Assays were performed by adding the test substance (solved in methanol, <1 vol%) into the growth medium and incubated as indicated.
After exposure, the test cells were stained with the fluorogenic dye JC-1 (for membrane potential) or calcein AM combined with propidium iodide (live/dead staining) and the nucleus stain Hoechst 33342 as described previously (48, 49), either as slide cultures or as suspensions in microtiter plate wells. Free dye was removed by washing with PBS, and the slides were immediately analyzed with a fluorescence microscope (Zeiss Axiovert 200; Carl Zeiss, Inc., Jena, Germany) using a band-pass filter at 450- to 490-nm excitation and a long-pass filter at 515-nm emission of the JC-1 green and orange fluorescence and propidium iodide red fluorescence simultaneously. Methanol (≤1% [vol/vol]), used as the vehicle, gave no measurable effect alone. All assays were done in triplicate. The standard deviation within a culture and within a cereulide preparation was <20%. Variation between biological replicates (new lot of cultured cells, new preparation of cereulide) was ≤40%.
Assay of potassium efflux from intact cells.
Monolayers of HaCaT keratinocytes were detached with trypsin and washed three to four times with RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) for trypsin inhibition. The cells were pelleted by centrifugation at 10,000 rpm (Rotofix 32; Hettich, Germany) for 2 min (HaCaT cells), 5 min (PBMC), or 10 min (sperm cells). The cell pellet was resuspended in 0.2 ml of K+-free medium and placed in a measurement cuvette (1.2 ml) provided with magnetic stirring, temperature control (24°C), and a potassium ion-selective electrode (Niko-Analit, Moscow, Russia) linked to PC recording software (Record 4; NIKO-ANALIT, Moscow, Russia). Efflux of K+ from the cells was recorded (once per second) by determining the extracellular concentration of potassium. The electrode signal was calibrated by adding 100 μmol of KCl (100 mM stock) into the cuvette at the end of each run and recording the corresponding rise in the electrode response for extracellular K+ concentration, as done in a previous study (40). An extracellular K+ concentration of 0.9 mM saturates cereulide with K+, whereas 0.3 mM K+ saturates only partially (40).
Media, reagents, and disposables.
Biosynthetic cereulide was purified from the B. cereus strains AB1A (potato endophyte) and NS58 (Norway spruce endophyte) as described previously (40).
K+-free medium contained 150 mM NaCl, 5 mM NaH2PO4, 1 mM MgCl2, 1 mM CaCl2, 5 mM glucose, and 10 mM HEPES (pH adjusted to 7.2 with Trizma base). RPMI 1640 (Lonza, Verviers, Belgium) was supplemented with 2 mM l-glutamine, 10% FBS, 50 IU of penicillin (Sigma, St. Louis, MO)/ml, and 50 μg of streptomycin (Sigma)/ml (complete medium). DMEM (Sigma) was supplemented with sterile-filtered solutions of 3.7 g NaHCO3/liter, 100 μl of 50 mM β-mercaptoethanol (Gibco, Paisley United Kingdom)/liter, 15% FBS, 10 ml of 1 M HEPES (adjusted to pH 7.4), 1.25 ml of penicillin-streptomycin solution/liter (Sigma; 10,000 U and 10 mg/ml, respectively), and HEPES buffer, glutamine, valinomycin, and PBS (Mg2+- and Ca2+-free; Sigma). Ficoll-Paque Plus (endotoxin < 0.12 endotoxin units ml−1) was from GE Healthcare Bioscience AB (Uppsala, Sweden), and TSA was from Scharlau Chemie (Barcelona, Spain). The fluorogenic dyes—JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide; a membrane-potential-responsive fluorogenic dye, dissolved in dimethyl sulfoxide [DMSO]), propidium iodide (dead stain propidium iodide, 2.4 mg ml−1 in water), and calcein AM (live stain, 1 mg ml−1 in DMSO)—were all obtained from Invitrogen (Carlsbad, CA). The other reagents were obtained from local suppliers and of analytical quality. The culture flasks and chamber slides were from Nunc (Roskilde, Denmark).
B. cereus reference strains and their ribopatterns.
Food poisoning isolates F4810/72 (HAMBI 2454/SMR-178/CWG52702) (20, 21, 50, 51, 52), F5881/94 (20, 21, 51), LMG17604 (51), B315 (52), and ML60 (54) and strains not connected to food-borne illness, including DSM31T (ATCC 14579) (51) and MIF1 (21), were obtained from the HAMBI laboratory collection (University of Helsinki). The ribopatterns (EcoRI and Pvu2) of reference strains can be found in references 20, 21, 50, and 51.
RESULTS
Heat-stable toxin-producing endophytic B. cereus from consumer potatoes.
Bacteria were aseptically cultured from the interior of healthy potato tubers. Bacillus-like (gram plus rods) colonies (<10% of all colonies on the plate) were pure cultured and screened for the presence of heat-stable substances toxic to mammalian cells. To assess for the presence of toxic metabolites, the biomass of each potato isolate was dispersed into methanol, heat treated, and tested for the ability to inhibit cell motility using boar spermatozoa as test cells. The cultures yielding heat-stable extracts that, at a high dilution, inhibited the motility of sperm cells distinguished themselves from the major colony type (on the same plate) by their small size (Fig. 1). The TSA plates were incubated for 7 to 10 days, because after a 2-day incubation, as prescribed in the standard isolation protocols for food-borne B. cereus (55, 56), the small colonies were barely visible. After extended cultivation of the plates, the difference between small and large B. cereus-like colonies on potato tuber-seeded plates was easy to see. DNA-fingerprinting (ribopatterns obtained with EcoRI and Pvu2) and physiological tests showed that the pure cultures obtained from the small colonies (Fig. 1) represented B. cereus sensu stricto, whereas the large B. cereus-like colonies were identified as B. cereus, B. mycoides, B. thuringiensis (i.e., B. cereus sensu lato), or B. subtilis (Fig. 2).
Fig 1.
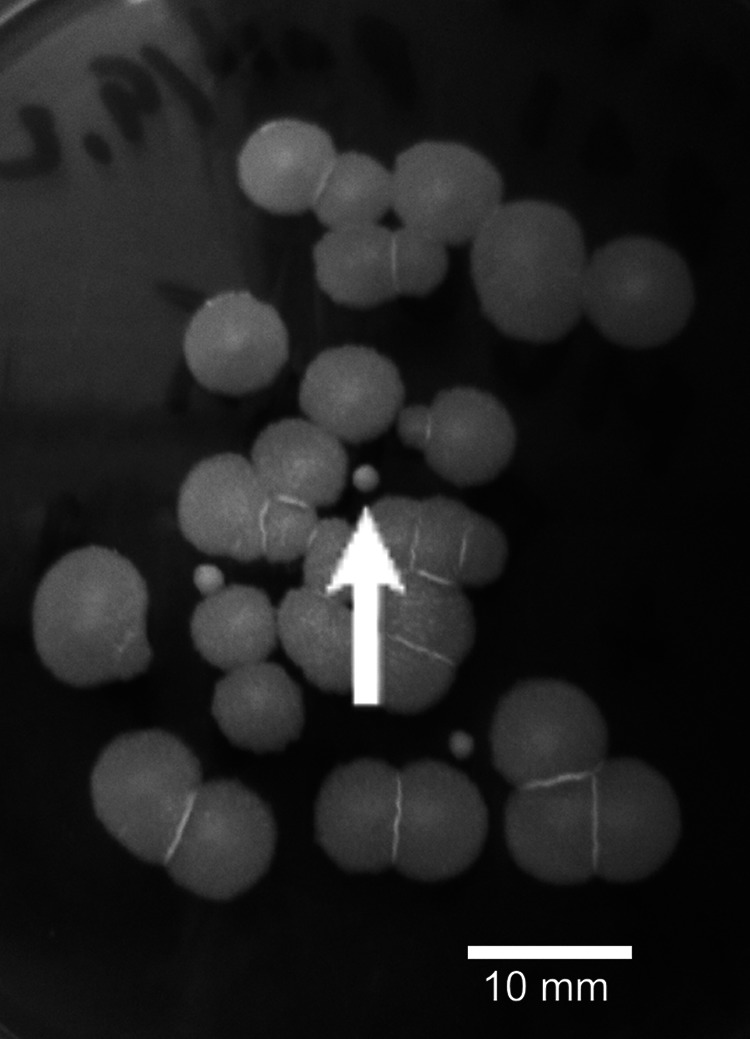
Typical appearance of cereulide-nonproducing (ML60, large colonies) and cereulide-producing (B315, small colonies) B. cereus isolates cultivated on the same tryptic soy agar plate.
Fig 2.
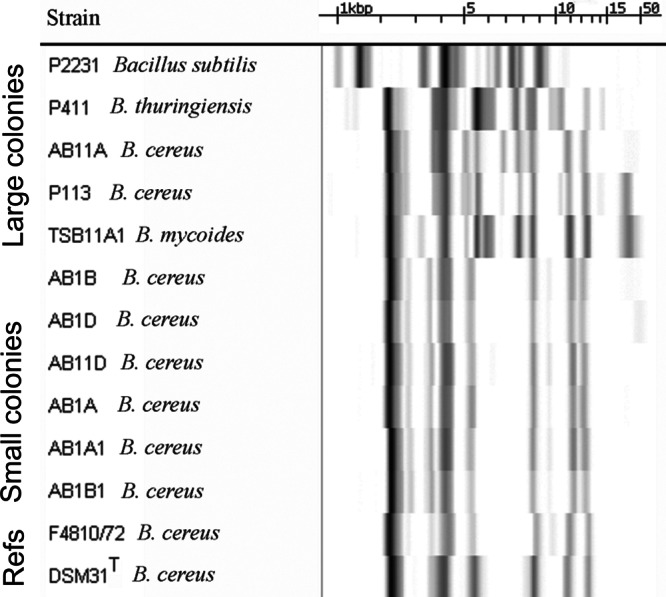
Fingerprints of the ribosomal operon area of Bacillus sp. isolated from potato tubers. Ribopatterns were generated with the automated Riboprinter using restriction enzyme EcoRI and visualized by hybridization to phosphorescence-labeled ribosomal operon of E. coli. The figure shows the identical ribopatterns of cereulide-producing, small colonies (Fig. 1), in contrast to the diversity of ribopatterns of the large-colony-forming B. cereus sensu lato that did not produce cereulide. The species names are indicated by the nearest matching ribopattern using a commercial library and an in-house database (120 well-characterized strains) of B. cereus. Patterns of strains B. cereus DSM31T (ATCC 14579T) and F4810/72 are shown as a reference. The measure bar shows the calibration of the fragment sizes in kilobases, obtained with molecular marker mixes of 1.1, 2.2, 3.2, 6.5, 9.6, and 48 kb.
Table 1 shows the toxic endpoints of lysates of 11 independent cultures of potato tuber endophytes. The toxic endpoint, EC100, here indicates the amount of bacterial biomass (in mg [wet weight] ml−1) required to inhibit the motility of 100% of the exposed spermatozoa (27 × 106 ml−1). Depolarization, i.e., loss of the mitochondrial transmembrane potential (ΔΨm), was recorded as a shift in fluorescence emission of the JC-1-stained mitochondrial sheath, located in the midpiece of the sperm tail, from orange-yellow to green. As seen in Table 1 and Fig. 2, only the isolates with a small-colony type yielded methanol lysates that inhibited the motility and concomitantly depolarized the mitochondria of the sperm cells at the same exposure concentrations, ranging from 0.0001 to 0.01 mg of the bacterial lysate ml−1, corresponding to 105 to 107 CFU equivalents.
Table 1.
Inhibition of motility, loss of mitochondrial membrane potential (ΔΨm), and damage to cell membrane of boar spermatozoa by exposure to lysates prepared from plate-grown cultures of endophytic isolates from potato tuber and reference strains of B. cereus
| Potato tuber isolate or reference B. cereus strain | Toxic dose (EC100) in mg of lysed bacteria ml−1 categorized by toxicity endpointa |
|||
|---|---|---|---|---|
| Motility inhibition |
Loss of ΔΨm | Cell membrane damage | ||
| 1 day | 4 days | |||
| Potato tuber strains | ||||
| P2231 | >1 | >1 | >1 | >1 |
| P411 | >1 | >1 | >1 | >1 |
| AB11A | >1 | >1 | >1 | >1 |
| P113 | >1 | >1 | >1 | >1 |
| TSB11A1 | 1 | 0.1 | 0.1 | 0.1 |
| AB1B | ≤0.01 | ≤0.01 | ≤0.01 | >1 |
| AB1D | ≤0.1 | 0.001 | 0.001 | >1 |
| AB11D | ≤0.01 | ≤0.01 | ≤0.01 | >1 |
| AB1A | 0.001 | ≤0.001 | ≤0.001 | >1 |
| AB1A1 | ≤0.01 | 0.0001 | ≤0.001 | >1 |
| AB1B1 | 0.01 | ≤0.01 | ≤0.01 | >1 |
| Reference B. cereus strains | ||||
| F4810/72b | 0.0001 | 0.0001 | 0.0001 | >1 |
| DSM31Tc | >1 | 1 | 1 | >1 |
EC100 indicates the concentration at which 100% of the exposed sperm cells (27 × 106 ml−1) were affected. The dilution step was step 10. The ΔΨm was measured by using the membrane potential sensor dye JC-1. Cell membrane damage was detected as relaxed permeation of the cells toward propidium iodide. The exposure time was 4 days, except as noted for the motility inhibition.
Cereulide producing (53).
Cereulide nonproducing.
Lysates of small-colony potato isolates (Fig. 2) and of B. cereus F4810/72 provoked the same effects in sperm cells, whereas the type strain of B. cereus, DSM31 (ATCC 14579, not producing cereulide), neither inhibited sperm motility nor depolarized the sperm mitochondria, with exposure concentrations up to 1 mg ml−1 (Table 1). General cytotoxic effects in the sperm cells, recorded as the emission of red fluorescence by propidium iodide, occurred only after exposure of up to 100- to 1,000-fold-higher concentrations of the same lysates (>1 mg ml−1; Table 1).
Identification of the heat-stable substance produced by potato tuber B. cereus isolates.
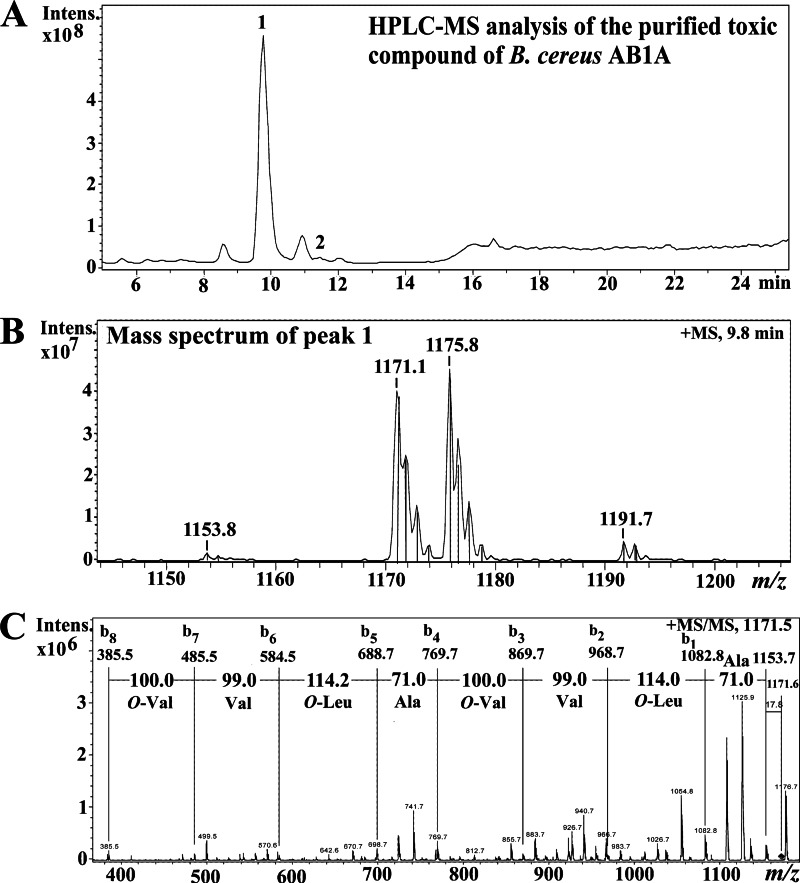
Figure 3 shows the results of liquid chromatography-MS (LC-MS) analysis and MS/MS of the toxic substance purified from the methanol extract of the potato isolate B. cereus AB1A. The total ion chromatogram of the toxic high-pressure liquid chromatography (HPLC) fractions is shown in Fig. 3A. The main peak at retention time 9.8 min had mass ions at m/z 1,153.8, m/z 1,171.1, m/z 1,175.8, and m/z 1,191.7 (Fig. 3B). These mass ions matched the [M+H]+, [M+NH4]+, [M+Na]+, and [M+K]+ ions, respectively, of cereulide from the emetic reference strain B. cereus F4810/72 (2). This was verified by MS/MS fragmentation of the purified toxic peak with m/z 1,171.1 of the potato isolate B. cereus AB1A (Fig. 3B) as a precursor ion (Fig. 3C). The b ion series (b1 to b8) observed corresponded to the depsipeptide sequence of O-Val-Val-O-Leu-Ala-O-Val-Val-O-Leu-Ala, and the mass ion at m/z 385 (b8) matched the protonated tetradepsipeptide O-Val-Val-O-Leu-Ala (at m/z 384) unit. Biosynthetic cereulide consists of three similar tetradepsipeptide (O-Val-Val-O-Leu-Ala) units forming a cyclic structure (O-Val-Val-O-Leu-Ala)3. The MS and MS/MS spectra of the purified toxic compound of B. cereus AB1A were similar to the previously published MS data for cereulide produced by the endophytic B. cereus NS58 (36). Therefore, the LC-MS and MS/MS analysis showed that the main toxic peak (peak 1 in Fig. 3A) of B. cereus AB1A was cereulide.
Fig 3.
HPLC-MS analysis of the purified toxic compound of B. cereus AB1A. (A) Total ion chromatogram of the purified toxic compound (peak 1) of B. cereus AB1A. (B) The mass spectrum of the purified toxic compound of B. cereus AB1A at retention time 9.8 min. (C) Tandem mass spectrum of peak 1 using mass ion at m/z 1,171.5 as a precursor ion and obtained ion series with the interpreted sequences. O-Val is 2-hydroxyisovaleric acid, and O-Leu is 2-hydroxyisocaproic acid.
The cereulide content of B. cereus AB1A and of the other five (Table 1) sperm toxic B. cereus isolates in Table 1 was quantified with the LC-MS using the area of mass ions [M+NH4]+ at m/z 1,171.1 and [M+Na]+ at m/z 1,175.8, using the corresponding ions of valinomycin (at m/z 1,129.1 and 1,133.8) as a reference. The results (Table 2) show that six of the eight B. cereus isolates produced cereulide, but productivities (measured from cultures grown 4 days on TSA plates by the quantitative LC-MS method) were lower than that of the cereulide-producing reference strain F4810/72 and that of the Norway spruce endophyte NS58 grown under the same conditions. Furthermore, Table 2 shows that the productivities of highest and lowest producers differed by 2 log units. The measured cereulide productivity divided potato isolates of B. cereus sensu stricto into three groups as follows: AB1A (medium) > AB1A1, AB11D, AB1B AB1D, and AB1B1 (low) and P113 and AB11A (no detectable cereulide). The measured toxicities (EC100 values, Table 1) divided the isolates similarly into three groups: highly toxic, EC100 = 0.001 mg/ml (AB1A); moderately toxic, EC100 = 0.01 to 0.1 mg/ml (AB1A1, AB11D, AB1B, AB1D, and AB1B1); and weakly toxic or none, EC100 ≥ 1 (P113 and AB11A). The synchrony between the toxic potential (Table 1) and the cereulide contents (Table 2) of the potato tuber isolates over a 2-log range supports the view that the main toxic agent was cereulide, and the differences between the isolates show that they were not clonal.
Table 2.
Cereulide content analyzed by LC-MS in methanol extracts of potato tuber Bacillus isolates and reference strainsa
| Potato tuber isolate or Bacillus strain | Cereulide content (ng/mg of biomass [wet wt])b |
|---|---|
| Potato tuber isolates | |
| AB11A | <0.2 |
| P113 | <0.2 |
| ABIB | 9 |
| AB1D | 1 |
| AB11D | 21 |
| AB1A | 96 |
| AB1A1 | 13 |
| AB1B1 | 3 |
| B. cereus reference strains | |
| F4810/72 (food poisoning)c | 350 |
| NS58 (endophyte of spruce tree) | 900 |
| DSM31Td (ATCC 14579) | <0.2 |
See also Table 1.
Tested with biomass harvested from tryptic soy agar plates at 7 days and 22°C. The variation between biological replicates was <40%.
Reference strain for producing cereulide.
Type strain; does not produce cereulide.
Genotyping of the potato endophytic cereulide-producing B. cereus.
Figure 2 shows the EcoRI cleavage ribopatterns of six cereulide-producing (toxic) isolates from five potato tubers. The six patterns were identical and distinct from nonproducers (strains P113 and AB11A). The patterns differed from that of B. cereus F4810/72 (cereulide-producing reference strain) in bands >10 kb in size. When the ribopatterns of the six cereulide-producing potato isolates were compared to those from 120 well-characterized B. cereus isolates in our laboratory database, a match was found (both in EcoRI and in Pvu2) with B. cereus F5881/94, a cereulide-producing isolate from a food-borne outbreak in United Kingdom (20, 50).
Detecting the toxicity of cereulide to somatic mammalian cells.
Table 3 summarizes the toxic endpoints of cereulide against five different human, porcine, and murine primary cell lines. The toxic endpoints of mitochondrial depolarization (i.e., the loss of ΔΨm) by 24 h of exposure to cereulide for human PBMC, human keratinocytes, porcine kidney proximal tubular epithelial cells, murine L-929 fibroblasts, and pancreatic β-cells (MIN-6) ranged from 0.0004 to 0.002 μg ml−1 (median, 0.002 μg ml−1). This finding is similar to that for boar sperm cells (0.001 μg ml−1), suggesting that boar spermatozoa, which are easy to handle and require no tissue culture facilities, have the potential of being a realistic surrogate toxicity sensor for observing cereulide-induced mammalian cell mitochondrial toxicity. In addition, boar sperm cell motility loss occurred at the same exposure concentration at which ΔΨm was lost (Table 3). The motility loss (EC100) can be observed with a phase-contrast or dark-field microscope, with no need for fluorogenic dyes and epifluorescence microscopy in a darkroom.
Table 3.
Toxicity endpoints of exposure to cereulide for human, porcine, and murine primary cells and selected cell lines
| Target cellsa | Toxicity endpointb |
|||
|---|---|---|---|---|
| Loss on ΔΨm |
Relaxed permeability to PI |
|||
| Exposure time | EC100 (μg ml−1) | Exposure time | EC100 (μg ml−1) | |
| Human | ||||
| PBMC | 30 min | 0.004 | 24 h | >0.4c |
| Keratinocytes (HaCaT) | 30 min | 0.004 | ||
| 24 h | 0.001 | 24 h | 1.5 | |
| Porcine | ||||
| Spermatozoad | 20 min | 0.007 | ||
| 24 h | 0.001 | 24 h | >1.0 | |
| KPTE cells (PK-15) | 30 min | 0.005 | ||
| 24 h | 0.0004 | 24 h | 2.0 | |
| Murine | ||||
| Insulinoma, MIN-6 cells | 20 min | 0.014 | 20 min | >0.14 |
| 8 h | 0.001 | 8 h | 0.013 | |
| 24 h | 0.002 | 24 h | ≤0.007 | |
| Fibroblast L-929 cells | 24 h | 0.001 | 24 h | >1.0 |
PBMC and sperm cells were exposed as suspensions, and MIN-6 cells were exposed as islets. KPTE cells, kidney proximal tubular epithelial cells.
ΔΨm, mitochondrial membrane potential. The EC100 indicates the exposure concentration causing an effect in 100% of the exposed cells. The variation between biological replicates was <40%. PI, propidium iodide.
When PBMC were labeled with propidium iodide, the result was similar, i.e., with <20% of the cells fluorescing red, for both the nonexposed sample (vehicle only) and the highest test concentrations, i.e., 400 ng of purified cereulide ml−1. A trypan blue assay showed 81 to 87% viability.
Sperm motility ceased at the same exposure concentration at which ΔΨm was lost.
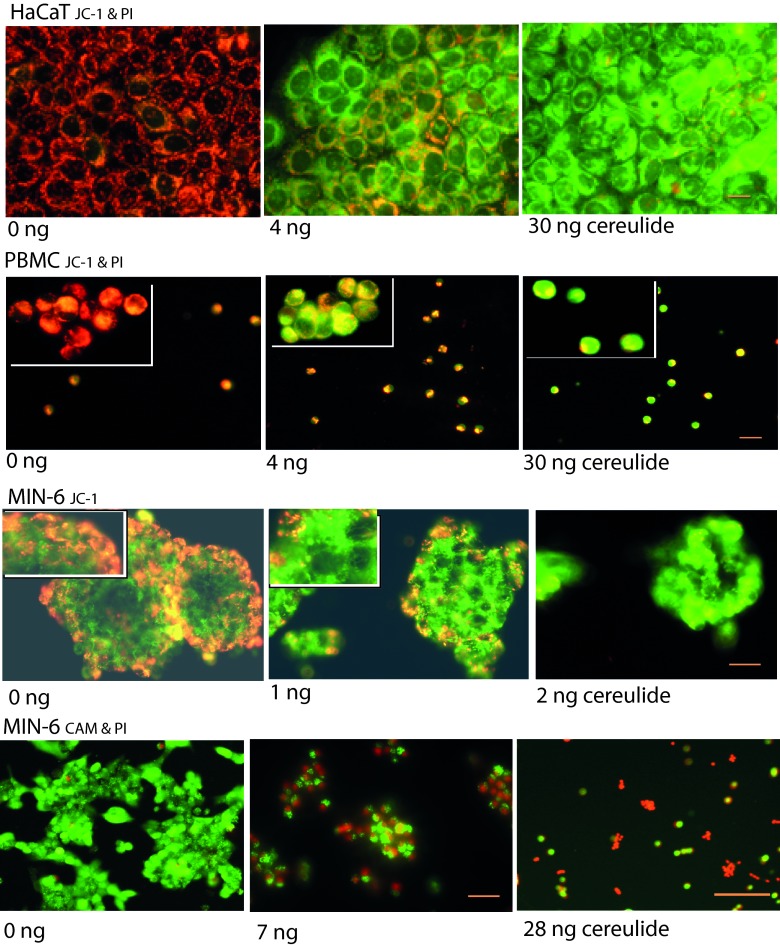
Table 3 further shows that necrotic cell death, visualized as relaxed cell membrane permeability to propidium iodide, was observed only at exposures 100- to 1,000-fold higher (>0.4 to 2 μg ml−1) than for mitochondrial depolarization. Thus, exposure to cereulide caused mitochondrial damage in keratinocytes (HaCaT), PBMC, kidney epithelial cells (PK-15), spermatozoa, and fibroblasts (L-929), whereas the cells were alive and able to exclude the propidium iodide dye. This is illustrated in Fig. 4 for the keratinocytes and the PBMC, double stained with the membrane potential indicator dye JC-1 and propidium iodide. The orange-red fluorescence in JC-1-stained cells indicates high mitochondrial membrane potential (ΔΨm > 140 mV), and green fluorescence indicates dissipated mitochondrial membrane potential (ΔΨm < 100 mV). The unexposed cells (solvent only, 0 ng of cereulide, left panels) show the JC-1 orange-red fluorescence of highly energized mitochondria that fill the cytoplasm around the cell nuclei. The cells exposed for 30 min to 4 ng of cereulide ml−1 lost a major part of the orange fluorescence, indicating depolarization (i.e., a decrease in ΔΨm) of the mitochondria. The cells exposed to 30 ng of cereulide ml−1 (right panels) have practically no energized mitochondria. However, none of the keratinocytes or PBMC cells showed any propidium iodide fluorescence staining (purple red), indicating that the cell membranes were intact and the cells were alive. This was confirmed using calcein AM (live stain) combined with propidium iodide (dead stain) (Fig. 4). The keratinocytes impermeable to propidium iodide exhibited fragmented nuclei visible by Hoechst 33342 staining after exposure to cereulide in amounts greater than 100 to 200 ng ml−1. This indicated early apoptosis in response to cereulide exposure.
Fig 4.
Epifluorescence micrographs of the effects of cereulide on human keratinocytes (HaCaT) and PBMC and murine pancreatic islet cells (insulin producing, MIN-6). The HaCaT and PBMC samples were double stained with the membrane potential responsive dye JC-1 and propidium iodide. The MIN-6 cells were stained with JC-1 (third row) or double stained with calcein AM (CAM) and propidium iodide (fourth row). The HaCaT and PBMC samples were exposed to cereulide for 30 min, and the MIN-6 cells were exposed to cereulide for 24 h. A value of “0 ng” indicates vehicle only. The orange-red fluorescence in JC-1-stained cells indicates a high membrane potential (ΔΨm > 140 mV), and green fluorescence indicates a dissipated membrane potential (ΔΨm < 100 mV). Unexposed pancreatic islets (MIN-6, 0 ng) stained with JC-1 fluoresce orange at the edges of the islet and green in the center. After exposure to cereulide, the islets fluoresce green (low ΔΨm). Propidium iodide-positive cells were only seen in MIN-6 cells under these exposure conditions. The images are representative of three independent microscopic views. Scale bar, 30 μm.
Pancreatic beta cells (MIN-6) responded to exposure to cereulide differently: the islets disintegrated, followed by necrotic cell death observed as a decrease in cell density, concomitant with the appearance of pyknotic cells and conversion to propidium iodide positivity (Fig. 4). This was observed after cereulide exposures as low as 10 ± 3 ng ml−1 (8 to 24 h exposure, Table 3), which is only ≤10-fold higher than that needed for depolarizing (loss of ΔΨm) the mitochondria in these cells.
We conclude from the data shown in Table 3 and Fig. 4 that cereulide, at concentrations relevant for food-borne illness, induced specific damage to mitochondria rather than causing general cytotoxicity to the human (PBMC and keratinocytes), porcine (spermatozoa and kidney tubular epithelial cells [PK-15]), and murine fibroblasts. The pancreatic beta cells (MIN-6) underwent necrotic cell death following exposure at concentrations 2 logs lower than those for the other cells.
Cereulide-dependent efflux of potassium from human cells.
Cereulide is known to act as a K+ ionophore in isolated mitochondria, leading to mitochondrial dysfunction (34, 57). Given the findings in Table 3 and Fig. 4 showing mitochondrial depolarization inside intact, live cells, we asked the question whether the mitochondrial damage involved trafficking of K+ ions from or to the intact mammalian cell. This was studied by monitoring cereulide imposed changes of the extracellular [K+] in a cuvette fitted with a K+ specific electrode and holding a suspension of live cells.
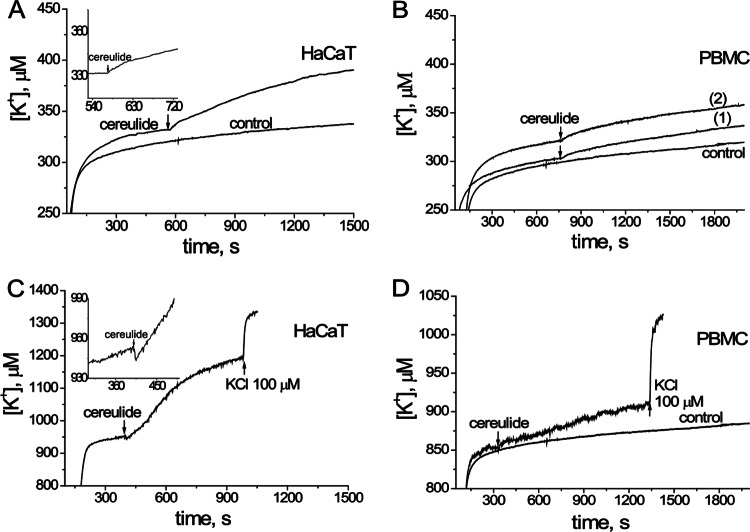
Figure 5 shows that exposing human keratinocytes (HaCaT) to 60 ng (Fig. 5A) and PBMC to 45 or 90 ng (Fig. 5B), respectively, of cereulide ml−1 provoked an efflux of K+ within seconds that was measurable as an increase in [K+] in the external medium. The efflux from 6 × 106 HaCaT cells into the external medium (1 ml) in the measurement cuvette raised the [K+] by 8 μM (Fig. 5A) per 60 s. Efflux from 15 × 106 PBMC raised the extracellular [K+] by 6 μM (trace 2 in Fig. 5B) per 60 s. These potassium fluxes were measured in a medium with [K+] of ca. 300 μM (Fig. 5A and B).
Fig 5.
Cereulide-driven efflux of K+ from human keratinocytes (HaCaT) and PBMC. Washed HaCaT cells (6 × 106 ml−1) or PBMC (15 × 106 ml−1) in 1 ml of K+-free medium were placed in a measurement chamber, and the extracellular [K+] was recorded in real time with a K+-selective electrode, with one reading per second. The effects of cereulide using 60 ng (panels A and C), 45 and 90 ng (traces 1 and 2, respectively, in panel B), and 30 ng (panel D) on the efflux of K+ are shown. The “control” trace indicates data for vehicle only (methanol). The inset in panel C shows that the exposure to cereulide transiently initiated the influx of K+, followed by a massive efflux of K+. At the end of each run, the electrode response was verified by adding KCl to increase the concentration in the cuvette by 100 μM.
When the efflux of K+ was monitored in a medium with a [K+] of 850 to 950 μM, the flux of K+ from HaCaT increased the extracellular [K+] by 50 μM and from PBMC by 4 μM per 60 s (Fig. 5C and D). The summed cell volume of 6 × 106 cells HaCaT cells (cell diameter, 30 μm) is ∼80 μl in the cuvette (1 ml) and that of the 15 × 106 PBMC (cell diameter, 7 μm) is ∼3 μl. Assuming the intracellular [K+] to be 150 mM, the summed potassium stores of HaCaT cells in the cuvette was 12 μmol. The efflux from HaCaT cells induced by exposure to 60 ng of cereulide ml−1 during the ∼10-min observation time was 250 nmol (Fig. 5C), representing roughly 2% of the cellular K+ stores. The summed cellular potassium stores of the PBMC in the cuvette were ∼0.45 μmol; thus, a 10-min exposure to 30 ng of cereulide ml−1 induced an efflux of ∼10% of the cellular K+ stores of the PBMC.
The measured effluxes of K+ from the PBMC were similar, whether the exposure dose of cereulide was 45 or 90 ng (Fig. 5B). This either indicates an on/off response or that the lower dose (∼106 cereulide molecules per PBMC) was a saturating one (Fig. 5B). Cereulide (130 ng/ml) also induced an efflux of K+ from porcine sperm cells (80 × 106 cells per ml) at similar exposure concentrations, i.e., ∼106 cereulide molecules per sperm cell. The resulting increase in extracellular [K+] was 50 μM in 8 min. This represents an efflux of ca. 10% of the cellular stores of the summed sperm cell volume (4 μl) in the cuvette (data not shown).
DISCUSSION
The extracellular spaces of vascular plants, including potato tubers, are not sterile (as animal tissues are) but contain microorganisms in the water channels and extracellular spaces (27, 28). In the present study we described the isolation and properties of B. cereus (sensu lato) from potato tubers. These environments are likely deficient in potassium for nonphytopathogens that do not penetrate into plant cells (27, 28). We recently showed that B. cereus benefits from its produced cereulide in a potassium-deficient environment (<1 mM) but not in potassium-rich environment (36). This may explain why potato endophytic B. cereus were frequently cereulide producers, similarly to what was earlier observed for endophytic B. cereus from Norway spruce (Picea abies), where four of the six B. cereus isolates were cereulide producers (NS58, NS88, NS115, and NS117 [20, 36, 50, 51]).
The potato tuber appeared not to suffer from its cereulide-producing endophytes. However, human, porcine, and murine cells the cereulides appeared highly toxic, indicating the endophyte isolates produced cereulide in its active, toxic form. Most of the B. cereus sensu stricto organisms from potato tubers were cereulide producers and displayed a genotype (both ribopatterns EcoRI and Pvu2) matching that reported for a cereulide-producing isolate from a food poisoning outbreak by B. cereus F5881/94 (fried rice, United Kingdom) (20, 50). The potato endophytic cereulide producers differed in ribopatterns from the nonproducers (Fig. 2). Endophytic cereulide-producing B. cereus organisms from spruce (Picea abies) were also reported to differ in ribopatterns from the nonproducers (NS61 and NS88 [20]). The potato isolates differed from the spruce tree producers in ribopatterns and modest cereulide production, 1 to 100 ng mg of biomass−1 compared 900 to 1,700 ng mg of biomass−1 for the NS strains (Norway spruce [51, 52, 54]). It might be that each plant species carries its own B. cereus cereulide-producing genotype, possibly adapted to the specific metabolic environment inside the host plant.
Vegetables may represent an emerging source of emetic food-borne illness by B. cereus, especially in mass catering. A large-scale food poisoning outbreak was reported from the Netherlands in which 116 students became ill with vomiting and nausea within 1.5 h after consuming a vegetarian meal. A total of 1,275 students participated in the meal, but only 120 vegetarian portions were served, and all of the students that became sick were from this group (57). Raw potato granules and potato flakes, constituents of many industrial foods, have been reported to contain log 1 to log 3.4 CFU of mesophilic aerobic spores g−1, including B. cereus (58).
In the present study, we demonstrated that cereulide provoked cross-membrane flux of potassium ions within seconds of exposure in intact primary human and porcine cells. The potassium efflux from live, healthy cells, driven by exposure to nanomolar concentrations of cereulide (45 to 60 ng ml−1) was observed to deplete the cells of 2 to 10% of the calculated cellular potassium stores within 10 min. This is a potential pathological trait, in addition to the observed mitochondrial toxicity (34, 47, 59), because transient cross cell-membrane gradients of potassium ions are known to operate as metabolic regulators in many mammalian cells, for example, the cells of innate and acquired immunity or voltage-gated potassium channel (hERG) heart muscle cells (60–64).
The mitochondrial toxic endpoints of cereulide were earlier shown in boar spermatozoa (30) and in many different tumor-derived cells lines (4, 48, 65). In the present study we showed that primary cells and nonmalignant immortalized cell lines of human and porcine origins were similarly sensitive to mitochondrial damage by cereulide, with EC100 (i.e., the concentration affecting 100% of the exposed cells) values of a few nanograms per milliliter. Sperm motility ceased at the same exposure concentration of cereulide at which the mitochondria of spermatozoa, as well as somatic mammalian cells, were depolarized. Thus, the outcome of the spermatozoan motility assay is relevant to mammalian cells for initial estimation of mitochondrial toxic concentrations of cereulide in various samples (4, 19, 21, 24, 65).
The detection limit of the assay is 0.2 ng of cereulide mg of biomass−1, and the response may be recorded within 30 min from sampling (38, 48). In a severe food poisoning case, the blood concentration of cereulide was reported as 4 ng ml−1 (3), which is in the range detectable by the sperm bioassay. One unit in the widely used Hep2 assay (66) was reported to correspond to 5 ng of biosynthetic cereulide (67). The sperm cell assay is therefore more sensitive; it also is simpler to execute since it requires no tissue culture facilities. The structurally related depsipeptides homocereulide (68) and paenilide (Paenibacillus tundrae) (40) are similarly toxic to cereulide and can be detected using the same method.
Mitochondrial damage by cereulide is presumed to impair the functioning of citric acid cycle and cell respiration, i.e., the aerobic pathways for the production of ATP. A consequence of dysfunctional mitochondria is that the body tissues must generate ATP from glycolysis, resulting in lactic acidosis. Where blood analysis data were available, all the serious cases of human emetic food poisoning by B. cereus displayed metabolic acidosis and increased plasma lactate levels and lowered the blood pH to 6.25 to 7.25 (3, 5, 7, 9, 10, 12). We recently showed that metabolic acidosis caused by mitochondrial toxicity from cereulide can be simulated by in vitro exposure using mammalian cultured cells (40). Human and murine insulin-producing β-cells are known in vivo and in vitro to be fully dependent on mitochondrial oxidative phosphorylation, since the beta cells do not possess a compensatory mechanism, e.g., glycolytic ATP from processing glucose to lactate (69, 70).
Here, we showed that insulin-producing pancreatic islet beta cells (MIN-6) differed from the other test cells by being highly sensitive to cytolytic killing by cereulide. Upon exposure, the pancreatic islets dispersed into pyknotic cells, followed by necrosis, at cereulide concentrations 2 log units below that required for other somatic human, porcine, or murine cell death. Earlier, we found that in vitro exposure of fetal porcine pancreatic islets to nanomolar concentration of cereulide from emetic potato isolates of B. cereus resulted in the necrosis of islet cells (71). Interestingly, a recent epidemiological survey from Finland indicated that the early introduction of root vegetables in infancy was associated with advanced β-cell autoimmunity in young children and increased susceptibility to type 1 diabetes (72). Cereulide-producing B. cereus is known to produce spores that are severalfold more resistant to heating at 90°C compared to B. cereus spores of cereulide-nonproducing B. cereus (52). Such spores are likely to survive and become enriched in foods processed by repeated heating. Endophytic B. cereus (strain NS117, spruce tree) and an isolate from a fatal food poisoning involving cereulide (strain 5964a) were shown to produce twice the amount of cereulide when inoculated in potato puree compared to that produced when inoculated into boiled rice (73). This finding indicates that potato is an environment favorable for cereulide production when cereulide producers are present.
The genotypic match between potato endophytes in the present study, strain F5881/94 (from a food-borne emetic outbreak involving fried rice in the United Kingdom [20, 50]), and cereulide-producing B. cereus from infant food formula (MIF1, not connected to food-borne illness [21]) suggests that vegetable crop endophytes may be one of the points of entry of emetic B. cereus into foods.
ACKNOWLEDGMENTS
This study was supported by the Finnish Graduate School for Applied Biosciences (D.H.), the Academy of Finland CoE “Photobiomics” (grant 118637), and the Finnish Medical Association.
MIN-6 cells were kindly provided by J. Miyazaki from Kumamoto University Medical School, Kumamoto, Japan. We thank Leena Kuoppasalmi, Tiiu Arumäe, and Petri Ylipaasto for expert contributions with cell preparations, the Viikki Science Library for the professional information service, Sarah Coleman for language editing, the Faculty Instrument Centre for technical services, and Leena Steininger, Hannele Tukiainen, Tuula Suortti, Mika Kalsi, and Riitta Saastamoinen for various types of assistance with this study.
Footnotes
Published ahead of print 22 March 2013
REFERENCES
- 1. Agata N, Ohta M, Mori M, Isobe M. 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129:17–20 [DOI] [PubMed] [Google Scholar]
- 2. Andersson MA, Mikkola R, Helin J, Andersson MC, Salkinoja-Salonen M. 1998. A novel sensitive bioassay for detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl. Environ. Microbiol. 64:1338–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shiota M, Saitou K, Matsusaka H, Agata N, Nakayama M, Kage M, Tatsumi S, Okamoto A, Yamaguchi S, Ohta M, Hata D. 2010. Rapid detoxification of cereulide in Bacillus cereus food poisoning. Pediatrics 125:e951–e955 [DOI] [PubMed] [Google Scholar]
- 4. Jääskeläinen EL, Teplova V, Andersson MA, Andersson LC, Tammela P, Andersson MC, Pirhonen TI, Saris NEL, Vuorela P, Salkinoja-Salonen MS. 2003. In vitro assay for human toxicity of cereulide, the emetic mitochondrial toxin produced by food poisoning Bacillus cereus. Toxicol. In Vitro 17:737–744 [DOI] [PubMed] [Google Scholar]
- 5. Mahler H, Pasi A, Kramer J, Schulte P, Scoging A, Baer W, Kraehenbuehl S. 1997. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 336:1143–1148 [DOI] [PubMed] [Google Scholar]
- 6. Shinagawa K, Konuma H, Sekita H, Sugii S. 1995. Emesis of rhesus monkeys induced by intragastric administration with the Hep-2 vacuolation factor (cereulide) produced by Bacillus cereus. FEMS Microbiol. Lett. 130:87–90 [DOI] [PubMed] [Google Scholar]
- 7. Pósfay-Barbe KM, Schrenzel J, Frey J, Studer R, Korff C, Belli DC, Parvex P, Rimensberger PC, Schäppi MG. 2008. Food poisoning as a cause of acute liver failure. Pediatr. Infect. Dis. J. 27:846–847 [DOI] [PubMed] [Google Scholar]
- 8. Yatsumoto Y, Hasegawa Y, Kobayashi H, Uchida Y. 2009. Acute encephalopathy caused by Bacillus cereus infection in a 5-year boy. J. Jpn. Pediatr. Soc. 113:75–78 (In Japanese, with abstract and tables in English.) [Google Scholar]
- 9. Naranjo M, Denayer S, Botteldoorn N, Delbrassinne L, Veys J, Waegenaere J, Sirtaine N, Driesen RB, Sipido KR, Mahillon J, Dierick K. 2011. Sudden death of a young adult associated with Bacillus cereus food poisoning. J. Clin. Microbiol. 49:4379–4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dierick K, Van Coillie E, Swiecicka I, Meyfroidt G, Devlieger H, Meulemans A, Hoedemaekers G, Fourie L, Heyndrickx M, Mahillon J. 2005. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 43:4277–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dirnhofer D, Sonnabend O, Sonnabend W. 1977. A fatal food poisoning by Bacillus cereus. Rechtsmedizin 80:139–151 (In German.) [DOI] [PubMed] [Google Scholar]
- 12. Takabe F, Oya M. 1976. An autopsy case of food poisoning associated with Bacillus cereus. Forensic Sci. 7:97–101 [DOI] [PubMed] [Google Scholar]
- 13. Temper K. 1963. Exitus Letalis nach Lebensmittelvergiftung durch Bacillus cereus. Z. Gesamte Hyg. Grenzgebiete 9:481–490 [Google Scholar]
- 14. Turnbull PCB, Nottingham JF, Ghosh AC. 1977. A severe necrotic enterotoxin produced by certain food, food poisoning, and other clinical isolates of Bacillus cereus. Br. J. Exp. Pathol. 58:273–280 [PMC free article] [PubMed] [Google Scholar]
- 15. Turnbull PCB, Kramer JM, Jörgensen Gilbert KRJ, Melling J. 1979. Properties and production characteristics of vomiting, diarrhea, and necrotizing toxins of Bacillus cereus. Am. J. Clin. Nutr. 32:219–228 [DOI] [PubMed] [Google Scholar]
- 16. Turnbull PCB. 1976. Studies on the production of enterotoxins by Bacillus cereus. J. Clin. Pathol. 29:941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ceuppens S, Rajkovic A, Heyndrickx M, Tsilia V, Van de Wiele T, Boon N, Uyttendaele M. 2011. Regulation of toxin production by Bacillus cereus and its food safety implications. Crit. Rev. Microbiol. 37:188–213 [DOI] [PubMed] [Google Scholar]
- 18. Messelhäusser U, Kämpf P, Fricker M, Ehling-Schulz M, Zucker R, Wagner B, Busch U, Höller C. 2010. Prevalence of emetic Bacillus cereus in different ice creams in Bavaria. J. Food Prot. 73:395–399 [DOI] [PubMed] [Google Scholar]
- 19. Hoton FM, Fornelos N, N′Guessan E, Hu X, Swiecicka I, Dierick K, Jääskeläinen E, Salkinoja-Salonen M, Mahillon J. 2009. Family portrait of Bacillus cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ. Microbiol. Rep. 1:177–183 [DOI] [PubMed] [Google Scholar]
- 20. Pirttijärvi TS, Andersson MA, Scoging AC, Salkinoja-Salonen MS. 1999. Evaluation of methods for recognizing strains of the Bacillus cereus group with food poisoning potential among industrial and environmental contaminants. Syst. Appl. Microbiol. 22:133–144 [DOI] [PubMed] [Google Scholar]
- 21. Shaheen R, Andersson MA, Apetroaie C, Schulz A, Ehling-Schulz M, Ollilainen VM, Salkinoja-Salonen MS. 2006. Potential of selected infant food formulas for production of Bacillus cereus emetic toxin, cereulide. Int. J. Food Microbiol. 107:287–294 [DOI] [PubMed] [Google Scholar]
- 22. Altayar M, Sutherland AD. 2006. Bacillus cereus is common in the environment but emetic toxin producing isolates are rare. J. Appl. Microbiol. 100:7–14 [DOI] [PubMed] [Google Scholar]
- 23. Ankolekar C, Rahmati T, Labbe RG. 2009. Detection of toxigenic Bacillus cereus and Bacillus thuringiensis spores in U.S. rice. Int. J. Food Microbiol. 128:460–466 [DOI] [PubMed] [Google Scholar]
- 24. Svensson B, Monthan A, Shaheen R, Andersson A, Salkinoja-Salonen M, Christiansson A. 2006. Occurrence of emetic toxin-producing Bacillus cereus in the dairy production chain. Int. Dairy J. 16:740–749 [Google Scholar]
- 25. Swiecicka I, Mahillon J. 2006. Diversity of commensal Bacillus cereus sensu lato isolated from the common sow bug (Porcellio scaber, Isopoda). FEMS Microbiol. Ecol. 56:132–140 [DOI] [PubMed] [Google Scholar]
- 26. Vassileva M, Torii K, Oshimoto M, Okamoto A, Agata N, Yamada K, Hasgawa T, Ohta M. 2007. A new phylogenetic cluster of cereulide producing Bacillus strains. J. Clin. Microbiol. 45:1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garbeva P, van Overbeek LS, van Vuurde JWL, van Elsas JD. 2001. Analysis of endophytic bacterial communities of potato by plating and denaturing gradient gel electrophoresis (DGGE) of 16S rDNA-based PCR fragments. Microb. Ecol. 41:369–383 [DOI] [PubMed] [Google Scholar]
- 28. Melnick RL, Testen AL, Poleatewich AM, Backman PA, Bailey BA. 2012. Detection and expression of enterotoxin genes in endophytic strains of Bacillus cereus. Lett. Appl. Microbiol. 54:468–474 [DOI] [PubMed] [Google Scholar]
- 29. Corratge-Faillie C, Jabnoune M, Zimmermann S, Very AA, Fizames C, Sentenac H. 2010. Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell. Mol. Life Sci. 67:2511–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leigh RA. 2001. Potassium homeostasis and membrane transport. J. Plant Nutr. Soil Sci. 164:193–198 [Google Scholar]
- 31. Rajkovic A, Uyttendaele M, Vermeulen A, Andjelkovic M, Fitz-James I, in't Veld P, Denon Q, Verhe R, Devebere J. 2008. Heat resistance of Bacillus cereus emetic toxin, cereulide. Lett. Appl. Microbiol. 46:536–541 [DOI] [PubMed] [Google Scholar]
- 32. Melling J, Capel BJ. 1978. Characteristics of Bacillus cereus emetic toxin. FEMS Microbiol. Lett. 4:133–135 [Google Scholar]
- 33. Mikkola R, Saris NE, Grigoriev PA, Andersson MA, Salkinoja-Salonen MS. 1999. Ionophoretic properties and mitochondrial effects of cereulide: the emetic toxin of Bacillus cereus. Eur. J. Biochem. 263:112–117 [DOI] [PubMed] [Google Scholar]
- 34. Teplova VV, Mikkola R, Tonshin AA, Saris NE, Salkinoja-Salonen MS. 2006. The higher toxicity of cereulide relative to valinomycin is due to its higher affinity for potassium at physiological plasma concentration. Toxicol. Appl. Pharmacol. 210:39–46 [DOI] [PubMed] [Google Scholar]
- 35. Makarasen A, Yoza K, Isobe M. 2009. Higher structure of cereulide, an emetic toxin from Bacillus cereus and special comparison with valinomycin, an antibiotic from Streptomyces fulvissimus. Chem. Asian J. 4:688–698 [DOI] [PubMed] [Google Scholar]
- 36. Ekman JV, Kruglov A, Andersson MA, Mikkola R, Raulio M, Salkinoja-Salonen MS. 2012. Cereulide produced by Bacillus cereus increases fitness of the producer organism in low potassium environment. Microbiology 158:1106–1116 [DOI] [PubMed] [Google Scholar]
- 37. Hallaksela AM, Väisänen O, Salkinoja-Salonen M. 1991. Identification of Bacillus species isolated from Picea abies by physiological tests, phage typing, and fatty acid analysis. Scand. J. Forest Res. 6:365–377 [Google Scholar]
- 38. Andersson MA, Jääskeläinen EL, Shaheen R, Pirhonen T, Wijnands LM, Salkinoja-Salonen MS. 2004. Sperm bioassay for rapid detection of cereulide producing Bacillus cereus in food and related environment. Int. J. Food Microbiol. 94:175–183 [DOI] [PubMed] [Google Scholar]
- 39. Smibert RM, Krieg NR. 1994. Phenotypic characterization, p 630 In Gerhardt P, Murray RGE, Wood WA, Krieg NR. (ed), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC [Google Scholar]
- 40. Rasimus S, Mikkola R, Andersson MA, Teplova VV, Venediktova N, Ek-Kommonen C, Salkinoja-Salonen M. 2012. Psychotolerant Paenibacillus tundrae from barley grains produces new cereulide-like depsipeptides, paenilide, and homopaenilide, highly toxic to mammalian cells. Appl. Environ. Microbiol. 78:3732–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Echard G. 1975. Chromosomal banding patterns and karyotype evolution in three pig kidney cell strains. Chromosoma (Berl.) 45:138–149 [DOI] [PubMed] [Google Scholar]
- 42. Bøyum A. 1968. Isolation of leucocytes from human blood: further observations on methylcellulose, dextran, and Ficoll as erythrocyte-aggregating agents. Scand. J. Clin. Lab. Invest. Suppl. 97:31–50 [PubMed] [Google Scholar]
- 43. Valmu L, Hilden TJ, van Willigen G, Gahmberg CG. 1999. Characterization of β2 (CD18) integrin phosphorylation in phorbol ester-activated T lymphocytes. Biochem. J. 339:119–125 [PMC free article] [PubMed] [Google Scholar]
- 44. Ihanus E, Uotila L, Toivanen A, Stefanidakis M, Bailly P, Cartron Gahmberg J-PCG. 2003. Characterization of ICAM-4 binding to the I domains of the CD11a/CD18 and CD11b/CD18 leukocyte integrins. Eur. J. Biochem. 270:1710–1723 [DOI] [PubMed] [Google Scholar]
- 45. Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. 1990. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127:126–132 [DOI] [PubMed] [Google Scholar]
- 47. Hoornstra D, Andersson MA, Mikkola R, Salkinoja-Salonen MS. 2003. A new method for in vitro detection of microbially produced mitochondrial toxins. Toxicol. In Vitro 17:745–751 [DOI] [PubMed] [Google Scholar]
- 48. Andersson MA, Hakulinen P, Honkalampi-Hämäläinen U, Hoornstra D, Lhuguenot JC, Mäki-Paakkanen J, Savolainen M, Severin I, Stammati AL, Turco L, Weber A, von Wright A, Zucco F, Salkinoja-Salonen M. 2007. Toxicological profile of cereulide, the Bacillus cereus emetic toxin, in functional assays with human, animal, and bacterial cells. Toxicon 49:351–367 [DOI] [PubMed] [Google Scholar]
- 49. Kruglov AG, Andersson MA, Mikkola R, Roivainen M, Kredics L, Saris NEL, Salkinoja-Salonen MS. 2009. Novel mycotoxin from Acremonium exuviarum is a powerful inhibitor of the mitochondrial respiratory chain complex III. Chem. Res. Toxicol. 22:565–573 [DOI] [PubMed] [Google Scholar]
- 50. Apetroaie C, Andersson MA, Spröer C, Tsitko I, Shaheen R, Jääskeläinen EL, Wijnands LM, Heikkilä R, Salkinoja-Salonen MS. 2005. Cereulide-producing strains of Bacillus cereus show diversity. Arch. Microbiol. 184:141–151 [DOI] [PubMed] [Google Scholar]
- 51. Apetroaie-Constantin C, Shaheen R, Andrup L, Smidt L, Rita H, Salkinoja-Salonen MS. 2008. Environment driven cereulide production by emetic strains of Bacillus cereus. Int. J. Food Microbiol. 127:60–67 [DOI] [PubMed] [Google Scholar]
- 52. Carlin F, Fricker M, Pielaat A, Heisterkamp S, Shaheen R, Salkinoja-Salonen M, Svensson B, Nguyen TH, Ehling-Schulz CM. 2006. Emetic toxin-producing strains of Bacillus cereus show distinct characteristics within the Bacillus cereus group. Int. J. Food Microbiol. 109:132–138 [DOI] [PubMed] [Google Scholar]
- 53. Häggblom MM, Apetroaie C, Andersson MA, Salkinoja-Salonen MS. 2002. Quantitative analysis of cereulide, the emetic toxin of Bacillus cereus, produced under various conditions. Appl. Environ. Microbiol. 68:2479–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shaheen R. 2009. Bacillus cereus spores and cereulide in food-borne illness. Ph.D thesis University of Helsinki, Department of Applied Chemistry and Microbiology, Helsinki, Finland [Google Scholar]
- 55. Anonymous 2004. Microbiology of food and animal feeding stuffs: horizontal method for the enumeration of a presumptive Bacillus cereus colony-count technique at 30°C. EN-ISO standard 7932, 3rd ed International Organisation for Standardization, Geneva, Switzerland [Google Scholar]
- 56. Tallent SM, Rhodehamel J, Harmon SM, Bennett R. 2012. Bacillus cereus in Food and Drug Administration bacteriological analytical manual, 8th ed, revision A. AOAC International, Gaithersburg, MD: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm070875.htm [Google Scholar]
- 57. Essen R, de Ruyter C, de Wit M. 2000. Massive food poisoning in Kotterbos at Almere. Infect. Bull. 11:205–207 (In Dutch.) [Google Scholar]
- 58. Rajkovic A, Uyttendaele M, Courtens T, Heyndrickx M, Debevere J. 2006. Prevalence and characterization of Bacillus cereus in vacuum packed potato puree. Int. J. Food Sci. Technol. 41:878–884 [Google Scholar]
- 59. Saris NEL, Andersson MA, Mikkola M, Andersson LC, Teplova VV, Grigoriev PA, Salkinoja-Salonen MS. 2009. Microbial toxins affect on mitochondrial survival by increasing mitochondrial uptake of K+. Toxicol. Ind. Health 25:441–446 [DOI] [PubMed] [Google Scholar]
- 60. Ceccarini L, Masetti M, Cavalli A, Recanatini M. 2012. Ion conduction through the hERG potassium channel. PLoS One doi: 10.1371/journal.pone.0049017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feske S, Skolnik EY, Prakriya M. 2012. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 12:532–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14:1583–1589 [DOI] [PubMed] [Google Scholar]
- 63. Qian J, Zhu L, Qiming L, Belevych N, Chen Q, Zhao F, Herness S, Quan N. 2012. Interleukin-1R3 mediates interleukin-1-induced potassium current increase through fast activation of Akt kinase. Proc. Natl. Acad. Sci. U. S. A. 109:12189–12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Unwin RJ, Luft FC, Shirley DG. 2011. Pathophysiology and management of hypokalemia: a clinical perspective. Nat. Rev. Nephrol. 7:75–84 [DOI] [PubMed] [Google Scholar]
- 65. Jääskeläinen EL, Häggblom-Andersson MM, Vanne MA, Salkinoja-Salonen L. 2003. Potential of Bacillus cereus for producing an emetic toxin, cereulide, in bakery products: quantitative analysis by chemical and biological methods. J. Food Prot. 66:1047–1054 [DOI] [PubMed] [Google Scholar]
- 66. Finlay WJJ, Logan NA, Sutherland AD. 1999. Semiautomated metabolic staining assay for Bacillus cereus emetic toxin. Appl. Environ. Microbiol. 65:1811–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Agata N, Ohta M, Yokoyama K. 2002. Production of Bacillus cereus emetic toxin (cereulide) in various foods. Int. J. Food Microbiol. 73:23–25 [DOI] [PubMed] [Google Scholar]
- 68. Pitchayawasin S, Isobe M, Kuse M, Franz T, Agata N, Ohta M. 2004. Molecular diversity of cereulide detected by means of nano-HPLC-ESI-Q-TOF-MS. Int. J. Mass Spectrom. 235:123–129 [Google Scholar]
- 69. Cantley J, Grey ST, Maxwell PH, Withers DJ. 2010. The hypoxia response pathway and β-cell function. Diabetes Obes. Metab. 2010(Suppl 2):159–167 [DOI] [PubMed] [Google Scholar]
- 70. Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. 2010. Regulation of insulin secretion: role of mitochondrial signaling. Diabetologia 53:1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Virtanen SM, Roivainen M, Andersson MA, Ylipaasto P, Hoornstra D, Mikkola R, Salkinoja-Salonen MS. 2008. In vitro toxicity of cereulide on porcine pancreatic Langerhans islets. Toxicon 51:1029–1037 [DOI] [PubMed] [Google Scholar]
- 72. Virtanen SM, Takkinen H-M, Nevalainen J, Kronberg-Kippilä C, Salmenhaara M, Uusitalo L, Kenward MG, Erkkola M, Veijola R, Simell O, Ilonen J, Knip M. 2011. Early introduction of root vegetables in infant associated with advanced β-cell autoimmunity in young children with human leukocyte antigen-conferred susceptibility to type 1 diabetes. Diabetic Med. 28:965–971 [DOI] [PubMed] [Google Scholar]
- 73. Rajkovic A, Uyttendaele M, Mbregt S-A, Jääskeläinen E, Salkinoja-Salonen M, Debevere J. 2006. Influence of type of food on the kinetics and overall production of Bacillus cereus emetic toxin. J. Food Prot. 69:847–852 [DOI] [PubMed] [Google Scholar]