Abstract
Heme oxygenase-1 (HO-1) is a stress-inducible rate-limiting enzyme in heme degradation that confers cytoprotection against oxidative injury and performs a vital function in the maintenance of cell hemostasis. Increasing numbers of reports have indicated that mycoplasma-derived membrane lipoproteins/lipopeptides, such as macrophage-activating lipopeptide-2 (MALP-2), function as agents that stimulate the immune system by producing various inflammatory mediators, such as cytokines and cyclooxygenase 2 (COX-2), which play roles in the pathogenesis of inflammatory responses during mycoplasma infection. Here, we report that MALP-2 induced HO-1 mRNA and protein expression and upregulated HO-1 enzyme activity in THP-1 cells. Specific inhibitors of mitogen-activated protein kinases (MAPKs), SB203580, PD98059, and SP600125, significantly abolished HO-1 expression. In addition, MALP-2 also induced NF-E2-related factor 2 (Nrf2) translocation, and the silencing of Nrf2 expression in THP-1 cells decreased the levels of MALP-2-mediated HO-1 expression. Furthermore, COX-2 protein expression levels were upregulated in THP-1 cells in response to MALP-2, and transfection with small interfering RNAs of HO-1 significantly increased COX-2 accumulation. These results demonstrate that MALP-2 induces HO-1 expression via MAPKs and Nrf2 pathways and, furthermore, that MALP-2-induced COX-2 expression was modulated by HO-1 in THP-1 cells.
INTRODUCTION
Heme oxygenase-1 (HO-1) is an inducible enzyme that catalyzes the first and rate-limiting step in the oxidative degradation of free heme into ferrous iron, carbon monoxide, and biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase (1, 2). In mammalian cells, three genetically distinct isozymes have been identified. HO-1 is an inducible form, whereas HO-2 and HO-3 are constitutively expressed forms. HO-1 expression is induced in various cell lines by a range of stress stimuli, including lipopolysaccharide (LPS), lipoteichoic acid, peptidoglycan, and proinflammatory cytokines (3–7). The increased HO-1 expression induced by these stress stimuli is thought to be an adaptive mechanism that protects the cells from immunopathogenesis or stress damage (8). For instance, Rushworth et al. reported that LPS-induced HO-1 and NAD(P)H:quinone oxidoreductase (NQO1) protected against excessive inflammatory responses in human monocytes (4, 9). Very recently, HO-1 has been reported to regulate the immune response to influenza virus infection and vaccination in aged mice (10). In addition, the HO-1 metabolites carbon monoxide, bilirubin, and ferritin play cytoprotective roles in many kinds of organ injury (11, 12). It has been reported that carbon monoxide, a product of HO-1, augments caveolin-1 (cav-1)/Toll-like receptor 4 (TLR4) interactions to downregulate proinflammatory signaling upon LPS stimulation (13). Furthermore, biliverdin from HO-1 protects against endotoxin-induced acute lung injury in rats (14). These studies suggest that HO-1 and its metabolites play important roles in suppressing deleterious increases in inflammation and oxidative injury.
Monocytes and macrophages play essential roles in inflammation and the mobilization of host defenses against mycoplasma infection. Mycoplasma lipoproteins are regarded as major virulence factors that contribute to the pathogenesis of mycoplasmas by the production of various inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) in monocytes and macrophages (15, 16). Macrophage-activating lipopeptide-2 (MALP-2), a molecular component of the surface membrane of Mycoplasma fermentans, is a potent inducer of chemokines and cytokines (17). MALP-2 activates gene transcription by binding to its membrane receptor, TLR2/6, on circulating monocytes and other cell lines, inducing the signal transduction pathways leading to the phosphorylation of kinases, including the IκB kinases and mitogen-activated protein kinases (MAPKs), and the activation of NF-κB and AP-1 families (18, 19). MALP-2 activation of monocytes results in a wide range of responses, including the secretion of proinflammatory factors, expression of chemoattractant proteins, and release of prostaglandins via cyclooxygenase (COX) pathways (17, 20). Nevertheless, MALP-2 is also reported to facilitate dendritic cell maturation and modulate proteasome composition and activity (21), and its synthetic derivative S-[2,3-bispalmitoyiloxy-(2R)-propyl]-R-cysteinyl-amido-monomethoxyl polyethylene glycol (BPP) exhibits some adjuvant effects for cross-priming against cellular antigens (22). Moreover, MALP-2 can induce endothelial cell proliferation and migration and a strong secretion of granulocyte-macrophage colony-stimulating factor to enhance or restore blood flow and recruit immune cells for pathogen defense and tissue regeneration (23). These studies have demonstrated that MALP-2 exhibits multiple effects on the immune system. However, whether mycoplasma-derived MALP-2 induces the expression of HO-1 to serve as a cytoprotective agent is still unknown.
HO-1 has been reported to have cytoprotective effects in mediating cellular homeostasis as a general inducible protein (24). For this reason, considerable research efforts have focused on characterizing the molecular mechanisms that regulate the expression of HO-1. When activated, NF-E2-related factor 2 (Nrf2), which belongs to the cap'n'collar family of basic leucine zipper transcription factors, induces the expression of a host of cytoprotective and detoxification genes such as HO-1 and NQO1 (4, 9). In this study, we investigated the mechanisms underlying MALP-2-induced HO-1 expression in THP-1 cells and the potential effects of HO-1 in modulating MALP-2-induced COX-2 expression. Our results demonstrate that MALP-2 can activate MAPKs and Nrf2 pathways to induce the expression of HO-1. Silencing the expression of HO-1 prolongs MALP-2-induced COX-2 expression.
MATERIALS AND METHODS
Materials.
MALP-2 was purchased from Alexis Biochemicals (product number ALX-162-027-C050). M. fermentans (PG18, ATCC 19989) was obtained from the ATCC. Real-time PCR primers for HO-1 and β-actin were synthesized by Invitrogen. The MAPK-specific inhibitors SB203580, SP600125, PD98059, and anti-β-actin antibody were purchased from Sigma-Aldrich. Anti-HO-1, anti-Nrf2, and anti-COX2 monoclonal antibodies were products of Cell Signaling Technology, Inc. Horseradish peroxidase (HRP)-labeled secondary antibodies (goat anti-rabbit IgG and goat anti-mouse IgG) and polyvinylidene difluoride (PVDF) membranes were purchased from Millipore. The TATA binding protein (TBP) polyclonal antibody was a product of Proteintech. The heme oxygenase-1 enzyme activity assay kit was obtained from GenMed Scientifics (Shanghai, China). Radioimmunoprecipitation assay (RIPA) buffer and NE-PER nuclear and cytoplasmic extraction reagents were purchased from Pierce Biotechnology. Protease and phosphatase inhibitors were purchased from Roche. Nrf2, HO-1 small interfering RNA (siRNA), and control (Con) siRNA were purchased from RiboBio Co. Ltd. (Guangzhou, China). All of the cell culture flasks and plates were purchased from Corning, and the endotoxin-free consumables were obtained from Axygen and Gilson.
Cell culture and MALP-2 stimulation.
Human monocytic THP-1 cells were purchased from ATCC and cultured in RPMI 1640 medium (HyClone) supplemented with 10% fetal bovine serum (FBS) (Gibco), 2 mM l-glutamine, 100 μg ml−1 penicillin, and 100 μg ml−1 streptomycin. Cells were maintained in a humidified atmosphere at 37°C and 5% CO2. For stimulation experiments, THP-1 cells were seeded in serum-free medium in 6-well plates (1 × 106 well−1) and allowed to cultivate overnight. Then cells were stimulated with MALP-2 for appropriate time intervals according to the protocols.
Mycoplasma culture and inactivation preparation.
M. fermentans cells were cultivated in medium containing 20% horse serum, 10% freshly prepared yeast extract, 1% glucose, and 1,000 U ml−1 penicillin G under the conditions of 37°C and 5% CO2 and quantified as described previously (25). For heat inactivation, the M. fermentans (106 color-changing units [CCU] ml−1) cells were isolated and resuspended in Hayflick medium, followed by incubation at 60°C for 30 min; no growth was observed over a 2-week period. The endotoxin levels of the heat-inactivated mycoplasma (HIM) preparations were <60 pg ml−1, as determined by a Limulus amebocyte lysate assay (Pyrochrome).
Inhibitor treatment.
THP-1 cells were cultivated in serum-free medium in 6-well plates (106 well−1) with SB203580 (30 μM), PD98059 (30 μM), SP600125 (30 μM), and a vehicle control (dimethyl sulfoxide [DMSO] <0.1%) for 30 min. Then the THP-1 cells were washed twice with warm phosphate-buffered saline (PBS) by centrifugation at 1,000 × g for 5 min, followed by further culturing for 16 h in complete medium in the presence or absence of MALP-2. For all inhibitors, the concentrations used were those recommended by Davies et al. (26). Cell viability was assessed by a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The inhibitors did not induce obvious cell toxicity.
RNA extraction and real-time PCR.
To extract total RNA from 1 × 106 cells, we used the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription of 3 μg total RNA was performed according to the instructions provided by Fermentas Life Sciences. Sequences of primers were as follows: HO-1, 5′-ATGGCCTCCCTGTACCACATC-3′ (forward) and 5′-TGTTGCGCTCAATCTCCTCCT-3′ (reverse); β-actin, 5′-CATCCTGCGTCTGGACCTGG-3′ (forward) and 5′-TAATGTCACGCACGATTTCC-3′ (reverse). Relative quantitative real-time PCR used SYBR green technology (ABI) on cDNA generated from the reverse transcription of purified RNA. After preamplification (50°C for 2 min and 95°C for 10 min), the PCR mixtures were amplified for 40 cycles (95°C for 15 s and 58°C for 1 min) on a real-time PCR detection system (ABI7500). We used the comparative cycle threshold method to normalize the expression levels for each mRNA against the expression levels for β-actin mRNA.
HO-1 enzyme activity assay.
We determined HO-1 activity in microsomal fractions from THP-1 cells by monitoring the conversion of heme into ferrous iron, carbon monoxide, and biliverdin according to the manufacturer's instructions. Briefly, cell pellets were centrifuged at 8,000 × g for 5 min in 1.5-ml tubes and homogenized in proper GenMed lysates. Homogenates were vortexed at 10-s intervals of 5 min, total 30 min. Supernatants were centrifuged at 10,000 × g for 15 min. When tested, the tubes were marked as sample and background. Then 340 μl of GenMed buffer, 20 μl of reaction liquid, and 20 μl of substrate were added to the sample tubes and a background tube. A GenMed negative control was then added to the background tube and the samples were added to the sample tubes, and then the tubes were vortexed and incubated at 37°C for 1 h. The reaction was stopped with the addition of 0.4 ml of chloroform, and the extraction of the chloroform layer was measured using a spectrophotometer. Bilirubin formation was calculated from the difference in absorption between 464 nm and 530 nm. The HO-1 enzyme activity level was indicated as nanomoles of bilirubin formed per milligram of protein per hour.
Western blotting.
Cells were pelleted by centrifugation at 2,500 × g for 5 min and then washed twice with ice-cold PBS, lysed on ice for 15 min in 100 μl of RIPA buffer, and centrifuged at 14,000 × g for 15 min. Cytoplasmic and nuclear extracts were prepared using the NE-PER nuclear and cytoplasmic extraction reagents according to the manufacturer's instructions. Supernatants were collected and protein concentrations were determined by a Bradford protein assay. Aliquots of cell lysates (80 μg of proteins) were boiled for 5 min in SDS-containing sample buffer and were separated by SDS-PAGE on a 10% to 12% polyacrylamide gel. Blots were transferred to a PVDF membrane and Western blot analyses were performed with the indicated antibody according to their manufacturer's guidelines. Proteins were detected using an enhanced chemiluminescence (ECL) Western blotting system (Thermo), and the band intensity was measured by densitometric analysis using ImageJ software.
Transient transfection with small interfering RNAs.
THP-1 cells (1 × 106 well−1) were transfected with a final concentration of 100 nM Nrf2 siRNA, HO-1 siRNA, or control siRNA for 24 h using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Transfected cells were left unstimulated or treated with MALP-2 (5 ng ml−1) for the indicated times. The samples were then prepared for Western blot analysis as described above.
Statistical analyses.
Data were assessed as means ± standard deviation (SD) of the results for at least three experiments. One-way analysis of variance (ANOVA) and Student's t test were used for statistical evaluation. Differences with P values of <0.05 were considered statistically significant.
RESULTS
MALP-2 induces HO-1 mRNA expression in human THP-1 cells.
MALP-2, with a structure of Pam2CGNNDESNISFKEK, has been shown to be a potent activator of macrophages and monocytes. To determine the effect of MALP-2 treatment on HO-1 mRNA expression, THP-1 cells were treated with various concentrations of MALP-2 for 12 h or treated with 5.0 ng ml−1 MALP-2 at various time points, and the mRNA expression levels of HO-1 were determined by real-time PCR analysis. As shown in Fig. 1A, MALP-2 induced HO-1 mRNA accumulation in a concentration-dependent manner. The level of HO-1 mRNA was highest when the concentration of MALP-2 reached 5 ng ml−1. HO-1 mRNA expression induced by MALP-2 was significantly elevated at 4 h, peaked at 8 h, and became attenuated at 12 to 24 h (Fig. 1B). As controls, THP-1 cells were treated with various concentrations of HIM for 12 h or infected with 106 CCU ml−1 of HIM at various time points. Similar results were obtained in HIM-induced HO-1 mRNA production (Fig. 1C and D).
Fig 1.
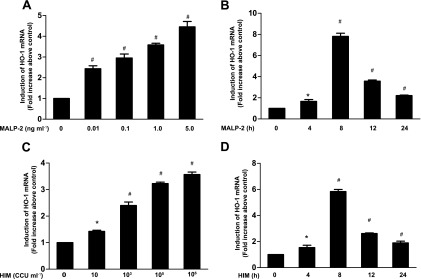
MALP-2 induces HO-1 mRNA expression in THP-1 cells. THP-1 cells were unstimulated or stimulated with different concentrations of MALP-2 or HIM for the indicated times and harvested for HO-1 mRNA analysis. (A and C) Cells exposed to different concentrations of MALP-2 (0, 0. 01, 0.1, 1.0, or 5.0 ng ml−1) or HIM (0, 10, 103, 105, or 106 CCU ml−1) for 12 h were harvested, and total RNA was prepared. The RNA samples were analyzed by real-time PCR as described in Materials and Methods. (B and D) THP-1 cells were stimulated by 5.0 ng ml−1 MALP-2 or 106 CCU ml−1 of HIM for 0, 4, 8, 12, or 24 h, and then induced HO-1 mRNA expression was measured by real-time PCR. HO-1 mRNA expression was normalized to β-actin mRNA levels. Data represent means ± SD from at least three independent experiments. *, P < 0.05, and #, P < 0.01, compared with the basal level.
MALP-2 induces HO-1 protein expression and upregulates its enzymatic activity in THP-1 cells.
HO-1 protein plays a pivotal role in monocytes activating inflammation, which counteracts immunopathogenic damage caused by stress stimuli (27, 28). To confirm the mRNA results, we examined HO-1 protein expression by Western blotting. As shown in Fig. 2A, the HO-1 protein level was very low in unstimulated cells; however, HO-1 protein expression was sharply increased in response to 0.01 to 5 ng ml−1 of MALP-2. In addition, the level of HO-1 protein peaked at 12 h, was sustained up to 16 h, and became attenuated at 24 h (Fig. 2B). Given that the HO-1 protein expression level does not always represent its enzymatic activity, we further determined the HO-1 enzymatic activity by measurement of the formation of bilirubin per milligram of protein. As shown in Fig. 2C, a significant increase in HO-1 activity caused by MALP-2 occurred in a dose-dependent manner. These data suggest that the increase in HO-1 protein expression is accompanied by enhanced enzymatic activity.
Fig 2.
MALP-2 induces HO-1 protein expression and upregulates its enzymatic activity in THP-1 cells. Cells were unstimulated or stimulated with various concentrations of MALP-2 (0, 0. 01, 0.1, 1.0, or 5.0 ng ml−1) for 16 h (A) or at a variety of time points (B). HO-1 protein expression was analyzed by Western blotting, and then densitometric analysis was performed after normalization with β-actin protein levels. (C) Cells were stimulated with various concentrations of MALP-2 (0, 0. 01, 0.1, 1.0, or 5.0 ng ml−1) for 16 h, and then HO-1 activity was detected by measurement of the formation of bilirubin (nmol per milligram of protein). The HO-1 enzymatic activity increased in a dose-dependent manner. All values are expressed as means ± SD obtained from three independent experiments. #, P < 0.01 compared with that at the basal level (A and B) or at the basal HO-1 activity level (C).
MALP-2-induced HO-1 expression requires ongoing transcription and translation.
How HO-1 is induced in response to MALP-2 is still unknown. To determine whether MALP-2-induced HO-1 expression required transcription or translation, we first examined the accumulation of HO-1 in response to MALP-2 in the absence or presence of a translational level inhibitor, cycloheximide (CHX), or the RNA synthesis inhibitor actinomycin D (ActD). As shown in Fig. 3, MALP-2-mediated induction of HO-1 expression was abolished by either CHX or ActD in a dose-dependent manner. Taken together, these results suggest that in response to MALP-2, HO-1 protein is synthesized de novo in THP-1 cells.
Fig 3.
Inhibition of MALP-2-induced HO-1 expression by cycloheximide (CHX; A) and actinomycin D (ActD; B). Cells were pretreated with or without cycloheximide (0.1 to 10 μmol liter−1) and actinomycin D (0.1 to 10 μmol liter−1) for 1 h and then incubated in the absence or presence of 5.0 ng ml−1 MALP-2 for 16 h. Cell lysates were subjected to Western blot analysis. Data are expressed as means ± SD of three independent experiments. #, P < 0.01 for significant differences between the groups.
MAPKs pathways involved in MALP-2-induced HO-1 expression in THP-1 cells.
MALP-2 has been reported to activate many kinase pathways in THP-1 cells, such as MAPKs (19, 20). The potential role of the MAPK pathways in MALP-2-induced HO-1 expression was examined in THP-1 cells using kinase-specific inhibitors. These included a p38 inhibitor (SB203580), a MEK1/2 inhibitor (PD98059), and a c-Jun N-terminal kinase (JNK) inhibitor (SP600125). THP-1 cells were pretreated with these inhibitors for 30 min prior to MALP-2 incubation. Total protein was extracted and determined by Western blotting. Figure 4 shows that the levels of HO-1 upregulation by MALP-2 were partially inhibited by SB203580 (38%), PD98059 (44%), and SP600125 (56%). These results indicate that the p38, ERK1/2, and JNK pathways participate in the MALP-2-mediated induction of HO-1.
Fig 4.
Involvement of MAPKs in the upregulation of HO-1 by MALP-2. Cells were preincubated for 30 min in the presence of SB203580 (30 μmol liter−1), PD98059 (30 μmol liter−1), or SP600125 (30 μmol liter−1) and then treated with 5.0 ng ml−1 MALP-2 for 16 h. HO-1 protein expression was analyzed by Western blotting. Densitometric analysis was performed after normalization with β-actin. Data are expressed as means ± SD of values obtained from three independent experiments. #, P < 0.01 for significant differences between the groups.
MALP-2-induced HO-1 upregulation is mediated via Nrf2 signaling.
Nrf2 is a redox-sensitive basic leucine zipper transcription factor of the NADPH oxidase complex that is activated by oxidative stresses and translocates into the nucleus (29). The activation of Nrf2 has been reported to play an important role in the antioxidant response element (ARE)-driven expression of several detoxifying and antioxidant enzymes, including HO-1 (9). To confirm whether MALP-2 induced Nrf2 translocation, THP-1 cells were treated with MALP-2 for various times, and then Nrf2 cytosolic and nuclear protein expression was prepared and analyzed by Western blotting. As illustrated in Fig. 5A, Nrf2 was present in the cytosol in unstimulated THP-1 cells but had almost disappeared by 120 min in response to MALP-2. Conversely, Nrf2 was undetectable in the nuclei of unstimulated THP-1 cells but, in response to MALP-2, significantly increased at 60 min and continued to increase at 120 min. To further determine the role of Nrf2 in MALP-2-dependent HO-1 induction, cells were transiently transfected with Nrf2 siRNA. As illustrated in Fig. 5B and C, transfection with Nrf2 siRNA downregulated the protein expression of Nrf2 and subsequently decreased the HO-1 expression induced by MALP-2.
Fig 5.
HO-1 expression was mediated by MALP-2-induced Nrf2 translocation. (A) THP-1 cells were stimulated with 5.0 ng ml−1 MALP-2 for various times, and cytoplasmic and nuclear lysates were prepared and analyzed by Western blotting. (B and C) Cells were transfected with Nrf2-specific siRNA prior to 5.0 ng ml−1 MALP-2 treatment. Total Nrf2 and HO-1 protein expression levels were detected by Western blotting. Densitometric analysis was performed after normalization with TBP or β-actin. All the values are expressed as means ± SD of three independent experiments. #, P < 0.01 for significant differences between the groups.
Silencing HO-1 enhances MALP-2-induced COX-2 expression.
MALP-2 has been shown to induce COX-2 accumulation in human placental trophoblast cells and macrophages (20, 30). Little is known, however, about whether HO-1 is involved in MALP-2-induced COX-2 expression. In this study, we found that MALP-2 significantly induced COX-2 expression in THP-1 cells (Fig. 6A). To confirm the effect of HO-1 on MALP-2-mediated COX-2 expression, THP-1 cells were transfected with HO-1 siRNA. As illustrated in Fig. 6B, transfection of THP-1 cells with HO-1 siRNA resulted in the significant knockdown of HO-1 protein upregulation and caused significant induction of COX-2 attributable to MALP-2.
Fig 6.
Silencing HO-1 enhances MALP-2-induced COX-2 expression. (A) MALP-2 (5.0 ng ml−1) was stimulated for 0 h, 4 h, 8 h, 12 h, and 16 h, and the level of COX-2 protein expression was detected by Western blotting. (B) Cells were transfected with HO-1 siRNA or control siRNA for 24 h, 5.0 ng ml−1 MALP-2 was stimulated for 16 h, and cell lysates were prepared and analyzed by Western blotting. The levels of COX-2 and HO-1 protein expression were then analyzed by Western blotting. Densitometric analysis was performed after normalization with β-actin. All of the values are expressed as means ± SD of three independent experiments. #, P < 0.01 for comparisons within groups.
DISCUSSION
LPS has been shown to be an important regulator of bacterial infections in monocytes and macrophages. In response to LPS, mononuclear phagocytes also synthesize intracellular cytoprotective proteins, including HO-1 and NQO1, to protect against excessive inflammatory responses in human monocytes (4, 9). In this study, our present data clearly demonstrate that MALP-2, like LPS, induces HO-1 mRNA and protein expression and increases its enzymatic activity levels in THP-1 cells. Moreover, MALP-2-dependent HO-1 expression is mediated by MAPKs and Nrf2 pathways to modulate MALP-2-induced COX-2 expression in human monocytes.
HO-1 expression in monocytic cells is thought to mediate potent anti-inflammatory effects, possibly by restraining their induction of tissue injury and by modulating their roles in the inflammatory response (31). We found that HO-1 mRNA and protein expression and enzymatic activity levels were increased in response to MALP-2 in a concentration-dependent manner. Moreover, Rushworth et al. reported that LPS-dependent HO-1 protein expression was undetectable within 8 h (4), a result that differed from our study. This difference may be due to differences in recognition receptors, i.e., monocytes recognize MALP-2 by TLR2 and -6, but this process is partially dependent on CD14, while TLR4 and CD14 are required for LPS (32, 33).
MAPKs pathways are central signaling pathways that regulate a wide variety of stimulated cellular processes, including stress responses (34, 35). Although HO-1 has previously been shown to be induced by various stimuli via activation of the MAPK-signaling pathway, the role of these protein kinases for HO-1 gene regulation is largely unknown. In this study, our results demonstrate that SB203580, PD98059, and SP600125 all significantly block MALP-2-induced HO-1 expression. This suggests that MAPK pathways may constitute an upstream signal necessary for MALP-2-dependent HO-1 expression. Furthermore, although all the inhibitors used in the study were dissolved in DMSO, we did not detect any significant increase of HO-1 expression caused by DMSO. Interestingly, Liang et al. reported that high concentrations of DMSO (0.2% to 0.8%) increased HO-1 protein expression in human umbilical vein endothelial cells (36). These discrepancies might be attributable to the fact that different cell lines and different concentrations of DMSO were used.
The HO-1 gene has been reported to contain many stress-activated response elements in the 5′-untranslated region, including antioxidant response elements (AREs) (4). Nrf2 has been shown to be a key factor in the ARE-mediated induction of antioxidant proteins and the regulation of inflammatory responses in response to various stimuli (37, 38). Rushworth et al. reported that Nrf2 regulates LPS-induced HO-1 and NQO1 protein production and thus modulates the LPS-induced proinflammatory response (9). Other studies have shown that Nrf2 activation inhibits proinflammatory cytokine-induced adhesion molecule expression in endothelial cells. Conversely, exposure to endotoxin leads to higher levels of TNF and interleukin-6 expression in Nrf2-deficient mice than in wild-type animals (39). In this study, we have demonstrated that MALP-2 also activates Nrf2 translocation to induce HO-1 accumulation, and furthermore, that suppressing Nrf2 production with siRNA attenuates HO-1 protein expression.
COX-2 is an inducible form of cyclooxygenase, and its expression level regulates endogenous prostaglandin synthesis. More and more studies have shown that COX-2-induced pathways play important roles in modulating inflammatory reactions (40). For example, the induction of COX-2 expression by cigarette smoke contributes to the proinflammatory effects of prostaglandin E2 (PGE2) in the airways of subjects with chronic obstructive pulmonary disease (COPD) (41). In human placental trophoblast cells, the expression of COX-2 and production of PGE2 caused by MALP-2 may be involved in the mechanism of preterm labor (30). COX-2-mediated pathways were involved in the production of CXC motif chemokine ligand 8 (CXCL8), CXCL1, CXCL5, and vascular endothelial growth factor (VEGF), which may have important roles in the pathogenesis of pulmonary fibrotic disorders (42). Therefore, elucidating the mechanism by which COX-2 expression is regulated will be crucial to our understanding of these COX2-regulated pathophysiological processes. Recently, the induction of HO-1 has been reported to attenuate LPS-induced COX-2 expression in mouse brain endothelial cells (43). Furthermore, some COX-2-selective inhibitors suppressed the expression of COX-2 by the induction of HO-1 expression (44, 45). In the current study, we found that HO-1 modulates MALP-2-induced COX-2 expression in THP-1 cells, suggesting a multifunctional role of HO-1 in balancing the relation of anti-inflammation and proinflammation.
The mechanism by which many inducers induce HO-1 production is a matter of much discussion (27, 36, 46, 47). This report adds new insight to the discussion because we have observed that upon activation with MALP-2, HO-1 expression is dependent on the activation of MAPKs and Nrf2. HO-1 protein subsequently contributes to the regulation of monocyte responses to MALP-2, so that monocytes are less likely to generate an excessive inflammatory response. Recently, MALP-2 has been found to improve lung host defenses against infections with mycobacteria (48) and improve reendothelialization in a murine model of experimental vascular injury (49). In addition, MALP-2 can reduce bacteremia and improve bacterial clearance in lung parenchyma (50) and can accelerate wound healing in diabetic mice (51). The induction of Nrf2 translocation to the nucleus to control excessive inflammatory responses is a process that might be important to our understanding of the pharmaceutical applications of MALP-2. In this regard, MALP-2 analogues developed without any inflammatory reactions to host cells are expected to eliminate the possibility of the emergence of some infections. Thus, further understanding of the functions of MALP-2 and its analogues that regulate Nrf2 and its gene products may lead to the identification of novel therapeutic targets for the treatment of diseases associated with inflammation.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 31000091) and the Science and Technology Planning Project of Hunan Province (grant no. 2010TD1007).
We thank Yuan Chunyang (University of South China) for his assistance in preparing the manuscript.
Footnotes
Published ahead of print 27 March 2013
REFERENCES
- 1. Pae HO, Chung HT. 2009. Heme oxygenase-1: its therapeutic roles in inflammatory diseases. Immune Netw. 9:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryter SW, Choi AM. 2009. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am. J. Respir. Cell Mol. Biol. 41:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Terry CM, Clikeman JA, Hoidal JR, Callahan KS. 1999. TNF-alpha and IL-1alpha induce heme oxygenase-1 via protein kinase C, Ca2+, and phospholipase A2 in endothelial cells. Am. J. Physiol. 276:H1493–H1501 [DOI] [PubMed] [Google Scholar]
- 4. Rushworth SA, Chen XL, Mackman N, Ogborne RM, O'Connell MA. 2005. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J. Immunol. 175:4408–4415 [DOI] [PubMed] [Google Scholar]
- 5. Lee IT, Wang SW, Lee CW, Chang CC, Lin CC, Luo SF, Yang CM. 2008. Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J. Immunol. 181:5098–5110 [DOI] [PubMed] [Google Scholar]
- 6. Kim YS, Pi SH, Lee YM, Lee SI, Kim EC. 2009. The anti-inflammatory role of heme oxygenase-1 in lipopolysaccharide and cytokine-stimulated inducible nitric oxide synthase and nitric oxide production in human periodontal ligament cells. J. Periodontol. 80:2045–2055 [DOI] [PubMed] [Google Scholar]
- 7. Hung CC, Liu X, Kwon MY, Kang YH, Chung SW, Perrella MA. 2010. Regulation of heme oxygenase-1 gene by peptidoglycan involves the interaction of Elk-1 and C/EBPalpha to increase expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 298:L870–L879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raval CM, Lee PJ. 2010. Heme oxygenase-1 in lung disease. Curr. Drug Targets 11:1532–1540 [DOI] [PubMed] [Google Scholar]
- 9. Rushworth SA, MacEwan DJ, O'Connell MA. 2008. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. 181:6730–6737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cummins NW, Weaver EA, May SM, Croatt AJ, Foreman O, Kennedy RB, Poland GA, Barry MA, Nath KA, Badley AD. 2012. Heme oxygenase-1 regulates the immune response to influenza virus infection and vaccination in aged mice. FASEB J. 26:2911–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. 2008. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Invest. 118:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirkby KA, Adin CA. 2006. Products of heme oxygenase and their potential therapeutic applications. Am. J. Physiol. Renal Physiol. 290:F563–F571 [DOI] [PubMed] [Google Scholar]
- 13. Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. 2009. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J. Immunol. 182:3809–3818 [DOI] [PubMed] [Google Scholar]
- 14. Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, Otterbein LE. 2005. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L1131–L1137 [DOI] [PubMed] [Google Scholar]
- 15. Wu Y, Qiu H, Zeng Y, You X, Deng Z, Yu M, Zhu C. 2008. Mycoplasma genitalium lipoproteins induce human monocytic cell expression of proinflammatory cytokines and apoptosis by activating nuclear factor kappaB. Mediators Inflamm. 2008:195427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimizu T, Kida Y, Kuwano K. 2008. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect. Immun. 76:270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaufmann A, Muhlradt PF, Gemsa D, Sprenger H. 1999. Induction of cytokines and chemokines in human monocytes by Mycoplasma fermentans-derived lipoprotein MALP-2. Infect. Immun. 67:6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia J, Lemercier B, Roman-Roman S, Rawadi G. 1998. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-kappaB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273:34391–34398 [DOI] [PubMed] [Google Scholar]
- 19. Gao F, Brant KA, Ward RM, Cattley RT, Barchowsky A, Fabisiak JP. 2010. Multiple protein kinase pathways mediate amplified IL-6 release by human lung fibroblasts co-exposed to nickel and TLR-2 agonist, MALP-2. Toxicol. Appl. Pharmacol. 247:146–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kandasamy P, Zarini S, Chan ED, Leslie CC, Murphy RC, Voelker DR. 2011. Pulmonary surfactant phosphatidylglycerol inhibits Mycoplasma pneumoniae-stimulated eicosanoid production from human and mouse macrophages. J. Biol. Chem. 286:7841–7853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Link C, Gavioli R, Ebensen T, Canella A, Reinhard E, Guzman CA. 2004. The Toll-like receptor ligand MALP-2 stimulates dendritic cell maturation and modulates proteasome composition and activity. Eur. J. Immunol. 34:899–907 [DOI] [PubMed] [Google Scholar]
- 22. Prajeeth CK, Jirmo AC, Krishnaswamy JK, Ebensen T, Guzman CA, Weiss S, Constabel H, Schmidt RE, Behrens GM. 2010. The synthetic TLR2 agonist BPPcysMPEG leads to efficient cross-priming against co-administered and linked antigens. Eur. J. Immunol. 40:1272–1283 [DOI] [PubMed] [Google Scholar]
- 23. Grote K, Schuett H, Salguero G, Grothusen C, Jagielska J, Drexler H, Muhlradt PF, Schieffer B. 2010. Toll-like receptor 2/6 stimulation promotes angiogenesis via GM-CSF as a potential strategy for immune defense and tissue regeneration. Blood 115:2543–2552 [DOI] [PubMed] [Google Scholar]
- 24. Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. 2007. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid. Redox Signal. 9:2157–2173 [DOI] [PubMed] [Google Scholar]
- 25. Rodwell AW, Whitcomb RH. 1983. Methods for direct and indirect measurement of mycoplasma growth, p 186–196 In Tully JG, Razin S. (ed), Methods in mycoplasmology, vol 1 Academic Press, New York, NY [Google Scholar]
- 26. Davies SP, Reddy H, Caivano M, Cohen P. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Weng CY, Wang YJ, Wu MJ. 2011. Lipoic acid ameliorates arsenic trioxide-induced HO-1 expression and oxidative stress in THP-1 monocytes and macrophages. Chem. Biol. Interact. 190:129–138 [DOI] [PubMed] [Google Scholar]
- 28. Park SY, Kim YH, Kim EK, Ryu EY, Lee SJ. 2010. Heme oxygenase-1 signals are involved in preferential inhibition of pro-inflammatory cytokine release by surfactin in cells activated with Porphyromonas gingivalis lipopolysaccharide. Chem. Biol. Interact. 188:437–445 [DOI] [PubMed] [Google Scholar]
- 29. Bloom DA, Jaiswal AK. 2003. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 278:44675–44682 [DOI] [PubMed] [Google Scholar]
- 30. Mitsunari M, Yoshida S, Shoji T, Tsukihara S, Iwabe T, Harada T, Terakawa N. 2006. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E(2) via toll-like receptor 2 in human placental trophoblast cells. J. Reprod. Immunol. 72:46–59 [DOI] [PubMed] [Google Scholar]
- 31. Soares MP, Bach FH. 2009. Heme oxygenase-1: from biology to therapeutic potential. Trends Mol. Med. 15:50–58 [DOI] [PubMed] [Google Scholar]
- 32. Schroder NW, Heine H, Alexander C, Manukyan M, Eckert J, Hamann L, Gobel UB, Schumann RR. 2004. Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses. J. Immunol. 173:2683–2691 [DOI] [PubMed] [Google Scholar]
- 33. Deiters U, Gumenscheimer M, Galanos C, Muhlradt PF. 2003. Toll-like receptor 2- and 6-mediated stimulation by macrophage-activating lipopeptide 2 induces lipopolysaccharide (LPS) cross tolerance in mice, which results in protection from tumor necrosis factor alpha but in only partial protection from lethal LPS doses. Infect. Immun. 71:4456–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plotnikov A, Zehorai E, Procaccia S, Seger R. 2011. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 1813:1619–1633 [DOI] [PubMed] [Google Scholar]
- 35. Cassidy H, Radford R, Slyne J, O'Connell S, Slattery C, Ryan MP, McMorrow T. 2012. The role of MAPK in drug-induced kidney injury. J. Signal Transduct. 2012:463617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang C, Xue Z, Cang J, Wang H, Li P. 2011. Dimethyl sulfoxide induces heme oxygenase-1 expression via JNKs and Nrf2 pathways in human umbilical vein endothelial cells. Mol. Cell. Biochem. 355:109–115 [DOI] [PubMed] [Google Scholar]
- 37. Jaiswal AK. 2004. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 36:1199–1207 [DOI] [PubMed] [Google Scholar]
- 38. Saidu NE, Touma R, Asali IA, Jacob C, Montenarh M. 2013. Diallyl tetrasulfane activates both the eIF2alpha and Nrf2/HO-1 pathways. Biochim. Biophys. Acta 1830:2214–2225 [DOI] [PubMed] [Google Scholar]
- 39. Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. 2006. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 116:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma W, St-Jacques B, Duarte PC. 2012. Targeting pain mediators induced by injured nerve-derived COX2 and PGE2 to treat neuropathic pain. Expert Opin. Ther. Targets 16:527–540 [DOI] [PubMed] [Google Scholar]
- 41. Profita M, Sala A, Bonanno A, Riccobono L, Ferraro M, La Grutta S, Albano GD, Montalbano AM, Gjomarkaj M. 2010. Chronic obstructive pulmonary disease and neutrophil infiltration: role of cigarette smoke and cyclooxygenase products. Am. J. Physiol. Lung Cell. Mol. Physiol. 298:L261–L269 [DOI] [PubMed] [Google Scholar]
- 42. Brant KA, Fabisiak JP. 2009. Nickel and the microbial toxin, MALP-2, stimulate proangiogenic mediators from human lung fibroblasts via a HIF-1alpha and COX-2-mediated pathway. Toxicol. Sci. 107:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shih RH, Yang CM. 2010. Induction of heme oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2 expression in mouse brain endothelial cells. J. Neuroinflammation 7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fernandez P, Guillen MI, Gomar F, Aller E, Molina P, Alcaraz MJ. 2004. A novel cyclo-oxygenase-2 inhibitor modulates catabolic and antiinflammatory mediators in osteoarthritis. Biochem. Pharmacol. 68:417–421 [DOI] [PubMed] [Google Scholar]
- 45. Han S, Roman J. 2006. COX-2 inhibitors suppress lung cancer cell growth by inducing p21 via COX-2 independent signals. Lung Cancer 51:283–296 [DOI] [PubMed] [Google Scholar]
- 46. Lu DY, Yeh WL, Huang SM, Tang CH, Lin HY, Chou SJ. 2012. Osteopontin increases heme oxygenase-1 expression and subsequently induces cell migration and invasion in glioma cells. Neuro Oncol. 14:1367–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen H, Li H, Cao F, Zhen L, Bai J, Yuan S, Mei Y. 2012. 1,2,3,4,6-penta-O-galloyl-beta-d-glucose protects PC12 cells from MPP(+)-mediated cell death by inducing heme oxygenase-1 in an ERK- and Akt-dependent manner. J. Huazhong Univ. Sci. Technolog. Med. Sci. 32:737–745 [DOI] [PubMed] [Google Scholar]
- 48. Hecke F, Steinwede K, Muhlradt P, Pabst R, Ehlers S, Dorsch M, Maus U, Tschernig T. 2009. The synthetic TLR-2/6 agonist MALP-2 reduces the bacterial load in the murine M. bovis BCG infection. Am. J. Respir. Crit. Care Med. 179:A5913 [Google Scholar]
- 49. Grote K, Sonnenschein K, Hillmer A, Kapopara P, Schuett H, Bavendiek U, Schieffer B. 2012. Abstract 11704: the Toll-like receptor 2/6 agonist MALP-2 promotes reendothelialization following vascular injury. Circulation 124:A11704. [DOI] [PubMed] [Google Scholar]
- 50. Reppe K, Tschernig T, Luhrmann A, van Laak V, Grote K, Zemlin MV, Gutbier B, Muller HC, Kursar M, Schutte H, Rosseau S, Pabst R, Suttorp N, Witzenrath M. 2009. Immunostimulation with macrophage-activating lipopeptide-2 increased survival in murine pneumonia. Am. J. Respir. Cell Mol. Biol. 40:474–481 [DOI] [PubMed] [Google Scholar]
- 51. Deiters U, Barsig J, Tawil B, Muhlradt PF. 2004. The macrophage-activating lipopeptide-2 accelerates wound healing in diabetic mice. Exp. Dermatol. 13:731–739 [DOI] [PubMed] [Google Scholar]







