Abstract
Enteric parasite infections around the world are a huge economic burden and decrease the quality of life for many people. The use of beneficial bacteria has attracted attention for their potential therapeutic applications in various diseases. However, the effects of beneficial bacteria in enteric parasitic infections remain largely unexplored. We investigated the effects of ingestion of Lactobacillus rhamnosus (JB-1) in a model of enteric nematode (Trichuris muris) infection. C57BL/6 (resistant to infection), AKR (susceptible to infection), interleukin 10 (IL-10) knockout (KO), and mucin Muc2 KO mice were infected with T. muris and treated orally with probiotic JB-1 or medium. The mice were sacrificed on various days postinfection to examine goblet cells, epithelial cell proliferation, cytokines, and worm burdens. Treatment with JB-1 significantly enhanced worm expulsion in resistant C57BL/6 mice, and this was associated with increases in IL-10 levels, goblet cell numbers, and epithelial cell proliferation. Beneficial effects of JB-1 were absent in IL-10 KO and resistant mice treated with γ-irradiated bacteria. Live JB-1 treatment also expedited worm expulsion in Muc2 KO mice and, more importantly, in AKR mice (susceptible to infection). Injection of IL-10 directly into the colonic tissue of uninfected mice induced goblet cell hyperplasia. These findings demonstrate that JB-1 modulates goblet cell biology and promotes parasite expulsion via an IL-10-mediated pathway and provide novel insights into probiotic effects on innate defense in nematode infection.
INTRODUCTION
Intestinal parasites, because of their widespread prevalence, are probably the most important parasites causing serious human diseases. Among these parasites, nematodes have a very high prevalence, infecting a large part of the human population. Trichuris trichiura affects over one billion people and is a significant economic burden on countries and their populations in areas where the parasite is endemic (1). Trichuris muris is a natural counterpart nematode that dwells in the large intestine of mice (2, 3). This parasite is very similar to the human parasite, and its larvae also embed in the epithelium of the cecum, causing alterations in the homeostasis of the epithelium as well as epithelial cell hyperplasia (3, 4).
Different strains of mice respond to T. muris infection differently depending on the type of immune response mounted. Some strains of mice are susceptible to a chronic infection, due to a Th1 immune response, and have high levels of gamma interferon (IFN-γ) (5, 6). On the other hand, some strains of mice are resistant to the infection and with a Th2 response characterized by high levels of interleukin 4 (IL-4) and IL-13 expel the worms in 21 to 35 days (5–8). It has been shown that in resistant mice, such as C57BL/6 and BALB/c, there is intestinal goblet cell hyperplasia under the control of Th2 responses (9–14). Goblet cells reside throughout the gastrointestinal (GI) tract and are the main source of mucins in the gut (13). The mucus layer coating the GI tract contains mucins and represents the front line of innate defense (15–17). The Muc2 and Muc3 mucin genes are found in large amounts in the GI tract of mice and play key roles in mucin production (15). Recently, it was shown that worm expulsion in the initial stage of T. muris infection is Muc2 dependent, whereas worm expulsion in the late stage is Muc2 independent (9).
IL-10 is one of the major anti-inflammatory regulatory cytokines which acts to inhibit both antigen presentation and the production of proinflammatory cytokines (18). IL-10 plays an important regulatory role in enteric infections while acting to maintain gut homeostasis. IL-10 knockout (KO) mice with a T. muris infection have a high mortality rate after a short period of time (19). It is evident from these and other findings that IL-10 plays a crucial role in host resistance to many enteric infections, including those caused by T. muris.
Beneficial bacteria such as probiotics have the ability to modulate the normal intestinal microbiome by direct action and also by signaling the host, which in turn adjusts the immune response (20, 21). Lactobacillus species are common probiotics that have been studied in many laboratories under various GI conditions (22, 23). Currently there are many outcomes of probiotic treatments that are known to be related to different diseases. Probiotic treatments might reverse or even prevent some GI pathological conditions through local effects such as promotion of barrier function or modification of the immune system. The exact mechanisms whereby probiotics benefit the host are not fully understood, and further research is required in order to elucidate the complete process of this complex interaction.
In this study, we used the well-defined T. muris infection model to investigate the effects of Lactobacillus rhamnosus (JB-1) treatment on host defense in nematode infections. Our study demonstrated that treatment with live JB-1 accelerates parasite expulsion and upregulates goblet cell hyperplasia in resistant mice via the IL-10 pathway. Importantly, this treatment promotes worm expulsion and goblet cell hyperplasia even in a susceptible strain of mice. Increases in goblet cells and beneficial effects on worm expulsion were not observed with γ-irradiated JB-1. We also showed that IL-10 itself promotes goblet cell hyperplasia. These novel findings provide evidence that this probiotic plays important beneficial roles in innate defense during parasitic infections and offer new insights into how this may occur.
MATERIALS AND METHODS
Mice.
Mice were housed in the Central Animal Facility (CAF) at McMaster University. Protocols were in accordance with the McMaster University Care Committee and guidelines set by the Canadian Council of the Use of Laboratory Animals. Mice (6 to 8 weeks old) were kept in sterilized, filter-topped cages under specific-pathogen-free (SPF) conditions. Male C57BL/6 (resistant to T. muris infection) and IL-10 KO (C57BL/6 background) mice were from Taconic (Germantown, NY) and Jackson Laboratory (Bay Harbor, ME), respectively. IL-10 KO mice have a targeted knockout of allele IL-10tm1Cgn (24). These mice spontaneously develop colitis under SPF conditions after 10 to 12 weeks but were monitored closely throughout the experiments to ensure that no colitis had developed in any of the mice that were used. Male AKR mice (susceptible to T. muris infection) were obtained from Jackson Laboratories (Bar Harbor, ME). Muc2 KO mice were created by gene mutation (25) and were a generous gift from A. Velcich (Albert Einstein Medical College, NY).
Bacteria and medium.
Lactobacillus rhamnosus (JB-1) was obtained from J. Bienenstock and P. Forsythe. This bacterium has been the subject of intense study, and was recently reclassified as a rhamnosus strain (26). Briefly, bacteria were grown at 37°C under anaerobic conditions, collected, centrifuged, washed, and stored at −20°C. Bacteria were measured using a Vitek colorimeter (bioMérieux, Hazelwood, MO) and were resuspended in deMan-Rogosa-Sharpe broth (MRS) (BD-Difco Laboratories, Sparks, MD) to give the optimal concentration of bacteria/ml. Resistant C57BL/6, IL-10 KO, and Muc2 KO mice were fed 1 × 109 CFU/day for a total of 15 days beginning 1 day before infection. Susceptible AKR mice were fed 1 × 109 CFU/day for a total of 36 days beginning 1 day before infection. JB-1 bacteria were killed by γ irradiation with cobalt 60 for 20 h at 8.05 Gy/min. We then determined viability by plating killed bacteria on MRS agar plates under anaerobic conditions for 72 h at 37°C. No bacterial growth was detected in γ-irradiated JB-1 preparations.
T. muris infection.
We used previously described techniques for maintenance of T. muris and transmission of T. muris infections (27). Mice were infected with approximately 400 eggs and worm burdens were assessed as described previously (28).
Histology.
Formalin-fixed, paraffin-embedded sections of intestines were stained with a combination of alcian blue and periodic acid-Schiff (AB-PAS) or PAS only. AB-PAS staining enables the differentiation between acid mucins and neutral mucins, with blue color showing acid mucins, purple color indicating a mixture of neutral and acid mucins, and red/magenta color indicating neutral mucins alone (29). PAS/AB-PAS-stained goblet cells were expressed per 10 villus-crypt units.
Enzyme-linked immunosorbent assays.
Colonic cytokines were assessed using commercially available kits (R&D Systems, Minneapolis, MN). Colon segments were homogenized in lysis buffer containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Assays were run according to the manufacturer's protocol.
Recombinant IL-10 injections into colonic tissue.
Naive C57BL/6 mice were anesthetized, and then the abdomen was opened surgically. We exposed the proximal colon, used a Hamilton syringe to inject 0.1 μg (in a volume of 100 μl) of recombinant IL-10 (rIL-10) (R&D Systems, Minneapolis, MN) or saline into the colon tissue, and marked the site of the injection with a black silk surgical suture. The incision was closed, and mice were administered saline parenterally with antibiotics (enrofloxacin [Baytril], 2.5 mg/kg) and an analgesic (buprenorphine, 0.05 mg/kg) while being closely monitored until wakening. The mice were sacrificed 48 h postsurgery to examine the goblet cells.
Proliferation analysis.
We injected mice intraperitoneally with 500 μl of bromo-2-deoxyuridine (BrdU) (Sigma, St. Louis, MO) diluted in phosphate-buffered saline (PBS) (20 mg/ml) 6 to 8 h before sacrifice. BrdU incorporates into newly synthesized DNA in proliferating cells to allow for the visualization of dividing epithelial cells (30). Tissue sections were stained with rat anti-BrdU antibody (1:100 dilution; AbD Serotec, Langford, United Kingdom). Sections were then incubated with secondary antibody (donkey anti-rat [Alexa Fluor 488; Invitrogen] at a 1:200 dilution) for 30 min at room temperature in the dark. Slides were examined using a fluorescence microscope and analyzed using NIS-Elements Basic Research (version 3.10, 2009; Nikon).
Statistical analysis.
Statistical significance was determined using GraphPad Prism software (version 4, 2004, San Diego, CA) using one-way analysis of variance (ANOVA) to compare more than two treatment groups or the unpaired Student's t test for two groups with normally distributed data. Differences were reported as significant at P values of <0.05. All data are presented as the mean ± standard error of the mean.
RESULTS
Live JB-1 promotes worm expulsion when IL-10 is present.
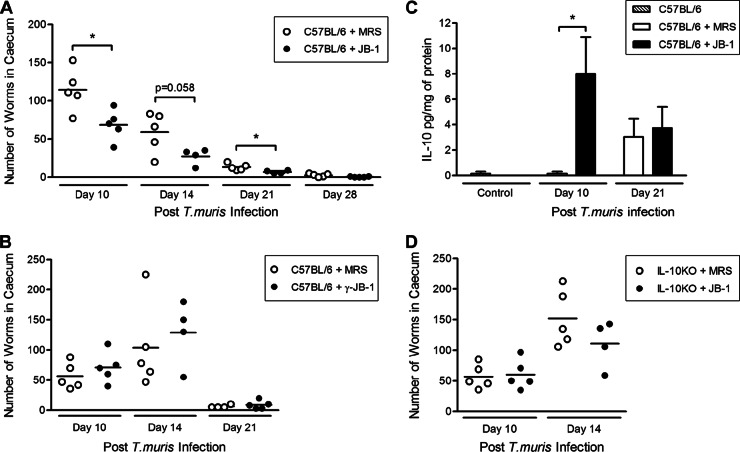
Resistant C57BL/6 mice naturally expel T. muris starting on day 14 postinfection (p.i.) and are fully cleared of the parasite by day 35 p.i. (6). Assessment of worm burden in the large intestines of live JB-1-treated infected C57BL/6 mice showed acceleration in worm expulsion in these mice. On days 10 and 21 p.i., we observed a significantly lower worm burden in mice treated with live bacteria than in mice treated with MRS medium (Fig. 1A). We saw no difference in worm burden in the mice treated with γ-irradiated JB-1 compared to the mice treated with MRS on any of the days p.i. (Fig. 1B). This suggests that live bacteria are needed to upregulate the host defense.
Fig 1.
Live Lactobacillus rhamnosus (JB-1) increased worm expulsion and colonic IL-10 production in resistant mice after T. muris infection. Resistant C57BL/6 mice were treated with either live or γ-irradiated JB-1 for 14 days after being infected with 400 embryonated T. muris eggs and sacrificed on different days to determine worm burden. (A) Live bacteria significantly increased worm expulsion, as mice on days 10 and 21 p.i. had lower numbers of worms. (B) Worm burden and expulsion rates showed no differences in mice treated with γ-irradiated JB-1 and mice treated with medium alone. (C) Colonic tissue IL-10 concentrations. Resistant mice treated with JB-1 during T. muris infection had significantly greater increases in IL-10 tissue concentrations on day 10 p.i. than mice treated with medium. (D) When IL-10 KO mice were treated with JB-1 from day −1 to day 14, the changes in the numbers of worms in these mice were not significantly different from those seen in the mice that were treated with MRS at either time point. Significance was determined using the parametric Student's t test for normal distribution. *, P < 0.05 (n = 5).
There was a significant increase in IL-10 concentrations in tissues on day 10 p.i. in mice treated with live JB-1 (Fig. 1C). In infected mice, the levels of colonic IL-4, IL-13, and IFN-γ in JB-1-treated mice were not significantly different than those in MRS-treated mice (data not shown). In uninfected mice, the levels of colonic IL-10 in mice treated with JB-1 for 15 days were not significantly different than the levels in mice treated with MRS for 15 days (4.6 ± 1.4 pg/mg of protein and 3.1 ± 0.4 pg/mg of protein, respectively). Schopf et al. showed that IL-10 KO mice were susceptible to T. muris infection and had a 100% mortality rate by day 25 p.i. (19). In T. muris-infected IL-10 KO mice there were no differences in worm burden at the latest time point (day 14 p.i.) in the mice treated with the probiotic JB-1 and those treated with the medium (Fig. 1D). These data suggest that JB-1 enhances worm expulsion from the intestine when IL-10 is present.
Live JB-1 increases goblet cell numbers in the colons of infected mice when IL-10 is present.
Previous studies have shown increases of goblet cell numbers in resistant mice during T. muris infection, which might play an important role in the expulsion of the worms (9, 31, 32). We also observed goblet cell hyperplasia in resistant mice after T. muris infection. Investigations of PAS-stained goblet cells revealed further augmentation of the infection-induced goblet cell hyperplasia on days 14 and 21 p.i. after treatment of resistant mice with JB-1 compared to that seen in mice treated with medium only, or in mice treated with γ-irradiated JB-1 (Fig. 2A to F). With AB-PAS staining, we observed that acid-neutral mucins in JB-1-treated mice were significantly upregulated compared to those in medium-treated mice on day 10 p.i. (Fig. 3). We observed more acid mucins in JB-1-treated mice on day 21 p.i. In the absence of infection, there were no differences in goblet cell numbers between groups (65 ± 8.4 goblet cells/10 crypts versus 57 ± 5.1 goblet cells/10 crypts in JB-1- and medium-treated groups, respectively).
Fig 2.
Intestinal goblet cells after PAS staining. (A) Goblet cell counts from the resistant C57BL/6 mice. Uninfected controls and T. muris-infected mice treated with either JB-1 or MRS. On days 14 and 21 postinfection, there were significant increases in the numbers of PAS-stained goblet cells in the infected mice treated with JB-1. (B and C) Corresponding histological sections of colon (stained with PAS) show the increases of goblet cells. (D) Goblet cell counts from the resistant C57BL/6 mice infected with T. muris and treated with either γ-irradiated JB-1 or MRS. There were no differences in the numbers of goblet cells in γ-irradiated JB-1- and MRS-treated mice. (E and F) Corresponding histological sections of the colon (stained with PAS) show that the numbers of goblet cells were similar in each group. (G) Goblet cell counts from the colons of IL-10 KO mice infected with T. muris and treated with either JB-1 or MRS. On any day, the goblet cell numbers in the infected mice treated with JB-1 did not differ from those in the mice treated with MRS. (H and I) Corresponding histological sections (stained with PAS) show similar numbers of goblet cells. *, P < 0.05 (n = 5 in each group).
Fig 3.
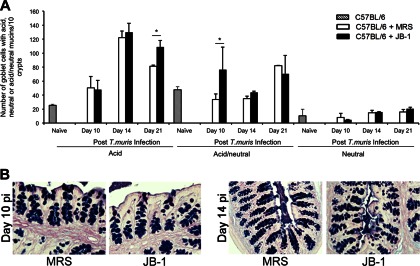
Intestinal goblet cells after AB-PAS staining. (A) Goblet cell counts from uninfected and T. muris-infected resistant C57BL/6 mice treated with either JB-1 or MRS. There were significant increases in the acid-neutral mucin-containing and acid mucin-containing goblet cell numbers in the infected mice treated with JB-1 on days 10 and 21 postinfection, respectively. (B) Corresponding histological sections of the colons (stained with AB-PAS) show the goblet cells containing acid, acid-neutral, and neutral mucins in the JB-1- and MRS-treated infected mice. *, P < 0.05 (n = 5 in each group).
In the IL-10 KO mice there were no overall increases of goblet cell numbers following the infection, and the JB-1 treatment did not have an effect on goblet cells (Fig. 2G to I). These observations further suggest that impairment in the upregulation of goblet cells plays a role in the increased susceptibility of IL-10 KO mice to T. muris infection and that the presence of IL-10 is necessary for goblet cell hyperplasia.
Administration of rIL-10 directly into gut upregulates goblet cell response.
With the significant increase in IL-10 early on during a T. muris infection in the mice treated with the probiotic, we set out to determine whether IL-10 has a direct role in the alteration of goblet cell numbers. Forty-eight hours after a direct surgical injection into the proximal colonic tissue of naive C57BL/6 mice, goblet cells were analyzed through PAS staining. We observed a direct effect of IL-10 on the goblet cell response during a state of homeostasis. In the rIL-10-injected colons, the numbers of goblet cells were significantly increased compared to those in the saline-injected colons (Fig. 4). This provides evidence in favor of an important role for IL-10 in goblet cell biology.
Fig 4.
Effects of direct administration of rIL-10 on intestinal goblet cell response. (A) Naive C57BL/6 mice with rIL-10 injected directly into colonic tissue showed increased numbers of PAS+ goblet cells. Mice were surgically injected with rIL-10 and sacrificed 48 h later. There was a significant increase in PAS+ goblet cell numbers in these mice compared to mice that were injected with saline (control) only. (B) Histology photographs of PAS+-stained goblet cells from colons of control C57BL/6 mice injected with saline. (C) Histology photographs of PAS+-stained goblet cells from colons of mice directly injected with rIL-10. *, P < 0.05 (n = 5 in each group).
JB-1 treatment enhances worm expulsion in Muc2 KO mice in T. muris infection.
The role of the Muc2 mucin in T. muris infection has been previously investigated, and it was shown to play a part in the initial expulsion but not to be critical at the late stage of parasite expulsion (9). After infection, Muc2 KO mice have minimal numbers of PAS+ goblet cells compared to wild-type mice. Muc2 KO mice expel the worms between days 21 and 30 p.i., rather than starting expulsion on day 15 like resistant C57BL/6 mice (9). When T. muris-infected Muc2 KO mice were treated with JB-1, they had significantly lower numbers of worms on day 21 p.i. than the mice treated with medium (Fig. 5A). There were no significant differences in worm burdens on days 10 and 14 p.i. These results are consistent with our previous results (9) and suggest that the beneficial effects of JB-1 in this infection are in part due to effects on Muc2 production. However, additional mucins may be involved in the late stage of worm expulsion, as Muc2 KO mice treated with bacteria had a significant increase of PAS+ goblet cells on day 21 p.i. (Fig. 5B to D), but the goblet cells were smaller than those in wild-type mice.
Fig 5.
(A) Worm burden in Muc2 KO mice treated with live JB-1 or MRS. Mice received treatment for 15 days beginning 1 day before infection. On day 21 postinfection, there was a significant decrease of worm burden in JB-1-treated mice compared with medium-treated mice. *, P < 0.05 (n = 3 to 5). (B) PAS+ goblet cell counts from the Muc2 KO mice infected with T. muris and treated with either JB-1 or MRS. A significant increase in the total goblet cell numbers in the infected mice treated with JB-1 was seen at day 21 p.i. *, P < 0.05. (C and D) Corresponding histological sections of the colon (stained with PAS) showing goblet cells.
Epithelial cell proliferation increases in mice treated with JB-1.
To examine the effects of JB-1 on proliferation of epithelial cells, we injected T. muris-infected C57BL/6 mice with BrdU and analyzed tissue sections. In accordance with other research, our examination demonstrated that an infection with T. muris in C57BL/6 mice significantly increased the number of proliferating cells by day 21. After infection, there were significantly higher numbers of proliferating cells in JB-1-treated mice than in medium-treated mice (Fig. 6).
Fig 6.
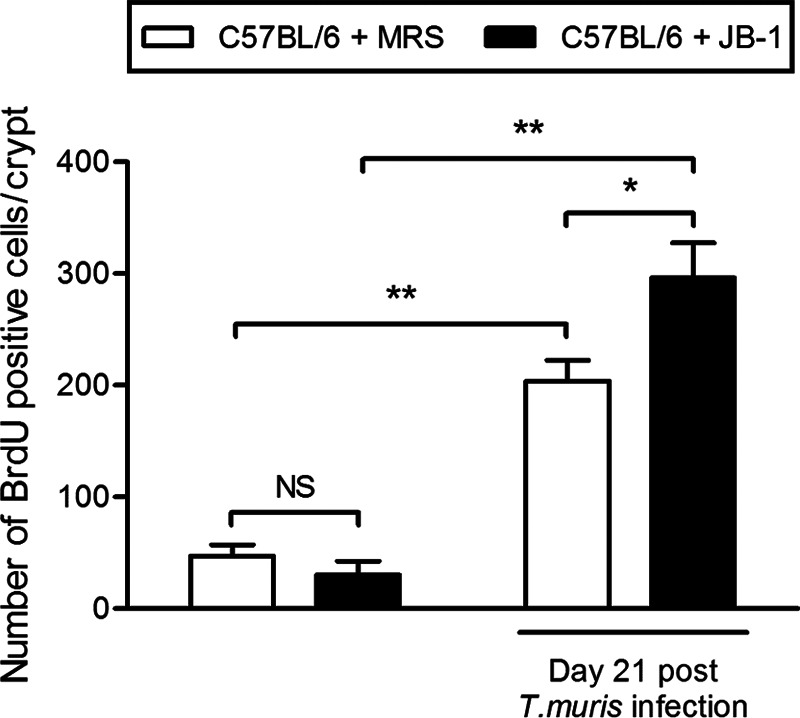
Numbers of proliferating cells per crypt in C57BL/6 mice injected with BrdU stain. Both groups infected with T. muris had significantly higher numbers of proliferating cells than groups that were not infected with the parasite. Noninfected and infected mice were treated with JB-1 for 15 days beginning 1 day before infection. Infected mice treated with JB-1 had significant increases in the numbers of proliferating cells compared with medium-treated mice. Seven to 10 crypts were examined in each group; all values are expressed as means ± SEM. *, P < 0.05; **, P < 0.001.
Treatment with live JB-1 promotes worm expulsion and upregulates goblet cell numbers in susceptible mice.
AKR mice are susceptible to T. muris infection and develop chronic infections, with the generation of a Th1 response. A previous study has demonstrated significantly lower numbers of goblet cells and lower levels of Muc2 expression in AKR mice after T. muris infection (9). Based on these findings of beneficial effects of JB-1 in modulating host protective responses in resistant mice, we next investigated whether this treatment modulates host responses in susceptible mice and promotes protection. We observed significant decreases in worm burden in AKR mice after treatment with JB-1 and this was accompanied with an upregulation of IL-10 (Fig. 7). There was attenuation of IFN-γ production, but IL-4 and IL-13 production did not differ in JB-1- and MRS-treated infected AKR mice (data not shown). We observed significantly higher numbers of goblet cells induced by JB-1 treatment in AKR mice after infection (Fig. 8). These observations further suggest that the bacteria act through an IL-10–goblet cell axis and exert their beneficial effects via this mechanism in this infection.
Fig 7.

Worm expulsion and intestinal IL-10 levels after probiotic treatment in susceptible AKR mice. AKR mice were infected with T. muris and were treated with either JB-1 or MRS for 36 days beginning 1 day before infection. Mice were sacrificed on day 35 p.i. to assess worm burden and IL-10 in colonic tissue. (A) Worm burden in AKR mice after JB-1 or MRS treatment. (B) Colonic tissue IL-10 concentrations from infected AKR mice after JB-1 or MRS treatment. *, P < 0.05 (n = 4 for controls and 6 to 8 for treatment groups).
Fig 8.
Intestinal goblet cell responses after live bacterial treatment in susceptible AKR mice. AKR mice were infected with T. muris and were treated with either JB-1 or MRS for 36 days beginning 1 day before infection. We sacrificed the mice on day 35 p.i. to assess intestinal goblet cells. (A) Goblet cell counts (after PAS staining) in T. muris-infected AKR mice after JB-1 or MRS treatment. (B and C) Corresponding histological sections of the colons (stained with PAS) showing goblet cells. *, P < 0.05 (n = 4 for controls and 6 to 8 for treatment groups).
DISCUSSION
The effects of treatment with a variety of commensal bacteria and probiotics under many different conditions have been studied experimentally and clinically, but the mechanisms whereby these bacteria act remain poorly understood. Beneficial bacteria have been shown to promote homeostatic conditions in the GI tract by acting on different aspects of tissue physiology, including epithelial integrity and function as well as the immune and enteric nervous systems (33–38). Despite a significant increase in this type of research in recent years, the effects of such bacterial treatment on host defense in enteric parasitic infections remain largely unexplored.
Enteric parasitic infections have a significant impact on the health status of the populations and the economies of the countries in which they occur. Nematode infections globally have widespread prevalence and cause acute and chronic diseases (3, 27, 39). These parasitic infections are responsible for extensive diseases that cause high rates of morbidity, anemia, nutrient deficiency, and poor cognition and may require surgical interventions (39–41). These parasites are thus responsible for decreased quality of life for billions of people and present a huge economic burden (42). While many antiparasitic medications are commercially available, resistance rates are increasing, which in turn leads to an increase in the numbers of people infected. Thus, there is a growing need for new research to provide alternate affordable therapeutic strategies.
In this comprehensive study, we observed a beneficial role of treatment with a potential probiotic, JB-1, in experiments involving the modulation of immune and goblet cell responses in the context of host defense during T. muris infections. The susceptibility of the host and its immune responses to this infection are determined by CD4+ T cells (1, 3). These T cells regulate mucosal alterations, such as epithelial cell turnover, goblet cell hyperplasia, and mucin production, which together determine the clearance of a T. muris infection (27, 43, 44).
We have shown that JB-1 administration modulated host responses to T. muris, which led to increased worm expulsion not only in a resistant strain of mice but also in a susceptible strain. Resistant mice treated with live probiotic had fewer parasites throughout the infection, and this effect was accompanied by an early increase in IL-10 and significant increases in goblet cells. These findings show that these bacteria are beneficial in promoting host defenses in this infection. Treatment with killed JB-1 did not have the same beneficial effect as the live bacteria in terms of worm expulsion and immunological changes. These data suggest that the beneficial effects are attributable not only to the bacterial structure but also perhaps to a secreted or diffusible product from the bacteria or to the interactions between the host and the live bacteria. Alternatively, it is possible that structural alterations in the bacteria generated by γ irradiation may lead to changes in the motifs recognized by pattern recognition receptors. Even minor chemical modifications in bacterial cell wall components can result in significantly different immunological consequences (45, 46). It was also shown that the standard methods to produce nonviable bacteria, γ irradiation and heat and UV inactivation, can reduce the capacity of lactobacilli to bind to mucins through as-yet-unknown mechanisms (47).
Clearance of intestinal parasites depends on different aspects of the immune system, some more critical than others. IL-10 plays a major role in regulating other cytokines that prevent the expulsion of the worm, such as IFN-γ and IL-12 (48). These two cytokines are upregulated in susceptible mice during T. muris infection (42). It is clear that IL-10 plays a role during a T. muris infection, as it was previously shown that IL-10 KO mice with T. muris infections had a 100% mortality rate caused by cytokine alterations, bacterial overgrowth, inflammation, and loss of mucus protection (19). The increase of IL-10 in the resistant infected mice after JB-1 treatment may have led to other changes that facilitated earlier expulsion of the parasites and achievement of intestinal homeostasis (18, 19). The early increase of IL-10 with JB-1 treatment during infection led us to use genetically altered mice to investigate the role of IL-10. IL-10 KO mice are extremely susceptible to T. muris infections (19), and when these infected mice were treated with JB-1, there was no decrease in worm burden and no increase in goblet cells throughout the infection. These findings strongly suggest that IL-10 plays a key role in mediating the beneficial effects of JB-1 in host defense in this infection. Although we have not investigated the source of the increased IL-10 production associated with JB-1 treatment, it is possible that JB-1 promotes IL-10 production by epithelial cells, which has been shown to occur with certain lactobacilli (49).
The increase in IL-10 in resistant mice treated with JB-1, and the increase of goblet cells in resistant mice but not in IL-10 KO mice, raised the question of whether IL-10 has a direct role in intestinal goblet cell biology. When rIL-10 was directly injected into the colons of naive mice there was a significant increase in goblet cell numbers, implying a direct role of IL-10 in the induction of goblet cell hyperplasia. However, JB-1 in the absence of infection did not promote goblet cell number increases. Furthermore, under these conditions, JB-1 had no effect on epithelial cell proliferation. Therefore, in order to produce their beneficial effects, these bacteria require the environmental changes induced by the responses to the nematodes.
Goblet cells have been previously shown to be upregulated in many types of parasitic infections and to be important determinants of defense against these infections, including those caused by T. muris (9–11, 32). Interestingly, our data showed further increases of goblet cells after JB-1 treatment in the infected mice. The numbers of goblet cells containing a mixture of acid and neutral mucins were significantly higher on day 10 p.i. in JB-1-treated mice, which correlates with the highest level of IL-10. The alteration in goblet cells after bacterial treatment may have provided benefits to the host defense, as mucins have been shown to help in worm expulsion by trapping the parasites in the mucus and preventing further attachment to the epithelial layer (32). The nature of the mucins in the goblet cells changes from neutral to acid around the time of worm expulsion (29). Intestinal mucus of the host was detected within the intestine of Nippostrongylus brasiliensis worms (32). With the development of immunity, ingestion of mucus by the parasites may have deleterious effects on the parasites. In addition to the goblet cell hyperplastic response, a qualitative change in mucins has been observed during N. brasiliensis infection. An increased quantity of mucus, along with qualitative changes in mucins, may trap the worms and prevent their attachment to the epithelial surface, and subsequently an enhancement in propulsive activity assists in the expulsion of the worms from the gut. Nevertheless, other components of host responses to nematode infection, such as mast cells and eosinophils, might also contribute to the process. We believe that multiple effector molecules, rather than a single one, act together in the efficient expulsion of the worms from the gut (13, 43). In addition to enhancing the mucus barrier, goblet cells might play a key role in immune function by presenting luminal antigens to lamina propria dendritic cells (50), and thus the increase in goblet cell numbers may promote improved adaptive immune responses.
Recently, we have shown that the initial expulsion of worms is delayed in Muc2 KO mice infected with T. muris (9). Around the time of expulsion there was an increase in Muc5ac in the Muc2 KO mice, triggered by the infection (9). Here, we observed that JB-1-treated Muc2 KO mice had a decreased parasite burden on day 21 p.i. This correlated with a surprising increase in the Th2 cytokine IL-4 and a decrease in IL-12, a Th1-promoting cytokine (data not shown). There was also an increase in goblet cells on day 21 p.i. in the treated mice. The alteration in cytokines may have benefited the host by altering mucins and creating a more resistant environment. The earlier expulsion of the worms from the Muc2 KO mice induced by treatment with live bacteria suggests that the benefits seen with JB-1 may include other mucins in addition to Muc2 (9).
During T. muris infection, cell proliferation is altered not only in goblet cells but also in other epithelial cells of the mucosa (51). In resistant mice, IL-13 has been shown to promote epithelial cell turnover during worm expulsion, and IFN-γ reduced this turnover. Delay in the proliferation and migration of cells may prevent the displacement of the smaller worms earlier on in the infection and thus may allow the larger worms to attach and burrow into the mucosa to create a niche for optimal survival (51). Our study on BrdU incorporation into cells revealed that JB-1 treatment in T. muris-infected mice promoted proliferation of epithelial cells. A recent study showed that treatment of mice with another probiotic, Lactobacillus reuteri, increased epithelial cell migration and proliferation (38). Taken together, these results seem to indicate that in addition to promoting the differentiation of goblet cells, JB-1 treatment might also increase the rate of epithelial cell turnover and thereby promote epithelial shedding, leading to increased expulsion of parasites.
Based on the compelling evidence for the beneficial role of JB-1 in the resistant mice, we carried out investigations of T. muris infection in the susceptible AKR mice. When infected with this parasite, AKR mice are known to have a Th1 response, which also correlates with nonexistent worm expulsion and the absence of goblet cell hyperplasia (4–9, 52). We observed a striking reduction in worm burden in infected AKR mice after JB-1 treatment. This was accompanied by the upregulation of goblet cell numbers and IL-10 levels and the downregulation of IFN-γ production. Recently, we have shown that an increase in IFN-γ levels is associated with impairment of goblet cell hyperplasia in T. muris infection (9). It has also been reported that IFN-γ specifically inhibited secretion of mucins in vitro, stimulated by cholera toxin or vasoactive intestinal peptide (53). Therefore, it is very likely that increases in IL-10 levels, along with decreases in IFN-γ levels, enhanced goblet cell responses in the mice in this study. Taken together, these findings further highlight the beneficial role of these bacteria in host defense in this infection, even in genetically susceptible mice.
The high numbers of parasitic infections and increasing resistance to antiparasitic medications impose a huge economic burden on affected populations (42, 54). This study provides us with important information on the actions of these types of bacteria in host defense against intestinal nematode infections and discloses a new area of research which may ultimately lead to the development of new strategies for prevention and therapy of nematode parasitic infections. As the data presented in this paper have shown that viable JB-1 provides significant beneficial effects to the host during a parasitic infection, future studies with bacterium-conditioned media may further enhance our understanding of beneficial probiotic-mediated effects in host defense in nematode infections.
ACKNOWLEDGMENTS
We thank Anna Velcich (Albert Einstein Cancer Center/Montefiore Medical Center, United States) for the Muc2 KO mice, Richard Grencis (University of Manchester, United Kingdom) for T. muris, and Marcus Manocha (McMaster University) for his valuable input.
This work is supported by grants from the Canadian Institutes of Health Research (CIHR) and the Crohn's and Colitis Foundation of Canada (CCFC) (to W.I.K.) and from the National Science and Engineering Council of Canada (NSERC) (to W.K.).
Footnotes
Published ahead of print 27 March 2013
REFERENCES
- 1. MacDonald AS, Araujo MI, Pearce EJ. 2002. Immunology of parasitic helminth infections. Infect. Immun. 70:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fahmy MA. 1954. An investigation on the life cycle of Trichuris muris. Parasitology 44:50–57 [DOI] [PubMed] [Google Scholar]
- 3. Grencis RK. 1993. Cytokine-mediated regulation of intestinal helminth infections: the Trichuris muris model. Ann. Trop. Med. Parasitol. 87:643–647 [DOI] [PubMed] [Google Scholar]
- 4. Cliffe LJ, Potten CS, Booth CE, Grencis RK. 2007. An increase in epithelial cell apoptosis is associated with chronic intestinal nematode infection. Infect. Immun. 75:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Else KJ, deSchoolmeester ML. 2003. Immunity to Trichuris muris in the laboratory mouse. J. Helminthol. 77:95–98 [DOI] [PubMed] [Google Scholar]
- 6. Deschoolmeester ML, Else KJ. 2002. Cytokine and chemokine responses underlying acute and chronic Trichuris muris infection. Int. Rev. Immunol. 21:439–467 [DOI] [PubMed] [Google Scholar]
- 7. Dixon H, Blanchard C, Deschoolmeester ML, Yuill NC, Christie JW, Rothenberg ME, Else KJ. 2006. The role of Th2 cytokines, chemokines and parasite products in eosinophil recruitment to the gastrointestinal mucosa during helminth infection. Eur. J. Immunol. 36:1753–1763 [DOI] [PubMed] [Google Scholar]
- 8. Motomura Y, Ghia JE, Wang H, Akiho H, El-Sharkawy RT, Collins M, Wan Y, McLaughlin JT, Khan WI. 2008. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut 57:475–481 [DOI] [PubMed] [Google Scholar]
- 9. Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, Grencis RK, Thornton DJ, Khan WI. 2010. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 138:1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishikawa N, Wakelin D, Mahida YR. 1997. Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 113:542–549 [DOI] [PubMed] [Google Scholar]
- 11. Khan WI, Abe T, Ishikawa N, Nawa Y, Yoshimura K. 1995. Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis-infection in mice. Parasite Immunol. 17:485–491 [DOI] [PubMed] [Google Scholar]
- 12. Khan WI, Blennerhasset P, Ma C, Matthaei KI, Collins SM. 2001. Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol. 23:39–42 [DOI] [PubMed] [Google Scholar]
- 13. Khan WI. 2008. Physiological changes in the gastrointestinal tract and host protective immunity: learning from the mouse-Trichinella spiralis model. Parasitology 135:671–682 [DOI] [PubMed] [Google Scholar]
- 14. McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. 1998. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 8:339–342 [DOI] [PubMed] [Google Scholar]
- 15. Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. 2000. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut 47:589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forstner JF. 1978. Intestinal mucins in health and disease. Digestion 17:234–263 [DOI] [PubMed] [Google Scholar]
- 17. Specian RD, Oliver MG. 1991. Functional biology of intestinal goblet cells. Am. J. Physiol. 260:C183–C93 [DOI] [PubMed] [Google Scholar]
- 18. Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. 2008. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 14:4280–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr, Wynn TA. 2002. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 168:2383–2392 [DOI] [PubMed] [Google Scholar]
- 20. Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet ME, Perdigon G. 2007. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin. Vaccine Immunol. 14:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. 2004. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig. Dis. Sci. 49:1095–1102 [DOI] [PubMed] [Google Scholar]
- 22. Herias MV, Koninkx JF, Vos JG, Huis in't Veld JH, van Dijk JE. 2005. Probiotic effects of Lactobacillus casei on DSS-induced ulcerative colitis in mice. Int. J. Food Microbiol. 103:143–155 [DOI] [PubMed] [Google Scholar]
- 23. Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. 1999. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116:1107–1114 [DOI] [PubMed] [Google Scholar]
- 24. Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274 [DOI] [PubMed] [Google Scholar]
- 25. Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. 2002. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295:1726–1729 [DOI] [PubMed] [Google Scholar]
- 26. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 108:16050–16055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wakelin D. 1967. Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology 57:515–524 [DOI] [PubMed] [Google Scholar]
- 28. Else KJ, Wakelin D, Wassom DL, Hauda KM. 1990. MHC-restricted antibody responses to Trichuris muris excretory/secretory (E/S) antigen. Parasite Immunol. 12:509–527 [DOI] [PubMed] [Google Scholar]
- 29. Koninkx JF, Mirck MH, Hendriks HG, Mouwen JM, van Dijk JE. 1988. Nippostrongylus brasiliensis: histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp. Parasitol. 65:84–90 [DOI] [PubMed] [Google Scholar]
- 30. Vega CJ, Peterson DA. 2005. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat. Methods 2:167–169 [DOI] [PubMed] [Google Scholar]
- 31. Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, Van Snick J, Collins SM. 2003. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect. Immun. 71:2430–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller HR. 1987. Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitology. 94(Suppl):S77–S100 [DOI] [PubMed] [Google Scholar]
- 33. Galdeano CM, Perdigon G. 2004. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 97:673–681 [DOI] [PubMed] [Google Scholar]
- 34. Forsythe P, Bienenstock J. 2010. Immunomodulation by commensal and probiotic bacteria. Immunol. Invest. 39:429–448 [DOI] [PubMed] [Google Scholar]
- 35. Wang B, Mao YK, Diorio C, Pasyk M, Wu RY, Bienenstock J, Kunze WA. 2010. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. FASEB J. 24:4078–4088 [DOI] [PubMed] [Google Scholar]
- 36. Ivanov II, de Llanos Frutos R, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4:337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yan F, Polk DB. 2012. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes 3:25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Preidis GA, Saulnier DM, Blutt SE, Mistretta TA, Riehle KP, Major AM, Venable SF, Finegold MJ, Petrosino JF, Conner ME, Versalovic J. 2012. Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. FASEB J. 26:1960–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Medlin CA, Chowdhury M, Jamison DT, Measham A. 2006. Improving the health of populations: lessons of experience. In Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P. (ed), Disease control priorities in developing countries, 2nd ed The International Bank for Reconstruction and Development/The World Bank Group, Washington, DC: [PubMed] [Google Scholar]
- 40. Crompton DWT, Montresor A, Nesheim MC, Savioli L. 2003. Controlling disease due to helminth infections. World Health Organization Press, Geneva, Switzerland [Google Scholar]
- 41. World Health Organization 2011. WHO partners for parasite control. Useful information on schistosoma and soil transmitted helminths. World Health Organization, Geneva, Switzerland [Google Scholar]
- 42. Artis D. 2006. New weapons in the war on worms: identification of putative mechanisms of immune-mediated expulsion of gastrointestinal nematodes. Int. J. Parasitol. 36:723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan WI, Collins SM. 2004. Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol. 26:319–326 [DOI] [PubMed] [Google Scholar]
- 44. Mahida YR. 2003. Host-parasite interactions in rodent nematode infections. J. Helminthol. 77:125–131 [DOI] [PubMed] [Google Scholar]
- 45. Lebeer S, Vanderleyden J, De Keersmaecker SC. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8:171–184 [DOI] [PubMed] [Google Scholar]
- 46. Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, Pot B, Hartung T, Hols P, Mercenier A. 2005. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc. Natl. Acad. Sci. U. S. A. 102:10321–10326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ouwehand AC, Tolkko S, Kulmala J, Salminen S, Salminen E. 2000. Adhesion of inactivated probiotic strains to intestinal mucus. Lett. Appl. Microbiol. 31:82–86 [DOI] [PubMed] [Google Scholar]
- 48. Hoffmann KF, Cheever AW, Wynn TA. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406–6416 [DOI] [PubMed] [Google Scholar]
- 49. Corr SC, Gahan CG, Hill C. 2007. Impact of selected Lactobacillus and Bifidobacterium species on Listeria monocytogenes infection and the mucosal immune response. FEMS Immunol. Med. Microbiol. 50:380–388 [DOI] [PubMed] [Google Scholar]
- 50. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. 2012. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483:345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. 2005. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308:1463–1465 [DOI] [PubMed] [Google Scholar]
- 52. Else KJ, Grencis RK. 1991. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology 72:508–513 [PMC free article] [PubMed] [Google Scholar]
- 53. Jarry A, Merlin D, Velcich A, Hopfer U, Augenlicht LH, Laboisse CL. 1994. Interferon-gamma modulates cAMP-induced mucin exocytosis without affecting mucin gene expression in a human colonic goblet cell line. Eur. J. Pharmacol. 267:95–103 [DOI] [PubMed] [Google Scholar]
- 54. Pullan R, Brooker S. 2008. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology 135:783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]