Abstract
Commercial bacterins for Glässer's disease are widely used for the prevention of this disease caused by Haemophilus parasuis; however, the protective efficacy varies depending on the strain and serovar. Bacterial ghosts (BGs) are empty bacterial envelopes that, unlike classic bacterins, suffer no denaturing steps during their production. These properties may lead to superior protection. In this study, a BG vaccine generated from the Haemophilus parasuis serovar 5 reference strain Nagasaki was prepared and used to inoculate piglets. The efficacy of the BG vaccine was evaluated by clinical, bacteriological, serological, and postmortem examinations. Inactivated bacterin (IB) and a placebo control (PC) were compared with the BG vaccine in this study. The results showed that the piglets inoculated with the BG vaccine developed higher antibody activity and higher gamma interferon and interleukin 4 levels than those vaccinated with IB or those in the PC group after primary and secondary exposure to the antigens and challenge. CD4+ T lymphocyte levels were observed to increase following secondary immunization more in the BG-vaccinated group than in the IB (P < 0.05) and PC (P < 0.05) groups. CD8+ T lymphocyte levels increased dramatically in all three groups after challenge, and the differences between groups were all significant (P < 0.05). There were fewer tissue lesions and lower bacterial loads in the tissue homogenates in the BG group after challenge. The results suggest that higher CD4+ T lymphocyte levels and both CD4+ major histocompatibility complex class II-restricted Th1-type and Th2-type immune responses in the BG group are relevant for protection.
INTRODUCTION
Haemophilus parasuis is a Gram-negative, nonhemolytic, NAD-dependent bacterium belonging to the Pasteurellaceae family. H. parasuis is a commensal organism of the upper respiratory tract of conventional pigs that causes Glässer's disease, which is characterized by fibrinous polyserositis, polyarthritis, and meningitis (1–3). This disease causes significant losses in the swine industry worldwide, due to its high mortality and morbidity rates (1, 3). Vaccination generally is considered to be the most effective means to control Glässer's disease; however, attempts to develop effective vaccines against H. parasuis have been hampered by a lack of overall knowledge regarding the virulence factors and protective antigens of this bacterium (1). Inactivated bacterin for H. parasuis is a traditional vaccine used around the world that can elicit efficient protection against homologous challenges; however, due to the serovar diversity of H. parasuis, inactivated bacterin is limited in cross-protection (2–4). In a previous study, subunit vaccines comprising recombinant transferrin-binding protein B (TbpB) or outer membrane protein (OMP) formulations enriched with TbpB conferred only partial protection (5). The OMPs PalA, Omp2, D15, and HPS-06257 induced protective responses against H. parasuis (SH0165) in pigs (3), and the OMPs SmpA, YgiW, and FOG have been shown to be protective both individually and synergistically against infection with the highly virulent H. parasuis in mice (6). However, as mentioned above, the major virulence factors and protective antigens of H. parasuis are still largely unknown and need to be identified and further characterized.
In recent years, a genetically inactivated vaccine—the ghost vaccine—has emerged as an alternative to traditional inactivated bacterin, which lacks cross-protection and fails to prevent subclinical or chronic infection and pathogen colonization (7–9). Bacterial ghosts (BGs) are empty cell envelopes from Gram-negative bacteria that can be produced by controlled expression of the cloned lysis gene E from bacteriophage ϕX174; the plasmid-encoded protein E leads to the formation of a transmembrane tunnel structure through the cell envelope of Gram-negative bacteria, through which the cytoplasmic contents are expelled (10, 11). The resulting ghosts share the function and antigenic determinants of the envelope with their replicating counterparts, because protein E does not cause any physical or chemical denaturation of the bacterial surfaces during the lysis processes (10–13). Owing to the particulate nature of the BGs and the fact that they contain many well-known immune response-stimulating compounds, the intrinsic adjuvant properties of BGs enhance T cell activation and systemic, mucosal, and cell-mediated immune responses to the envelope structures (12–15). This useful property makes BGs a promising versatile delivery vehicle for heterologous antigens, drugs, and other biologically active substances (14–19). Another attractive feature of BGs is the easy-to-produce platform technology, requiring merely expression of lysis gene E within the bacterial cells without the need for information on the genome (12, 16), which is especially useful for bacteria that have not been thoroughly studied regarding functional genes, e.g., H. parasuis.
The objective of the present study was to assess the efficacy of intramuscular immunization with H. parasuis ghosts to confer protection against a homologous challenge in an intraperitoneal infection model. The protective effect was compared with that induced by a formalin-inactivated bacterin. In this study, we describe for the first time the protective efficacy of H. parasuis ghosts.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The H. parasuis serovar 5 reference strain Nagasaki was cultured in tryptic soy broth (TSB) (Difco) or on tryptic soy agar (TSA) supplemented with 10 μg/ml NAD and 5% fetal calf serum (Gibco) and was incubated at 37°C in a 5% CO2 incubator. The Escherichia coli strain DH5α (Novagen) was grown in Luria-Bertani (LB) broth at 37°C in an orbital shaking incubator; for cultivation of E. coli β2155, diaminopimelic acid (dapA) (1 mM; Sigma Chemical Co.) was added. The medium was supplemented with the appropriate antibiotic (ampicillin, 100 μg/ml) as needed for selection or stabilization of the plasmids. The bacterial strains, plasmids, and primers used in this study are listed in Table 1.
Table 1.
Bacterial strains, plasmids, and primers used in this study
| Bacterial strain, plasmid, or primer | Characteristics | Source or conditions |
|---|---|---|
| Strains | ||
| E. coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Novagen |
| E. coli β2155 | thrB1004 pro thi hsdS lacZ M15 (F′ lacZ M15 lacIq traD36 proA+ proB+) dap::erm (Ermr)a | Gerald-F. Gerlachb |
| Bacteriophage ϕX174 | Novagen | |
| H. parasuis Nagasaki | H. parasuis serovar 5 reference strain | P. J. Blackallc |
| Plasmids | ||
| pMD-18T | TA cloning vector | TaKaRa |
| pMD-E | pMD-18T carrying gene mE | This study |
| pBV220 | Expression vector carrying the λ bacteriophage PR/PL promoter | Guihong Zhangd |
| pBV-E | pBV-220 carrying gene mE | This study |
| pGZRS-18 | A. pleuropneumoniae-E. coli shuttle vector | Susan E. H. Weste |
| pGZRS-E | pGZRS-18 carrying gene mE | This study |
| pBBR1MCS | Broad-host-range cloning vector | M. E. Kovachf |
| pBBR-E | pBBR1MCS carrying gene mE | This study |
| pPBA1100 | P. multocida-E. coli shuttle vector | Ben Alderg |
| pPBA-E | pPBA1100 carrying gene mE | This study |
| Primers | 95°C for 5 min, 30 cycles of 94°C for 30 s | |
| LysisE-U | 5′-AGGGAATTCTGGTACGCTGGACTTTGTGG-3′(EcoRI site) | 59°C for 30 s |
| LysisE-L | 5′-AGGGGATCCGAGCTCTCACTCCTTCCG-3′(BamHI site, for amplification of the 275-bp lysis gene mE) | 72°C for 30 s |
| cI-E-U | 5′-GGGGGAGCTCTCAGCCAAACGTCTC-3′ (SacI site) | 95°C for 5 min, 30 cycles of 94°C for 30 s |
| LysisE-L | 5′-AGGGGATCCGAGCTCTCACTCCTTCCG-3′(BamHI site) | 56°C for 30 s, 72°C for 30 s |
Ermr, erythromycin resistance.
Tierärztliche Hochschule Hannover, Institut für Mikrobiologie und Tierseuchen.
Department of Primary Industries and Fisheries Queensland, Animal Research Institute.
Department of Veterinary Microbiology, School of Veterinary Medicine, South China Agricultural University.
Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin-Madison.
Department of Microbiology and Immunology, Louisiana State University Medical Center.
Australian Research Council Center of Excellence in Structure and Functional Microbial Genomics, Monash University.
Construction of bacteriolytic plasmid pMD-mE and electroporation of E. coli β2155.
A mutant lysis gene E with a single-base mutation at the 5′-terminal start codon ATG (A/C) and a truncated 201-base-pair (bp) lysis gene was inserted into plasmid pBV220, which harbored a thermosensitive regulator system, pR/pL-cI857, from a lambda bacteriophage (9). The lysis cassette gene pR/pL-cI857-mE was amplified by PCR and cloned into plasmid pMD-18T (TaKaRa) to create the bacteriolytic plasmid pMD-mE. To construct recombinant E. coli β2155 harboring a bacteriolytic plasmid, pMD-mE was electroporated into competent E. coli β2155 cells with an electroporator (MicroPulser; Bio-Rad) set at 12.5 kV/cm, 200 Ω, and 25 μF (20). The positive clone was identified by clone PCR and restriction enzyme digestion, and the E. coli β2155 strain containing the bacteriolytic plasmid pMD-mE was designated rEC-mE.
Transconjugation of rEC-mE with H. parasuis strain Nagasaki.
The bacteriolytic plasmid pMD-mE was mobilized from rEC-mE to H. parasuis serovar 5 reference strain Nagasaki by using a filter mating technique, as described previously (20). Briefly, donor and acceptor strains were grown overnight on an appropriate solid medium and resuspended in TSB, and then optical density at 600 nm (OD600) values were determined. Aliquots corresponding to 1 ml of donor and 0.1 ml of acceptor (each at OD600 = 0.4) were mixed and filtered onto a nitrocellulose disc (pore size, 0.45 μm; diameter, 2.5 cm; Millipore). The filters were placed on solid medium to support the growth of the donor and acceptor (TSB supplemented as described above, containing 1 mM dapA and 10 μg/ml NAD) and were incubated for 24 h. Next, the bacteria were washed off the filters with supplemented TSB, plated onto selective medium (supplemented TSB with 100 μg/ml ampicillin and 10 μg/ml NAD), and incubated for 36 h. The positive H. parasuis clone harboring bacteriolytic plasmid pMD-mE was identified by clone PCR and was designated rHps-mE.
Preparation of H. parasuis ghost vaccine and inactivated bacterin.
The rHps-mE clone was inoculated into supplemented TSB (with 100 μg/ml ampicillin and 10 μg/ml NAD), and the culture was incubated at 28°C to an OD600 of 0.4 to 0.5. The temperature was subsequently raised to 42°C to induce mutant lysis protein mE-mediated lysis (9–11). The OD600 was measured every 30 min until no further decrease in OD600 was detectable. After the completion of lysis, the BGs were harvested by centrifugation (4,000 × g for 10 min at 4°C), washed with phosphate-buffered saline (PBS) (pH 7.2), resuspended in sterile distilled water, lyophilized, and stored at −20°C. Loss of viability was assessed by plating the BG samples onto supplemented TSA and into supplemented TSB (with 10 μg/ml NAD). The incubations were performed at 28°C for 36 h. The lyophilized BGs were dissolved in PBS (pH 7.2) to an appropriate concentration (equivalent to 1 × 1011 CFU/ml) and were used directly as a BG vaccine to inoculate piglets. To prepare the inactivated bacterin, the H. parasuis serovar 5 reference strain Nagasaki was cultured, and 8 to 10 single colonies were selected and spread onto TSA plates (with 10 μg/ml NAD). After incubation at 37°C for 16 to 18 h, the bacteria were harvested into sterile 0.01 M PBS, diluted to 2 × 1011 CFU/ml, inactivated with 0.3% formalin for 24 h, washed three times with PBS (pH 7.2), and finally resuspended in PBS to the original volume, at 4°C. Two hundred microliters of the prepared inactivated cultures was randomly sampled and cultured on LB plates overnight to determine bacterial growth. The sterile cultures were emulsified with equivalent amounts of complete Freund's adjuvant (catalog no. F5881; Sigma-Aldrich) for the first injection or incomplete Freund's adjuvant (catalog no. F5506; Sigma-Aldrich) for the booster injection.
Animals and study design.
A total of 18 piglets (5 to 6 weeks of age) from an H. parasuis- and other respiratory pathogen-free farm (PCR- or reverse transcription-PCR-negative results with nasal and tonsillar swabs and no serological responses in relevant enzyme-linked immunosorbent assays [ELISAs]) were selected from two litters of the same ages and weights (10 piglets from one litter and 8 piglets from the other) and were blocked according to sex. The 18 piglets were randomly allotted to three groups of equal size according to sex and were housed separately, with identical feeding conditions. Thus, there were equal proportions of each sex among the groups, to avoid sex-based effects. The selection and allotting of the piglets were blinded. All of the experimental procedures involving animals in this study were conducted with approval from the Harbin Veterinary Research Institute Animal Ethics Committee, in accordance with guidelines for the use of animals in research based on animal welfare and ethical considerations.
Vaccination and challenge of piglets.
The piglets in groups I and II were injected with BGs (H. parasuis ghosts, 1 × 1011 CFU/piglet) and inactivated bacterin (IB) (1 × 1011 CFU/piglet), respectively. The placebo control (PC) group was injected with the same dose of PBS. Over a 2-week period, the vaccines were administered twice by deep intramuscular injection into the pig's neck. Fourteen days after the second immunization, all of the piglets received an intraperitoneal challenge with a dose of 5 × 109 CFU/piglet of H. parasuis strain Nagasaki. The challenged pigs which showed clinical signs of severe Glässer's disease, e.g., dyspnea, prostration, and incoordination, were subjected to euthanasia, and the surviving pigs in all of the experimental groups were euthanized on day 7 postinfection. Rectal temperatures and clinical signs were recorded twice a day during the first 3 days postinfection (dpi) and then once a day until the end of the study. A clinical scoring system was used to assess clinical signs for each animal, as follows. A score of 1 was given for each case of coughing, anorexia, and lameness, resulting in a minimal clinical score of 0 and a maximal score of 3 per day; pigs euthanized because of the disease were assigned a score of 4. The added daily clinical scores for days 1 to 7 after the challenge for each piglet were designated the total clinical scores, and the arithmetic mean and standard deviation were determined for each group. The average temperature value for each piglet during the observation period was calculated, and the mean temperature value for each group was determined. All animals were subjected to necropsy, and gross lesions were recorded. The severity of the pathological changes was scored blindly, as follows: a score of 1 was given for each case of pleuritis, peritonitis, arthritis, meningitis, and pericarditis, resulting in a minimal pathological score of 0 and a maximal score of 5 for each piglet. The arithmetic mean and standard deviation were determined for each group.
Serological analysis.
Blood and serum samples were collected prior to and following the primary and secondary immunizations and the challenge. Serum antibody activities were measured using an indirect ELISA based on inactivated whole-cell antigens, as described previously (8). Briefly, 96-microwell plates (Costar; eBioscience) were coated with 5 × 107 bacteria per well in carbonate buffer (pH 9.6) overnight at room temperature. Next, the plates were blocked overnight at 4°C. After blocking, the plates were incubated with the sera at 37°C for 1 h. The sera of the immunized pigs, diluted (1:100, 50 μl/well) with PBS with 0.1% Tween 20 (PBST), were used as the primary antibodies, and horseradish peroxidase-conjugated goat anti-pig IgG(H+L) (1:5,000 dilution, 50 μl/well; Sigma-Aldrich) was used as the secondary antibody. Convalescent-phase sera from H. parasuis infections and preimmune sera were used as the positive and negative controls, respectively. The enzymatic reaction product was developed for 10 min with chromogen containing 20 μl of 1% H2O2 (vol/vol) in 10 ml of 0.1 M citrate buffer (pH 5.0) and 0.1 mg/ml 3,3′,5,5′-tetramethylbenzidine (TMB), and development was stopped by the addition of 2 M sulfuric acid. Between the steps, the plates were washed 3 times with PBST. The results were read on a Dynatech MR 7000 ELISA reader (Bio-Rad model 680; Bio-Rad Laboratories). OD450 values were recorded to determine the serum antibody activities in the piglets. All of the reaction mixtures were prepared in triplicate, and the average values were used for recording and calculation. The mean values for 6 pigs in each group were reported. The amounts of gamma interferon (IFN-γ) and interleukin 4 (IL-4) were determined using IFN-γ and IL-4 ELISA kits (Dalian Pan-State Biotechnology Co.). These two cytokine detection kits were based on a solid-phase sandwich ELISA using paired cytokine-specific monoclonal antibodies (MAbs). Reaction products were detected with TMB substrate, and the results were read at a wavelength of 450 nm using an ELISA reader. The concentrations of IFN-γ or IL-4 in the samples were read from a standard curve based on the values for the serially diluted standard. The detection limit for IFN-γ was <2.0 pg/ml and the standard curve range was 0 to 500 pg/ml; the detection limit for IL-4 was 2.0 pg/ml and the standard curve range was 0 to 1,000 pg/ml. Each serum sample was tested in triplicate, and the mean value for each group was calculated.
CD4+/CD8+ T cell subset counts.
Blood samples were collected by aseptic venipuncture from the jugular vein, into commercial tubes containing heparin sodium as an anticoagulant. The blood samples were analyzed within 8 h. A three-color staining method was used in this study. One microliter each of fluorescein isothiocyanate (FITC)-conjugated CD4 MAb, phycoerythrin (PE)-conjugated CD8 MAb, and Spectral Red (SPRD)-conjugated CD3 MAb (SouthernBiotech) was added to 0.1 ml of a blood suspension, and the mixture was incubated in the dark at room temperature for 20 min. Next, 2 ml of fluorescence-activated cell sorting (FACS) lysing solution (BD Biosciences), prewarmed to 37°C, was added, and the resulting mixture was incubated in the dark at room temperature for 15 min. The cell suspension was centrifuged at 200 × g for 5 min, and the supernatant was discarded. The pellets were washed twice with PBS at 200 × g for 5 min, and the cells were subsequently resuspended in 400 μl of PBS. The FACS analysis was performed using a flow cytometer (BD Biosciences) equipped with CellQuest software (BD Biosciences). Before analysis, an unstained cell sample and FITC-, PE-, and SPRD-labeled cells were analyzed to set proper compensation values and to define quadrants. The lymphocytes were selected for analysis by gating to exclude both macrophages and cellular debris, based on size and granularity. A total of 10,000 events were measured per sample, and the percentage of each lymphocyte subpopulation was recorded.
Bacteriological analysis.
At necropsy immediately after euthanasia, tissues from the lungs, spleen, and inguinal lymph nodes were aseptically collected from each animal, weighed, and homogenized with aseptic glass dismembrators. The tissue homogenates were serially diluted with PBS, and 100 μl each of 103-, 104-, and 105-fold dilutions was plated onto selective medium and incubated at 37°C for 36 h. Confirmation of H. parasuis was carried out by colony PCR, and values of CFU per mg of homogenized tissue were determined.
Histopathological examination.
At necropsy, the liver, spleen, lung, kidney, and small intestine were collected, processed into samples of 0.5 to 1.0 cm3, and fixed in 10% Formol saline for 6 to 24 h. The tissue blocks were then dehydrated with ethanol, treated with xylene, and embedded in paraffin. Sections measuring 5 to 6 μm in thickness were prepared using a microtome, placed onto slides, and dried. After washing with xylene and dehydration with ethanol, the slides were stained with hematoxylin and eosin and were observed under a microscope.
Statistical analysis.
A statistical analysis was conducted using SAS v. 9.0 software. Analysis of variance with post hoc Bonferroni adjustments was used to determine the significance of the differences in means between multiple experimental groups. The data were expressed as means ± standard deviations, and values of P < 0.05 were considered significant.
RESULTS
Preparation of H. parasuis ghosts and lysis efficacy.
As shown in the lysis efficacy curve (Fig. 1), the lysis plasmid, pMD-mE, demonstrated high efficiency in the H. parasuis strain Nagasaki. When the culture temperature was shifted from 28°C to 42°C, the OD600 values of the recombinant H. parasuis (rHps-mE) that harbored the lysis plasmid pMD-mE decreased after 30 min. The OD600 values decreased continuously until lysis was complete after induction for 180 min, and lysis efficiency as high as 99.99% was subsequently obtained. In comparison, the OD600 values for the parent strain of H. parasuis Nagasaki increased continuously during the observation period. Although there were few viable cells after lysis, no viable cells were observed from the lyophilized ghosts. Transmission electron microscopy photographs of the H. parasuis parent strain and its ghost are shown in Fig. 2A and B, respectively.
Fig 1.
Growth curves for the Haemophilus parasuis strain Nagasaki and the recombinant strain rHps-mE under induction conditions. rHps-mE, recombinant strain of Haemophilus parasuis Nagasaki that harbors the lysis plasmid pMD-mE; ps Hps, parent strain Haemophilus parasuis Nagasaki. The culture was incubated at 28°C to an OD600 of 0.4 to 0.5, and the temperature then was raised to 42°C to induce mutant lysis protein mE-mediated lysis. The OD600 values of rHps-mE decreased after induction for 30 min; the OD600 values decreased continuously until lysis was complete after induction for 180 min. In comparison, the OD600 values of the parent strain Haemophilus parasuis Nagasaki increased continuously during the observation period.
Fig 2.
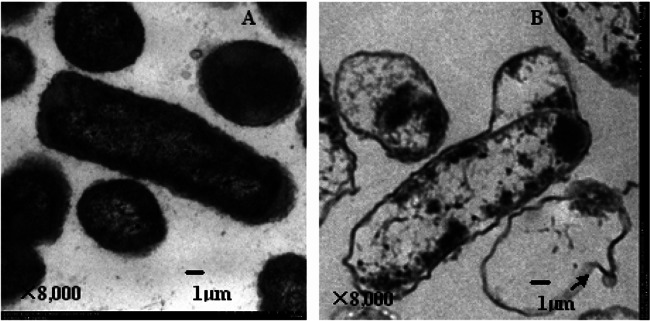
Transmission electron microscopic photographs of Haemophilus parasuis strain Nagasaki (A) and Haemophilus parasuis ghosts in which the cytoplasmic contents had been expelled from Haemophilus parasuis cells, leaving the empty cell envelope (B). Arrow, transmembrane lysis tunnel.
Clinical monitoring.
All of the BG-vaccinated pigs and four of the pigs vaccinated with IB gave the best clinical performance. A slight transient rise in rectal temperature (<40.5°C) for several of the piglets was recorded within 24 h postinfection (hpi), and moderate clinical signs such as cough and anorexia were observed for individual pigs. However, all of the pigs in the PC group and two pigs in the IB group were euthanized due to severe clinical signs suggesting Glässer's disease, from 6 hpi to 5 dpi. All of the euthanized pigs with severe clinical signs had shown elevated temperatures from 40.5 to 42.1°C and clinical signs including prostration, incoordination, and severe dyspnea. Summaries of the clinical signs are listed in Table 2.
Table 2.
Protective effect in each group upon Haemophilus parasuis serovar 5 reference strain Nagasaki challenge
| Group (n)a | No. of animals with clinical signsb | Clinical sign scorec | Temperature (°C)d | No. of animals with histopathological lesionse | Lesion scoref | Survival time (days)g |
|---|---|---|---|---|---|---|
| BG-vaccinated (6) | 3 | 2.6 ± 2.3h,i | 39.6 ± 0.3h,i | 3 | 0.7 ± 0.5h,i | 7.0 ± 0.0i |
| IB-vaccinated (6) | 5 | 9.3 ± 4.6j | 40.3 ± 0.6j | 6 | 2.0 ± 0.3j | 5.8 ± 3.2i |
| PC (6) | 6 | 23.8 ± 3.2 | 41.3 ± 0.8 | 6 | 3.0 ± 0.1 | 1.1 ± 0.6 |
A total of 18 postweaning piglets from a Haemophilus parasuis- and other-respiratory-pathogen-free farm were randomly divided into three groups, with 6 pigs in each group. The groups were designated as follows: BG vaccinated, vaccinated with Haemophilus parasuis ghost; IB vaccinated, vaccinated with inactivated bacterin; PC, placebo control.
The assessed clinical signs included coughing, anorexia, and lameness.
A score of 1 was given for each case of a clinical sign for each individual piglet, the added daily clinical scores for days 1 to 7 after challenge for each piglet were designated the total clinical scores, and the arithmetic mean and standard deviation were determined for each group.
The average temperature value for each piglet during the observation period was calculated, and the mean temperature value for each group was determined.
The assessed histopathological lesions included pleuritis, peritonitis, arthritis, meningitis, and pericarditis.
A score of 1 was given for each case of a histopathological lesion for each individual piglet, and the arithmetic mean and standard deviation were determined in each group.
The days of survival after challenge for each piglet were recorded, and the arithmetic mean and standard deviation were determined in each group.
Significant difference, compared with the IB-vaccinated group (P < 0.05).
Significant difference, compared with the PC group (P < 0.01).
Significant difference, compared with the PC group (P < 0.05).
Histopathological examination.
The pigs that were euthanized on day 7 postchallenge had no or moderate lesions when examined; focal pneumonia and mild peritonitis were observed in individual pigs. However, in the pigs that were euthanized due to severe clinical signs, the lesions were severe, with at least one of the following fibrinous inflammations being observed in each of the pigs: fibrinosuppurative pneumonia, pericarditis, pleuritis, peritonitis, arthritis, and meningitis, characterized by the presence of fibrin strands or layers on serosal surfaces. The microscopic lesions showed comprehensive fibrinous exudative inflammation in the organ samples examined, with a mixed inflammatory exudate composed of variable amounts of fibrin, macrophages, neutrophils, and lymphocytes. Vascular alterations, such as edema, congestion, hemorrhage, and thrombosis, were observed in most of the organs (heart, lungs, lymph nodes, kidneys, liver, spleen, and brain) and blood vessels. Summaries of the histopathological findings are listed in Table 2.
Serological analysis.
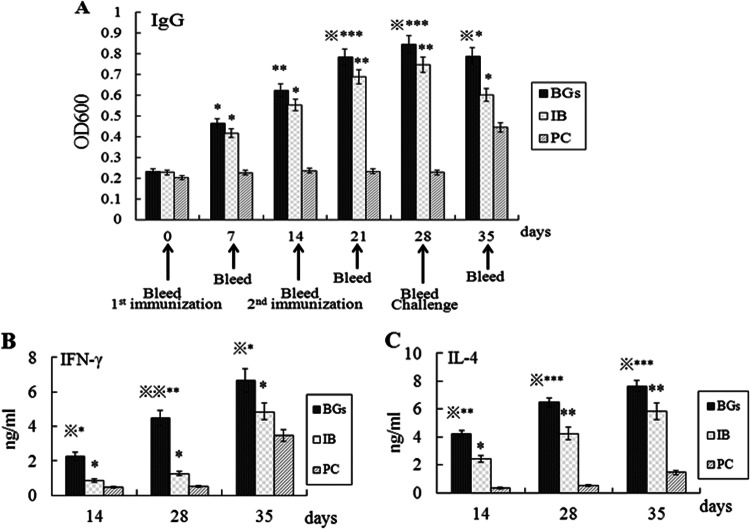
The serum antibody against the whole-cell antigen of H. parasuis was observed in both immunized groups following primary and secondary immunization, and the antibody activity increased over time (Fig. 3A). On days 7 and 14 after the initial injection, the IB group showed significant differences in antibody activity, compared with the nonvaccinated PC group (P < 0.05); the differences between the BG group and the PC group became significant on day 7 (P < 0.05) and on day 14 (P < 0.01). On days 21 and 28, the piglets in the BG group showed significant differences in antibody activity, compared with the IB group (P < 0.05), and the differences between the BG group and the PC group were significant (P = 0.0008). The differences between the IB group and the PC group also were significant (P < 0.01). On day 35, there were significant differences in antibody activity between the BG and IB groups (P < 0.05) and between the BG and PC groups (P < 0.05). The difference between the IB and PC groups also was significant (P < 0.05) on day 35.
Fig 3.
Levels of IgG antibody (A), IFN-γ (B), and IL-4 (C) in the sera of piglets from each group. The BG group was vaccinated with Haemophilus parasuis ghosts, the IB group was vaccinated with Haemophilus parasuis inactivated bacterin, and the PC group (the placebo control group) was injected with PBS. ∗, Significant difference, compared with the PC group (P < 0.05); ∗∗, significant difference, compared with the PC group (P < 0.01); ∗∗∗, significant difference, compared with the PC group (P < 0.001);  , significant difference, compared with the IB group (P < 0.05);
, significant difference, compared with the IB group (P < 0.05); 
 , significant difference, compared with the IB group (P < 0.01).
, significant difference, compared with the IB group (P < 0.01).
The concentrations of IFN-γ in the different groups are summarized in Fig. 3B. The IFN-γ concentrations in the sera of vaccinated piglets in the BG group were significantly higher than those in the IB and PC groups on day 14 (P < 0.05) and on day 28 (P < 0.01). On day 35, the IFN-γ concentrations increased in all of the experimental groups and the differences between the BG and IB groups, the BG and PC groups, and the IB and PC groups all were significant (P < 0.05).
The concentrations of IL-4 in the different groups are summarized in Fig. 3C. On day 14, the IL-4 concentrations in the sera of piglets in the BG group showed a significant difference from those in the PC group (P < 0.01), and the differences between the BG and IB groups and between the IB and PC groups also were significant (P < 0.05). From the results collected on days 28 and 35, the differences in IL-4 concentrations between the BG and PC groups (P < 0.001), the IB and PC groups (P < 0.01), and the BG and IB groups (P < 0.05) all were significant.
Relative proportions of CD4+ and CD8+ T cell subsets.
The relative proportions of CD4+ and CD8+ T cell subsets in the peripheral blood of the piglets in each group were analyzed using FACS. On day 14, the numbers of CD4+ T cells in the groups showed no significant difference; on day 28, however, the proportion of CD4+ T cells in the BG group increased and was significantly higher than that observed in the IB and PC groups (P < 0.05). On day 35, the percentage of CD4+ T cells in the BG group was significantly higher than those in the IB group (P < 0.05) and the PC group (P < 0.01). Although the CD4+ T cells in the IB group showed no significant difference in comparison with the PC group on days 14 and 28 (P > 0.05), the difference was significant on day 35 (P < 0.05) (Fig. 4A). On days 14 and 28, there were no significant differences in the proportions of the CD8+ T cell subset among the experimental groups. On day 35, 1 week after challenge, the percentages of CD8+ T cells in all three groups increased dramatically, there were significantly more CD8+ T cells in the IB and PC groups than in the BG group (P < 0.05), and the difference between the IB and PC groups also was significant (P < 0.05) at this time point (Fig. 4B).
Fig 4.

FACS analysis of CD4+ (A) and CD8+ (B) T lymphocyte numbers. Peripheral blood samples from piglets in each group were collected prior to and following immunization on days 0, 14, 28, and 35, and the CD3+CD4+ and CD3+CD8+ T lymphocyte populations were analyzed by fluorescence-activated cell sorting (FACS). The BG group was vaccinated with Haemophilus parasuis ghosts, the IB group was vaccinated with Haemophilus parasuis inactivated bacterin, and the PC group (the placebo control group) was injected with PBS. ∗, Significant difference, compared with the PC group (P < 0.05); ∗∗, significant difference, compared with the PC group (P < 0.01);  , significant difference, compared with the IB group (P < 0.05).
, significant difference, compared with the IB group (P < 0.05).
Bacteriological analysis of tissue homogenates.
The numbers of recovered CFU in the homogenized inguinal lymph nodes, spleen, and lung tissues were significantly lower in the BG group than in the IB group (P < 0.05) and the PC group (P < 0.01), and the differences in bacterial loads between the IB group and the PC group also were significant (P < 0.05) (Fig. 5).
Fig 5.
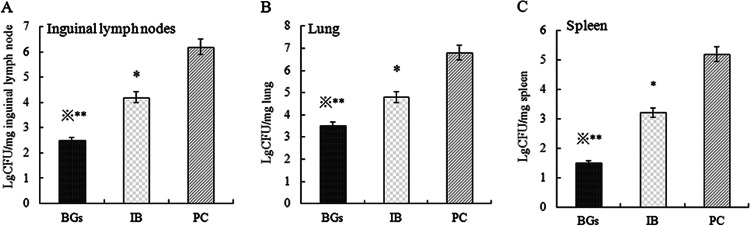
Bacterial loads in inguinal lymph nodes (A), spleen (B), and lung (C) homogenates after a Haemophilus parasuis strain Nagasaki challenge. The logarithm value (Lg) of the CFU in each milligram of tissue sample was recorded. The BG group was vaccinated with Haemophilus parasuis ghosts, the IB group was vaccinated with Haemophilus parasuis inactivated bacterin, and the PC group (the placebo control group) was injected with PBS. ∗, Significant difference, compared with the PC group (P < 0.05); ∗∗, significant difference, compared with the PC group (P < 0.01);  , significant difference, compared with the IB group (P < 0.05).
, significant difference, compared with the IB group (P < 0.05).
DISCUSSION
In this study, we constructed a series of bacteriolytic plasmids based on different plasmids, including the Actinobacillus pleuropneumoniae-Escherichia coli shuttle vector pGZRS-18 (isolated from A. pleuropneumoniae), the broad-host-range cloning vector pBBR1MCS-4 (isolated from Bordetella bronchiseptica), the Pasteurella multocida-Escherichia coli shuttle vector pPBA1100 (isolated from P. multocida), and the TA cloning vector pMD-18T (derived from plasmids isolated from E. coli). Because A. pleuropneumoniae, B. bronchiseptica, and P. multocida have closer relationships with H. parasuis than E. coli, we anticipate that the pGZRS-18-, pBBR1MCS-4-, and pPBA1100-based bacteriolytic plasmids should have better replicative ability than the pMD-18T-based bacteriolytic plasmid in H. parasuis and should express and yield more E protein, thereby leading to higher lysis efficiency. However, the lysis efficiencies of the bacteriolytic plasmids pGZRS-E, pBBR-E, and pPBA-E were all lower than 90% when E protein expression in H. parasuis was induced (data not shown), whereas the lysis efficiency of pMD-E reached as high as 99.9966%. Thus, the bacteriolytic plasmid pMD-E was used to prepare the H. parasuis ghosts in this study.
Our study investigated the dynamic changes in CD4+ and CD8+ T lymphocyte subsets, the secretion of cytokines, and the humoral immune responses to H. parasuis in BG- and IB-vaccinated piglets. Our data showed that there were no significant differences in the CD4+ and CD8+ T cell populations between the treatment groups at early time points (days 0, 14, and 28) except for CD4+ T cells in the BG group, which apparently increased on day 28 (P < 0.05, compared with the IB group and the PC group). However, greater differences were observed following a high-dose challenge; the proportions of CD4+ T cells increased significantly in both the BG- and IB-vaccinated groups, the differences compared with the PC group were significant for the IB group (P < 0.05) and for the BG group (P < 0.01), and the difference between these two vaccinated groups also was significant (P < 0.05). Our result was similar to that observed in a previous study with another Gram-negative organism, A. pleuropneumoniae, which also showed a greater cell-mediated immune response after infection than after immunization (21). The CD8+ T cell subset also was observed to increase dramatically in all three experimental groups 1 week after challenge (day 35). The ranking of the numbers of CD8+ T cells in each group was PC group, IB group, and BG group, and the differences between the groups all were significant (P < 0.05). From our results, the better protective efficacy of the BG vaccine appeared to be correlated with the increased numbers of CD4+ T cells and thus the increased CD4+/CD8+ ratios prior to and following challenge. In contrast, in the unprotected PC group, which had the highest numbers of CD8+ T cells after challenge, the CD4+ T cell levels and the CD4+/CD8+ ratios were the lowest. The CD4+/CD8+ ratio was considered to be relevant to protection in a previous immunization and challenge study on Actinobacillus pleuropneumoniae (21). In that study, the CD4+/CD8+ ratio in the BG-vaccinated group was 1.5-fold higher than those in the IB-vaccinated group and the PC group on day 28. On day 35, the ratio became 1.8-fold and 5.6-fold higher than those in the IB-vaccinated group and the PC group, respectively. Similarly, in the present study, the CD4+/CD8+ ratio in the BG group was 1.5-fold higher than those in the IB group and the PC group, respectively, on day 28 and the ratio became 1.8-fold and 5.7-fold higher on day 35.
In combination with the results on cytokine IFN-γ and IL-4 levels, in which the BG group showed much higher levels than the IB group and the PC group throughout the whole experimental period, these data indicate that both the CD4+ major histocompatibility complex class II (MHC-II)-restricted Th1-type cell-mediated immune responses (produced with IFN-γ) and the Th2-type humoral immune responses (produced with IL-4) may play important roles in BG vaccine-induced immune protection. Similarly, a previous study (22) reported that, when bacterial ghosts were used to vaccinate animals, significant activation of IL-12 was observed. IFN-γ and IL-12 are known to be of special importance in the activation of cellular Th1 immune responses. Because of the unique structure of the BG envelope, with preserved pathogen-associated molecular patterns, including the outer membrane proteins, adhesins, lipopolysaccharide (LPS), and peptidoglycan, BGs can be recognized by pattern recognition receptors of the innate immune system and consequently can effectively stimulate the antigen-presenting cells (APCs) to trigger immune responses (15). Dendritic cells (DCs) are potent antigen-presenting cells that are essential for the initiation of primary immune responses. A previous study found that DCs were highly effective in the uptake of A. pleuropneumoniae BGs, as well as in the activation of cellular immune responses (22). This study showed that APCs were able not only to internalize BGs but also to process them and to present them to T cells. The process of internalization was related to increased expression of MHC-II molecules on the cell surface that might be recognized by CD4+ T cells.
In contrast to the BG vaccine, the Th1-type cell-mediated immune responses and Th2-type humoral immune responses appeared to be induced in the inactivated bacterin (IB)-vaccinated group; however, both of these immune responses in the IB group were inferior to those in the BG group. The levels of IFN-γ in the IB group were much lower than those in the BG group on days 14 (P < 0.05) and 28 (P < 0.01); for IL-4, the difference also was significant on days 14 and 28 (P < 0.05).
In accordance with the results on the T cell phenotype and cytokines, the humoral antibody levels also showed that the BG vaccine was superior to the inactivated vaccine in promoting antibody production. Although there were no significant differences in the antibody levels between the BG-vaccinated group and the IB-vaccinated group on days 7 and 14, the antibody levels in the BG-vaccinated group were continuously higher than those in the IB-vaccinated group after secondary exposure to the antigen (days 21 to 35). The superior protection effect of the BG vaccine also was seen, as the vaccinated animals exhibited better clinical performance and fewer pathological lesions. The effective protection imparted by the BG vaccine indicates that this vaccine may be a promising candidate for the prevention of Glässer's disease caused by H. parasuis. However, as mentioned above, due to the serovar diversity and lack of overall knowledge regarding the virulence factors and protective antigens for this germ, the differences in recognized antigens between the BG vaccine and the inactivated bacterin and the cross-protective ability of the BG vaccine were not examined in this work and need to be further studied and clarified in the future.
ACKNOWLEDGMENTS
This work was supported by the National High Technology Research and Development Program of China (grant 2011AA10A210), the Special Fund for Agro-scientific Research in the Public Interest (grant 201303034), the Harbin Science and Technology Innovative Talents project (grant 2012RFLYN009), and the State Key Laboratory of Veterinary Biotechnology program (grant SKLVBP200822).
Footnotes
Published ahead of print 27 March 2013
REFERENCES
- 1. Oliveira S, Pijoan C. 2004. Haemophilus parasuis: new trends on diagnosis, epidemiology and control. Vet. Microbiol. 99:1–12 [DOI] [PubMed] [Google Scholar]
- 2. de la Fuente AJ, Gutierrez-Martin CB, Rodriguez-Barbosa JI, Martinez-Martinez S, Frandoloso R, Tejerina F, Rodriguez-Ferri EF. 2009. Blood cellular immune response in pigs immunized and challenged with Haemophilus parasuis. Res. Vet. Sci. 86:230–234 [DOI] [PubMed] [Google Scholar]
- 3. Zhou M, Guo Y, Zhao J, Hu Q, Hu Y, Zhang A, Chen H, Jin M. 2009. Identification and characterization of novel immunogenic outer membrane proteins of Haemophilus parasuis serovar 5. Vaccine 27:5271–5277 [DOI] [PubMed] [Google Scholar]
- 4. Rapp-Gabrielson VG, Kocur GJ, Clark JT, Muir SK. 1997. Haemophilus parasuis: immunity in swine after vaccination. Vet. Med. 92:83–90 [Google Scholar]
- 5. Martin de la Fuente AJ, Gutierrez Martin CB, Perez Martinez C, Garcia Iglesias MJ, Tejerina F, Rodriguez Ferri EF. 2009. Effect of different vaccine formulations on the development of Glasser's disease induced in pigs by experimental Haemophilus parasuis infection. J. Comp. Pathol. 140:169–176 [DOI] [PubMed] [Google Scholar]
- 6. Yuan F, Fu S, Hu J, Li J, Chang H, Hu L, Chen H, Tian Y, Bei W. 2012. Evaluation of recombinant proteins of Haemophilus parasuis strain SH0165 as vaccine candidates in a mouse model. Res. Vet. Sci. 93:51–56 [DOI] [PubMed] [Google Scholar]
- 7. Huter V, Hensel A, Brand E, Lubitz W. 2000. Improved protection against lung colonization by Actinobacillus pleuropneumoniae ghosts: characterization of a genetically inactivated vaccine. J. Biotechnol. 83:161–172 [DOI] [PubMed] [Google Scholar]
- 8. Hensel A, Huter V, Katinger A, Raza P, Strnistschie C, Roesler U, Brand E, Lubitz W. 2000. Intramuscular immunization with genetically inactivated (ghosts) Actinobacillus pleuropneumoniae serotype 9 protects pigs against homologous aerosol challenge and prevents carrier state. Vaccine 18:2945–2955 [DOI] [PubMed] [Google Scholar]
- 9. Peng W, Si W, Yin L, Liu H, Yu S, Liu S, Wang C, Chang Y, Zhang Z, Hu S, Du Y. 2011. Salmonella enteritidis ghost vaccine induces effective protection against lethal challenge in specific-pathogen-free chicks. Immunobiology 216:558–565 [DOI] [PubMed] [Google Scholar]
- 10. Witte A, Wanner G, Blasi U, Halfmann G, Szostak M, Lubitz W. 1990. Endogenous transmembrane tunnel formation mediated by phi X174 lysis protein E. J. Bacteriol. 172:4109–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Witte A, Wanner G, Sulzner M, Lubitz W. 1992. Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli. Arch. Microbiol. 157:381–388 [DOI] [PubMed] [Google Scholar]
- 12. Szostak MP, Hensel A, Eko FO, Klein R, Auer T, Mader H, Haslberger A, Bunka S, Wanner G, Lubitz W. 1996. Bacterial ghosts: non-living candidate vaccines. J. Biotechnol. 44:161–170 [DOI] [PubMed] [Google Scholar]
- 13. Lubitz W, Witte A, Eko FO, Kamal M, Jechlinger W, Brand E, Marchart J, Haidinger W, Huter V, Felnerova D, Stralis-Alves N, Lechleitner S, Melzer H, Szostak MP, Resch S, Mader H, Kuen B, Mayr B, Mayrhofer P, Geretschlager R, Haslberger A, Hensel A. 1999. Extended recombinant bacterial ghost system. J. Biotechnol. 73:261–273 [DOI] [PubMed] [Google Scholar]
- 14. Jalava K, Eko FO, Riedmann E, Lubitz W. 2003. Bacterial ghosts as carrier and targeting systems for mucosal antigen delivery. Expert Rev. Vaccines 2:45–51 [DOI] [PubMed] [Google Scholar]
- 15. Riedmann EM, Kyd JM, Cripps AW, Lubitz W. 2007. Bacterial ghosts as adjuvant particles. Expert Rev. Vaccines 6:241–253 [DOI] [PubMed] [Google Scholar]
- 16. Lubitz P, Mayr UB, Lubitz W. 2009. Applications of bacterial ghosts in biomedicine. Adv. Exp. Med. Biol. 655:159–170 [DOI] [PubMed] [Google Scholar]
- 17. Ebensen T, Paukner S, Link C, Kudela P, de Domenico C, Lubitz W, Guzman CA. 2004. Bacterial ghosts are an efficient delivery system for DNA vaccines. J. Immunol. 172:6858–6865 [DOI] [PubMed] [Google Scholar]
- 18. Mayr UB, Walcher P, Azimpour C, Riedmann E, Haller C, Lubitz W. 2005. Bacterial ghosts as antigen delivery vehicles. Adv. Drug Deliv. Rev. 57:1381–1391 [DOI] [PubMed] [Google Scholar]
- 19. Walcher P, Mayr UB, Azimpour-Tabrizi C, Eko FO, Jechlinger W, Mayrhofer P, Alefantis T, Mujer CV, DelVecchio VG, Lubitz W. 2004. Antigen discovery and delivery of subunit vaccines by nonliving bacterial ghost vectors. Expert Rev. Vaccines 3:681–691 [DOI] [PubMed] [Google Scholar]
- 20. Oswald W, Tonpitak W, Ohrt G, Gerlach G. 1999. A single-step transconjugation system for the introduction of unmarked deletions into Actinobacillus pleuropneumoniae serotype 7 using a sucrose sensitivity marker. FEMS Microbiol. Lett. 179:153–160 [DOI] [PubMed] [Google Scholar]
- 21. Appleyard GD, Furesz SE, Wilkie BN. 2002. Blood lymphocyte subsets in pigs vaccinated and challenged with Actinobacillus pleuropneumoniae. Vet. Immunol. Immunopathol. 86:221–228 [DOI] [PubMed] [Google Scholar]
- 22. Haslberger AG, Kohl G, Felnerova D, Mayr UB, Fürst-Ladani S, Lubitz W. 2000. Activation, stimulation and uptake of bacterial ghosts in antigen presenting cells. J. Biotechnol. 83:57–66 [DOI] [PubMed] [Google Scholar]