Abstract
Despite the substantial beneficial effects of incorporating the 7-valent pneumococcal conjugate vaccine (PCV7) into immunization programs, serotype replacement has been observed after its widespread use. As there are many serotypes currently documented, the use of a conjugate vaccine relying on protective pneumococcal proteins as active carriers is a promising alternative to expand PCV coverage. In this study, capsular polysaccharide serotype 6B (PS6B) and recombinant pneumococcal surface protein A (rPspA), a well-known protective antigen from Streptococcus pneumoniae, were covalently attached by two conjugation methods. The conjugation methodology developed by our laboratory, employing 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) as an activating agent through carboxamide formation, was compared with reductive amination, a classical methodology. DMT-MM-mediated conjugation was shown to be more efficient in coupling PS6B to rPspA clade 1 (rPspA1): 55.0% of PS6B was in the conjugate fraction, whereas 24% was observed in the conjugate fraction with reductive amination. The influence of the conjugation process on the rPspA1 structure was assessed by circular dichroism. According to our results, both conjugation processes reduced the alpha-helical content of rPspA; reduction was more pronounced when the reaction between the polysaccharide capsule and rPspA1 was promoted between the carboxyl groups than the amine groups (46% and 13%, respectively). Regarding the immune response, both conjugates induced functional anti-rPspA1 and anti-PS6B antibodies. These results suggest that the secondary structure of PspA1, as well as its reactive groups (amine or carboxyl) involved in the linkage to PS6B, may not play an important role in eliciting a protective immune response to the antigens.
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) remains a leading cause of bacterial infectious diseases, particularly in children less than 2 years of age. About 800,000 children die annually due to pneumococcal disease, especially in emerging countries (1). The increasing number of antibiotic-resistant strains (2) and the severity of pneumococcal diseases make vaccination the most effective intervention.
Polysaccharide (PS) capsules are the main virulence factor of the pneumococci, which function by preventing phagocytosis and hampering bacterial clearance. Due to their high immunogenicity and importance in bacterial pathogenesis, PSs have been the antigens of choice in all current vaccines. The 23-valent pneumococcal polysaccharide vaccine (PPV23; Merck) has been shown to cover 80% to 90% of the serotypes responsible for invasive pneumococcal disease (IPD) in developed countries (3). According to a meta-analysis of randomized trials, the administration of PPV in immunocompetent adults can reduce the incidence of IPD and death due to pneumonia in this population by 71% and 32%, respectively. Conversely, pneumococcal polysaccharide vaccines are not effective in children under 2 years of age (4). The inefficacy of PS vaccines in this population has been attributed to the immaturity of the infant immune system in the expression of B cell receptors, including complement receptor type 2 (CR2) (5, 6).
Conjugation of PSs to carrier proteins converts it from a T cell-independent to a T cell-dependent antigen. As a T cell-dependent antigen, PS can raise a response with isotype switching, generation of memory cells, and a boosting effect (7).
The first pneumococcal conjugate vaccine (PCV) was licensed in 2000 as a 7-valent formulation (PCV7; Pfizer), which included capsular polysaccharides 4, 6B, 9V, 14, 18C, 19F, and 23F conjugated to the nontoxic variant of diphtheria toxin (CRM197). In spite of the high degree of effectiveness of PCV7 in reducing pneumococcal diseases (8–12), recent reports have described an increase in the rate of disease caused by serotypes not included in this vaccine (13–15). The current pneumococcal vaccine strategy involves extending protection against emerging serotypes by increasing the valence to target additional serotypes (PCV13 [Pfizer], PCV10 [GlaxoSmithKline], PCV15 [in development by Merck]). An alternative to this trend could be the use of pneumococcal surface proteins as carriers conjugated to PSs from a few of the most common serotypes. The replacement of the same universal carrier proteins, such as tetanus toxoid (TT) or CRM197, by a pneumococcal protein, besides broadening the vaccine coverage, would also prevent the impairment of immune responses caused by the excessive use of the same proteins in commercial vaccines (16, 17). In this study, we reinforce the use of pneumococcal surface protein A (PspA) as a promising carrier protein.
PspA is described to be an important pneumococcal virulence factor for inhibiting complement deposition (18, 19) and for protecting pneumococci from killing by apolactoferrin (20). This protein is widely known to be immunogenic and protective (21, 22) and is present in all pneumococcal strains (23). According to sequence identities, PspA molecules have been classified into families and clades: family 1 (clades 1 and 2), family 2 (clades 3, 4, and 5), and family 3 (clade 6) (24). More than 90% of clinical isolates are distributed in family 1 or family 2 (25, 26).
Our group has previously demonstrated that conjugation of recombinant PspA (rPspA) to different PSs either maintains or increases its immunogenicity: (i) rPspA family 1, clade 1, conjugated to PS23F induced higher protection against lethal challenge than the nonconjugated rPspA (52), and (ii) rPspA family 2, clade 3, conjugated to polysaccharide serotype 14 (PS14) induced antibodies with a higher efficiency in complement deposition and higher opsonophagocytic activity than the nonconjugated protein (27). To extend these studies, PS6B was conjugated to rPspA family 1, clade 1, using two different methods of conjugation: the chemical linkage of PS6B either to the carboxyl groups or to the amine groups of rPspA. The focus of this study was to elucidate the influence of the method of conjugation on the efficiency of coupling PS6B to rPspA, on the secondary structure of the protein, and on the protective immune response induced against each antigen (PS6B and rPspA).
The improvement of conjugation yields also represents a current effort in the development of new conjugates. Improved conjugation yields increase the possibility of achieving an affordable manufacturing process. In the studies described herein, the method of conjugation through the carboxyl groups previously described by us (27, 52) was extended to the conjugation of PS6B with to rPspA clade 1 (rPspA1), and its efficiency was compared with that of reductive amination, a classical method used to obtain PCV7 and PCV13.
MATERIALS AND METHODS
Materials.
Recombinant PspA clade 1 (rPspA1) and S. pneumoniae polysaccharide serotype 6B (PS6B) were produced in the Fermentation Laboratory of the Instituto Butantan (29–32). 1,8-Diaminooctane (OCT) and 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) were from Sigma-Aldrich (St. Louis, MO). S. pneumoniae strains (245/00 and 679/99) were generously supplied by Instituto Adolfo Lutz (São Paulo, Brazil). The strains were maintained as frozen stocks (−80°C) in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) with 10% glycerol.
Polysaccharide activation.
Before activation, PS6B (10 mg/ml) was hydrolyzed with HCl (0.5 M) under agitation at 80°C for 1 h in a reflux system, followed by neutralization with NaOH to pH 7.5. Hydrolyzed PS6B was oxidized with NaIO4 at a final concentration of 10 mM in 10 mM phosphate buffer (pH 7.5) for 30 min in the dark (3:2 molar ratio of the PS6B repeating unit to NaIO4). The reaction was quenched by adding glycerol (10 eq). Oxidized PS6B was purified from the remaining glycerol and from low-molecular-weight oxidation products through chromatography using Sephadex G-25 gel filtration medium packed in an XK 26/40 column (GE Healthcare) and elution with 10 mM phosphate buffer (pH 7.5). The purified oxidized PS6B was lyophilized and resuspended to a final concentration of 10 mg/ml. The extent of oxidation was estimated by the bicinchoninic acid (BCA) colorimetric method (33) with glucose as the standard.
Polysaccharide derivatization.
Oxidized PS6B (10 mg/ml) was incubated with OCT in a ratio of 100 mol of OCT per mol of aldehyde in PS. The reaction proceeded for 24 h in 10 mM phosphate buffer (pH 7.5). Sodium cyanoborohydride (NaBH3CN) was then added at the same proportion used for OCT in order to reduce the Schiff's base generated and to favor the formation of the PS6B-OCT product. Sodium borohydride in a ratio of 100 mol per mol of aldehyde in PS was dissolved in 2% NaOH (final volume, 100 μl) and added to the solution to stop the reaction. The product, PS6B-OCT, was purified by gel filtration chromatography using Sephadex G-25 packed in an XK 26/40 column (GE Healthcare) and elution with 10 mM phosphate buffer (pH 7.5). The extent of the reaction with OCT was estimated by the trinitrobenzenesulfonic acid (TNBS) method (34), using TNBS (Sigma-Aldrich) with OCT as the standard. After purification, the PS6B-OCT was lyophilized and stored at −20°C.
rPspA1 modification.
rPspA1 (15 mg/ml) was treated with formaldehyde (5%) in the presence of a 5 M solution of sodium cyanoborohydride in 2% NaOH (10 μl per ml of reaction mixture) for 5 days at room temperature. Modified PspA1 (mPspA1) was purified by gel filtration chromatography using Sephadex G-25 packed in an XK 26/40 column (GE Healthcare) and eluted with 10 mM phosphate buffer (pH 7.5). The rPspA1 lysine content after the modification reaction was compared to that of rPspA1 by estimation using the TNBS method (34). After purification, mPspA1 was lyophilized and stored at −20°C.
PS6B-rPspA1 conjugation. (i) Conjugation using DMT-MM.
mPspA1 (10 mg/ml) was activated with 0.1 M DMT-MM, followed by the addition of PS6B-OCT (15 mg/ml) (mass ratio, 1:1) in 10 mM phosphate buffer (pH 7.5) for 48 h. The PS6B-OCT-mPspA1 conjugate was dialyzed against 10 mM phosphate buffer (pH 7.5) and purified by hydrophobic chromatography in phenyl-Sepharose 6 Fast Flow High Sub packed in an XK 16/20 column (GE Healthcare), using descending gradient elution from 1 M to 0 M (NH4)2SO4, starting in 50 ml and ending in 189 ml of the elution volume. The chromatography was performed using an ÄKTA Prime system (GE Healthcare) with a flow rate of 3 ml/min. The conjugate fraction was dialyzed against 1 mM sodium phosphate buffer (pH 7.5) and stored lyophilized at −20°C.
(ii) Reductive amination method.
Oxidized PS6B was incubated with rPspA1 (recombinant PspA1 in its native form) for 15 days in a ratio of 1:1 (wt/wt) and final concentration of 5.5 mg/ml each in the presence of sodium cyanoborohydride (twice the PS mass) and 0.1% phenol. After 15 days, sodium borohydride was added to reduce the remaining aldehyde groups. The PS6B-rPspA1 conjugate was dialyzed against 10 mM sodium phosphate buffer (pH 7.5). The product was purified by hydrophobic chromatography in phenyl-Sepharose 6 Fast Flow High Sub packed in an XK 16/20 column (GE Healthcare), using descending gradient elution from 1 M to 0 M (NH4)2SO4, starting in 50 ml and ending in 180 ml of the elution volume. The chromatography was performed using an ÄKTA Prime system (GE Healthcare) with a flow rate of 3 ml/min. The conjugate fraction was dialyzed against 1 mM sodium phosphate buffer (pH 7.5) and stored lyophilized at −20°C.
Analytical procedures. (i) Measurement of PS.
The quantities of PS6B were measured by the phenol-sulfuric acid method (35) with a small modification: the reaction volumes were reduced 5 times, but the proportions of the reagents were maintained. Rhamnose was used as the standard.
(ii) Measurement of protein.
The concentration of rPspA1 was assayed by the method of Lowry (36), using a Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA) and bovine serum albumin as the standard.
(iii) Determination of molecular size.
The molecular size of hydrolyzed PS6B was determined in Sephacryl S-400 packed in an XK16/100 column (GE Healthcare), using 0.2 M NaCl as the mobile phase at 1 ml/min. The column was calibrated with dextrans (Sigma-Aldrich) of known sizes (2,000 kDa, 229 kDa, 70 kDa, 40 kDa, and 10 kDa).
CD analysis.
The circular dichroism (CD) spectra were obtained on a Jasco J-810 spectropolarimeter (Japan Spectroscopic, Tokyo, Japan) at 20°C. The measurements were performed at wavelengths from 185 to 260 nm and intervals of 0.1 nm in a 0.1-cm-path cell. All samples were previously dialyzed against 10 mM sodium phosphate buffer, pH 7.5. The spectra presented are the averages of five scans, and the data obtained were reported as molar ellipticity (degrees·cm2·dmol−1). A baseline measurement with sodium phosphate buffer was subtracted from each spectrum; for each PS-protein conjugate, a measurement with the same amount of PS was also subtracted from the spectrum (the measurements for oxidized PS6B and PS6B-OCT were subtracted from the PS6B-rPspA1 and PS6B-OCT-mPspA1 spectra, respectively). The secondary structure deconvolution analyses were performed with Dichroweb software (37), using the CDSSTR algorithm (38).
Immunization procedures.
BALB/c mice (female, 8 weeks old, and 6 per group) were obtained from the local breeding facility of the Universidade Federal de São Paulo (UNIFESP) and were immunized intraperitoneally (i.p.) on days 1, 14, and 28. The same dose of PS6B (15 μg) was established for both conjugates, and as the mass ratio of PS6B/rPspA1 varied in the conjugates produced, the rPspA1 dose also varied (described in detail in Table 1). The controls (coadministered compounds) were prepared to contain the same mass of protein and PS contained in their respective conjugates. All samples (500 μl per mouse) were prepared in 0.9% saline solution with 50 μg of Al(OH)3 as the adjuvant. Sera were collected from mice on the 41st day by retro-orbital bleeding and kept at −20°C before use.
Table 1.
PS6B and rPspA1 doses used in the immunization protocola
| Group tested | Amt (μg)/dose |
|
|---|---|---|
| PS6B | rPspA1 | |
| Saline + adjuvant | 0 | 0 |
| Coadministered PS6B + rPspA1 (control 1) | 15 | 45 |
| PS6B-rPspA1 (test 1) | 15 | 45 |
| Coadministered PS6B + mPspA1 (control 2) | 15 | 30 |
| PS6B-OCT-mPspA1 (test 2) | 15 | 30 |
The active carrier protein was rPspA1 (family 1, clade 1).
ELISA.
Antibodies to rPspA1 were determined by conventional direct enzyme-linked immunosorbent assay (ELISA). PolySorp 96-well plates (Nunc) were coated with 0.1 μg per well of rPspA1 in 0.05 M sodium bicarbonate buffer (pH 9.6) overnight at 4°C. The plates were then washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) and blocked for 1 h at 37°C with PBS containing nonfat dried milk (10%). After this incubation time, the plates were washed with PBS-T and then incubated with serial dilutions of serum from individual mice in PBS for 1 h at 37°C. The plates were then washed with PBS-T and loaded with peroxidase-conjugated goat anti-mouse IgG (Sigma-Aldrich, St. Louis, MO) in PBS. After a new incubation for 1 h at 37°C, the plates were washed and incubated for 15 min at room temperature in the dark with 40 μg of o-phenylenediamine (Sigma) and 0.5 μl of 3% hydrogen peroxide in 0.1 M citrate buffer (pH 5.0). The reaction was stopped by addition of 50 μl of 4 M sulfuric acid. The optical density was measured at 492 nm using an ELISA reader (Multiskan EX; Labsystems Uniscience) (39). The titer was defined as the dilution of serum that measured 0.1 at an optical density at 492 nm (OD492).
Complement deposition assay.
S. pneumoniae strain 245/00, bearing homologous PspA (serotype 14, PspA clade 1), was plated on blood agar, followed by growth in THY to an OD600 of 0.4 to 0.5 (concentration, approximately 108 CFU/ml). The samples were centrifuged at 4,000 × g for 3 min, and the pellets were washed once with PBS and resuspended in the same buffer. Sera from mice immunized with conjugates or control samples had their complement previously inactivated by heating at 56°C for 30 min and were added to the pneumococcus suspension at a final concentration of 10%; the mixture was incubated for 30 min at 37°C. After this incubation period, the bacteria were washed once with PBS and then incubated with 100 μl per well of 10% fresh-frozen normal mouse serum (NMS) from naive BALB/c mice in gelatin Veronal buffer (Sigma) for 30 min at 37°C. The bacteria were washed again with PBS, followed by another incubation with 100 μl of fluorescein isothiocyanate (FITC)-conjugated goat antiserum to mouse complement C3 (MP Biomedicals) at a dilution of 1:500 on ice for 30 min in the dark. In the last step, the bacteria were washed twice with PBS and then resuspended in 1% formaldehyde for analysis in a FACSCanto flow cytometer (BD Biosciences).
Opsonophagocytic assay (OPA).
S. pneumoniae strains 245/00 (serotype 14, PspA clade 1, used to evaluate the opsonic activity of anti-PspA antibodies) and 679/99 (serotype 6B, PspA clade 3, used to evaluate the opsonic activity of anti-PS6B antibodies) were grown in THY to an OD600 of 0.4 to 0.5 (concentration, approximately 108 CFU/ml) and harvested by centrifugation at 4,000 × g for 3 min. The pellets were washed once with PBS and resuspended in Hank's buffer (Invitrogen) containing 0.1% gelatin. Aliquots of bacteria containing 2.5 × 106 CFU were incubated with heat-inactivated pooled test sera at a final dilution of 1:16 for 30 min at 37°C. Sera from mice injected with saline plus Al(OH)3 were used as a control for all the assays. The samples were then incubated with 10% NMS diluted in opsono-buffer (Hank's buffer containing 0.1% gelatin) at 37°C for 30 min. Following incubation, the samples were washed once with PBS and then incubated with 4 × 105 peritoneal cells diluted in opsono-buffer at 37°C for 30 min with shaking (200 rpm). Peritoneal cells were obtained as previously described (40). The reaction was stopped by cooling on ice for 5 min. Tenfold dilutions of the samples were plated on blood agar plates in triplicate. The plates were incubated at 37°C in a 5% CO2 incubator, and the numbers of pneumococcal CFU recovered were counted after 20 h (40).
Statistical analysis.
The significance of differences in the final pneumococcal counts in the protection studies was assessed using a one-way analysis of variance (ANOVA), followed by Tukey's multiple-comparison test. For all comparisons, a P value of <0.05 was considered to represent statistical significance.
RESULTS
Conjugation and purification of PS6B-rPspA1.
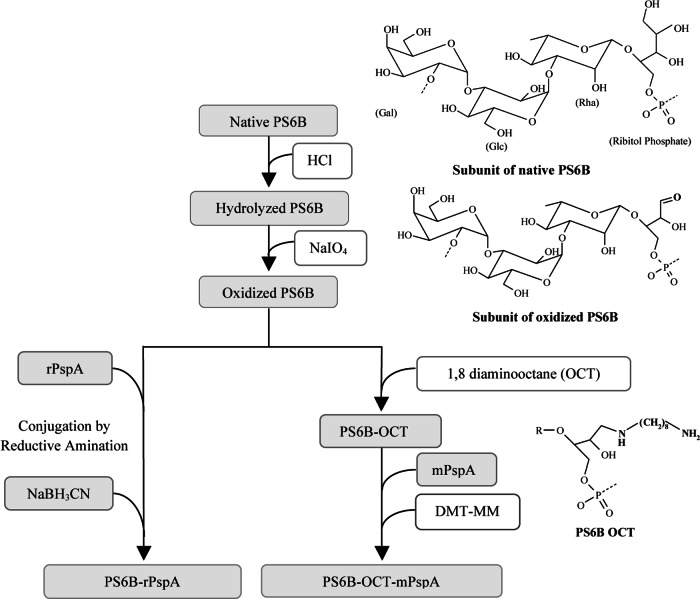
Two different conjugates were synthesized: PS6B-OCT-mPspA1 (with an eight-carbon spacer molecule) and PS6B-rPspA1 (with no spacer molecule). PS6B-OCT-mPspA1 was prepared by the method developed in our laboratory (28). The steps of the conjugation process are represented in Fig. 1 and described above in detail in the experimental protocols. The acid hydrolysis of native PS6B reduced its size from 1,000 kDa to approximately 20 kDa. The aldehyde groups were obtained by a mild oxidation condition with NaIO4 that resulted in 5 aldehydes per PS6B molecule (approximately 0.16 aldehyde per PS6B repeating unit). Eighty percent of the aldehydes inserted in the PS6B molecule were linked to the spacer molecule OCT, resulting in 4 OCT molecules per PS6B (approximately 0.128 OCT molecule per PS6B repeating unit). PS6B-rPspA1 was obtained by the currently used reductive amination method (41), using the same hydrolyzed and oxidized PS.
Fig 1.
Conjugation steps. Native PS6B (1,000 kDa) has its size reduced to 20 kDa by acid hydrolysis. Then, aldehyde groups are produced by oxidation of the PS molecule. This reactive group (aldehyde) reacts directly with amine groups on rPspA by reductive amination (the method applied in commercial vaccines) or with amine groups present in OCT. In the second case, the product, PS6B-OCT, is subsequently reacted with carboxyl groups on mPspA, intermediated by DMT-MM, to form the conjugate. In order to increase the specificity of conjugation with PS6B-OCT, PspA's lysine was previously modified with formaldehyde (mPspA).
The rPspA1 employed in the DMT-MM-mediated conjugation was previously treated with formaldehyde in order to avoid intermolecular reactions and precipitation during conjugate synthesis. This modification process incorporates methyl groups in about 70% of the ε-amine groups of PspA lysine residues; methyl incorporation has been proven not to interfere with rPspA immunogenicity (27).
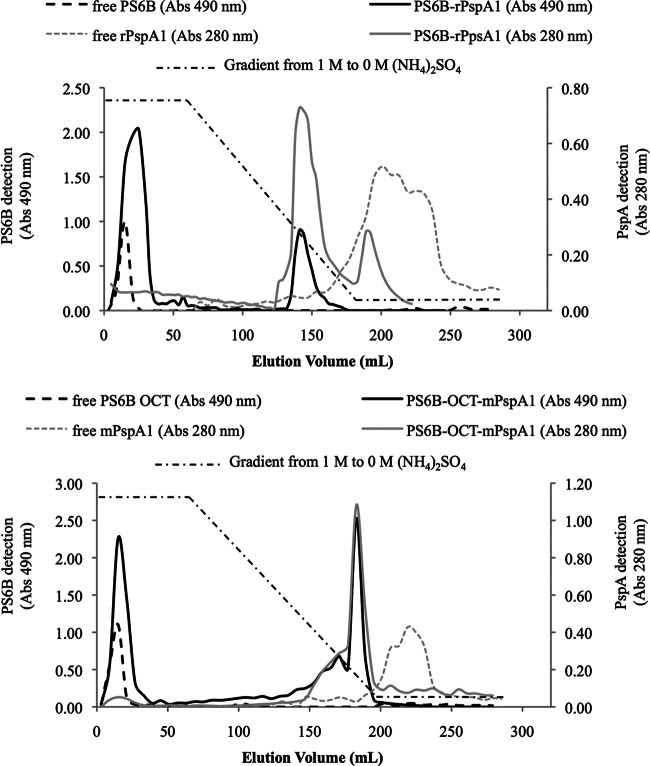
The conjugates were purified using hydrophobic interaction chromatography (HIC) (Fig. 2). PS6B, a hydrophilic molecule, did not bind to phenyl-Sepharose and eluted in the flowthrough fraction. rPspA1, which contains hydrophobic domains, interacted strongly with the resin and eluted after the end of the gradient. The conjugates combine the characteristics of both PS6B and rPspA and eluted in the last third of the decreasing ammonium sulfate gradient, allowing separation of the reagents and products. The conjugate elution was characterized by the coincidence of PS and protein detection in the same elution volume. Figure 2 shows the chromatograms of PS6B-OCT-mPspA (top) and PS6B-PspA (bottom) with 3 overlapping chromatograms each: (i) nonconjugated rPspA1, (ii) nonconjugated PS6B, and (iii) the conjugates. The relative amounts of the conjugated and nonconjugated PSs were measured from the column elution for calculation of the conjugation yields (42). The chromatograms shown for the nonconjugated compounds, rPspA1 and PS6B, are representative of a column loaded with 5 mg, and the chromatograms shown for the conjugates are representative of 25 mg of PS6B.
Fig 2.
Purification of conjugates. Hydrophobic interaction chromatography (phenyl-Sepharose 6 Fast Flow High Sub) of PS6B-rPspA1 conjugate: PS6B-rPspA1 produced by reductive amination (top) and PS6B-OCT-mPspA1 produced by conjugation using DMT-MM (bottom). The chromatograms of PS6B and free rPspA1 are displayed in both panels. The amounts of free PS6B and conjugated PS6B loaded in the column were 5 mg and 25 mg, respectively. Elution was with a decreasing gradient from 1 M to 0 M (NH4)2SO4. The absorbance (Abs) at 490 nm and the absorbance at 280 nm correspond to the measurements for PS6B and rPspA1, respectively.
Using the methodology employing DMT-MM, 55.0% ± 6.0% of the PS was in the PS6B-OCT-mPspA peak, whereas in the reductive amination method, 24.0% ± 2.6% of the PS was associated with the conjugated PS6B-rPspA1 peak. The mass ratios of PS6B/rPspA1 obtained in the conjugates PS6B-OCT-mPspA1 and PS6B-rPspA1 were 1:2 and 1:3, respectively.
CD analysis.
The effect of PS6B conjugation on rPspA secondary structure was analyzed by CD, comparing the CD spectra of conjugates and controls (rPspA1 and mPspA1). As shown in Fig. 3, there was a predominance of alpha-helix structures (78%) in rPspA1. After the treatment of rPspA1 with formaldehyde (mPspA1), the alpha-helix content changed from 78% (rPpsA1) to 61% (mPspA1), a reduction of approximately 22%. The conjugation processes were also shown to disrupt the secondary structure of conjugated rPspA1 compared to that for the rPspA1 control: the reductive amination led to an alpha-helix reduction of 13%, while the process of conjugation by carboxamide formation reduced the alpha-helix content in the protein by 46% (a 22% reduction associated with treatment with formaldehyde and a 24% reduction associated with its conjugation with PS6B). The level of unordered structures increased proportionally to the reduction in the alpha-helix content of the protein.
Fig 3.
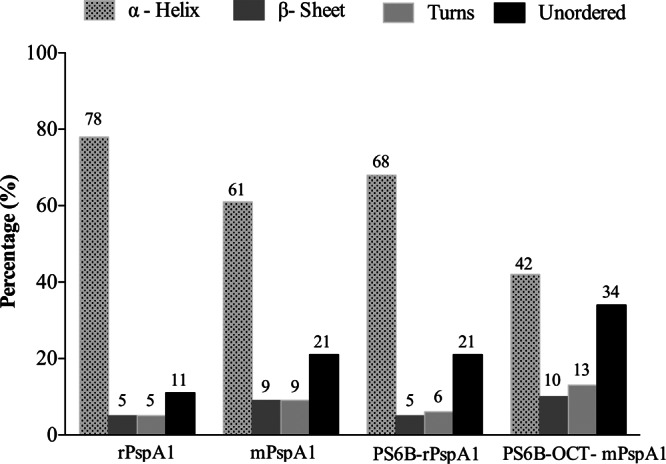
rPspA1 secondary structure following conjugation. The protein secondary structure was assessed by CD. rPspA1 was compared to rPspA1 after modification with formaldehyde (mPspA1) and to rPspA1 after conjugation to PS6B by reductive amination (PS6B-rPspA1) or by conjugation using DMT-MM (PS6B-OCT-mPspA1). CD spectra were obtained on a Jasco J-810 spectropolarimeter at 20°C. The measurements were performed at wavelengths from 185 to 260 nm and intervals of 0.1 nm in a 0.1-cm-path cell. The secondary structure deconvolution analysis was performed with Dichroweb software, using the CDSSTR algorithm.
Immunogenicity of conjugates.
PS6B exhibits low immunogenicity in murine models (43), and its optimal dose ranges from 10 to 20 μg (44). In our immunization protocol, we used the conjugate dose equivalent to 15 μg of PS6B. This implied having different concentrations of rPspA1 per dose in the conjugate groups, since the PS6B/rPspA1 ratio varied in each conjugate. In order to compare the response induced against both antigens before and after conjugation, the controls (native PS6B plus rPspA1 or mPspA1) contained the same amount of PS6B and rPspA1 as the corresponding conjugate.
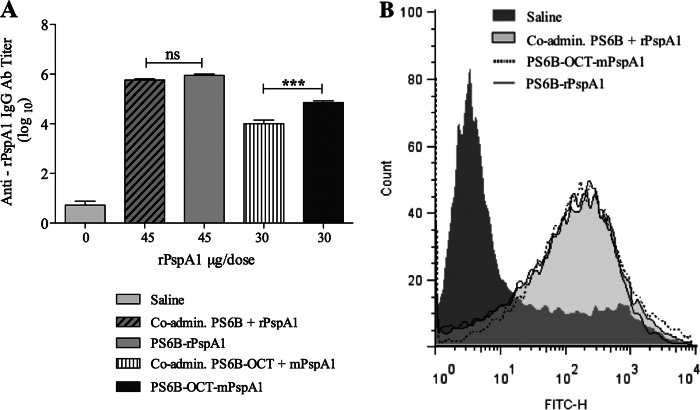
The anti-rPspA1 IgG titer induced by the conjugates and their control groups was measured by ELISA (Fig. 4A). The conjugate obtained by reductive amination (PS6B-rPspA1) induced the same anti-rPspA1 IgG titer as rPspA1 coadministered with PS6B (Fig. 4A). On the other hand, the conjugate synthesized by carboxamide formation (PS6B-OCT-mPspA1) displayed an anti-rPspA1 antibody titer higher than that induced with the coadministered antigens.
Fig 4.
Anti-PspA immune response. (A) IgG antibody titer to rPspA1. Individual serum samples from mice (n = 6) immunized i.p. with rPspA1 conjugated to PS6B by reductive amination (PS6B-rPspA1) or with mPspA1 conjugated to PS6B-OCT (PS6B-OCT-mPspA1) were analyzed by ELISA and compared by one-way ANOVA with Tukey's multiple-comparison test. Sera from mice immunized with the respective coadministered components or with saline plus Al(OH)3 were used as controls. Asterisks indicate statistically significant differences (***, P < 0.0001). ns, nonsignificant differences. The results for all groups were significantly different from those for the saline-treated group (P < 0.0001). (B) Complement deposition on S. pneumoniae bacteria. An example of a flow cytometry histogram for C3 deposition is shown. S. pneumoniae strain 245/00 (serotype 14 and PspA clade 1) was incubated with sera from mice immunized with PspA clade 1 conjugated to PS6B or to PS6B-OCT. Sera from mice immunized with the respective coadministered components or with saline plus Al(OH)3 were used as controls.
The functionality of these antibodies was evaluated by their ability to mediate complement deposition on the pneumococcal surface and their opsonophagocytic activity. The flow cytometry histograms of the complement deposition obtained when a serotype 14 strain bearing a PspA homologous to the conjugate was incubated with antisera from mice immunized with the conjugates were shown to be comparable to those obtained with the coadministered antigens (Fig. 4B). According to our results, the ability to induce opsonizing antibodies that mediate C3 complement deposition on the pneumococcus was preserved after conjugation, showing that the partial loss in the secondary structure of the rPspA molecule did not impair its ability to elicit opsonizing antibodies.
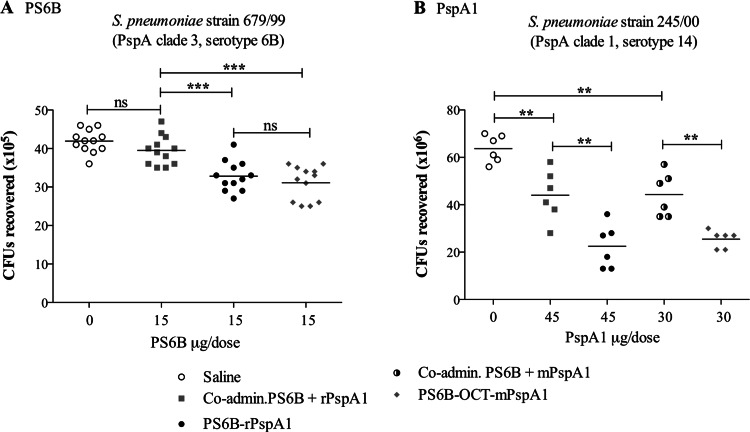
The opsonophagocytic activities exhibited by anti-PS6B antibodies and anti-rPspA antibodies in the sera of mice immunized with PS6B-OCT-mPspA1 and PS6B-rPspA1 were evaluated in two separate assays: (i) an assay using a pneumococcus serotype 6B strain carrying a PspA heterologous to the conjugate to assess the protective immunogenicity of anti-PS6B antibodies and (ii) an assay using a pneumococcus serotype 14 strain bearing a PspA homologous to the conjugate to measure the protective immunogenicity of anti-rPspA1 antibodies. Despite having different conformations, both conjugates were equally efficient in inducing opsonophagocytic antibodies against PS6B (shown by a significant reduction in the number of CFU recovered) (Fig. 5A). As expected, the sera of mice immunized with the nonconjugated rPspA1 or mPspA1 were efficient in reducing the number of CFU recovered compared to the efficiency for the sera of mice in the negative-control group immunized with saline and Al(OH)3 (Fig. 5B). The conjugation of the protein to PS6B did not result in the loss of opsonophagocytic activity of the anti-rPspA1 antibodies. On the contrary, the conjugation seemed to improve the protective activity of anti-rPspA antibodies, reducing bacterial survival and inducing the recovery of lower numbers of CFU (P < 0.001) (Fig. 5B).
Fig 5.
Opsonophagocytic assay. Pneumococcal strain 679/99 (PspA clade 3, serotype 6B, used to test the opsonic activity of anti-PS6B antibodies [Ab]) (A) and pneumococcal strain 245/00 (PspA clade 1, serotype 14, used to test the opsonic activity of anti-PspA1 antibodies) (B) were incubated with the sera from mice immunized with PS6B-rPspA1 or with PS6B-OCT-mPspA1 and a complement source. The opsonized pneumococci were incubated with peritoneal cells and plated on blood agar plates. Sera from mice immunized with saline plus Al(OH)3 or with PS6B coadministered with rPspA1 or mPspA1 were used as controls. The numbers of CFU recovered after 20 h were compared by one-way ANOVA with Tukey's multiple-comparison test. The lines on the graph represent means. Asterisks indicate statistically significant differences (**, P < 0.001; ***, P < 0.0001). ns, nonsignificant differences.
DISCUSSION
In this study, we have compared the effect of two conjugation methodologies on the immunogenicity of both antigens present in the conjugate, PS6B and rPspA1. Both methods start by oxidation of the vicinal hydroxyls of the polysaccharides, creating aldehyde groups. In the classical reductive amination method, the aldehyde is bound directly to an ε-amine group of lysine in the rPspA1 molecule. In the DMT-MM-mediated method, the aldehyde was first coupled with OCT and then the free amine group of derivatized PS (PS-OCT) reacted with the carboxyl groups of mPspA1 (41). The reaction mechanism of conjugation mediated by DMT-MM is shown in Fig. 6: the carboxyl group in the carrier protein (A) is activated by DMT-MM (B), resulting in an acyloxytriazine intermediate (A-B). The NH2, a nucleophilic group from PS-OCT (C), reacts with the intermediate (A-B) through a carboxamide linkage, generating the conjugate (A-C). The use of this reagent in the production of a conjugate vaccine was first proposed by our laboratory (29). The majority of the conjugation methods that are based on the reaction of carboxyl groups employ 1-ethyl-3-(3-dimethylaminpropyl) carbodiimide hydrochloride (EDC) in their activation step. We introduced DMT-MM as a more efficient activating reagent than EDC due to its higher stability in aqueous solution, especially when using phosphate buffer (45). Studies have shown that the use of DMT-MM in coupling PS with small bioactive molecules or with microspheres resulted in higher reaction yields than conventional methodologies (46, 47). Likewise, as shown by the HIC chromatograms of the conjugates, the amount of PS6B conjugated to rPspA1 is higher in the method that uses DMT-MM-mediated conjugation (55.0% ± 6.0%) than in the reductive amination (24.0% ± 2.6%). When using adipic acid dihydrazide (ADH; a currently used spacer with 6 molecules) instead of OCT, no difference in the yield of the reaction between the PS and protein was observed (results not shown).
Fig 6.
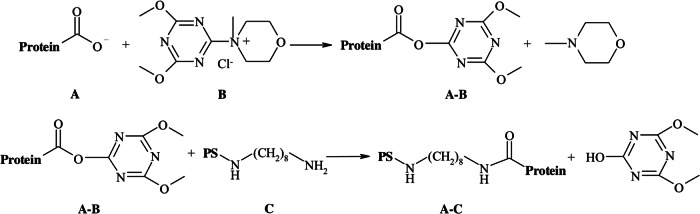
Mechanism of conjugation mediated by DMT-MM: the carboxyl group (A) is activated by DMT-MM (B) and an acyloxytriazine intermediate is obtained (A-B). This intermediate (A-B) is susceptible to attack by a nucleophile, leading to the formation of A-C.
Serotype 6B strains are epidemiologically important, and PS6B is in the currently licensed PCVs. PS6B appears at double the concentration of the other serotypes in the 7-valent and 13-valent formulations. Despite being present at a higher dose, the levels of antibodies induced against PS6B are the lowest among the levels of antibodies induced against the serotypes included in the PCVs (48). Regardless of the antibody concentrations, PS6B induces a protective response, demonstrated by the ability of the anti-PS6B antibodies to induce opsonophagocytosis of pneumococci (48). Due to its correlation with protection, we compared the effectiveness of our conjugates by OPA. The serum of mice immunized with the conjugates showed antibodies equally able to opsonize and mediate the phagocytosis of S. pneumoniae serotype 6B expressing a heterologous PspA. This demonstrates that the changes in chemical structure of the conjugates (spacer molecules and different types of linkages between PS and the carrier protein) did not influence the immune response induced against the PS.
The incorporation of PspA as a carrier protein in PCVs more than confers a T cell-dependent identity to the PS, and PspA is expected to act as an immunogenic antigen. Therefore, we analyzed the effect of the conjugation reaction on the secondary structure of the protein and on the immune response induced. The recombinant fragment of PspA used in this study contains the two main regions related to protection: the N-terminal alpha-helical domain and the proline-rich region. The N-terminal alpha-helical region of PspA is long known for its protective potential (22–24, 49, 50). Recently, the proline region has also been shown to induce protection against pneumococcal infections (51). The secondary structure of conjugated rPspA1 was assessed by CD, and rPspA1 and mPspA1 molecules were used as controls. According to our results, both conjugates showed a reduction in the content of the alpha-helical structure and an increased amount of unordered structure in comparison to the rPspA1 molecule. The process of conjugation using DMT-MM led to a 31% reduction in alpha-helical content in comparison to that of its immediate nonconjugated precursor, mPspA1; a global reduction of 46% was obtained when losses due to both the rPspA1 modification and the conjugation are considered. The reductive amination had a minor effect on the alpha-helical content, leading to a reduction of only 13%.
The protective immunity conferred by immunization with PspA has usually been assessed in pneumococcal disease models. A restricted repertoire of pneumococcal strains is virulent in murine models, and these strains usually bear capsular polysaccharides 3, 6A, and 6B (21). In the present case, the selection of a strain virulent for mice mainly relies on strains bearing capsular type 3. The selection of a strain bearing serogroup 6 would impair the analysis of the immune response induced by PspA. The strains carrying PspA clade 1 and serotype 3 were shown to be highly pathogenic, causing rapid sepsis and death in the mice, while tests using serotypes different from serotypes 3, 6A, and 6B did not cause disease in the animals (data not shown). In the absence of a suitable pneumococcal disease model, the OPA was the assay of choice for evaluating the functional activity of anti-PspA antibodies (27, 40).
Notably, both conjugation processes preserved PspA's antigenic properties, including the ability to induce antibodies capable of mediating complement deposition and phagocytosis. These results would indicate that the primary sequence of amino acid residues in rPspA1, rather than its secondary structure, is probably associated with the induction of protective antibodies.
Our main goals with this study were to investigate whether rPspA1 could act as a carrier protein for PS6B and whether the conjugation would disrupt rPspA1's structure, affecting its immunogenicity. We observed that, when conjugated, rPspA1 induced lower levels of antibodies, although it had higher opsonophagocytic activity than when it was nonconjugated (Fig. 5B). The ratio of the opsonophagocytic activity per unit of antibody titer to rPspA1 was 15% higher for the conjugated rPspA1 than for the coadministered rPspA1 (data from Fig. 4A and 5A). A hypothesis for this observation is that surface PspA1, contrary to rPspA1, interacts with other pneumococcal surface components, including the capsular polysaccharide, and the charge distribution of conjugated rPspA1 (positively charged protein and negatively charged PS) more closely resembles that of the conformational structure of PspA expressed on the bacterial surface.
In conclusion, despite the fact that the circular dichroism analysis has shown that the conjugation alters the secondary structure of rPspA1, the immunological assays have demonstrated that these alterations do not affect its ability to induce a protective immune response. Furthermore, the conjugation strategy using different chemical linkages does not seem to impair the immunogenicity of rPspA1 or PS6B and, consequently, does not impose an obstacle to implementation of the more economical methodology. Therefore, our results support the use of rPspA1 as an antigenic carrier protein and reinforce the use of DMT-MM-mediated conjugation as a valuable strategy to be considered in conjugation processes.
ACKNOWLEDGMENT
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Footnotes
Published ahead of print 3 April 2013
REFERENCES
- 1. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 2. Linares J, Ardanuy C, Pallares R, Fenoll A. 2010. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin. Microbiol. Infect. 16:402–410 [DOI] [PubMed] [Google Scholar]
- 3. Fedson DS, Nicolas-Spony L, Klemets P, van der Linden M, Marques A, Salleras L, Samson SI. 2011. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev. Vaccines 10:1143–1167 [DOI] [PubMed] [Google Scholar]
- 4. Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M. 2001. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 19:4780–4790 [DOI] [PubMed] [Google Scholar]
- 5. Griffioen AW, Rijkers GT, Janssens-Korpela P, Zegers BJ. 1991. Pneumococcal polysaccharides complexed with C3d bind to human B lymphocytes via complement receptor type 2. Infect. Immun. 59:1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. 1989. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J. Immunol. 143:3200–3206 [PubMed] [Google Scholar]
- 7. Guttormsen HK, Sharpe AH, Chandraker AK, Brigtsen AK, Sayegh MH, Kasper DL. 1999. Cognate stimulatory B-cell–T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect. Immun. 67:6375–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Redelings MD, Sorvillo F, Simon P, Mascola L. 2005. Declining early childhood mortality from invasive pneumococcal disease: the impact of vaccination. Arch. Pediatr. Adolesc. Med. 159:195–196 [DOI] [PubMed] [Google Scholar]
- 9. Roush SW, Murphy TV. 2007. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 298:2155–2163 [DOI] [PubMed] [Google Scholar]
- 10. Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. 2009. Changing epidemiology of invasive pneumococcal disease in Canada, 1998-2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin. Infect. Dis. 49:205–212 [DOI] [PubMed] [Google Scholar]
- 11. Ruckinger S, van der Linden M, Reinert RR, von Kries R, Burckhardt F, Siedler A. 2009. Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine 27:4136–4141 [DOI] [PubMed] [Google Scholar]
- 12. Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. 2007. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 369:1179–1186 [DOI] [PubMed] [Google Scholar]
- 13. Chibuk TK, Robinson JL, Hartfield DS. 2010. Pediatric complicated pneumonia and pneumococcal serotype replacement: trends in hospitalized children pre and post introduction of routine vaccination with pneumococcal conjugate vaccine (PCV7). Eur. J. Pediatr. 169:1123–1128 [DOI] [PubMed] [Google Scholar]
- 14. Leach AJ, Morris PS, McCallum GB, Wilson CA, Stubbs L, Beissbarth J, Jacups S, Hare K, Smith-Vaughan HC. 2009. Emerging pneumococcal carriage serotypes in a high-risk population receiving universal 7-valent pneumococcal conjugate vaccine and 23-valent polysaccharide vaccine since 2001. BMC Infect. Dis. 9:121 doi: 10.1186/1471-2334-9-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia JJ, Pallares R. 2008. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin. Infect. Dis. 46:174–182 [DOI] [PubMed] [Google Scholar]
- 16. Borrow R, Dagan R, Zepp F, Hallander H, Poolman J. 2011. Glycoconjugate vaccines and immunointeractions and implication for vaccination schedules. Expert Rev. Vaccines 10:1621–1631 [DOI] [PubMed] [Google Scholar]
- 17. Dagan R, Poolman J, Siegrist CA. 2010. Glycoconjugate vaccines and immune interference: a review. Vaccine 28:5513–5523 [DOI] [PubMed] [Google Scholar]
- 18. Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 71:75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tu AH, Fulgham RL, McCrory MA, Briles DE, Szalai AJ. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaper M, Hollingshead SK, Benjamin WH, Jr, Briles DE. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin [corrected]. Infect. Immun. 72:5031–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, Ferguson LM, Nahm MH, Nabors GS. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694–1701 [DOI] [PubMed] [Google Scholar]
- 22. Wu HY, Nahm MH, Guo Y, Russell MW, Briles DE. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839–846 [DOI] [PubMed] [Google Scholar]
- 23. Crain MJ, Waltman WD, II, Turner JS, Yother J, Talkington DF, McDaniel LS, Gray BM, Briles DE. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hollingshead SK, Becker R, Briles DE. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hollingshead SK, Baril L, Ferro S, King J, Coan P, Briles DE. 2006. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol. 55:215–221 [DOI] [PubMed] [Google Scholar]
- 26. Pimenta FC, Ribeiro-Dias F, Brandileone MC, Miyaji EN, Leite LC, Sgambatti de Andrade AL. 2006. Genetic diversity of PspA types among nasopharyngeal isolates collected during an ongoing surveillance study of children in Brazil. J. Clin. Microbiol. 44:2838–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santamaria R, Goulart C, Perciani CT, Barazzone GC, Carvalho R, Goncalves VM, Leite LC, Tanizaki MM. 2011. Humoral immune response of a pneumococcal conjugate vaccine: capsular polysaccharide serotype 14-lysine modified PspA. Vaccine 29:8689–8695 [DOI] [PubMed] [Google Scholar]
- 28. Barazzone GC, Perciani CT, Raw I, Tanizaki MM. July 2011. Método de conjugação de polissacarídeo capsular a uma proteína carregadora, para uso como antígeno vacinal contra bactérias encapsuladas, utilizando o reagente cloreto de 4-(4,6-dimetoxi-1,3,5-triazin-2-il)-4-metilmorfolino (DMT-MM). Brazilian patent PI0904528-7 [Google Scholar]
- 29. Barazzone GC, Carvalho RJ, Kraschowetz S, Horta ACL, Sargo C, Silva AJ, Tanizaki MM, Cabrera-Crespo J, Gonçalves VM. 2011. Production and purification of recombinant fragment of pneumococcal surface protein A (PspA) in Escherichia coli. Proc. Vaccinol. 4:27–35 [Google Scholar]
- 30. Carvalho RJ, Cabrera-Crespo J, Tanizaki MM, Gonçalves VM. 2012. Development of production and purification processes of recombinant fragment of pneumococcal surface protein A in Escherichia coli using different carbon sources and chromatography sequences. Appl. Microbiol. Biotechnol. 94:683–694 [DOI] [PubMed] [Google Scholar]
- 31. Silva M, Cabrera-Crespo J, Sbrogio-Almeida ME, Miyaji EN, Ho PL, Leite LC, Lopes AP. 2007. Optimizing expression of Streptococcus pneumoniae surface protein a, PspA: serocross-reactivity within families of antisera induced against clades 1 and 3. Mol. Biotechnol. 37:146–154 [DOI] [PubMed] [Google Scholar]
- 32. Gonçalves VM, Takagi M, Carmo TS, Albani SMF, Pinto JV, Zangirolami TC, Giordano RC, Tanizaki MM, Cabrera-Crespo J. 2007. Simple and efficient method of bacterial polysaccharides purification for vaccines production using hydrolytic enzymes and tangential flow ultrafiltration, p 250–257 In Mendez-Vilas A. (ed), Communicating current research and educational topics and trends in applied microbiology. Formatex; Badajoz, Spain [Google Scholar]
- 33. Tyllianakis PE, Kakabakos SE, Evangelatos GP, Ithakissios DS. 1994. Direct colorimetric determination of solid-supported functional groups and ligands using bicinchoninic acid. Anal. Biochem. 219:335–340 [DOI] [PubMed] [Google Scholar]
- 34. Qi XY, Keyhani NO, Lee YC. 1988. Spectrophotometric determination of hydrazine, hydrazides, and their mixtures with trinitrobenzenesulfonic acid. Anal. Biochem. 175:139–144 [DOI] [PubMed] [Google Scholar]
- 35. Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350–356 [Google Scholar]
- 36. Fryer HJ, Davis GE, Manthorpe M, Varon S. 1986. Lowry protein assay using an automatic microtiter plate spectrophotometer. Anal. Biochem. 153:262–266 [DOI] [PubMed] [Google Scholar]
- 37. Whitmore L, Wallace BA. 2008. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers 89:392–400 [DOI] [PubMed] [Google Scholar]
- 38. Compton LA, Johnson WC., Jr 1986. Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal. Biochem. 155:155–167 [DOI] [PubMed] [Google Scholar]
- 39. Darrieux M, Moreno AT, Ferreira DM, Pimenta FC, de Andrade AL, Lopes AP, Leite LC, Miyaji EN. 2008. Recognition of pneumococcal isolates by antisera raised against PspA fragments from different clades. J. Med. Microbiol. 57:273–278 [DOI] [PubMed] [Google Scholar]
- 40. Goulart C, Darrieux M, Rodriguez D, Pimenta FC, Brandileone MC, de Andrade AL, Leite LC. 2011. Selection of family 1 PspA molecules capable of inducing broad-ranging cross-reactivity by complement deposition and opsonophagocytosis by murine peritoneal cells. Vaccine 29:1634–1642 [DOI] [PubMed] [Google Scholar]
- 41. Anderson P, Pichichero ME, Insel RA. 1985. Immunogens consisting of oligosaccharides from the capsule of Haemophilus influenzae type b coupled to diphtheria toxoid or the toxin protein CRM197. J. Clin. Invest. 76:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee CH, Kuo WC, Beri S, Kapre S, Joshi JS, Bouveret N, LaForce FM, Frasch CE. 2009. Preparation and characterization of an immunogenic meningococcal group A conjugate vaccine for use in Africa. Vaccine 27:726–732 [DOI] [PubMed] [Google Scholar]
- 43. Fairchild RL, Braley-Mullen H. 1983. Characterization of the murine immune response to type 6 pneumococcal polysaccharide. Infect. Immun. 39:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chu RS, McCool T, Greenspan NS, Schreiber JR, Harding CV. 2000. CpG oligodeoxynucleotides act as adjuvants for pneumococcal polysaccharide-protein conjugate vaccines and enhance antipolysaccharide immunoglobulin G2a (IgG2a) and IgG3 antibodies. Infect. Immun. 68:1450–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gilles MA, Hudson AQ, Borders CL., Jr 1990. Stability of water-soluble carbodiimides in aqueous solution. Anal. Biochem. 184:244–248 [DOI] [PubMed] [Google Scholar]
- 46. Farkas P, Bystricky S. 2007. Efficient activation of carboxyl polysaccharides for the preparation of conjugates. Carbohydr. Polymers 68:187–190 [Google Scholar]
- 47. Schlottmann SA, Jain N, Chirmule N, Esser MT. 2006. A novel chemistry for conjugating pneumococcal polysaccharides to Luminex microspheres. J. Immunol. Methods 309:75–85 [DOI] [PubMed] [Google Scholar]
- 48. Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène JP, Lommel P, Dieussaert I, Schuerman L. 2009. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr. Infect. Dis. J. 28(4 Suppl):S66–S76 [DOI] [PubMed] [Google Scholar]
- 49. McDaniel LS, Ralph BA, McDaniel DO, Briles DE. 1994. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 17:323–337 [DOI] [PubMed] [Google Scholar]
- 50. McDaniel LS, Sheffield JS, Swiatlo E, Yother J, Crain MJ, Briles DE. 1992. Molecular localization of variable and conserved regions of pspA and identification of additional pspA homologous sequences in Streptococcus pneumoniae. Microb. Pathog. 13:261–269 [DOI] [PubMed] [Google Scholar]
- 51. Daniels CC, Coan P, King J, Hale J, Benton KA, Briles DE, Hollingshead SK. 2010. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect. Immun. 78:2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Csordas FC, Perciani CT, Darrieux M, Goncalves VM, Cabrera-Crespo J, Takagi M, Takagi M, Sbrogio-Almeida ME, Leite LC, Tanizaki MM. 2008. Protection induced by pneumococcal surface protein A (PspA) is enhanced by conjugation to a Streptococcus pneumoniae capsular polysaccharide. Vaccine 26:2925–2929 [DOI] [PubMed] [Google Scholar]