Abstract
Melissococcus plutonius is a fastidious honeybee pathogen, and the addition of KH2PO4 to culture medium is required for its growth. Using genome sequences and a newly developed vector, we showed that mutations in genes encoding Na+/H+ antiporter and cation-transporting ATPase are involved in the potassium requirement for growth.
TEXT
European foulbrood is an important bacterial disease of honeybee larvae. The causative agent, Melissococcus plutonius, is a fastidious organism, requiring microaerophilic to anaerobic conditions and carbon dioxide for growth. In addition, the Na/K ratio required for growth is described to be 1 or less (1), and thus, the addition of KH2PO4 to culture medium is required for the growth of typical M. plutonius strains.
Although M. plutonius had been thought to be remarkably homogeneous (2–4), Arai et al. (5) recently reported atypical M. plutonius strains and demonstrated that M. plutonius is a more heterogeneous species than previously believed. The atypical M. plutonius was not fastidious, and the addition of KH2PO4 was not required for its normal growth. Moreover, unlike typical M. plutonius, it was positive for β-glucosidase activity, hydrolyzed esculin, and produced acid from l-arabinose, d-cellobiose, and salicin (5). Furthermore, although typical M. plutonius is known to lose its virulence quickly when subcultured in vitro, atypical M. plutonius maintained virulence even after repeated subculture (5). Because of these interesting phenotypic differences, comparative analysis of typical and atypical M. plutonius is expected to be a major breakthrough for research on the physiology and pathogenesis of M. plutonius. For such analysis, genome sequencing of M. plutonius type strain ATCC 35311 (typical) and strain DAT561 (atypical) has recently been completed (6, 7). However, the genetic factors responsible for the phenotypic differences between them have yet to be investigated.
In our studies on M. plutonius, we noted that culture variants arise at low frequencies (4.62 × 10−7 to 3.52 × 10−6) from ATCC 35311. Unlike ATCC 35311, the variants grew well even on media not supplemented with KH2PO4 (medium 6 and brain heart infusion [BHI] agar; see Table S1 in the supplemental material), implying that, in these variants, mutations have occurred in genes related to the cultural characteristics. Therefore, in this study, to identify genes involved in the potassium requirement, we determined the draft genome sequence of a culture variant (DAT628) and compared the data with that of the parental strain, ATCC 35311.
Selection of candidate genes involved in the potassium requirement for growth.
Chromosomal DNA of DAT628 was extracted as described previously (5), and draft genome sequencing was performed at Hokkaido System Science (Sapporo, Japan), using an Illumina GA II sequencing system. Obtained sequences were 2,051,268 kb (991.6-fold coverage) and were assembled into 40 contigs. Sequencher version 4.8 (Hitachi Software Engineering, Yokohama, Japan) and the BLAST programs (http://www.ncbi.nlm.nih.gov/BLAST) were used for the sequence analysis.
Comparative analysis of the draft genome sequence and reported genome sequences (DDBJ/EMBL/GenBank accession numbers AP012200 and AP012201) revealed that, in DAT628, putative Na+/H+ antiporter genes (MPTP_0420 and MPTP_0421) of ATCC 35311 were fused to a single open reading frame (ORF) (MPTP_0420-0421) due to a single nucleotide insertion in MPTP_0421 (Fig. 1A) (accession number AB778545). Interestingly, the same insertion also occurred in other variants independently obtained from ATCC 35311, while such an insertion was not present in all typical M. plutonius strains used in a previous study (5) (data not shown). Comparison of the deduced amino acid sequences with the homologues of other bacteria by BLASTp suggested that the fused gene (designated napA according to the name of the homologues of other species) is intact and functional, whereas MPTP_0420 and MPTP_0421 of ATCC 35311 are disrupted. In Enterococcus hirae, activities of sodium pumps, including Na+/H+ antiporter and Na+-ATPase, are necessary for normal growth in Na+-rich medium, whereas K+-rich medium is required for normal growth of a mutant lacking these sodium pumps (8). Therefore, disruption of napA might cause the inability of typical M. plutonius to grow in Na+-rich medium, while restoration of the gene might give DAT628 the ability to grow without the addition of KH2PO4.
Fig 1.
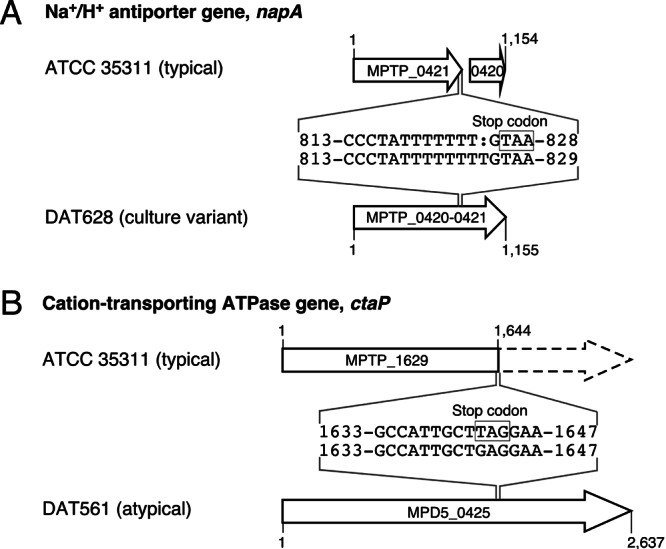
Genetic maps of napA (MPTP_0420 and MPTP_0421) and ctaP (MPTP_1629) regions of M. plutonius ATCC 35311 (typical M. plutonius) and the corresponding regions of DAT628 (culture variant of ATCC 35311) and DAT561 (atypical M. plutonius). The numbers indicate nucleotide positions in the ORFs. MPTP_0420 and MPTP_0421 of ATCC 35311 were fused to a single ORF in DAT628 due to a single nucleotide insertion in MPTP_0421. MPTP_1629 of ATCC 35311 was truncated due to a nonsense mutation, whereas the corresponding gene of DAT561 (MPD5_0425) was expected to be intact.
On the basis of these results, we supposed that atypical M. plutonius possesses the intact napA gene. However, the genome sequence data of atypical strain DAT561 (accession numbers AP012282 and AP012283) showed that DAT561 lacks genes corresponding to napA (see Fig. S1A in the supplemental material). Genomic Southern hybridization analysis confirmed the absence of the napA homologue in all atypical strains tested (see Fig. S1B). Therefore, to identify genes involved in the growth of atypical M. plutonius in Na+-rich medium such as medium 6 and BHI (see Table S1), we selected three additional genes of ATCC 35311 (MPTP_1078, MPTP_1579, and MPTP_1629) and their corresponding genes in DAT561 (MPD5_0870, MPD5_0470, and MPD5_0425, respectively) from genome sequence data of the strains (Table 1). MPTP_1078/MPD5_0870 is annotated as Na+/H+ antiporter, whereas MPTP_1579/MPD5_0470 and MPTP_1629/MPD5_0425 are annotated as cation transport and cation-transporting ATPase, respectively. Although there were several differences in deduced amino acid sequences of MPTP_1078/MPD5_0870 (designated nhaP according to the name of the homologues of other species) and MPTP_1579/MPD5_0470 (designated ctaM for putative cation transport ATPase of Melissococcus) between ATCC 35311 and DAT561, these genes seemed to be intact in both strains. On the other hand, although the cation-transporting ATPase gene (MPD5_0425) of DAT561 seemed to be intact, the corresponding gene (MPTP_1629) of ATCC 35311 had a nonsense mutation (Fig. 1B). The same nonsense mutation was also present in all typical M. plutonius strains and the culture variant DAT628, whereas such mutation was not present in all atypical M. plutonius strains used in our previous study (5) (data not shown), implying that the mutation in MPTP_1629 is another factor that makes typical M. plutonius fastidious. Therefore, we designated MPTP_1629/MPD5_0425 ctaP (for putative cation-transporting ATPase involved in potassium requirement).
Table 1.
State of genes that might be involved in the potassium requirement of M. plutonius
| Gene | Locus tag(s) in ATCC 35311 (accession no.)a | Annotation in ATCC 35311 | Locus tag in DAT561 (accession no.)a | State |
||
|---|---|---|---|---|---|---|
| ATCC 35311/DAT584 (typical) | DAT628 (variant of ATCC 35311) | DAT561 (atypical) | ||||
| napA | MPTP_0420 and MPTP_0421 (AB778538) | Na+/H+ antiporter | Truncated due to a frameshift mutation | Presentb | Absent | |
| nhaP | MPTP_1078 (AB778539) | Na+/H+ antiporter | MPD5_0870c (AB778542) | Presentd | Presentd | Presentd |
| ctaM | MPTP_1579 (AB778540) | Cation transport ATPase | MPD5_0470 (AB778543) | Presente | Presente | Presente |
| ctaP | MPTP_1629 (AB778541) | Cation-transporting ATPase, E1-E2 family | MPD5_0425 (AB778544) | Truncated due to a nonsense mutation | Truncated due to a nonsense mutation | Present |
Selected genes were resequenced with the primers listed in Table S2 in the supplemental material by the Sanger method.
MPTP_0420 and MPTP_0421 of ATCC 35311 were fused to a single open reading frame due to a single nucleotide insertion in MPTP_0421 (Fig. 1A).
Although MPD5_0870 of DAT561 is annotated as a pseudogene in the genome sequence data (accession number AP012282), resequencing of the region by the Sanger method revealed that there is a single nucleotide sequence error in MPD5_0870, and so this gene was considered an intact gene.
Amino acid sequence identity: 100% between ATCC 35311/DAT584 and DAT628 and 99.4% between ATCC 35311/DAT584 and DAT561.
Amino acid sequence identity: 100% between ATCC 35311/DAT584 and DAT628 and 99.6% between ATCC 35311/DAT584 and DAT561.
Development of a novel gene expression system for M. plutonius.
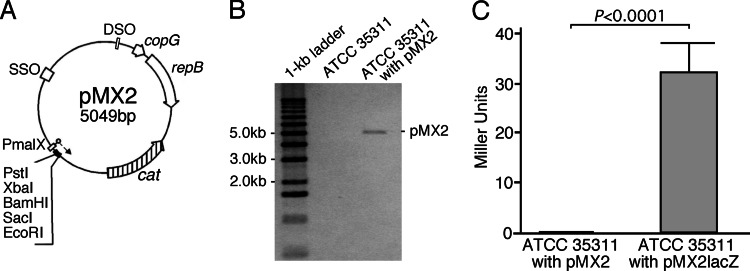
To investigate the effect of the mutations found in ATCC 35311 on the cultural characteristics, complementation analyses using gene expression systems are necessary. However, because no one has ever performed gene manipulations in M. plutonius, we constructed a novel gene expression vector on the basis of Streptococcus suis gene expression vector pMX1 (9). pMX1 consists of the broad-host-range replication origin, S. suis malX promoter, multiple cloning sites, and spectinomycin resistance gene. However, because M. plutonius strains were relatively resistant to spectinomycin (MIC, 128 to 512 μg/ml on KSBHI agar) but susceptible to chloramphenicol (MIC, 2 to 4 μg/ml on KSBHI agar) (D. Takamatsu, unpublished data), we replaced the spectinomycin resistance gene with the chloramphenicol resistance gene (cat). A fragment containing the replication origin, malX promoter, and multiple cloning sites of pMX1 and the cat gene of pSET6s (10) was amplified by the primers listed in Table S2 in the supplemental material. Amplified fragments were digested with XhoI, ligated to each other using a DNA ligation kit (TaKaRa Bio, Otsu, Japan), and introduced into Escherichia coli MC1061 by electroporation using standard procedures (11). Transformants were then selected on Luria-Bertani agar (Becton, Dickinson, Sparks, MD) containing 15 μg/ml chloramphenicol, and one of the candidate plasmids, the structure of which was confirmed by sequencing, was designated pMX2 (Fig. 2A).
Fig 2.
(A) Physical map of pMX2. The unique restriction cleavage sites are indicated in the map. SSO, single-strand origin; DSO, double-strand origin; copG, transcriptional repressor protein gene; repB, replication initiation-termination protein gene; cat, chloramphenicol resistance gene; PmalX, malX promoter of S. suis. (B) Genomic Southern hybridization analysis to confirm introduction of pMX2 into M. plutonius. Genomic DNAs of ATCC 35311 and ATCC 35311 transformed with pMX2 were digested with EcoRI, separated by agarose gel electrophoresis, and probed with the cat probe. (C) β-Galactosidase activity of ATCC 35311 transformed with pMX2 and pMX2lacZ. In pMX2lacZ, the ribosomal binding site of the B. subtilis spoVG gene and the promoterless lacZ reporter gene of E. coli are located downstream of PmalX. Data were collected from six independent experiments. β-Galactosidase activity is expressed as Miller units (mean ± standard deviation). Differences in β-galactosidase activity were compared by the unpaired t test, using the Welch modification.
We then designed an electroporation protocol for M. plutonius (Table 2) on the basis of the protocol for S. suis (12) and the results of our preliminary experiments (data not shown). Transformation of M. plutonius ATCC 35311 with pMX2 by this method yielded chloramphenicol-resistant colonies at an average efficiency of 1.76 × 103 colonies/μg DNA in five independent trials. The colonies were positive for M. plutonius-specific PCR (13). In addition, genomic Southern hybridization performed as described previously (14) using the cat probe amplified from pMX2 by primers catIF and catIR (see Table S2 in the supplemental material) confirmed the presence of pMX2 in the M. plutonius cells (Fig. 2B); that is, M. plutonius was successfully transformed by this method.
Table 2.
Electroporation protocol for M. plutonius developed in this study
| Step no. | Protocol |
|---|---|
| 1 | Grow M. plutonius strains on KSBHI agar at 35°C for 3 days under anaerobic conditions |
| 2 | Harvest cells, suspend them in sterilized deionized distilled water to an OD600e of 0.8, and inoculate 2 ml of the bacterial suspension into 500 mla of KSBHI broth containing 40 mM dl-threonine |
| 3 | Grow M. plutonius cells at 35°C under anaerobic conditions |
| 4 | Stop culturing M. plutonius at early logarithmic phase (OD600 = 0.05 to 0.1) |
| 5 | Chill the culture on ice for 15 to 30 min and collect cells by centrifugation at 12,000 × g for 15 min at 4°Cb |
| 6 | Wash the cells once with 1/50 to 1/100 culture vol of ice-cold CTB (55 mM MnCl2, 15 mM CaCl2, 250 mM KCl, and 10 mM PIPES, pH 6.7), resuspend the cells in 1/50 to 1/100 culture vol of ice-cold CTB, and incubate the suspension on ice for 30 min |
| 7 | Collect the cells by centrifugation at 12,000 × g for 5 to 15 min at 4°C |
| 8 | Wash the cells three times with 1/50 to 1/100 culture vol of ice-cold EB (2 mM potassium phosphate containing 10% sucrose, pH 8.4) and once with 1/Xc culture vol of ice-cold EB containing 15% glycerol and resuspend the final cell pellet in 1/Xc culture vol of ice-cold EB containing 15% glycerold |
| 9 | Add plasmid DNA (1 μg) to an 80-μl aliquot of ice-cold cells and mix gently |
| 10 | Pulse the cell-DNA mixture (20.0 kV/cm, 200 Ω, and 25 μF) in a cold 0.1-cm-gap electroporation cuvette, add 920 μl of room-temp KSBHI broth containing 10% glucose, and disperse the cells with gentle pipetting |
| 11 | Plate on KSBHI agar containing 4 μg/ml chloramphenicol immediately and incubate at 35°C for 4 to 5 days under anaerobic conditions |
Scale up or scale down, depending on the application.
It is important to keep the cells at 4°C or on ice for the remainder of the procedure. The cells, and any bottles or solutions that they come in contact with, must be prechilled on ice.
X = 50/OD600 of M. plutonius culture at step 4.
Although cells are suspended in the buffer containing glycerol, freeze-thaw procedures drastically reduce the transformation efficiency.
OD600, optical density at 600 nm.
To investigate if the malX promoter in pMX2 could work in M. plutonius, the ribosomal binding site (RBS) of the Bacillus subtilis spoVG gene and the promoterless β-galactosidase gene (lacZ) of E. coli were amplified from pEVP3 (15) by primers LacZ1 and LacZ2.2 (see Table S2 in the supplemental material) and cloned into BamHI and EcoRI sites of pMX2. The resultant plasmid pMX2lacZ was introduced into ATCC 35311, and production of β-galactosidase was assessed according to the method of Miller (16) after growing the transformant for 3 days at 37°C on KSBHI agar containing 6 μg/ml chloramphenicol. As shown in Fig. 2C, β-galactosidase activity of ATCC 35311 transformed with pMX2lacZ was significantly higher than that with pMX2, confirming that the malX promoter can work in M. plutonius. These results suggest that the gene expression system developed in this study can be used for complementation of M. plutonius.
Complementation analysis.
We then constructed Na+/H+ antiporter and cation-transporting ATPase gene expression vectors using pMX2. For construction of the napA expression vector (pDAT628NapA), a DNA fragment containing the coding region and RBS of napA was amplified from DAT628 by the primers listed in Table S2 in the supplemental material, cloned into pCR2.1 (Invitrogen, Carlsbad, CA), and then subcloned into PstI and EcoRI sites of pMX2. For construction of ctaP, ctaM, and nhaP expression vectors (pDAT561CtaP, pDAT561CtaM, and pDAT561NhaP, respectively), fragments containing the coding regions and RBSs were amplified from DAT561 by the primers listed in Table S2 and cloned into PstI and BamHI sites (ctaP) or the PstI site (ctaM and nhaP) of pMX2. The resultant plasmids were introduced into a typical M. plutonius strain, and the cultural characteristics were examined under anaerobic conditions. Because culture variants arise spontaneously from ATCC 35311, this strain is not suitable as a host for complementation. On the other hand, because such variants rarely arose from typical strain DAT584 (frequency, <3.53 × 10−9), DAT584 was used as the host. As controls, DAT584 and atypical strain DAT561 were also transformed with pMX2.
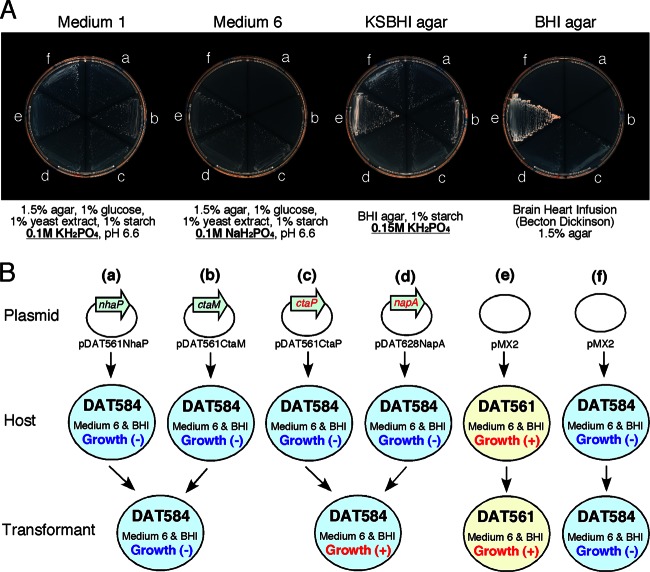
As expected, although DAT561 transformed with pMX2 grew under all conditions tested, DAT584 transformed with pMX2 grew only on media supplemented with KH2PO4 (medium 1 and KSBHI agar) (Fig. 3). However, when DAT584 was transformed with pDAT628NapA or pDAT561CtaP, the transformants grew not only on medium supplemented with KH2PO4 but also on medium not supplemented with KH2PO4 (BHI) or supplemented with NaH2PO4 (medium 6). On the other hand, the cultural characteristics of DAT584 were not changed by transformation with pDAT561CtaM or pDAT561NhaP (Fig. 3). Because chromosomal genes corresponding to napA and ctaP were still disrupted in the DAT584 transformants (data not shown), these results demonstrated that introduced functional napA or ctaP affected the cultural characteristics of DAT584.
Fig 3.
Complementation analysis. (A) Growth of M. plutonius strains transformed with various expression vectors on various agar plates. M. plutonius strains were cultured at 35°C for 1 week under anaerobic conditions. a, DAT584 + pDAT561NhaP (Na+/H+ antiporter gene expression vector); b, DAT584 + pDAT561CtaM (cation transport ATPase gene expression vector); c, DAT584 + pDAT561CtaP (cation-transporting ATPase gene expression vector); d, DAT584 + pDAT628NapA (Na+/H+ antiporter gene expression vector); e, DAT561 + pMX2 (control); f, DAT584 + pMX2 (control). (B) Schematic representations of the procedures and results of the complementation analysis.
Conclusion.
Our results indicate that the potassium requirement for the growth of typical M. plutonius is associated with loss of function of a putative Na+/H+ antiporter gene (napA) and a cation-transporting ATPase gene (ctaP) and that imparting either functional napA or ctaP to typical M. plutonius is sufficient to remove the potassium requirement of the strains. Potassium is the major intracellular cation in bacteria as well as in eukaryotic cells (17), and the accumulation of K+ is known to play a primary role in maintaining the osmotic balance of the cell in some bacteria (18). In E. hirae, it was speculated that sodium extrusion systems eliminate Na+ from cytoplasm to make room for K+ accumulation (8). Although further analysis is needed to show if products encoded by napA and ctaP really function as sodium pumps, typical M. plutonius, which may not be able to eliminate Na+ from the cytoplasm, may require the addition of potassium salts to make the environmental K+ concentration high and take K+ in efficiently by osmotic pressure. In nature, M. plutonius multiplies in the larval gut of the honeybee (19). Honeybee larvae are fed with royal (or worker) jelly, honey, and pollen, and they are known to contain more potassium than sodium (20–22). Therefore, larval gut contents are considered to represent high-K+ conditions. Because of this environment, typical M. plutonius may be able to multiply in larvae.
In this study, we identified mutations involved in a phenotypic difference between typical and atypical M. plutonius using a newly developed plasmid vector. Although M. plutonius was originally described a century ago (23), our understanding of its physiology and pathogenesis remains very limited. A lack of tools and genome information for molecular approaches was a factor that hampered studies of M. plutonius. Although there is room for improvement in the transformation efficiency, our genetic tools in combination with the genome sequences of typical and atypical M. plutonius should promote future research on this important honeybee pathogen.
Nucleotide sequence accession numbers.
The sequences determined in this study have been deposited in the DDBJ/EMBL/GenBank database under the accession numbers AB778538 to AB778545.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a Grant-in-Aid for Scientific Research (C) (22580345) from the Japan Society for the Promotion of Science and by a Sasakawa Scientific Research Grant from The Japan Science Society.
pEVP3 was a gift from Donald A. Morrison. We thank Toshio Fujisawa for preparing photographs.
Footnotes
Published ahead of print 12 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00598-13.
REFERENCES
- 1. Bailey L, Collins MD. 1982. Reclassification of ‘Streptococcus pluton’ (White) in a new genus Melissococcus, as Melissococcus pluton nom. rev.; comb. nov. J. Appl. Bacteriol. 53:215–217 [Google Scholar]
- 2. Allen MF, Ball BV. 1993. The cultural characteristics and serological relationships of isolates of Melissococcus pluton. J. Apic. Res. 32:80–88 [Google Scholar]
- 3. Bailey L, Gibbs AJ. 1962. Cultural characters of Streptococcus pluton and its differentiation from associated enterococci. J. Gen. Microbiol. 28:385–391 [DOI] [PubMed] [Google Scholar]
- 4. Djordjevic SP, Smith LA, Forbes WA, Hornitzky MA. 1999. Geographically diverse Australian isolates of Melissococcus pluton exhibit minimal genotypic diversity by restriction endonuclease analysis. FEMS Microbiol. Lett. 173:311–318 [DOI] [PubMed] [Google Scholar]
- 5. Arai R, Tominaga K, Wu M, Okura M, Ito K, Okamura N, Onishi H, Osaki M, Sugimura Y, Yoshiyama M, Takamatsu D. 2012. Diversity of Melissococcus plutonius from honeybee larvae in Japan and experimental reproduction of European foulbrood with cultured atypical isolates. PLoS One 7:e33708 doi: 10.1371/journal.pone.0033708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okumura K, Arai R, Okura M, Kirikae T, Takamatsu D, Osaki M, Miyoshi-Akiyama T. 2011. Complete genome sequence of Melissococcus plutonius ATCC 35311. J. Bacteriol. 193:4029–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okumura K, Arai R, Okura M, Kirikae T, Takamatsu D, Osaki M, Miyoshi-Akiyama T. 2012. Complete genome sequence of Melissococcus plutonius DAT561, a strain that shows an unusual growth profile and is representative of an endemic cluster in Japan. J. Bacteriol. 194:3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikegami M, Takahashi H, Igarashi K, Kakinuma Y. 2000. Sodium ATPase and sodium/proton antiporter are not obligatory for sodium homeostasis of Enterococcus hirae at acid pH. Biosci. Biotechnol. Biochem. 64:1088–1092 [DOI] [PubMed] [Google Scholar]
- 9. Okura M, Osaki M, Fittipaldi N, Gottschalk M, Sekizaki T, Takamatsu D. 2011. The minor pilin subunit Sgp2 is necessary for assembly of the pilus encoded by the srtG cluster of Streptococcus suis. J. Bacteriol. 193:822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takamatsu D, Osaki M, Sekizaki T. 2001. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46:140–148 [DOI] [PubMed] [Google Scholar]
- 11. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 12. Takamatsu D, Osaki M, Sekizaki T. 2001. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid 45:101–113 [DOI] [PubMed] [Google Scholar]
- 13. Govan VA, Brözel V, Allsopp MH, Davison S. 1998. A PCR detection method for rapid identification of Melissococcus pluton in honeybee larvae. Appl. Environ. Microbiol. 64:1983–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sekizaki T, Otani Y, Osaki M, Takamatsu D, Shimoji Y. 2001. Evidence for horizontal transfer of SsuDAT1I restriction-modification genes to the Streptococcus suis genome. J. Bacteriol. 183:500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Claverys JP, Dintilhac A, Pestova EV, Martin B, Morrison DA. 1995. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164:123–128 [DOI] [PubMed] [Google Scholar]
- 16. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 17. Epstein W. 2003. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 75:293–320 [DOI] [PubMed] [Google Scholar]
- 18. Sutherland L, Cairney J, Elmore MJ, Booth IR, Higgins CF. 1986. Osmotic regulation of transcription: induction of the proU betaine transport gene is dependent on accumulation of intracellular potassium. J. Bacteriol. 168:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forsgren E. 2010. European foulbrood in honey bees. J. Invertebr. Pathol. 103(Suppl 1):S5–S9 doi: 10.1016/j.jip.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 20. Chua LS, Abdul-Rahaman NL, Sarmidi MR, Aziz R. 2012. Multi-elemental composition and physical properties of honey samples from Malaysia. Food Chem. 135:880–887 [DOI] [PubMed] [Google Scholar]
- 21. Orzáez Villanueva MT, Díaz Marquina A, Bravo Serrano R, Blázquez Abellán G. 2001. Mineral content of commercial pollen. Int. J. Food Sci. Nutr. 52:243–249 [DOI] [PubMed] [Google Scholar]
- 22. Stocker A, Schramel P, Kettrup A, Bengsch E. 2005. Trace and mineral elements in royal jelly and homeostatic effects. J. Trace Elem. Med. Biol. 19:183–189 [DOI] [PubMed] [Google Scholar]
- 23. White GF. 1912. The cause of European foulbrood. US Department of Agriculture Bureau of Entomology circular no. 157. US Department of Agriculture, Washington, DC [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.