Abstract
The objective of this study was to investigate a new potential photosensitizer (PS) in the photodynamic inactivation (PDI) of microorganisms in vitro (11 reference strains and 13 clinical isolates, representing common Gram-positive and Gram-negative human pathogens), with special emphasis on Candida albicans. We studied the light-induced cytotoxicity of the imidazoacridinone derivative C1330 toward fungal cells grown in planktonic form. We examined the influence of various parameters (time of incubation, PDI quencher effect, and C1330 accumulation in C. albicans cells) on the efficacy of light-dependent cytotoxicity. Additionally, we checked for the potential cyto- and phototoxic activity of C1330 against human dermal keratinocytes. In our research, we used a broadband incoherent blue light source (380 to 470 nm) with an output power of 100 mW/cm2. In vitro studies showed that the C1330 action against C. albicans was a light-dependent process. C1330 was an efficient photosensitizer in the photodynamic inactivation of C. albicans, which reduced the growth of planktonic cells by 6.1 log10 units. Efficient accumulation of PS in the nucleus and vacuoles was observed after 30 min of incubation, which correlated with the highest photokilling efficacy. Significant changes in intracellular structure were observed upon illumination of C1330-incubated C. albicans cells. In the case of the human HaCaT cell line, approximately 40% of cells survived the treatment, which indicates the potential benefit of further study of the application of C1330 in photoantimicrobial chemotherapy. These data suggest that PDI may be a viable approach for the treatment of localized C. albicans infections.
INTRODUCTION
Imidazoacridinones (IAs) (Table 1) are chemical compounds synthesized by J. Konopa and coworkers at the Technical University of Gdansk, Poland (1). Originally, the compounds were developed as broad-spectrum antitumor agents, expressing cytotoxicity against cancers of the colon, lung, and ovaries as well as gastric cancers, melanoma, and leukemia (2). Several compounds within this group are cytotoxic against fast-dividing cells; a much less cytotoxic effect was reported with respect to slowly dividing or confluent cells. Imidazoacridinones were shown to accumulate efficiently in the eukaryotic cell nucleus and to inhibit DNA synthesis (3). These compounds were shown to trap topoisomerase II cleavage complexes (4). Several biophysical characteristics of imidazoacridinones were described (5). It was also reported that in an in vitro transformation assay, which showed correlation with carcinogenicity in vivo, these compounds were devoid of any transforming activity (3). The most potent antineoplastic agent among imidazoacridinones, C1311, was in phase II clinical trials for the treatment of women with metastatic breast cancer. Imidazoacridinones manifest strong fluorescence (maximum emission at 500 to 540 nm) when excited with 420 to 440 nm of light. This particular characteristic prompted us to test a group of the imidazoacridinone derivatives as photosensitizers (PSs) using a photodynamic method.
Table 1.
General structure and physical properties of the tested IA derivativesa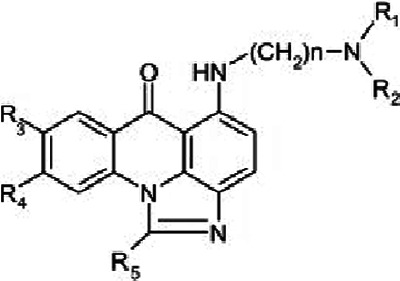
| IA derivative | R1,2 | R3 | R4 | R5 | Absorption (λmax) (nm) | Emission (λmax) (nm) |
|---|---|---|---|---|---|---|
| C1310 | Et | OH | H | Me | 428 | 534 |
| C1311 | Et | OH | H | H | 420 | 524 |
| C1330 | Et | OMe | H | H | 423 | 524 |
| C1371 | Me | OH | H | H | 429 | 533 |
| C1375 | Me | OMe | H | Me | 436 | 553 |
| C1379 | Et | OMe | H | Me | 437 | 554 |
| C1415 | Et | H | H | H | 420 | 520 |
| C1419 | Et | H | OH | H | 409 | 483 |
| C1492 | Me | OH | H | H | 439 | 534 |
| C1558 | Et | t-Butyl | H | H | 421 | 518 |
Based on data by Dziegielewski et al. (5). Shading indicates the compounds most active in photodynamic inactivation of C. albicans. Et, CH2CH3; Me, CH3; OMe, OCH3.
The photodynamic method is based on the concept that highly metabolically active cells (e.g., tumor cells or microbial cells) accumulate a photosensitizer, a small molecular compound which is excited upon exposure to a specific wavelength of light. In the oxygen-rich environment, either a surplus of energy or an electron is passed to an oxygen atom, giving rise to a highly reactive singlet oxygen as well as other reactive oxygen species (ROS). This action causes a cytotoxic effect on living cells (6). The most widely studied application of the photodynamic method is the killing of tumor cells (photodynamic therapy [PDT]), whereas in recent years, antimicrobial photodynamic inactivation (PDI) has become more popular as a method for effectively limiting the growth of microorganisms (7). Photodynamic inactivation of various microorganisms using different photosensitizers and different light sources has been described in the literature (8–11). Photodynamic inactivation has been further studied to demonstrate its effectiveness for the eradication of both Gram-negative and Gram-positive antibiotic-resistant bacteria. So far, induction of resistance to this form of treatment, which is very important from a clinical point of view, has not been demonstrated.
The majority of the human population are hosts for the harmless commensal yeast Candida albicans. It commonly colonizes the epithelial surfaces of the body as well as the oropharyngeal cavity and the vaginal tract, primary sites of mucosal colonization. Chronic exposure to excessive moisture is a predisposing factor for cutaneous candidiasis, and keratinized surfaces are also susceptible to infection. Additionally, in some cases, e.g., patients with immunodeficiency (AIDS and chemotherapy, etc.) or imbalance of the normal microbial flora, C. albicans can cause life-threatening systemic infections (12, 13). The rate of mortality caused by systemic fungal infections is approximately 40%, higher than that observed for any bacterial sepsis (14, 15). The fungus C. albicans grows as a biofilm on epithelial surfaces (16) and prosthetic devices, contributing to the failure of antifungal therapy and recurrent infection. Candida albicans, together with Staphylococcus aureus and Pseudomonas aeruginosa, is known to be involved in burn wound infections. The therapeutic options for treatment of such infections are limited, due mainly to increasing drug resistance.
Here, we present the results of experiments showing that a representative of the imidazoacridinones, namely, C1330, is a very active anti-C. albicans agent, and its mechanism of action involves a light-dependent pathway. Moreover, we present the microbicidal potential of the compound against other human pathogens, mainly S. aureus and P. aeruginosa.
MATERIALS AND METHODS
Microorganism strains.
Reference strains included Enterococcus faecium ATCC 27270, Acinetobacter baumannii ATCC 17978, Staphylococcus aureus Newman, Pseudomonas aeruginosa ATCC 10145, Streptococcus agalactiae ATCC 27956, Candida glabrata ATCC 2001, Candida albicans ATCC 2091, Klebsiella pneumoniae ATCC 2733, Escherichia coli ATCC 25922, Salmonella enterica serotype Anatum ATCC 9270, and C. albicans B311 (strain was provided by Kevin Francis, Calpier LifeSciences). Staphylococcus aureus clinical strains 472, 56/A5, 6347, 1397, and 2002 were isolated from infected wounds from patients of the Provincial Hospital in Gdansk, Poland. The isolates were characterized by Gram staining and their ability to produce coagulase and clumping factor by using Slidex Staph Plus (bioMérieux). Additionally, the species were identified by using the ID 32 Staph biochemical identification system (bioMérieux), Pseudomonas aeruginosa clinical strains K3 (blood isolate), AI, and AW (urine isolates) were obtained from Public Clinical Hospital Number 1 in Zabrze, Poland. The isolates were characterized by using a Vitek 2 identification system (bioMérieux). Candida albicans strains 2, 9, 151, 166, and 257 were isolated from nail infections from patients of the Department of Dermatology, Venereology and Allergology, Medical University of Gdansk. The yeasts were identified by microscopic examination, colony morphology on CHROMagar Candida (Becton, Dickinson), and the API ID 32C identification kit for yeasts (bioMérieux).
Photosensitizer and light source.
Imidazoacridinone (IA) derivatives (Table 1), obtained from A. Składanowski of Gdansk University of Technology, Poland, were dissolved in sterile dimethyl sulfoxide (DMSO) (Sigma-Aldrich) to obtain 10 mM and 1 mM stock solutions, which were kept at −20°C until use.
The light source used was the Q.Light 70 NT phototherapy system (Q.Products AG, Switzerland), with an output power of 100 mW, a fluence of 0.127 J/cm2 (energy of 50 J in a time of 396 s), and a filter which emits incoherent blue light (385 to 480 nm).
Photodynamic inactivation of planktonic culture.
Microorganisms were grown overnight in brain heart infusion (BHI) broth (bioMérieux) at 37°C with shaking (200 rpm), and a standardized suspension in BHI broth was prepared: a 5 McFarland standard (106 CFU/ml) for Candida spp. and a 0.5 McFarland standard (104 to 106 CFU/ml) for other microorganisms. The suspension (0.2 ml) was added to a sterile 96-well flat-bottom microtiter plate, and IA derivatives were added to obtain final concentrations of 10, 50, and 100 μM for Candida albicans B311 and 50 μM for the remaining microorganisms. The plate with samples was incubated in the dark at 37°C for 30 min without shaking, and 100 μl of the suspensions was transferred onto a new plate and irradiated with 50, 100, and 200 J/cm2 with shaking (400 rpm). The 100-μl suspension in the original plate was used as a control and kept for the time of irradiation in the dark at room temperature. After irradiation, serial dilutions from 10−1 to 10−6 were obtained from every sample in phosphate-buffered saline (PBS), and aliquots of 10 μl were streaked horizontally onto BHI agar plates to determine CFU. Plates were incubated overnight at 37°C, the CFU were counted, and the results were statistically analyzed.
According to the particular experiments, glycerol, sodium azide, and tryptophan were added at final concentrations of 300 mM, 1 mM, and 3.3 mM, respectively. The results of each experiment are presented as the means of at least three independent replicates with standard deviations of the means.
Fluorescence microscopy.
The cells were incubated with IA C1330 for 15 to 180 min, washed with PBS, transferred onto a microscope slide with a coverslip, and observed by using an Olympus BX51 fluorescence microscope with an F-View-II charge-coupled-device (CCD) camera. The excitation wavelength was 360 to 370 nm, and the emission wavelength was >420 nm. Measurements and image analysis were conducted by using AnalySIS software.
Cell culture.
The human keratinocyte (HaCaT) cell line was cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units ml/liter penicillin, and 100 μg/ml streptomycin. Cultures were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Cyto- and phototoxicity of C1330.
Cells (3 × 104) were seeded into 96-well plates and allowed to adhere overnight. C1330 was examined at the following concentrations: 1 μM, 10 μM, and 50 μM. Compounds were added to the medium, and cells to be used for cyto- and phototoxicity analyses were incubated for 30 min at 37°C in the dark. Prior to irradiation, the medium was replaced with new medium. Irradiation was performed by using the Q.Light 70 NT phototherapy system. Cells for both the cyto- and phototoxicity analyses were incubated for 24 h, and cell survival was determined with an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Briefly, MTT (0.5 mg/ml) was added, and cells were incubated for 3 h at 37°C. Cells were lysed with DMSO, and the absorbance of the formazan solution was measured at 550 nm with a plate reader (Victor 1420 multilabel counter).
Statistical analysis.
Each experiment was performed at least in triplicate. All primary data are presented as means with standard deviations of the means. Statistical analysis was performed with one-way analysis of variance (ANOVA) with the Tukey post hoc test. Hypotheses were tested at a significant level of a P value of 0.05. All analysis were performed by using STATISTICA version 8.0 software (2008; StatSoft Inc., Tulsa, OK).
RESULTS
Screening of IA derivatives with respect to their phototoxicity against C. albicans.
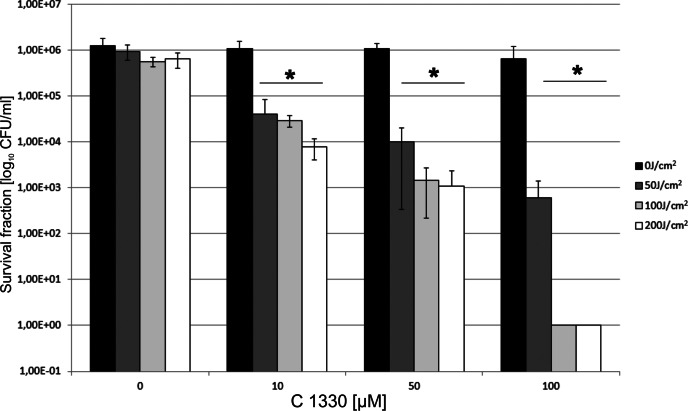
In our initial experiment, we screened 10 various randomly chosen IA derivatives (Table 1). We chose those IA molecules for which some biophysical and biological data were known. As there have so far been no data on the light-dependent microbicidal action of the compounds, we were interested in whether this is a general feature of this group of molecules. We tested their potential phototoxic effect on C. albicans, as there is a limited number of effective antifungal therapies for this species, and some evidence indicated that this particular species was the most resistant to phthalocyanine-based PDI among various Candida species (17). We used two types of controls: cells incubated with IA without irradiation (dark control) and cells subjected to light without IA. None of the agents studied showed dark toxicity. Some representatives of the screened IA group, namely, C1330, C1415, and C1558, were very active antimicrobial agents, expressing their mechanism of action through a light-dependent pathway (Table 2). The most pronounced phototoxic activity was observed in the case of the C1330 compound (reduction in survival of 6.1 log10 units). For the latter two compounds, reductions of 3.6 and 2.9 log10 units were noticed for a concentration of 100 μM IA and a light dose of 200 J/cm2. Imidazoacridinone C1330 has an excitation maximum of 420 nm, and photodynamic inactivation was performed at a wavelength range of 385 to 480 nm (Table 1). It was clearly demonstrated that with the range of C1330 concentrations tested (0 to 100 μM), no dark toxicity was observed (Fig. 1). The same was true for samples that were treated with increasing light doses in the absence of C1330. The fungicidal effect was observed only for samples where C1330 was combined with illumination. The observed effect was dependent on both concentrations of C1330 in the range of 0 to 100 μM and light doses in the range of 0 to 200 J/cm2. A fungicidal effect of a 3.1-log10 drop in the surviving fraction (99.9%) was observed at 50 μM C1330 and a light dose of 200 J/cm2. An increase of the concentration of C1330 to 100 μM resulted in a 6.1-log10 reduction in the number of surviving C. albicans cells under our experimental conditions (Fig. 1).
Table 2.
Cyto- and phototoxic effects of IA derivatives on C. albicans planktonic cultures
| IA derivative | Light dose (J/cm2) | Log10 reduction in survival at IA concn (μM) ofa: |
|||
|---|---|---|---|---|---|
| 0 | 10 | 50 | 100 | ||
| C1311 | 0 | 0.0 | 0.0 | 0.1 | 0.0 |
| 100 | 0.2 | 0.2 | 0.2 | 0.2 | |
| 200 | 0.1 | 0.3 | 0.3 | 0.3 | |
| C1330 | 0 | 0.0 | 0.1 | 0.1 | 0.3 |
| 100 | 0.3 | 1.6 | 2.9 | 6.1 | |
| 200 | 0.3 | 2.2 | 3.1 | 6.1 | |
| C1375 | 0 | 0.0 | 0.1 | 0.1 | 0.3 |
| 100 | 0.3 | 0.3 | 0.3 | 0.3 | |
| 200 | 0.1 | 0.2 | 0.3 | 0.2 | |
| C1415 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 100 | 0.2 | 0.7 | 1.6 | 1.7 | |
| 200 | 0.0 | 1.0 | 1.9 | 3.6 | |
| C1558 | 0 | 0.0 | 0.2 | 0.3 | 0.3 |
| 100 | 0.2 | 0.6 | 1.1 | 2.1 | |
| 200 | 0.2 | 0.4 | 0.3 | 2.9 | |
The values were calculated by subtracting log10 CFU/ml of tested samples from those of untreated controls (0 J/cm2; 0 μM IA). Shading indicates dark toxicity (samples treated with PS in the dark). Underlined values indicate noticeable phototoxicity.
Fig 1.
Effect of different concentrations of C1330 and different light doses on C. albicans planktonic cultures. Two types of controls were present: cells kept in the dark (0 J/cm2) with increasing concentrations of C1330 and cells irradiated with increasing light doses without the presence of C1330. *, significant at the level of a P value of <0.05.
Only phototoxic IA derivatives accumulate in C. albicans cells.
As the accumulation of a PS is the critical element of an efficient photodynamic process, we used fluorescence microscopy in order to observe IA derivative accumulation in C. albicans cells. Efficient accumulation of IA was observed only in the case of the three derivatives that caused a growth reduction of C. albicans, namely, C1330, C1415, and C1558 (Fig. 2). The phototoxic effect of IA derivatives was in accordance with the accumulation of the compounds in C. albicans cells. No cyto- or phototoxic effect was observed in the case of the IA derivatives which did not accumulate in the cell.
Fig 2.
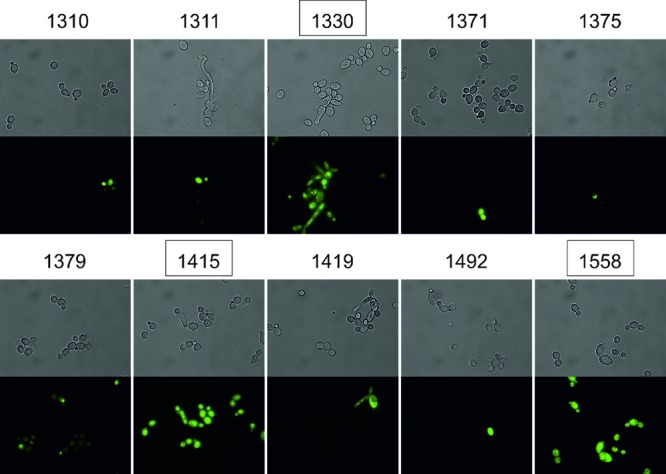
Accumulation of IA derivatives in C. albicans cells. The compounds showing phototoxic activity are boxed. Top panels represent light microscopy pictures, and bottom panels represent pictures taken with the use of filters (excitation at 360 to 370 nm and emission at >420 nm).
Imidazoacridinone C1330 is more active against Gram-positive species.
As C1330 was the most effective compound against C. albicans, we further tested it on other microorganisms in order to check its potential action against other microbial species. To verify that the observed effects were not dependent on the isolates, thus limiting the potential usefulness of this work, we applied a larger population of strains in our analysis. We chose Gram-positive representatives of human pathogens, namely, Staphylococcus aureus (1 reference strain and 5 clinical isolates), the Gram-negative organism Pseudomonas aeruginosa (1 reference strain and 3 clinical isolates), C. albicans (1 reference strain and 5 clinical isolates), as well as representatives of other common human pathogens (Table 3). The tested microorganisms (Table 3) were treated by using the same methodology as that described above, with an IA concentration of 50 μM and light doses of 100 and 200 J/cm2. In the case of fungi and Gram-positive bacteria, the reduction of survival was >3 log10 CFU. According to the CLSI standard (47), such a result entitles C1330 to be recognized as an effective antimicrobial agent, as a minimum of a 3-log10 reduction in CFU/ml is biologically relevant. For Gram-negative bacteria, the phototoxic effect in the case of P. aeruginosa was 3 log10 units for the reference strain and >4 log10 units for clinical isolates, and in the case of the remaining Gram-negative bacteria, it was lower and accounted for at most a 1.5-log10 reduction in survival (Table 3). It is known that Gram-negative bacteria are more resistant to photodynamic therapy than Gram-positive species (18). Previous studies by Hamblin's group have shown that even a 10-times-higher concentration of an antimicrobial is needed to inactivate Gram-negative than Gram-positive microorganisms (19, 20).
Table 3.
Phototoxic effects of 50 μM C1330 on microorganisms
| Microorganism | Light dose (J/cm2) | Mean reduction of survivala (log10 CFU) ± SD |
|---|---|---|
| Candida albicans ATCC 2091 | 100 | 2 ± 0.45 |
| Candida albicans B311 | 200 | 3.1 ± 0.01 |
| Candida albicans clinical strain 2 | 100 | 4 ± 0.06 |
| Candida albicans clinical strain 9 | 100 | 2 ± 0.36 |
| Candida albicans clinical strain 151 | 200 | 4 ± 0.01 |
| Candida albicans clinical strain 166 | 100 | 6 ± 0.01 |
| Candida albicans clinical strain 257 | 100 | 4 ± 0.4 |
| Candida glabrata ATCC 2001 | 100 | 4.5 ± 0.3 |
| Staphylococcus aureus Newman | 100 | 5 ± 0.01 |
| Staphylococcus aureus clinical strain 472 | 200 | 2 ± 0.2 |
| Staphylococcus aureus clinical strain 56/A5 | 100 | 3.2 ± 0,17 |
| Staphylococcus aureus clinical strain 6347 | 100 | 4.5 ± 0.01 |
| Staphylococcus aureus clinical strain 1397 | 100 | 4.5 ± 0.11 |
| Staphylococcus aureus clinical strain 2002 | 100 | 5 ± 0.01 |
| Enterococcus faecium ATCC 27270 | 100 | 4.5 ± 0.11 |
| Streptococcus agalactiae ATCC 27956 | 100 | 4 ± 0.01 |
| Pseudomonas aeruginosa ATCC 10145 | 200 | 3 ± 0.1 |
| Pseudomonas aeruginosa clinical strain K3 | 200 | 4 ± 0.1 |
| Pseudomonas aeruginosa clinical strain AI | 200 | 5 ± 0.02 |
| Pseudomonas aeruginosa clinical strain AW | 200 | 4 ± 0.06 |
| Salmonella enterica serotype Anatum ATCC 9270 | 200 | 0.5 ± 0.12 |
| Klebsiella pneumoniae ATCC 2733 | 200 | 0.9 ± 0.07 |
| Acinetobacter baumannii ATCC 17978 | 100 | 1 ± 0.2 |
| Escherichia coli ATCC 25922 | 200 | 1.5 ± 0.15 |
The values were calculated by subtracting log10 CFU/ml of tested samples from those of untreated controls (0 J/cm2; 0 μM IA). At least three biological replicates were used for calculation of the mean reduction values.
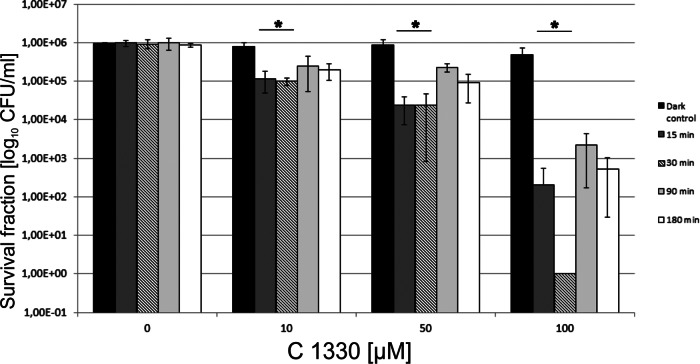
Incubation time influences the efficacy of the light-dependent action of C1330.
To evaluate the influence of incubation time on the PDI effect, we performed PDI experiments in a C1330 concentration-dependent manner, but the C. albicans cells were treated with a single light dose of 100 J/cm2. The overall protocol for this particular experiment was the same as that for the PDI studies, with the exception of the time of incubation. Cells were incubated with 10, 50, and 100 μM C1330 in the dark for the following time periods: 15, 30, 90, and 180 min. The maximum effect of PDI on C. albicans planktonic forms was achieved when the preirradiation time (PIT) was 30 min (the maximum reduction was 6 log10 CFU/ml) (Fig. 3). When the PIT was 15 min, a fungicidal effect was also observed, but the reduction was 2-fold lower and accounted for 3.1 log10 units. In the case of PITs of 90 and 180 min, the maximum reductions in CFU/ml were 2 and 2.5 log10 units, respectively. Based on the results obtained, one can conclude that time of incubation is a critical factor in PDI of C. albicans.
Fig 3.
Time of incubation with C1330 affects PDI outcome of C. albicans planktonic cultures. Time of incubation with C1330 in the dark is indicated in the key. After incubation, cells were subjected to PDI treatment (10, 50, and 100 μM C1330 and a light dose of 100 J/cm2). Two types of controls were present: cells kept in the dark with increasing concentrations of C1330 and cells irradiated with increasing light doses without the presence of C1330. *, significant at the level of a P value of <0.05.
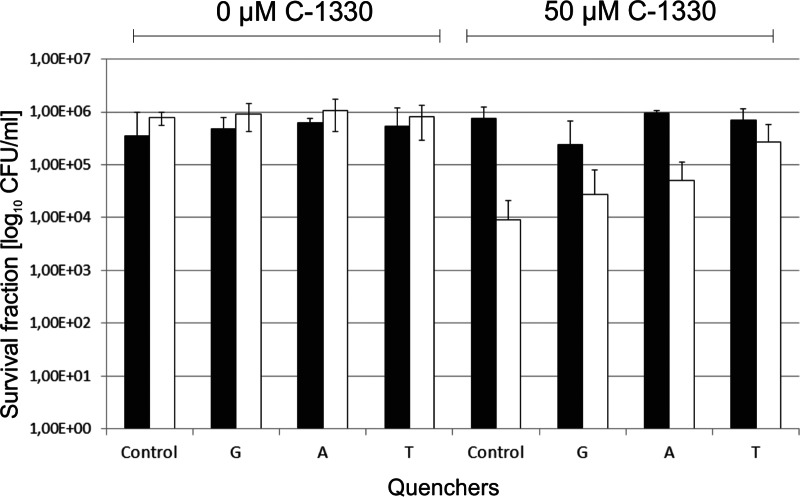
Application of various reactive oxygen species quenchers increases the survival of C. albicans cells after PDI treatment.
To check whether particular reactive oxygen species play a role in C1330-based PDI treatment, we applied several types of ROS quenchers. Glycerol, sodium azide, and tryptophan were used at concentrations of 300 mM, 1 mM, and 3.3 mM, respectively. Each of the studied quenchers was added to the cell suspension together with C1330. The addition of glycerol and sodium azide increased the surviving fraction by 0.5 log10 CFU/ml, whereas the addition of tryptophan increased this number by 1 log10 unit compared to the value for PDI-treated cells without any quenchers added (Fig. 4). Azide ions and glycerol are quenchers of PDI mechanism types II and I, respectively, while tryptophan is a quencher for both types of PDI reactions. The results presented indicate that in the case of C1330, there are two PDI mechanisms that act simultaneously.
Fig 4.
Photodynamic inactivation of C. albicans treated with 0 or 50 μM C1330 and a light dose of 0 J/cm2 (black bars) or 50 J/cm2 (white bars) in the presence of ROS quenchers (glycerol [G], sodium azide [A], and tryptophan [T]).
Imidazoacridinone efficiently accumulates in C. albicans cells.
The process of PS accumulation in the cell is a critical factor for efficient photokilling of a microorganism. To check if C. albicans accumulates IA molecules in a time-dependent manner, we incubated the cells in the dark for 15, 30, 90, and 180 min and further observed IA fluorescence under a fluorescence microscope. Based on the results obtained, it can be clearly seen that C1330 efficiently enters yeast cells. At the moment, the mechanism by which the analyzed molecules enter the cells cannot be elucidated. However, one can notice that the efficiency depends on both the IA concentration as well as the time of incubation. With 50 μM PS, the IA-based fluorescence intensity was lower than when a concentration of 100 μM used. This can be observed by both fluorescence photographs of C1330-treated C. albicans cells as well as direct fluorescence measurements of C. albicans cells in suspensions (Fig. 5A and B). The second pattern noticeable from the results is that incubation times of 15 min and 30 min resulted in more intense IA-based fluorescence in C. albicans cells when cells were treated with 100 μM IA. When a concentration lower than 50 μM was used for IA, this relationship was true only for an incubation time of 15 min (Fig. 5B). Based on the observed effects, we assume that C1330 enters the cells, and in the first stage (15 min), we observed an intense fluorescent signal dispersed throughout the cell (Fig. 5A). In the next stage (30 min), C1330 accumulated more efficiently in cellular structures, preferentially in the nucleus, which was clearly observed at a C1330 concentration of 50 μM (Fig. 5A). At C1330 concentrations higher than 100 μM, other structures also accumulated with the compound, most probably those containing DNA. After 90 and 180 min of incubation in the dark, C1330 was again dispersed throughout the cell, and the observed fluorescence was less intense. This observation also correlates with direct fluorescence measurements of C. albicans-bound C1330 in suspension (Fig. 5B).
Fig 5.
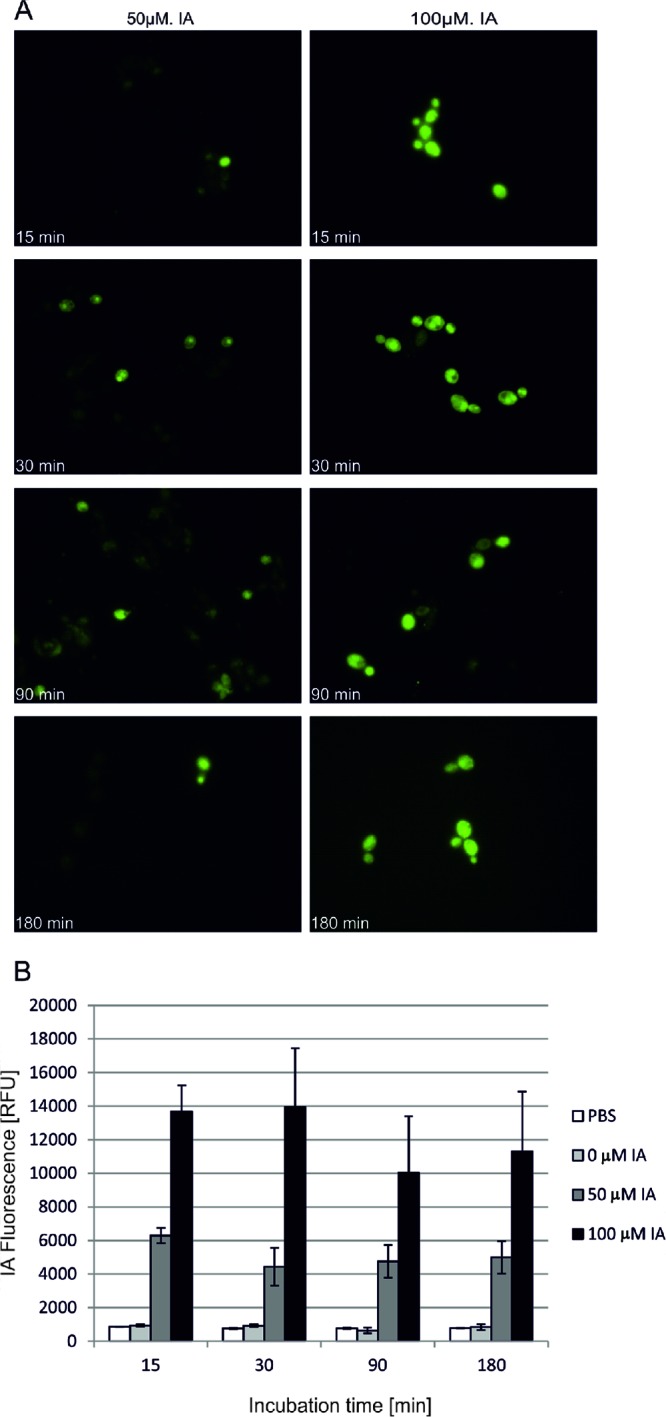
Accumulation of C1330 in C. albicans strains. Cells were incubated in the dark with a C1330 concentration of 50 or 100 μM at 37°C. At the time intervals indicated, cells were taken up and C1330 fluorescence was observed (excitation wavelength of 360 to 370 nm and emission wavelength of >420 nm) under a fluorescence microscope (A), or the cells were washed and resuspended in PBS prior to fluorescence measurements in a microplate reader (excitation wavelength of 405 nm and emission wavelength of 525 to 551 nm) (B). RFU, relative fluorescence units.
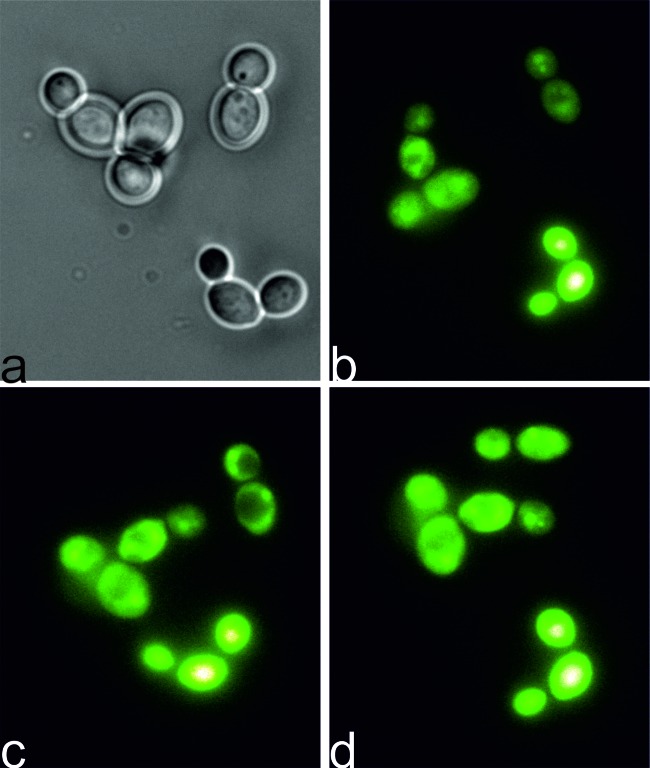
As IA derivatives express toxicity through light-dependent mechanisms, we were interested in what happens to C1330-incubated C. albicans cells when they are exposed to light. We applied a fluorescence microscopy technique in order to observe possible changes in fungal intracellular structures upon microscope light exposure. We therefore incubated the cells in the dark in the presence of IA C1330 (100 μM) for 30 min and further placed the cells on a glass slide and exposed them to 360 to 370 nm of light for another 30 min. At particular times, pictures were taken to visualize the dramatic changes in the C. albicans cells upon increasing light doses (Fig. 6). Following 15 min of microscopic light exposure (Fig. 6c), changes in the integrity of cellular structures were observed, compared to what was observed with 5 min of light exposure (Fig. 6b). Regular nonfluorescing round shapes were seen in the cells, probably representing vacuoles (Fig. 6c). In the next step (20 min), vacuoles were destroyed, which was manifested by a redispersing of the fluorescing signal throughout the cells (Fig. 6d).
Fig 6.
PDI effect on C. albicans cells during illumination. Cells were incubated in the dark with IA C1330 (100 μM at 37°C). After this, cells were observed under a fluorescence microscope for 30 min. At particular times, pictures were taken at time zero, with white light, directly after incubation in the dark (a) and 5 min (b), 15 min (c), and 20 min (d) after illumination. Panels b to d represent IA fluorescence (excitation wavelength of 360 to 370 nm and emission wavelength of >420 nm).
C1330 causes moderate cyto- and phototoxic effects on human keratinocytes.
To assess the clinical potential of C1330 as an anti-C. albicans agent, we applied a cytotoxicity and phototoxicity assay. The fraction of surviving cells of the human HaCaT cell line after C1330 accumulation and light treatment was determined. Eukaryotic cells were exposed to similar photodynamic treatment conditions as microbial cells. When HaCaT cells were incubated with 50 μM C1330 and exposed to a light dose of 50, 100, or 200 J/cm2, approximately 43% of cells survived the treatment. At this particular concentration, the surviving fraction was independent of the light dose applied. Under the same conditions (50 μM C1330 with a light dose of 50, 100, or 200 J/cm2), the C. albicans cell numbers were reduced by 2, 2.9, and 3.1 log10 units for the three light doses applied, respectively (Fig. 7). This result points out that C1330 may potentially be used as a candidate photosensitizer in the light-dependent killing of C. albicans cells.
Fig 7.
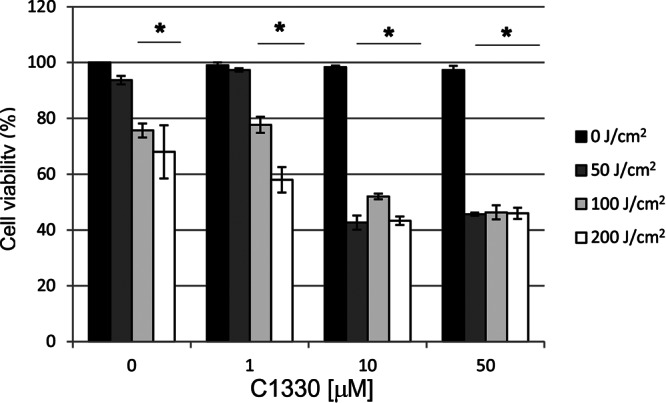
Cytotoxic and phototoxic effect of C1330 on the human HaCaT cell line. Two types of controls were present: cells kept in the dark (0 J/cm2) with increasing concentrations of C1330 and cells irradiated with increasing light doses without the presence of C1330. Cell survival was measured with the use of the MTT assay. *, significant at the level of a P value of <0.05.
DISCUSSION
Imidazoacridinone-based photodynamic inactivation.
Candida species are recognized as the main opportunistic fungi causing invasive mycoses in humans. This is caused by both the increasing number of immunocompromised patients as well as selection of fungicide-resistant strains (21). PDI treatment proved to be an efficient method for eradication of fungi independently of their chemoresistance pattern (22). Several attempts to investigate the possibilities of clinical application of PDI against local candidiasis have been undertaken (23, 24). Photodynamic therapy was proposed for the treatment of buccal candidiasis in rats (9, 25). In antifungal photodynamic chemotherapy, three main groups of photosensitizers are used, namely, phenothiazine dyes (e.g., methylene blue [MB] and toluidine blue O [TBO]), porphyrins (e.g., hematoporphyrin derivative [HpD] and Photogem), and phthalocyanines (e.g., Pc4 and RPL068) (Table 4). Here, we present data concerning a novel representative of a group of photosensitizing agents that so far have been studied as anticancer drugs exhibiting their mechanism of action through DNA binding and trapping the DNA-topoisomerase II-cleavable complexes. It was only recently found that the compounds from the imidazoacridinone group, exemplified by C1330, are also able to act through the light-dependent lysosome-destructive pathway, thus overcoming the MDR (multidrug resistance) phenotype in several cancer cell lines (26). This light-dependent action encouraged us to study the potential fungicidal action of imidazoacridinone agents against C. albicans in a light-dependent way. Under our experimental conditions, we checked whether one of the IA derivatives, namely, C1330 (Table 1), expresses a cytotoxic effect in the dark as well as potential phototoxicity toward C. albicans cells. The light-dependent cytotoxicity of C1330 toward C. albicans cells was efficient and accounted for a 2.2- or 3.1-log10 reduction in CFU/ml of the studied cells when a 200-J/cm2 light dose was applied and the PS concentrations were 10 and 50 μM, respectively (Fig. 1). However, even a 6.1-log10 reduction was possible at a higher PS concentration. We claim that such a result is very satisfactory and comparable to the results obtained for other groups of PSs used in antimicrobial PDI or is even better, assuming that the light source used under our experimental conditions is a broadband incoherent light (385 to 480 nm) (Table 1). Currently, research concerning antimicrobial photoinactivation employs mostly light-emitting diodes (LEDs) or lasers as light sources. One specific wavelength being in accordance with a maximum absorption of a PS results in much more efficient excitation of a particular PS than with broadband light, where only energy resulting from a particular wavelength within the spectrum is efficient in PS excitation. This explains why we used quite high light doses (e.g., 200 J/cm2) under our experimental conditions. When we compared a range of PS concentrations used in various in vitro experiments (Table 4), we observed that the IA concentration under our experimental conditions was within the range of concentrations of phenothiazine-based dyes in photodynamic inactivation studies (0.027 to 320 μM); however, the efficiencies of light-dependent killing are more similar to porphyrin-based PDI efficiencies (Table 4).
Table 4.
Comparison of the photokilling efficiencies with the use of three groups of photosensitizersa
| PS group | Photosensitizer(s) | Photosensitizer concn | Log10 reduction in survival | Wavelength(s) (nm), light source | Light dose (J/cm2) | Reference |
|---|---|---|---|---|---|---|
| Phthalocyanine-based PS | ZnPcM, ZnPcS | 6 μM | 4–7 | 675, laser | 12–60 | 32 |
| BAM-SiPc | 0.0025–0.08 μM | 0.9 | 675, laser | 12 | 33 | |
| GaPcs | 3 μM | 4–5 | 635, LED | 50 | 34 | |
| Pc4 | 1 μM | 4–5 | 670–675, LED | 2 | 35 | |
| SiPc1, GePc1 | 1.8 μM | Eradication | 635, LED | 50 | 36 | |
| Porphyrin-based PS | Photofrin | 1 μg/ml | 400–700, Hg lamp | 9 | 37 | |
| PEI-ce6 | 1–10 μM | 4–6 | 665, laser | 16 | 38 | |
| TFAP(3+), TMAP(4+), TPPS(4−) | 1–5 μM | 5 | 300–800 | 162 | 39 | |
| Photofrin | 25 μg/ml | 630, laser | 45–135 | 40 | ||
| TMpyP | 5 μM | 5 | 300–800 | 162 | 41 | |
| Photogem | 50 μg/ml | 1.79–3.99 | 37.5 | 42 | ||
| SSO-P | Visible | 300 | 43 | |||
| Phenothiazine-based PS | TBO | 50 μM | 7 | 620–650, noncoherent | 10–20 | 20 |
| MB | 0.027 μM | 0.9 | 683, laser | 28 | 44 | |
| TBO, MB, MG | 320, 310, 270 μM | 3, 3, 2.5 | 660, laser | 39.5 | 45 | |
| TBO, MB, NMB | 20 μM | 0, 0, 4.43 | 635, 660, noncoherent | 10 | 9 | |
| TBO, MB | 320, 310 μM | 685 | 53 | 46 | ||
| Imidazoacridinone | C1330 | 100 μM | 6 | 385–480, noncoherent | 100 | This work |
ZnPcM, tetrakis-(3-methylpyridyloxy)phthalocyanine zinc(II); ZnPcS, tetrakis-(4-sulfophenoxy)phthalocyanine zinc(II); BAM-SiPc, silicon(IV) phthalocyanine; GaPcs, gallium(III) phthalocyanine; PEI-ce6, polyethyleneimine and chlorin(e6) conjugate; TFAP(3+), 5-(4-trifluorophenyl)-10,15,20-tris(4-trimethylammoniumphenyl)porphyrin iodide; TMAP(4+), 5,10,15,20-tetra(4-N,N,N-trimethylammonium phenyl)porphyrin p-tosylate; TPPS(4−), 5,10,15,20-tetra(4-sulfonatophenyl)porphyrin; TMpyP, 5,10,15,20-tetra(4-N-methylpyridyl)porphyrin; SSO-P, 5-(4-carboxyphenyl)-10,15,20-tris(4-methylphenyl)porphyrin conjugated with polysilsesquioxane; MG, malachite green; NMB, new methylene blue.
Mechanism of C1330 action in C. albicans cells.
Photosensitizer accumulation in microbial cells is a prerequisite for efficient photodynamic action after light exposure. We observed that only those IA derivatives that entered fungal cells showed a light-dependent phototoxic effect. The photosensitizer either binds to its cell wall-associated structures (e.g., membrane in the case of porphyrin PS) or enters the cells and accumulates in particular intracellular fractions. After light exposure, PS excitation takes place to its long-lived triplet state. Next, in a type I reaction, an electron is transferred to the nearest-located substrate (e.g., unsaturated lipids in a membrane) and further to oxygen (O2). Alternatively, in the type II pathway, the energy is transferred directly to O2, thus producing a singlet oxygen, a molecule against which there is no detoxifying enzyme known. For porphyrin- and phenothiazine-based PSs, it is generally accepted that they act mainly via singlet oxygen formation; however, O2− (superoxide anion) and OH (hydroxyl radical) are also implicated (27, 28). Bacteriochlorin a (BCA), a structural analog of protoporphyrin IX, was shown to be a 50%/50% type I/type II photosensitizer (28). Our experiments concerning survival of C. albicans after PDI in the presence of type I (glycerol) and type II (sodium azide) or mixed (type I/type II) quenchers pointed out that C1330-based PDI acted via both mechanism types (Fig. 4). However, we observed only a trend, and more detailed analyses are needed to estimate an input of a particular mechanism type into the overall outcome of C1330-based PDI. Explanation of a particular photosensitizer with respect to its mode of photodynamic action (type I or type II) is important in the sense that a combination with various quenching agents may enhance the efficacy of the photodynamic process.
Imidazoacridinones are planar structures which interact with DNA in vitro (5). Imidazoacridinones accumulate effectively in lysosomes of tumor cells, due to their weak base nature. Alkalization of the cells with bafilomycin or ammonium chloride abolished selective accumulation of IA in cell lysosomes and promoted accumulation in the nucleus (26). Under our experimental conditions, we observed that in C. albicans cells, C1330 accumulated efficiently in the nucleus. This accumulation was observed after approximately 30 min of incubation in the dark at 50 μM (Fig. 6). The cytotoxic activity of C1336, another IA derivative studied for its action against cancer cell lines, correlated with inhibition of DNA synthesis (3). In our case, DNA binding may be important for the phototoxic action against C. albicans, as we observed C1330 accumulation in the nuclear fraction; however, it is not the only factor responsible for cellular death. At the higher C1330 concentrations used, when it also accumulated in other cellular structures, photokilling was most effective after application of the appropriate light dose. Intercalation of IA derivatives to DNA was shown to be reversible (5). We observed that when a prolonged incubation of C. albicans cells with C1330 was applied, the photokilling efficacy decreased (Fig. 3). This remains in accordance with the lowered fluorescence intensity of C1330 accumulated in the nucleus after 90 min versus 30 min (Fig. 5). The results obtained point out that accumulation of C1330 in particular structures is crucial for the effective photokilling of C. albicans cells. In accordance with observations made by others (3, 26, 29), we observed that the nucleus is not the only structure where C1330 accumulates (30 min at 100 μM) (Fig. 6A). The compound is also probably directed to vacuoles, which are analogs of higher-eukaryotic lysosomes in yeasts, responsible for cell detoxification and characterized by low pH. At this point of analysis, one can only speculate that nuclear accumulation of C1330 occurs first and that, at higher concentrations, “the surplus” of the compound then accumulates in the vacuoles. In the case of photodynamic inactivation, cellular toxicity depends on both the photosensitizer concentration as well as the light dose applied. Under our experimental conditions, we clearly saw PS concentration dependence with relation to photokilling efficacy (Fig. 1). Less pronounced was the light-dependent action of IA C1330. This may result from the limitations of our equipment, which is a halogen lamp with an output power of 100 mW and a fluence of 0.127 J/cm2. To achieve a 100-J light dose, we had to illuminate the cells for 13 min; a 200-J light dose application requires 26 min of illumination. However, the C1330 efflux from C. albicans cells is very fast, and after several minutes, it drops back to the background value (data not shown). Therefore, it is a challenging task to apply the most optimal light dose for an appropriate duration. Once the active compound is accumulated in an intracellular structure, the effect of the same total light dose applied in a short time, in contrast to when it is applied for a long time, may result in a different efficacy of photokilling of C. albicans cells. Additionally, manipulation of the IA incubation time together with the total light dose application time may be an important issue in lowering C1330 cytotoxicity toward human cells. This may further result in optimization of PDI of C. albicans in in vivo models. The question of photosensitizer toxicity to healthy human cells is of great importance in situations where the photodynamic method is proposed as a treatment option for microbial infections. Here, we present data indicating that 43% of HaCaT cells survived PDI treatment conditions in which cell numbers of various C. albicans isolates, S. aureus isolates, as well as P. aeruginosa strains were reduced by >3 log10 units. In the case of C. albicans, for 2 out of 7 isolates analyzed, we observed a reduction of only 2 log10 units (Table 3); we must remember, however, that the starting inocula in our experiments were higher than the ones used in standardized methodologies for MIC determinations (106 CFU versus 104 CFU), which may influence the efficacy of photodynamic treatment. We should also remember that PDI is almost from its definition a localized treatment; therefore, the approach to human cell toxicity is slightly different from, e.g., systemic treatment. In the literature, a 78.9% reduction in viable fibroblasts was shown after porphyrin-based PDI treatment, and under the same conditions, 99.999% of S. aureus cells were killed. This was further interpreted as acceptable and not causing too much collateral damage (30). It is worth mentioning that in our PDI experiments, we incubated and illuminated cells under protein-rich conditions, thus limiting the efficacy of the PDI outcome. Our intent was to reflect the conditions in the body (e.g., skin). In a similar study, Lambrechts et al. observed a increasing protein dose-dependent protective action against the PDI directed toward C. albicans. The strongest protection was observed for C. albicans suspended in plasma or in 4.5% albumin. Under such conditions, either no significant reduction in viable counts was observed for C. albicans or the reduction was smaller than 1 log10 unit, respectively (31). Dark toxicity toward human cells in our experimental model was not observed, in contrast to previously published results, where some dark toxicity was noticed. Cells incubated with C1330 at a concentration of 10 μM, upon illumination, decreased their survival to 30% and 50% of the controls (nonilluminated cells) for the human cancer cell line A549 and its multidrug resistance phenotype counterpart, A549/K1.5 (26). Under our experimental conditions, the studied HaCaT cell line survived the dark treatment, even at a C1330 concentration of 50 μM (Fig. 7). The lack of dark toxicity in our model of noncancerous healthy keratinocytes may be connected to differences in metabolic pathways of healthy versus cancer cells.
In summary, we conclude that C1330 is a potentially effective photosensitizer molecule with light-dependent photodynamic action against C. albicans, S. aureus, and P. aeruginosa. In the future, we plan to use the IA structure for rational design of effective PSs characterized by increased selectivity for microbial cells.
ACKNOWLEDGMENTS
This work was supported by a University of Gdansk grant for young investigators (A.T.), National Science Centre grant no. 1640/B/P01/2010/39 (J.N.), and a Lider/32/36/L-384 2/10/NCBiR2011 grant from the National Centre for Research and Development (M.G.).
We are grateful to Andrzej Składanowski (Gdansk University of Technology, Poland) for kindly providing the imidazoacridinone derivatives. We acknowledge Anna Gwizdek-Wisniewska for statistical analysis. We also thank Barbara Bykowska for providing us with C. albicans clinical isolates.
Footnotes
Published ahead of print 5 April 2013
REFERENCES
- 1. Cholody WM, Martelli S, Paradziej-Lukowicz J, Konopa J. 1990. 5-[(Aminoalkyl)amino]imidazo[4,5,1-de]acridin-6-ones as a novel class of antineoplastic agents. Synthesis and biological activity. J. Med. Chem. 33:49–52 [DOI] [PubMed] [Google Scholar]
- 2. De Marco C, Zaffaroni N, Comijn E, Tesei A, Zoli W, Peters GJ. 2007. Comparative evaluation of C1311 cytotoxic activity and interference with cell cycle progression in a panel of human solid tumour and leukaemia cell lines. Int. J. Oncol. 31:907–913 [PubMed] [Google Scholar]
- 3. Berger B, Marquardt H, Westendorf J. 1996. Pharmacological and toxicological aspects of new imidazoacridinone antitumor agents. Cancer Res. 56:2094–2104 [PubMed] [Google Scholar]
- 4. Skladanowski A, Plisov SY, Konopa J, Larsen AK. 1996. Inhibition of DNA topoisomerase II by imidazoacridinones, new antineoplastic agents with strong activity against solid tumors. Mol. Pharmacol. 49:772–780 [PubMed] [Google Scholar]
- 5. Dziegielewski J, Slusarski B, Konitz A, Skladanowski A, Konopa J. 2002. Intercalation of imidazoacridinones to DNA and its relevance to cytotoxic and antitumor activity. Biochem. Pharmacol. 63:1653–1662 [DOI] [PubMed] [Google Scholar]
- 6. Wainwright M. 1998. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 42:13–28 [DOI] [PubMed] [Google Scholar]
- 7. Hamblin MR, Hasan T. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang L, Terakawa M, Zhiyentayev T, Huang YY, Sawayama Y, Jahnke A, Tegos GP, Wharton T, Hamblin MR. 2010. Innovative cationic fullerenes as broad-spectrum light-activated antimicrobials. Nanomedicine 6:442–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dai T, Bil de Arce VJ, Tegos GP, Hamblin MR. 2011. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob. Agents Chemother. 55:5710–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hashimoto MC, Prates RA, Kato IT, Nunez SC, Courrol LC, Ribeiro MS. 2012. Antimicrobial photodynamic therapy on drug-resistant Pseudomonas aeruginosa-induced infection. An in vivo study. Photochem. Photobiol. 88:590–595 [DOI] [PubMed] [Google Scholar]
- 11. Eichner A, Gonzales FP, Felgentrager A, Regensburger J, Holzmann T, Schneider-Brachert W, Baumler W, Maisch T. 2013. Dirty hands: photodynamic killing of human pathogens like EHEC, MRSA and Candida within seconds. Photochem. Photobiol. Sci. 12:135–147 [DOI] [PubMed] [Google Scholar]
- 12. Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin R, Wachtler B, Schaller M, Wilson D, Hube B. 2011. Host-pathogen interactions and virulence-associated genes during Candida albicans oral infections. Int. J. Med. Microbiol. 301:417–422 [DOI] [PubMed] [Google Scholar]
- 14. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 15. Picazo JJ, Gonzalez-Romo F, Candel FJ. 2008. Candidemia in the critically ill patient. Int. J. Antimicrob. Agents 32(Suppl 2):S83–S85 doi: 10.1016/S0924-8579(08)70005-0 [DOI] [PubMed] [Google Scholar]
- 16. Jarvensivu A, Hietanen J, Rautemaa R, Sorsa T, Richardson M. 2004. Candida yeasts in chronic periodontitis tissues and subgingival microbial biofilms in vivo. Oral Dis. 10:106–112 [DOI] [PubMed] [Google Scholar]
- 17. Junqueira JC, Jorge AO, Barbosa JO, Rossoni RD, Vilela SF, Costa AC, Primo FL, Goncalves JM, Tedesco AC, Suleiman JM. 2012. Photodynamic inactivation of biofilms formed by Candida spp., Trichosporon mucoides, and Kodamaea ohmeri by cationic nanoemulsion of zinc 2,9,16,23-tetrakis(phenylthio)-29H,31H-phthalocyanine (ZnPc). Lasers Med. Sci. 27:1205–1212 [DOI] [PubMed] [Google Scholar]
- 18. Malik Z, Ladan H, Nitzan Y. 1992. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B 14:262–266 [DOI] [PubMed] [Google Scholar]
- 19. Huang L, Huang YY, Mroz P, Tegos GP, Zhiyentayev T, Sharma SK, Lu Z, Balasubramanian T, Krayer M, Ruzie C, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. 2010. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob. Agents Chemother. 54:3834–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Demidova TN, Hamblin MR. 2005. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 49:2329–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson JB. 2005. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat. Rev. Microbiol. 3:547–556 [DOI] [PubMed] [Google Scholar]
- 22. Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, van Lier JE. 1992. Photosensitizing activity of water- and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells. Microbios 71:33–46 [PubMed] [Google Scholar]
- 23. Donnelly RF, McCarron PA, Tunney MM, David WA. 2007. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J. Photochem. Photobiol. B 86:59–69 [DOI] [PubMed] [Google Scholar]
- 24. Teichert MC, Jones JW, Usacheva MN, Biel MA. 2002. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 93:155–160 [DOI] [PubMed] [Google Scholar]
- 25. Junqueira JC, Martins JS, Faria RL, Colombo CE, Jorge AO. 2009. Photodynamic therapy for the treatment of buccal candidiasis in rats. Lasers Med. Sci. 24:877–884 [DOI] [PubMed] [Google Scholar]
- 26. Adar Y, Stark M, Bram EE, Nowak-Sliwinska P, van den Bergh H, Szewczyk G, Sarna T, Skladanowski A, Griffioen AW, Assaraf YG. 2012. Imidazoacridinone-dependent lysosomal photodestruction: a pharmacological Trojan horse approach to eradicate multidrug-resistant cancers. Cell Death Dis. 3:e293 doi: 10.1038/cddis.2012.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chekulayeva LV, Shevchuk IN, Chekulayev VA, Ilmarinen K. 2006. Hydrogen peroxide, superoxide, and hydroxyl radicals are involved in the phototoxic action of hematoporphyrin derivative against tumor cells. J. Environ. Pathol. Toxicol. Oncol. 25:51–77 [DOI] [PubMed] [Google Scholar]
- 28. Hoebeke M, Schuitmaker HJ, Jannink LE, Dubbelman TM, Jakobs A, Van de Vorst A. 1997. Electron spin resonance evidence of the generation of superoxide anion, hydroxyl radical and singlet oxygen during the photohemolysis of human erythrocytes with bacteriochlorin a. Photochem. Photobiol. 66:502–508 [DOI] [PubMed] [Google Scholar]
- 29. Burger AM, Jenkins TC, Double JA, Bibby MC. 1999. Cellular uptake, cytotoxicity and DNA-binding studies of the novel imidazoacridinone antineoplastic agent C1311. Br. J. Cancer 81:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lambrechts SA, Schwartz KR, Aalders MC, Dankert JB. 2005. Photodynamic inactivation of fibroblasts by a cationic porphyrin. Lasers Med. Sci. 20:62–67 [DOI] [PubMed] [Google Scholar]
- 31. Lambrechts SA, Aalders MC, Verbraak FD, Lagerberg JW, Dankert JB, Schuitmaker JJ. 2005. Effect of albumin on the photodynamic inactivation of microorganisms by a cationic porphyrin. J. Photochem. Photobiol. B 79:51–57 [DOI] [PubMed] [Google Scholar]
- 32. Mantareva V, Kussovski V, Angelov I, Borisova E, Avramov L, Schnurpfeil G, Wohrle D. 2007. Photodynamic activity of water-soluble phthalocyanine zinc(II) complexes against pathogenic microorganisms. Bioorg. Med. Chem. 15:4829–4835 [DOI] [PubMed] [Google Scholar]
- 33. So CW, Tsang PW, Lo PC, Seneviratne CJ, Samaranayake LP, Fong WP. 2010. Photodynamic inactivation of Candida albicans by BAM-SiPc. Mycoses 53:215–220 [DOI] [PubMed] [Google Scholar]
- 34. Mantareva V, Kussovski V, Angelov I, Wohrle D, Dimitrov R, Popova E, Dimitrov S. 2011. Non-aggregated Ga(III)-phthalocyanines in the photodynamic inactivation of planktonic and biofilm cultures of pathogenic microorganisms. Photochem. Photobiol. Sci. 10:91–102 [DOI] [PubMed] [Google Scholar]
- 35. Lam M, Jou PC, Lattif AA, Lee Y, Malbasa CL, Mukherjee PK, Oleinick NL, Ghannoum MA, Cooper KD, Baron ED. 2011. Photodynamic therapy with Pc 4 induces apoptosis of Candida albicans. Photochem. Photobiol. 87:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mantareva V, Angelov I, Kussovski V, Dimitrov R, Lapok L, Wohrle D. 2011. Photodynamic efficacy of water-soluble Si(IV) and Ge(IV) phthalocyanines towards Candida albicans planktonic and biofilm cultures. Eur. J. Med. Chem. 46:4430–4440 [DOI] [PubMed] [Google Scholar]
- 37. Chabrier-Rosello Y, Foster TH, Perez-Nazario N, Mitra S, Haidaris CG. 2005. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob. Agents Chemother. 49:4288–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tegos GP, Anbe M, Yang C, Demidova TN, Satti M, Mroz P, Janjua S, Gad F, Hamblin MR. 2006. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 50:1402–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cormick MP, Alvarez MG, Rovera M, Durantini EN. 2009. Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. Eur. J. Med. Chem. 44:1592–1599 [DOI] [PubMed] [Google Scholar]
- 40. Mang TS, Mikulski L, Hall RE. 2010. Photodynamic inactivation of normal and antifungal resistant Candida species. Photodiagnosis Photodyn. Ther. 7:98–105 [DOI] [PubMed] [Google Scholar]
- 41. Quiroga ED, Alvarez MG, Durantini EN. 2010. Susceptibility of Candida albicans to photodynamic action of 5,10,15,20-tetra(4-N-methylpyridyl)porphyrin in different media. FEMS Immunol. Med. Microbiol. 60:123–131 [DOI] [PubMed] [Google Scholar]
- 42. Mima EG, Pavarina AC, Ribeiro DG, Dovigo LN, Vergani CE, Bagnato VS. 2011. Effectiveness of photodynamic therapy for the inactivation of Candida spp. on dentures: in vitro study. Photomed. Laser Surg. 29:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alvarez MG, Gomez ML, Mora SJ, Milanesio ME, Durantini EN. 2012. Photodynamic inactivation of Candida albicans using bridged polysilsesquioxane films doped with porphyrin. Bioorg. Med. Chem. 20:4032–4039 [DOI] [PubMed] [Google Scholar]
- 44. Munin E, Giroldo LM, Alves LP, Costa MS. 2007. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J. Photochem. Photobiol. B 88:16–20 [DOI] [PubMed] [Google Scholar]
- 45. Souza RC, Junqueira JC, Rossoni RD, Pereira CA, Munin E, Jorge AO. 2010. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against Candida albicans. Lasers Med. Sci. 25:385–389 [DOI] [PubMed] [Google Scholar]
- 46. Pupo YM, Gomes GM, Santos EB, Chaves L, Michel MD, Kozlowski VA, Jr, Gomes OM, Gomes JC. 2011. Susceptibility of Candida albicans to photodynamic therapy using methylene blue and toluidine blue as photosensitizing dyes. Acta Odontol. Latinoam. 24:188–192 [PubMed] [Google Scholar]
- 47. CLSI 2007. Performance standards for antimicrobial susceptibility testing. CLSI approval standard M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]