Abstract
In the last few years the need to produce food with added value has fueled the search for new ingredients and health-promoting compounds. In particular, to improve the quality of bakery products with distinct nutritional properties, the identification of new raw materials, appropriate technologies, and specific microbial strains is necessary. In this study, different doughs were prepared, with 10% and 20% flour from immature wheat grain blended with type “0 America” wheat flour. Immature flour was obtained from durum wheat grains harvested 1 to 2 weeks after anthesis. Doughs were obtained by both the straight-dough and sourdough processes. Two selected exopolysaccharide-producing strains of lactic acid bacteria (LAB), Leuconostoc lactis A95 and Lactobacillus curvatus 69B2, were used as starters. Immature flour contained 2.21 g/100 g (dry weight) of fructo-oligosaccharides. Twenty percent immature flour in dough resulted in a shorter leavening time (4.23 ± 0.03 h) than with the control and dough with 10% immature flour. The total titratable acidity of sourdough with 20% immature flour was higher (12.75 ± 0.15 ml 0.1 N NaOH) than in the control and sourdough with 10% immature wheat flour (9.20 ml 0.1 N NaOH). Molecular analysis showed that all samples contained three LAB species identified as L. lactis, L. curvatus, and Pediococcus acidilactici. A larger amount of exopolysaccharide was found in sourdough obtained with 20% immature flour (5.33 ± 0.032 g/kg), positively influencing the exopolysaccharide content of the bread prepared by the sourdough process (1.70 ± 0.03 g/kg). The addition of 20% immature flour also led to a greater presence of fructo-oligosaccharides in the bread (900 mg/100 g dry weight), which improved its nutritional characteristics. While bread volume decreased as the concentration of immature wheat flour increased, its mechanical characteristics (stress at a strain of 30%) were the same in all samples obtained with different percentages of fructo-oligosaccharides. These data support the use of immature wheat grain flour, and exopolysaccaride-producing lactic acid bacteria in formulating functional prebiotic baked goods whose nutritional value can be suitably improved.
INTRODUCTION
Cereals are a source of oligosaccharides, such as fructo-oligosaccharides (FOS) and transgalacto-oligosaccharides (1). Oligosaccharides are defined as carbohydrates with a degree of polymerization from 3 to 10. They are soluble in water and moderately sweet. Oligosaccharides have higher molecular weights than mono- and disaccharides and can be used to increase viscosity. Furthermore, oligosaccharides have a high water-holding capacity, preventing excessive drying, and are also inhibitors of starch retrogradation (2). FOS are oligomers formed by chains of fructose obtained from fructosyl-nystose with increasing lengths, namely, kestose, nistose, and fructosilnistose, normally present in the tissues of many plants. FOS are the category of nondigestible carbohydrates most often used as a prebiotic food ingredient. They withstand hydrolysis due to human digestive juices and reach the colon intact, where they promote beneficial physiological effects in the human gut (3–9).
Among the dietary sources of FOS, the immature kernels of cereals, harvested at the stage of milky maturation, have a high FOS content, up to 10 times higher than that of mature wheat grain (10–12). For these reasons, they have recently generated interest as a raw material with a prebiotic function.
In the last few years, the need to improve the quality of bakery products (13) with distinct nutritional properties has boosted the search to identify raw materials, technologies, and microbial strains capable of responding to new market requirements. Studies on the exopolysaccharide (EPS)-producing LAB strains used as starters in bread technology have been developed with the aim of obtaining new standardized baked products with higher quality and a reduced need for additives (8). EPSs are considered biothickeners or hydrocolloids, which represent a good alternative to additives (14). The formation of EPS in situ from sucrose has been reported to promote the production of additional metabolites, such as mannitol, glucose, and acetate, which contribute to the quality of the finished product (15). In addition, it was demonstrated that EPSs provide additional nutritional properties as a prebiotic attribute (16, 17). As reported by Escalada and Mos (12), D'Egidio et al. (18), and Mujoo and Ng (19), the high FOS content in immature wheat flour (IWF) suggested its use as a prebiotic ingredient for the development of new functional foods. It could represent added value and an alternative to the traditional uses of durum wheat (20). From an agronomic point of view, the soil released after early harvesting may be allocated to new production. Moreover, the presence of FOS may stimulate the production of EPS by LAB. Therefore, the combined use of flour obtained from immature wheat seeds and selected EPS-producing LAB strains may enable the production of bread with prebiotic properties and acceptable technological characteristics (19).
Based on the above considerations, our research focused on the study of dough and bread obtained with sourdough and straight-dough technologies, using immature flour and selected dextran-producing LAB. For this purpose, we assessed the effects of different concentrations of IWF and different baking technologies on microbiological and acidimetric characteristics of dough, on in situ EPS production, and on the nutritional and physical properties of putative prebiotic bread.
MATERIALS AND METHODS
Flours and microbial strains.
IWF was obtained from immature seeds harvested during milk ripeness (1 to 2 weeks after anthesis) of durum wheat (Triticum durum Desf.) variety Grace cultivated on the Torre Lama experimental farm (Campania, Italy; 40°37′N, 14°58′E, 30 m above sea level), located in an area suitable for the production of good-quality wheat and pasta (20). Wheat flour type 0 America was used for the preparation of flour mixtures employed in dough and sourdough making. To prepare the IWF, seeds were ground and accurately mixed with 0 America flour as described below to obtain a homogeneous batch of IWF.
Two strains of dextran-producing LAB (21) were used for the preparation of bread: Leuconostoc lactis A95 and Lactobacillus curvatus 69B2, isolated from sourdough for sweet baked goods (22). Saccharomyces cerevisiae T22, isolated from pizza dough (23), was included in the starter as a leavening agent for bread preparation.
Dough, sourdough, and bread preparation.
Flour mixtures were prepared by repeated steps of mixing IWF with 0 America flour in a 1:1 ratio. Each mixing step was performed with a professional mixer (model 50 KPM; Kitchenaid, St. Joseph, MI) for 2 min at room temperature.
Two different types of dough were prepared using type 0 America wheat flour blended with 10% and 20% IWF. In addition, a dough obtained with only the 0 America flour was used as a control. Each type of dough was produced by using both the straight-dough and sourdough processes. For the straight-dough process, the LAB starter and yeast were added at the same time (23), while in the sourdough process, the inoculum consisted only of LAB strains used in a prolonged fermentation (15 h) at 30°C. The yeast was added at a different time for the preparation of the sourdough for bread (21). LAB and yeast achieved viable counts of approximately 5 × 107 CFU g−1 and 5 × 106, respectively. In particular, the LAB were grown in MRS Broth (Oxoid) and the yeast in malt extract (Oxoid). After overnight incubation at 30°C, the broth cultures were subjected to direct counting in count chambers (Thoma Counting Chambers; depth, 0.02 mm; area, 1/400 mm2; Hawksley, United Kingdom). After centrifugation at 5,200 × g for 15 min, the pellets were used for the dough- and bread-making experiments. For the dough obtained with the sourdough process, 30% sourdough was added to the other ingredients. All dough contained 5% (wt/vol) sucrose for EPS production. The dough was prepared by mixing all ingredients in a mixer (model 50 Professional KPM; Kitchenaid, St Joseph, MI) for 5 min at room temperature and at a speed of 1. The dough was shaped into loaves of 400 g each, placed in aluminum pans, and incubated at 30°C until twice the initial volume was reached. The dough was baked in a preheated oven at 180°C for 35 min and then cooled at room temperature for 2 h.
Microbiological, acidimetric analysis and leavening ability.
Twenty grams of fermented dough or sourdough was diluted with 180 ml of 0.1% peptone water in a Stomacher 400 blender (PBI, Milan, Italy) and serially diluted with sterile quarter-strength Ringer's solution (Oxoid). Differential microbial counts of LAB strains were determined on duplicate plates of modified Chalmers agar plates (24). The pH and total titratable acidity (TTA) were determined by standard methods, and the acid equivalent was expressed as the amount of 0.1 N NaOH/10g consumed in milliliters (25). The leavening ability, defined as the leavening time to raise the initial volume 2-fold, was evaluated by incubating part of the dough (150 g) in graduated glass containers at 30°C.
Quantification of FOS and EPS in flour, dough, and bread.
The quantification of FOS in flour and bread samples with 0%, 10%, and 20% IWF was carried out using the Fructan Assay Kit K-FRUC 5/2008 (Megazyme International Ireland Ltd.). Extraction of EPS was performed by following the method previously described (21). Enzymatic hydrolysis of the extracts was performed to remove starch through treatments with thermostable α-amylase for 10 min at 100°C, followed by amyloglucosidase for 1 h at 50°C (total starch assay kit; Megazyme) (26). The glucose liberated from the degraded starch was removed by repeated washes with 2 volumes of chilled 98% (vol/vol) ethanol. The concentration of EPS in the wheat sourdough was determined according to the phenol-sulfuric method (27, 28).
DGGE analysis.
Twenty grams of fermented dough or sourdough was diluted (1/10) in quarter-strength Ringer's solution (Oxoid). Two milliliters of the dilution was centrifuged at 14,000 × g for 5 min, and the resulting pellets were used for DNA isolations. The DNA was extracted by using Nucleo Spin Food (Macherey-Nagel, Germany) according to the supplier's recommendation. The primers V3f and V3r, spanning the 200-bp V3 region of the 16S rRNA of Escherichia coli (29), were used for PCR-denaturing gradient gel electrophoresis (DGGE) analysis. The PCR mixture and conditions were as described by Ercolini et al. (30). PCR products were analyzed by DGGE as described by Palomba et al. (21). Bands were excised from the gel, eluted in sterile water, and reamplified. The PCR products were checked by electrophoresis of 12 μl of amplicons in DGGE gels; DNA amplified from dough was used as a control. PCR products that gave a single band comigrating with the control were purified with a QIAquick PCR Purification kit (Qiagen, Milan, Italy) and sequenced. The sequences were analyzed with MacDNasis Pro v3.0.7 (Hitachi Software Engineering Europe S.A., Olivet, France) and compared to the GenBank nucleotide data library using the BLAST software at the National Centre for Biotechnology Information (31) in order to determine their closest phylogenetic relatives.
Volume of bread samples by image analysis.
The loaves with rectangular bases, after cooling to room temperature, were sliced transversely, and from each, the central slice (1 cm thick) was chosen. A Casio Exilim camera (EX-Z35) acquired the image of the bread, and the program Adobe Photoshop CS2 reported its size. Eight different heights were measured using the Adobe program, corresponding to four points on the right and four on the left of the center (x = 0), all equidistant from each other. The height measurements were plotted on a scatter plot, and a polynomial trend line fitted to the data with Microsoft Excel software (Microsoft Office 2000 Professional; Microsoft Corp., Redmond, WA) was drawn. The volume was calculated using the following formula: V = ∫ 2π f(x)dx [a,b], where f(x) is the equation of the linear polynomial, a is the 0 value of x at maximum height, and b is the value of x corresponding to half the width of the dough (32).
Mechanical analysis.
All samples were submitted to a uniaxial compression test by using an Instron Universal Testing Machine (model 4467; Instron Ltd., High Wycombe, United Kingdom) equipped with a 1-kN load cell. Cylindrical crumb samples (diameter, 17 mm; height, 17 mm) were placed between parallel plates and compressed to a final deformation of 80% at a crosshead speed of 10 mm/min. For each sample, seven measurements were made. Crumb firmness was expressed as the stress corresponding to 30% deformation.
Statistical analysis.
The statistical analysis of data (mean ± standard deviation [SD], analysis of variance, and t tests) was performed using the software Macintosh Systat version 5.2.1. All analyses were carried out in duplicate, and each sample was analyzed from two to four times.
RESULTS
FOS in the flours and characterization of dough and sourdough.
Grains harvested 1 week after anthesis had the highest percentage of FOS (2.83 ± 0.06 g/100 g dry weight) (Table 1). The value decreased to 1.41 ± 0.00/100 g dry weight with the prolonged maturation of the seeds (2 weeks). The IWF batch obtained by grains harvested 1 and 2 weeks after anthesis (2.21 g/100 g dry weight) was used to prepare flour containing 10 and 20% IWF (Table 1). Flour type 0 America did not exceed 0.52 ± 0.04 g/100 g dry weight of FOS.
Table 1.
FOS contents in different flour samples
| Type of floura | FOS content (g/100 g dry wt) |
|---|---|
| 0 America | 0.52 ± 0.04 |
| Immature wheat grains 1 | 2.83 ± 0.06 |
| Immature wheat grains 2 | 1.41 ± 0.00 |
| IWF from wheat grains 1 and 2 | 2.21 ± 0.07 |
| IWF 10% | 0.69 ± 0.06 |
| IWF 20% | 0.86 ± 0.05 |
Immature wheat grains 1, grains harvested 1 week after anthesis; immature wheat grains 2, grains harvested 2 weeks after anthesis; IWF, mixture of immature wheat flour from grains harvested 1 and 2 weeks after anthesis; IWF 10% and 20%, flour with 10 or 20% IWF.
Microbiological, acidimetric, and leavening characterization of dough obtained by the straight-dough process.
On analyzing the dough immediately after mixing (time zero), no significant differences (P ≥ 0.01) among the different samples were detected. In contrast, after dough leavening, differences were shown (P ≤ 0.01) both between the different technologies (straight-dough and sourdough processes) and between the doughs with different amounts of IWF. To highlight the differences, the t test was carried out on every single parameter.
Immediately after mixing (time zero), the cell concentrations of L. lactis A95 in the different doughs ranged from 7.2 ± 0.2 to 7.6 ± 0.1 log CFU g−1, whereas L. curvatus 69B2 ranged from 8.2 ± 0.1 to 8.4 ± 0.1 log CFU g−1. The yeast had a microbial concentration from 5.7 ± 0.3 and 6.1 ± 0.1 log CFU g−1. After leavening, the cellular concentrations of LAB and yeast strains showed no significant differences (P ≥ 0.01) (Table 2).
Table 2.
Microbial contents and acidification properties (pH and TTA) of sourdoughs and doughs obtained with different preparation methodsa
| Sampleb | pH | TTA (ml 0.1 N NaOH/10 g) | Microbial count (log CFU g−1) |
Leavening time (h) | EPS (g/kg) | ||
|---|---|---|---|---|---|---|---|
| L. lactis A95 | L. curvatus 69B2 | S. cerevisiae T22 | |||||
| Straight dough control | 4.7 ± 0.1AB | 6.75 ± 0.11A | 8.9 ± 0.1A | 8.9 ± 0.5A | 6.4 ± 0.3A | 6.30 ± 0.04A | ND |
| Straight dough with 10% IWF | 4.6 ± 0.1AC | 7.50 ± 0.06B | 8.7 ± 0.1A | 9.3 ± 0.3A | 6.4 ± 0.2A | 6.55 ± 0.05A | ND |
| Straight dough with 20% IWF | 5.8 ± 0.1D | 3.65 ± 0.09C | 8.3 ± 0.6AB | 8.9 ± 0.1A | 6.8 ± 0.1A | 4.20 ± 0.03B | ND |
| Sourdough dough control | 4.4 ± 0.1C | 7.20 ± 0.15D | 8.2 ± 0.1B | 9.2 ± 0.1A | 6.6 ± 0.2A | 5.20 ± 0.04C | ND |
| Sourdough dough with 10% IWF | 4.6 ± 0.0BC | 7.65 ± 0.05B | 8.0 ± 0.1B | 9.3 ± 0.6A | 6.6 ± 0.2A | 6.10 ± 0.04D | ND |
| Sourdough dough with 20% IWF | 4.8 ± 0.0A | 9.05 ± 0.25E | 8.1 ± 0.1B | 9.1 ± 0.1A | 6.7 ± 0.2A | 4.25 ± 0.04B | ND |
| Sourdough control | 4.1 ± 0.0A | 9.20 ± 0.32A | 8.8 ± 0.1A | 9.4 ± 0.1A | ND | ND | 1.32 ± 0.051A |
| Sourdough with 10% IWF | 4.2 ± 0.1A | 9.20 ± 0.11A | 8.7 ± 0.1A | 9.5 ± 0.1A | ND | ND | 0.90 ± 0.071B |
| Sourdough with 20% IWF | 4.2 ± 0.0A | 12.75 ± 0.15B | 9.5 ± 0.1B | 9.4 ± 0.0A | ND | ND | 5.33 ± 0.032C |
The doughs were analyzed after the leavening time required to reach twice the initial volume. The sourdoughs were analyzed after 15 h of fermentation at 30°C. The leavening time of each dough was defined as the leavening time required to raise the initial volume 2-fold. The values represent the means ± SD of two replicates of two independent experiments. Different letters after the values indicate significant differences (P ≤ 0.01; t test). ND, not determined. The initial values immediately after dough mixing were as follows: pH = 6.8 ± 0.5; TTA = 6.9 ± 0.17 ml 0.1 N NaOH/10g; microbial count of L. lactis A95, 7.2 ± 0.2 to 7.6 ± 0.14 log CFU ml−1; microbial count of L. curvatus 69B2, 8.2 ± 0.1 to 8.4 ± 0.1 log CFU ml−1; microbial count of S. cerevisiae T22, 5.7 ± 0.3 to 6.1 ± 0.1 log CFU ml−1.
Statistical analysis of the samples in lightface was carried out by comparing only the dough samples; statistical analysis of the samples in boldface was carried out by comparing only the sourdough samples.
Immediately after kneading (time zero), the pH and TTA showed no significant differences (P ≥ 0.01) between the control and the dough with 10% and 20% IWF, showing pH values from 6.9 ± 0.5 to 6.8 ± 0.2 and TTA from 1.20 ± 0.06 to 1.60 ± 0.15 ml 0.1 N NaOH/10 g. After leavening, however, the dough containing 20% IWF was less acidic (pH 5.8 ± 0.1; TTA, 3.65 ± 0.09) than the control and the dough with 10% IWF (pH 4.6 ± 0.1 and 4.7 ± 0.1; TTA, 6.75 ± 0.11 and 7.50 ± 0.06 ml 0.1 N NaOH/10 g, respectively). Dough obtained with 20% IWF showed a significant reduction in leavening time (4.20 ± 0.03 h) with respect to the control and dough containing 10% IWF (6.30 ± 0.04 and 6.55 ± 0.05 h, respectively; P ≤ 0.01) (Table 2).
Microbiological and acidimetric characterization and EPS concentration of sourdough.
The cell concentration of LAB varied depending on the concentration of the IWF used to obtain the sourdough (Table 2). L. curvatus 69B2 reached around 9.4 log CFU g−1 after 15 h of incubation at 30°C in all samples of sourdough analyzed. L. lactis A95, however, reached a concentration equal to 9.5 log CFU g−1 only when 20% IWF was added, approximately 1 log unit higher than the control and the dough obtained with 10% IWF (8.7 ± 0.1 log CFU g−1). Also, the TTA value of the sample with 20% IWF was greater (12.75 ± 0.15 ml 0.1 N NaOH) than the control and the dough with 10% IWF (9.20 ml 0.1 N NaOH). In the sourdough containing 20% IWF, a larger amount of EPS (5.33 ± 0.032 g/kg) was observed than in the control and the sample containing 10% IWF (1.32 ± 0.051 g/kg and 0.90 ± 0.0071 g/kg, respectively) (Table 2).
Microbiological, acidimetric, and leavening characterization of dough obtained by the sourdough process.
The cell concentration of the LAB and yeast strains before and after dough leavening (Table 2) showed a slight increase that did not exceed 0.5 log CFU g−1 (L. lactis A95 increased from 7.8 log CFU g−1 to 8.1 log CFU g−1, L. curvatus 69B2 from 8.9 log CFU g−1 to 9.2 log CFU g−1, and S. cerevisiae T22 by 5.9 log CFU g−1 to 6.6 log CFU g−1).
The pH values after kneading (time zero) showed no significant differences between the dough samples obtained with 10% and 20% IWF (6.2 ± 0.0 and 6.0 ± 0.1, respectively), but they were higher (P ≤ 0.01) than those of the control (5.5 ± 0.0). In contrast, at the beginning of fermentation (time zero), all samples analyzed showed the same acidity (P ≥ 0.01), about 3.5 ml 0.1 N NaOH (Table 2). After fermentation, the dough with 20% IWF had a higher TTA value (9.05 ± 0.05 0.1 N NaOH) than the control and the dough with 10% IWF (7.20 ± 0.15 and 7.65 ± 0.05 0.1 N NaOH). The leavening time of sourdough obtained with 20% IWF was significantly shorter (4.25 ± 0.04 h; P ≤ 0.01) than that of the control (5.20 ± 0.04 h) (Table 2). A longer rising time was shown by dough obtained with 10% IWF (6.10 ± 0.04 h).
Molecular analysis.
PCR-DGGE was carried out on sourdough and dough. All samples showed the same profile, consisting of four bands (data not shown). Identification of the bands performed by sequencing indicated the presence of the two LAB strains used as starters, L. lactis A95 (99% identity) and L. curvatus 69B2 (100% identity) (accession numbers of closest-relative species, EU676346.1 and JX979220.1, respectively). Cereal mitochondrial DNA/uncultured bacteria (100% identity; accession number of the closest related species, JQ013040.1) and a band corresponding to the species Pediococcus acidilactici (99% identity; accession number of closest related species, AB627837.1) were also found.
Volume, consistency, and nutritional characterization of bread.
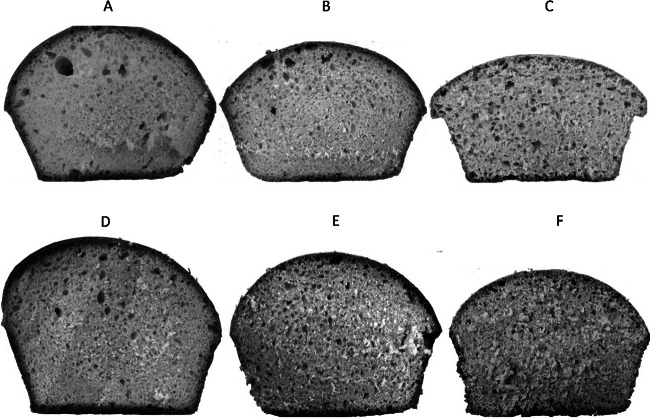
To assess the technological characteristics of the bread in question, the sample volume (cm3) and the resistance offered by the specimen to an imposed deformation of 30% were considered. Comparing the values of the volumes of the loaves with 0%, 10%, and 20% IWF, it can be seen that the control samples (0%), prepared by the straight and sourdough processes had larger volumes (690.4 ± 7.1 cm3 and 642.9 ± 4.0 cm3, respectively) than the other samples obtained with the addition of IWF (Table 3 and Fig. 1). In particular, the values decreased (from 50 to 100 cm3) with an increasing amount of IWF used: with 10% IWF, the maximum volume did not exceed 590.7 ± 4.9 cm3 with both the straight-dough and sourdough processes; with the addition of 20% IWF, the maximum value suffered a further contraction (556.6 ± 8.3 cm3 in straight dough).
Table 3.
Technological characteristics of bread obtained with 0%, 10%, and 20% immature wheat floura
| Bread sample | Vol of bread (cm3) | Firmness (kN/m2)b | FOS content (g/100 g dry wt) | EPS content (g/kg) |
|---|---|---|---|---|
| Straight-dough bread control | 690.4 ± 7.1A | 13.1 ± 1.2A | 0.25 ± 0.08A | ND |
| Straight-dough bread with 10% IWF | 590.7 ± 4.9B | 12.5 ± 0.9A | 0.70 ± 0.05B | ND |
| Straight-dough bread with 20% IWF | 556.6 ± 8.3C | 13.0 ± 2.1A | 0.93 ± 0.04C | ND |
| Sourdough bread control | 642.9 ± 4.0D | 15.6 ± 2.1B | 0.32 ± 0.06A | 0.30 ± 0.10A |
| Sourdough bread with 10% IWF | 506.6 ± 6.5E | 15.3 ± 1.2B | 0.74 ± 0.02B | 0.25 ± 0.03A |
| Sourdough bread with 20% IWF | 462.3 ± 9.2F | 15.7 ± 2.2B | 0.90 ± 0.03C | 1.70 ± 0.10B |
The values represent the means ± SD of two replicates of two independent experiments. Different letters after the values indicate significant differences (P ≤ 0.01; t test). ND, not determined.
Firmness was measured as the stress at 30% deformation.
Fig 1.
(A to C) Bread obtained by the straight-dough process. (A) Bread control. (B) Bread with 10% IWF. (C) Bread with 20% IWF. (D to F) Bread obtained by the sourdough process. (D) Bread control. (E) Bread with 10% IWF. (F) Bread with 20% IWF.
The volumes of the bread samples also differed (P ≤ 0.01) between the straight-dough and sourdough processes. When the sourdough process was used, the volume of the bread was always lower in all cases tested, showing values of 506.0 ± 4.9 and 462.3 ± 9.2 cm3 with 10 and 20% IWF, respectively. In addition, breads produced through the use of sourdough had a volume of less than about 50 to 90 cm3 compared with loaves obtained with direct inoculation (Table 3).
Bread loaves with different percentages of IWF showed the same firmness (P ≥ 0.01) for both the straight-dough and sourdough processes (Table 3). However, the samples prepared with direct inoculation had a lower firmness (12.7 kN/m2) than those prepared with sourdough (15.6 kN/m2).
The breads obtained with only 0 America flour (products with and without the use of sourdough) presented FOS contents of 0.25 to 0.32 g/100 g dry weight. The samples with 10% and 20% IWF had concentrations of 0.70 to 0.74 g/100 g dry weight and 0.90 to 0.93 g/100 g dry weight, respectively (Table 3).
As in the case of sourdoughs, the amount of EPS in the bread was also greater in the sample with 20% immature flour (1.70 ± 0.10 g/kg) than in the control (0.30 ± 0.10 g/kg) and the bread with 10% IWF (0.25 ± 0.03 g/kg). Therefore, the bread obtained with 20% immature flour and the use of sourdough showed larger amounts of both FOS and dextran (Table 3).
DISCUSSION
The high content of FOS in IWF was similar to that found in previous studies by Escalada and Mos (12), D'Egidio et al. (18), and Mujoo and Ng (19) and suggested its use as a prebiotic ingredient for the development of new functional foods. The presence of 20% IWF influenced the growth and metabolic activities of L. lactis A95 and L. curvatus 69B2, previously selected as dextran-producing LAB (21). A higher concentration of IWF drastically reduced the leavening time due to the increased amount of soluble carbohydrates (FOS) (10, 12) potentially available to the yeast as a carbon source for CO2 release. This result is significant, since one of the critical points in the production of baked goods is the competition between LAB and yeasts for the use of soluble carbohydrates (33). Reduced fermentation time resulted in lower metabolic activity of LAB and hence reduced release of acids within the dough (23). However, the shorter leavening time is an important condition for the bakery industry, since excessively long times may slow down production and have negative economic effects.
The acidimetric results confirmed once again the strong influence of FOS on microbial activity, contributing to the increase in acidification of sourdough. The increase in the acidity of the bakery product is necessary for good leavening and bread baking, for the control of enzyme activities, for the elasticity and softness of the crumb, and to prolong the shelf life of the product by preventing the growth of mold and of spoilage bacteria (34–38). Moreover, from experimental evidence (21), the higher concentration of EPS detected in sourdough with 20% IWF also led to the stimulation of LAB, notably in regard to the acidifying activity. Some of the benefits due to the use of sourdough in the preparation of dough are due to the production of EPS by LAB during fermentation, since they can improve the dough's mechanical, sensory, and nutritional properties. This is the first study of the oligosaccharides' effect on EPS production, which seems to be stimulated by larger amounts of FOS present in the IWF. These soluble carbohydrates can be a further source of monomers for the formation of EPS by glycosyltransferases (39).
The methods of dough preparation did not affect the final concentrations of LAB and yeast strains, since the counts were similar in all samples analyzed. Values of pH and TTA immediately after kneading showed no significant differences between the dough samples obtained with the straight-dough and sourdough processes with IWF. As observed above, the use of IWF with a higher FOS concentration stimulated the metabolism of LAB and yeast, resulting in an increase in TTA and a decrease in leavening time in dough obtained by the sourdough process. A previous study reported pseudoplastic behavior and increase in viscosity in sourdough obtained with a large EPS concentration (21). It is reasonable to assume that the use of sourdough obtained with dextran-producing LAB allowed a more interconnected structure that promoted the speed of leavening.
PCR-DGGE profiles demonstrated that IWF addition did not influence the colonization capacity or the dominance of the selected LAB strains used as starter components for dough and sourdough preparation. Moreover, according to a previous study, cereal mitochondrial DNA was also found (22), together with the species P. acidilactici, a bacterium commonly found in plant products and fermented dairy and meat (40).
IWF used in this study was obtained by grains harvested 1 to 2 weeks after anthesis, during milk ripeness (10) of durum wheat, which contains the highest level of FOS. Physiologically, for the industrial use of immature kernels as a natural source of FOS, the most appropriate harvest time is between 13 and 17 days after anthesis (1). In this study, the bread samples obtained with 10% and 20% IWF showed a percentage of FOS three times higher than those of other samples. The use of IWF in bread preparation led to a significant increase in FOS. FOS are naturally present in many foods, such as bananas (0.3 g/100 g dry weight), onion (0.23 g/100 g dry weight), ripe grain (0.50 g/100 g dry weight), honey (0.75 g/100 g dry weight), tomatoes (0.75 g/100 g dry weight), and garlic (0.6 g/100 g dry weight), but in much smaller quantities than in immature wheat (2 to 7.5 g/100 g dry weight) (18, 19, 41). It has been estimated that to ensure their pronutritional effects (4, 42–45), the daily dose of FOS should be about 3 g (41). In particular, FOS intake (1, 3, and 5 g/day) for 2 weeks significantly increased the number of Bifidobacteria organisms from 2.5 to 3.5 times and improved intestinal function (46, 47). A daily consumption of 200 g of bread produced with 20% IWF ensured an intake of FOS equal to about 30% of the daily requirement. This result is very important, since common foods normally present in the human diet have low FOS contents. Moreover, the larger amount of FOS and the sourdough technology stimulated the biosynthesis of dextran by selected LAB strains employed as starters. Recently, it was demonstrated that EPSs have a prebiotic effect on the human intestinal microbiota (14, 16, 48–50). Furthermore, Olano-Martin et al. (17) have shown by in vitro studies that dextran is a good substrate for butyric acid production by intestinal microorganisms. Though the molecular characteristics differentiating prebiotics from EPS are not yet known (51), the presence of certain glycoside bonds could affect their prebiotic potential (52).
According to Mujoo and Ng (19), the addition of increasing amounts of IWF leads to a decrease in bread volume. The total nitrogen content of the kernels at milk ripeness is very similar to that of fully mature grain, while there is a higher content of albumins (water-soluble fraction) and lower contents of gliadins and glutenins (11, 53). Bread prepared with IWF had a low gluten content that negatively influenced gas retention during cooking, resulting in its lower volume (26). The sourdough process also led to a lower volume, as well as greater firmness of the bread. This could be due to the increase in acidity, resulting in stimulation of proteolytic activity that weakens the structure of the gluten network, making the dough softer with a consequent effect on CO2 retention and hence on the volume and rheological properties of the final product (21, 25).
In conclusion, the research demonstrated that the combined use of 20% IWF, dextran-producing LAB strains, and the sourdough process stimulated LAB and yeast metabolism and led to shorter leavening times and higher EPS production in dough. As a consequence, it was possible to develop prebiotic bread able to satisfy about 30% of the daily requirement for FOS and supplement the intake of prebiotics in the daily diet.
Footnotes
Published ahead of print 12 April 2013
REFERENCES
- 1. Costa A, Oliviero D, D'Egidio MG. 2001. Fructo-oligosaccharides in wheat kernel: effect of genotype and environmental factors on the accumulation and degradation kinetic. Tec. Molitoria 52:849–853 [Google Scholar]
- 2. Nakakuki T. (ed). 1993. Oligosaccharides. Production, properties and application. Gordon and Breach Science Publishers, Yverdon-les-Bains, Switzerland [Google Scholar]
- 3. Bijlani RL. 1985. Dietary fibre: consensus and controversy. Prog. Food Nutr. Sci. Field 9:343–393 [PubMed] [Google Scholar]
- 4. Gibson GR, Beatty ER, Wang X, Cummings JH. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975–982 [DOI] [PubMed] [Google Scholar]
- 5. Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutri. 125:1401–1412 [DOI] [PubMed] [Google Scholar]
- 6. Iwata N, Ishiwatari K. 2001. Additives to food for controlling diabetes and obesity. Jpn. Kokai Tokyo Koho 4 [Google Scholar]
- 7. McCann SE, Moysich KB, Mettlin C. 2001. Intakes of selected nutrients and food groups and risk of ovarian cancer. Nutr. Cancer 39:19–28 [DOI] [PubMed] [Google Scholar]
- 8. Schley PD, Field CJ. 2002. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 87:S221–S230 [DOI] [PubMed] [Google Scholar]
- 9. Spiller RC. 1994. Pharmacology of dietary fibre. Pharmacol. Ther. Field 62:407–427 [DOI] [PubMed] [Google Scholar]
- 10. D'Egidio MG, Cecchini C. 1997. Cariossidi di grano immature come alimento funzionale, p 478–483 Atti III Convegno CISETA “Ricerche e innovazioni nell'industria alimentare.” [Google Scholar]
- 11. D'Egidio MG, Cecchini C. 1998. Cariossidi di grano immature come alimento funzionale. Tecnica Molitoria 12:1304–1310 [Google Scholar]
- 12. Escalada JA, Mos DN. 1976. Change in nonstructural carbohydrate fraction of developing spring wheat kernels. Crop Sci. 16:627–631 [Google Scholar]
- 13. Hammes WP, Ganzle MG. 1998. Sourdough breads and related products, p 199–216 In Woods BJB. (ed), Microbiology of fermented foods, 2nd ed, vol 1 Chapman and Hall, London, United Kingdom [Google Scholar]
- 14. Korakli M, Pavlovic M, Gänzle MG, Vogel RF. 2003. Exopolysaccharide and ketose production by Lactobacillus sanfranciscensis LTH2590. Appl. Environ. Microbiol. 69:2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Cagno R, De Angelis M, Limotone A, Minervini F, Carnevali P, Corsetti A, Gaenzle M, Ciati R, Gobbetti M. 2006. Glucan and fructan production by sourdough Weissella cibaria and Lactobacillus plantarum. J. Agric. Food Chem. 54:9873–9881 [DOI] [PubMed] [Google Scholar]
- 16. Djouzi Z, Andlueux C. 1997. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with a human faecal flora. Br. J. Nutr. 78:313–324 [DOI] [PubMed] [Google Scholar]
- 17. Olano-Martin E, Gibson GR, Rastall RA. 2002. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 93:505–511 [DOI] [PubMed] [Google Scholar]
- 18. D'Egidio MG, Cecchini C, Nardi S, Chianese L, Pizzano R, Pugliano G, Siciliano R. 1995. Caratterizzazione biochimica delle proteine del frumento duro durante la maturazione della granella, p 208 Atti 2nd Congr. Nazionale Chim. Aliment [Google Scholar]
- 19. Mujoo R, Ng PKW. 2003. Physicochemical properties of bread baked from flour blended with immature wheat meal rich in fructooligosaccharides. J. Food Sci. 68:2448–2452 [Google Scholar]
- 20. Fagnano M, Fiorentino N, D'Egidio MG, Quaranta F, Ritieni A, Ferracane R, Raimondi G. 2012. Durum wheat in conventional and organic farming: yield amount and pasta quality in southern Italy. ScientificWorldJournal 2012:973058 doi: 10.1100/2012/973058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palomba S, Cavella S, Torrieri E, Piccolo A, Mazzei P, Blaiotta G, Ventorino V, Pepe O. 2012. Wheat sourdough from Leuconostoc lactis and Lactobacillus curvatus exopolysaccharide-producing starter culture: polyphasic screening, homopolysaccharide composition and viscoelastic behavior. Appl. Environ. Microbiol. 78:2737–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palomba S, Blaiotta G, Ventorino V, Saccone A, Pepe O. 2011. Microbial characterization of sourdough for sweet baked products in the Campania region (southern Italy) by a polyphasic approach. Ann. Microbiol. 61:307–314 [Google Scholar]
- 23. Coppola S, Pepe O, Mauriello G. 1998. Effect of leavening microflora on pizza dough properties. J. Appl. Microbiol. 85:891–897 [DOI] [PubMed] [Google Scholar]
- 24. Pepe O, Villani F, Coppola S. 2001. Differential viable count of mixed starter cultures of lactic acid bacteria by using modified Chalmers medium. Microb. Res. 155:351–354 [DOI] [PubMed] [Google Scholar]
- 25. Pepe O, Villani F, Oliviero D, Greco T, Coppola S. 2003. Effect of proteolytic starter cultures as leavening agents of pizza dough. Int. J. Food Microbiol. 84:319–326 [DOI] [PubMed] [Google Scholar]
- 26. Pritchard JR, Lawrence GJ, Larroque O, Li Z, Laidlaw HKC, Morell MK, Rahman S. 2011. A survey of β-glucan and arabinoxylan content in wheat. J. Sci. Food Agric. 91:1298–1303 [DOI] [PubMed] [Google Scholar]
- 27. Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350–356 [Google Scholar]
- 28. Minervini F, De Angelis M, Surico RF, Di Cagno R, Ganzle M, Gobbetti M. 2010. Highly efficient synthesis of exopolysaccharides by Lactobacillus curvatus DPPMA10 during growth in hydrolyzed wheat flour agar. Int. J. Food Microbiol. 141:130–135 [DOI] [PubMed] [Google Scholar]
- 29. Muyzer G, De Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ercolini D, Blaiotta G, Moschetti G, Coppola S. 2002. Evaluation of PCR-DGGE analysis for molecular typing of cheeses. Ann. Microbiol. 52:81–87 [Google Scholar]
- 31. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whitaker M, Barringer SA. 2004. Measurement of contour and volume changes during cake baking. Cereal Chem. 81:177–181 [Google Scholar]
- 33. Gobbetti M, Corsetti A, Rossi J. 1994. The sourdough microflora: interactions between lactic acid bacteria and yeast: metabolism of carbohydrates. Appl. Microbiol. Biotechnol. 41:456–460 [DOI] [PubMed] [Google Scholar]
- 34. Corsetti A, Gobbetti M, Smacchi E. 1996. Antibacterial activity of sourdough lactic acid bacteria: isolation of a bacteriocin-like inhibitory substance from Lactobacillus sanfrancisco C57. Food Microbiol. 13:447–456 [Google Scholar]
- 35. Lavermicocca P, Valerio F, Evidente A, Lazzaroni S, Corsetti A, Gobetti M. 2000. Purification and characterization of antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 66:2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lonner C, Preve-Åkesson K. 1989. Effects of lactic acid bacteria on the properties of sourdough bread. Food Microbiol. 6:19–35 [Google Scholar]
- 37. Pepe O, Blaiotta G, Moschetti G, Greco T, Villani F. 2003. Rope-producing strains of Bacillus spp. from wheat bread and strategy for their control by lactic acid bacteria. Appl. Environ. Microbiol. 69:2321–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Röcken W. 1996. Applied aspects of sourdough fermentation. Adv. Food Sci. 18:212–216 [Google Scholar]
- 39. Monchois V, Willemot RM, Monsan P. 1999. Glucansucrases: mechanism of action and structure-function relationships. FEMS Microbiol. Rev. 23:131–151 [DOI] [PubMed] [Google Scholar]
- 40. Barros RR, Carvalho MG, Peralta JM, Facklam RR, Teixeira LM. 2001. Phenotypic and genotypic characterization of Pediococcus strains isolated from human clinical source. J. Clin. Microbiol. 39:1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spiegel JE, Rose R, Karabell P, Frankos VH, Shmiddt F. 1994. Safety and benefits of fructooligosaccharides as food ingredients. Food Technol. 1:85–89 [Google Scholar]
- 42. McKellar RC, Modler HW. 1989. Metabolism of fructooligosaccharides by Bifidobacterium spp. Appl. Microbiol. Biotechnol. 31:537–541 [Google Scholar]
- 43. Pomeranz Y, Shogren M, Finney KF, Bechtel DB. 1977. Fiber in bread making—effect on functional properties. Cereal Chem. 54:25–41 [Google Scholar]
- 44. Tomomatsu H. 1994. Health effects of oligosaccharides. Food Technol. 10:61–65 [Google Scholar]
- 45. Wang Y, Li C. 2001. Effect of dietary fibre on diabetes patient. Shipin Gongye Keji 22:25–27 [Google Scholar]
- 46. Bouhnik Y, Flouriè B, D'Agay-Abensour L, Pochert P, Gramet G, Durand M, Rambaud JC. 1997. Administration of transgalactooligosaccharides increases faecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J. Nutr. 127:444–448 [DOI] [PubMed] [Google Scholar]
- 47. Tokunaga T, Nakada Y, Yasuhito T, Hirayama M, Hidemasa H. 1993. Effects of fructooligosaccharides intake on the intestinal microflora and defecation in healthy volunteers. Bifidus 6:143–150 [Google Scholar]
- 48. Cinquin C, Gwenae LB, Ismail F, Lacroix C. 2006. Comparative effects of exopolysaccharides from lactic acid bacteria and fructo-oligosaccharides on infant gut microbiota tested in an in vitro colonic model with immobilized cells. FEMS Microbiol. Ecol. 57:226–238 [DOI] [PubMed] [Google Scholar]
- 49. Dal Bello G, Padin S, Lopez Lastra C, Fabrizio M. 2000. Laboratory evaluation of chemical-biological control of the rice weevil (Sitophilus oryzae L.) in stored grains. J. Stored Prod. Res. 37:77–84 [DOI] [PubMed] [Google Scholar]
- 50. Ruijssenaars HJ, Hartmans S, Verdoes JC. 2000. A novel gene encoding xanthan lyase of Paenibacillus alginolyticus strain XL-1. Appl. Environ. Microbiol. 66:3945–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ruas-Madiedo P, de los Reyes-Gavilá CG. 2005. Methods for the screening, isolation and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 88:843–856 [DOI] [PubMed] [Google Scholar]
- 52. Mountzouris KC, Tsistsikos P, Kalamara E, Nitsh S, Schatzmayr G, Fegeros K. 2007. Evaluation of efficacy of a prebiotic containing Lactobacillus, Bifidobacterium, Enterococcus and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 86:309–317 [DOI] [PubMed] [Google Scholar]
- 53. Ng PKW, Slominski E, Johonson WJ, Bushuk W. 1990. Changes in wheat endosperm proteins during grain maturation, p 740–754 In Bushuk W, Tkachuk R. (ed), Gluten proteins. American Association of Cereal Chemists, St. Paul, MN [Google Scholar]