Abstract
Monoclonal antibodies (MAbs) are potential therapeutic agents against Bacillus anthracis toxins, since there is no current treatment to counteract the detrimental effects of toxemia. In hopes of isolating new protective MAbs to the toxin component lethal factor (LF), we used a strain of mice (C57BL/6) that had not been used in previous studies, generating MAbs to LF. Six LF-binding MAbs were obtained, representing 3 IgG isotypes and one IgM. One MAb (20C1) provided protection from lethal toxin (LeTx) in an in vitro mouse macrophage system but did not provide significant protection in vivo. However, the combination of two MAbs to LF (17F1 and 20C1) provided synergistic increases in protection both in vitro and in vivo. In addition, when these MAbs were mixed with MAbs to protective antigen (PA) previously generated in our laboratory, these MAb combinations produced synergistic toxin neutralization in vitro. But when 17F1 was combined with another MAb to LF, 19C9, the combination resulted in enhanced lethal toxicity. While no single MAb to LF provided significant toxin neutralization, LF-immunized mice were completely protected from infection with B. anthracis strain Sterne, which suggested that a polyclonal response is required for effective toxin neutralization. In total, these studies show that while a single MAb against LeTx may not be effective, combinations of multiple MAbs may provide the most effective form of passive immunotherapy, with the caveat that these may demonstrate emergent properties with regard to protective efficacy.
INTRODUCTION
Bacillus anthracis, the causative agent of anthrax, has been studied extensively, especially after it was demonstrated to be a powerful biological weapon during the bioterrorism attacks of 2001. B. anthracis virulence is largely due to its ability to produce a tripartite toxin consisting of protective antigen (PA), edema factor (EF), and lethal factor (LF). EF is an adenylate cyclase (1), which binds with PA to form edema toxin, while LF is a zinc metalloprotease that disrupts host cell signaling via cleavage of mitogen-activated protein kinase kinases (as reviewed in reference 2) and combines with PA to form lethal toxin (LeTx).
Anthrax vaccine adsorbed (AVA) has long been the only vaccine available for protection against B. anthracis in the United States. This vaccine consists of an acellular filtrate from an acapsular strain of B. anthracis (3). Albeit effective, the exact antigenic composition of this vaccine remains unknown and varies from batch to batch (4). Although vaccine-elicited antibodies to PA are thought to be the major mediators of protection, it is unclear whether immune responses to other toxin components also contribute to induce immunity (5–7). The vaccine has several other shortcomings, including a burdensome schedule of vaccinations and a requirement for annual boosts (8). In addition, while antibiotics such as ciprofloxacin can control the bacterial infection, there is currently no effective treatment to counter the effects of anthrax toxin. Antimicrobial therapy can clear the infection but does not affect toxemia.
Over the past 2 decades, passive immunotherapy has been widely explored as an alternative approach to protection from and treatment of B. anthracis infections and other microbial pathogens and their toxins and has been reviewed extensively (7, 9–13). Specifically, there have been many studies reporting the generation and characterization of monoclonal antibodies specific to the individual components of anthrax toxin (for a comprehensive summary of these studies, see references 11 and 13). Most of these studies have focused on monoclonal antibodies (MAbs) to PA. There have been several MAbs to LF generated from splenocytes derived from BALB/c or A/J mice (14–18). Consequently, a goal of our study was to use a genetically different mouse strain (C57BL/6) with the hope of isolating novel MAbs to LF, since the genetic background affects the susceptibility to anthrax toxins (19). In addition, we sought to further characterize the protective efficacy of these MAbs to LF in combinations, since serum is a polyclonal mix of antibodies and the context of a MAb in the presence of other antibodies may affect its interactions with LeTx. To our knowledge, only one study has explored antibodies to LF in combinations with MAbs to PA (20). Together, the combination of these two MAbs provided increased protection against B. anthracis Sterne challenge in mice. A subsequent study (21) tested two LF MAbs with one PA MAb in a Fischer F344 rat model and showed “synergistic” protection with one of the two combinations. Here we show that combinations of MAbs to LF can manifest properties different from those of their individual components to enhance or abrogate MAb-mediated LeTx protection both in vitro and in vivo.
MATERIALS AND METHODS
B. anthracis and toxin components.
B. anthracis Sterne 34F2 (pXO1+, pXO2−) was obtained from Alex Hoffmaster at the Centers for Disease Control and Prevention (Atlanta, GA). Bacterial cultures were grown from frozen stock in brain heart infusion (BHI) broth (Difco, Detroit, MI) at 37°C for 18 h with shaking. Recombinant, endotoxin-reduced protective antigen (rPA), edema factor (rEF), and lethal factor (rLF) proteins were obtained from the Northeast Biodefense Center Expression Core, New York State Department of Health (Albany, NY).
Murine immunization with purified LF.
Female 6- to 8-week-old C57BL/6 mice were obtained from the National Cancer Institute (Bethesda, MD). Five mice were immunized with 10 μg rLF in Freund's complete adjuvant (CFA) (Sigma, St. Louis, MO). At 2 and 4 weeks after the initial immunization, mice were boosted with 10 μg of LF in incomplete Freund's adjuvant (IFA). Six weeks following the initial immunization, one mouse was boosted once a day for 2 days with 100 μg of rLF in IFA and was then sacrificed 2 days later to collect splenocytes for the hybridoma fusion assay. As a control, two mice were immunized with CFA alone. Sera from the mice were collected by retro-orbital bleeding and stored at −20°C. Antibody titers were determined by standard enzyme-linked immunosorbent assay (ELISA), as described below.
Generation of MAbs to LF.
Splenocytes from mouse LF5 were recovered and fused with myeloma cells by standard techniques (22) to generate hybridomas, which were then plated on 96-well plates and screened for reactivity to LF by ELISA. Positive clones were isolated and purified by two rounds of soft-agar cloning and also screened for nonspecific binding to bovine serum albumin (BSA).
ELISA.
Binding of Abs to LF, EF, or PA was detected using standardized ELISAs as previously described (23). Briefly, polystyrene plates were coated with 50 μl of 2 μg/ml purified rLF, rEF, or rPA, suspended in phosphate-buffered saline (PBS), and blocked for 1 h at 37°C with 1% BSA–PBS. Antibody binding to immobilized toxin components was detected using anti-mouse alkaline-phosphatase (AP)-conjugated secondary antibodies, diluted 1:1,000 (∼0.5 μg/ml). For competition ELISAs, plates were coated and blocked as described above and then incubated with 2 μg/ml of the constant MAb mixed with a 1:3 titration of the variable MAb across the plate starting with 90 μg/ml. Binding of the primary Abs was detected as described above.
Western blotting.
rLF (15 μg/ml) was submitted to electrophoresis on a 12% SDS polyacrylamide gel, transferred to a nitrocellulose membrane using the iBlot Semi-Dry Transfer system (Invitrogen, Carslbad, CA), blocked with 5% milk–PBS, and then incubated with each MAb to LF diluted to ∼0.5 μg/ml. Primary Ab binding was detected with isotype-matched goat anti-mouse horseradish peroxidase (HRP)-conjugated Abs and chemiluminescence using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Waltham, MA).
In vitro cell viability assay.
Cell viability assays were performed as described previously (24) using MTT [3,(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide]. In brief, J774 macrophage cells were seeded in 96-well plates at a density of 3 × 104 cells/well and incubated overnight at 37°C. To assay the toxin neutralization capability of polyclonal sera (Fig. 1C), a toxin concentration of 1 μg/ml was added to each well and cells were incubated for 4 h. For all other MTT assays, a solution containing 0.1 μg/ml of PA and LF (a concentration that yields approximately 50% cell killing) was added to each well and incubated 4 h. Then, 25 μl of 5 mg/ml MTT substrate was added to each well, and cells were incubated at 37°C for an additional 2 h. Lastly, culture medium was aspirated, and 200 μl of 12.5% SDS–45% N,N-dimethylformamide (DMF) was added to each well. Optical densities were measured at 570 nm with a spectrophotometer (Multiskan; Labsystems, Franklin, MA). For the Fc receptor (FcR) blocking experiment, 100 μl of 10 μg/ml mouse BD Fc Block (rat anti-mouse CD16/CD32, MAb 2.4G2) (BD Biosciences, San Jose, CA) was added to each well, and wells were incubated for 15 min prior to addition of toxin and MAbs. Fc Block was present in the Ab/toxin media for the duration of the experiment.
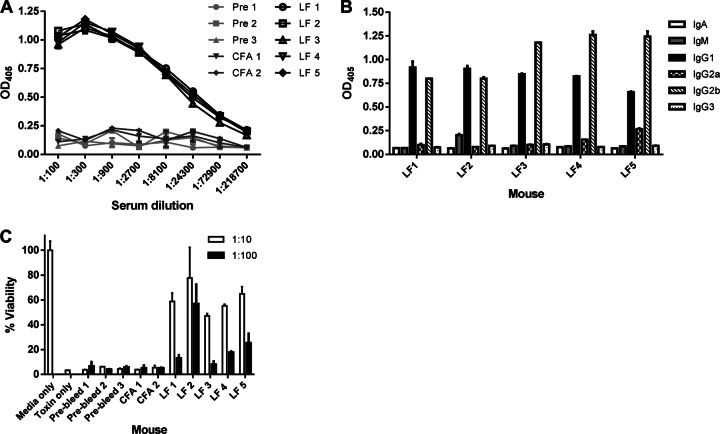
Fig 1.
Characterization of serum from LF-immunized mice 6 weeks after initial immunization. Five C57BL/6 mice were immunized with 10 μg of LF and boosted every 2 weeks with 10 μg for 6 weeks. (A) Titers of antibodies to LF in 6-week serum. (B) Subtyping of 6-week sera. LF-coated polystyrene plates were incubated with sera diluted 1:1,000 and then assayed with isotype-specific, alkaline phosphatase-conjugated secondary antibodies. (C) MTT cell viability assay to measure protective ability of 6-week sera on J774 macrophage cells against LeTx. Macrophages were incubated with 1 μg/ml LeTx with sera diluted 1:10 or 1:100 in duplicate for each mouse. Percent viability was calculated based on cells incubated with medium alone. Prebleed, preimmune mice; CFA, mice immunized with CFA only; LF, mice immunized with LF.
SPR.
Binding constants of MAbs to LF were measured by surface plasmon resonance (SPR) using a BIAcore 3000 analyzer (Biacore, GE Healthcare, Piscataway, NJ). Immobilization of MAbs to a CM5 sensor chip was achieved as described previously (25). Serially diluted rLF was run over the immobilized MAbs.
Sequence analysis of immunoglobulin variable genes.
Hybridoma cell lines 12H11 and 20C1 were grown to confluence in 6-well plates and then collected in TRIzol reagent (Invitrogen), and RNA was isolated according to the manufacturer's specifications. cDNA was generated using the Superscript III First-Strand Synthesis system (Invitrogen). PCR amplification of the variable light (VL) and heavy (VH) genes was performed with primers and under reaction conditions previously described (26). Sequencing was performed at the Genomics Core at Albert Einstein College of Medicine.
Mouse infections with B. anthracis strain Sterne.
C57BL/6 mice (n = 10) were immunized with rLF or CFA as described above. Mice were boosted with rLF every 2 weeks for 6 weeks and then challenged with 105 cells of strain Sterne per mouse. For passive transfer experiments, immunized mice were serially bled retro-orbitally and the sera from each group pooled. Mice were given 200 μl sera from the LF-immunized mice 2 h prior to infection with strain Sterne. To establish whether MAbs to LF alone or in combination were protective in vivo, five C57BL/6 mice/group were given 10, 50, or 100 μg MAb intraperitoneally (i.p.) 2 h before challenge with 105 cells of strain Sterne/mouse. Mice were monitored daily for mortality. All animal work was done in accordance with the regulations of the Institute for Animal Studies at Albert Einstein College of Medicine.
Screen for interactions between MAbs to LeTx.
MTT assays were performed as described above, combining two MAbs to LF. One MAb was kept at a constant concentration of 30 μg/ml and mixed with another MAb to LF, which was serially diluted 1:3 across the plate, starting with 90 μg/ml. MTT assays were also performed to screen for interesting multiple MAb interactions between the LF MAbs generated in this study and MAbs previously generated in our laboratory (24, 39). For each combination, one MAb was kept at a constant concentration of 5 μg/ml, while the other was serially diluted 1:3 across the plate, starting with 30 μg/ml. Results of these assays were used to calculate fractional inhibitory concentration (FIC) indices, as described below.
Statistical and mathematical analyses.
To characterize multiple MAb interactions, we used the following equation for calculating the FICs: MAb concentration yielding 50% of the maximum cell survival for two Abs in combination divided by the MAb concentration yielding 50% of the maximum cell survival of an antibody alone, as adapted from references 27, 28, and 29). The FIC index is a sum of the FICs for each antibody, and the interaction between two Abs was considered synergistic if the FIC index was <1.0, additive if the FIC index was equal to 1, indifferent if the FIC index was between 1 and 2, and antagonistic if it was >2. The Student t test was used when appropriate, and the log rank test was used for mouse survival experiments (GraphPad Prism version 5.04; GraphPad Software, San Diego CA, USA).
Nucleotide sequence accession number.
The VH and VL gene sequences of MAbs 12H11 and 20C1 determined in this study have been submitted to GenBank under accession numbers KC592294 and KC592295.
RESULTS
Murine antibody response to immunization with LF.
All five LF-immunized mice demonstrated similar, robust antibody responses to LF (Fig. 1A), and as expected, the CFA and preimmune mice showed no reactivity to LF. Immunoglobulin subtyping of 6-week sera revealed that the majority of the anti-LF response was IgG, with little to no IgA or IgM present in the sera (Fig. 1B). The IgG response consisted of a mix of IgG1 and IgG2b and very low or absent levels of IgG2a and IgG3 (Fig. 1B). Next, we tested the sera's ability to neutralize lethal toxin in vivo using a macrophage viability assay. Sera from the preimmune and CFA mice offered no protection, while the sera from the LF-immunized mice all showed similar levels of toxin neutralization of about 50% or more (Fig. 1C). These results show that over the course of 6 weeks, the mice mounted a strong, specific polyclonal Ab response to the rLF antigen and produced toxin-neutralizing antibodies.
Generation and characterization of LF-specific MAbs.
When titers of the LF-immunized mice were deemed sufficiently high (at least 2.5 times greater than baseline titers of CFA-only immunized mice), mouse 5 was sacrificed, and splenocytes were collected and subjected to a hybridoma fusion assay. We recovered six hybridomas producing 6 LF-specific MAbs: 17E7, 17F1, 19C9, 20C1, 12H11, and 11D7. Isotyping revealed that this set consisted of one IgG1 (19C9), one IgG2a (17F1), three IgG2b (17E7, 12H11, and 20C1), and one IgM (11D7). Since there are regions of homology between LF and EF (30) and some MAbs have been reported to bind both antigens (15, 18), we evaluated the ability of our MAbs to bind to all three anthrax toxin components. ELISA results showed that while all MAbs strongly bound to LF (Fig. 2B), 17E7, 17F1, and 19C9 had reactivity for all three antigens. All MAbs were also reactive with LF by Western blotting (Fig. 2A), but binding to PA and EF could not be detected by immunoblotting (data not shown). The relative binding affinity of these MAbs for LF was measured by ELISA, which showed that 17E7 bound the strongest, followed by 20C1, 12H11, 17F1, and 19C9 (Fig. 2C). To further investigate the binding properties of these MAbs, SPR techniques were employed on 12H11, 17F1, and 20C1. The two-state kinetic reaction model yielded results that corresponded with the binding ELISA: 12H11 and 20C1 showed similar affinities, both higher than that of 17F1 (Table 1).
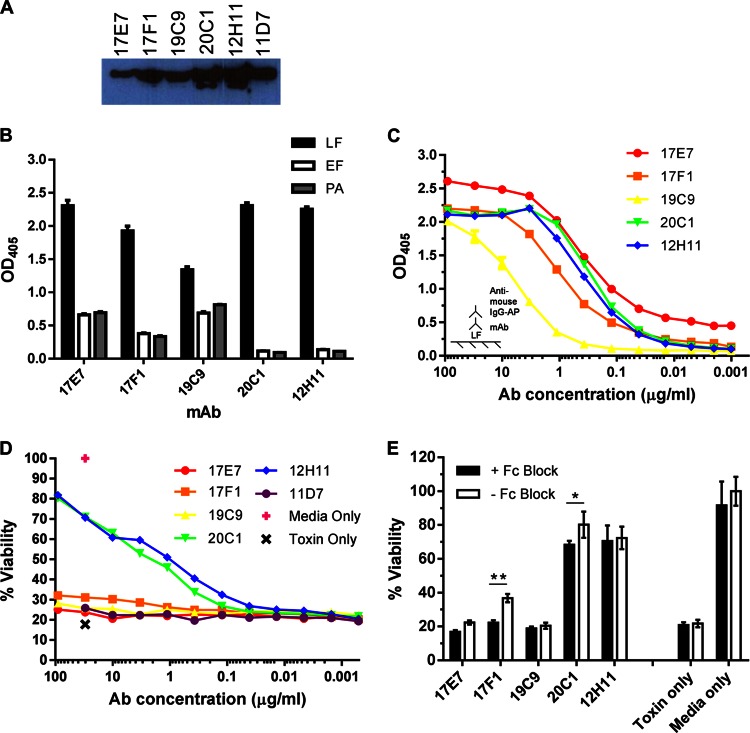
Fig 2.
In vitro characterization of MAbs to LF. Six LF-specific MAbs were purified from cell lines generated from a standard splenocyte-myeloma fusion. (A) Immunodetection of LF. MAbs, diluted 1:500, were incubated with purified LF on a nitrocellulose membrane and detected by chemiluminescence with HRP-conjugated secondary Abs. (B) IgG MAb reactivity to anthrax toxin components. Binding to LF, EF, and PA was measured by ELISAs in triplicate for each Ab; plates were coated with 2 μg/ml antigen and incubated with 10 μg/ml antibody. Ab binding was detected with AP-conjugated secondary Abs. (C) Relative binding capacity of IgG MAbs to LF as determined by ELISA. The ELISA plate was coated with 2 μg/ml LF, and MAb concentrations were titrated 1:3 from 90 μg/ml. (D) In vitro cytotoxicity assay to measure MAb-mediated toxin protection. J774 macrophages were incubated with 0.1 μg/ml LeTx with or without MAbs at various concentrations. Cells were incubated with toxin alone or toxin-free medium as negative and positive controls, respectively, for viability. (E) Cell viability assay with Fc receptors blocked. The assay was performed as described above, with 0.1 μg/ml LeTx, except cells were incubated with 100 μl of 10 μg/ml Fc Block for 15 min prior to incubation with LeTx and MAbs. *, cell viability was significantly lower with Fc Block for 20C1 (P = 0.0077); **, P < 0.0001 for 17F1.
Table 1.
Binding affinities of MAbs to LFa
| MAb | Ka (1/Ms) | Kd (1/s) | K (1/M) |
|---|---|---|---|
| 12H11 | 1.25e5 | 2.26e−3 | 8.03e9 |
| 17F1 | 1.36e4 | 3.22e−3 | 2.75e7 |
| 20C1 | 1.34e5 | 2.71e−3 | 4.55e9 |
Ka, binding affinity constant; Kd, dissociation constant; K, equilibrium constant.
Next, we determined the toxin neutralization capacity of these MAbs by macrophage cell viability assay. These results showed that MAbs 12H11 and 20C1conferred the highest levels of protection and 17F1 showed slight protection while the remaining MAbs showed little to no toxin neutralization (Fig. 2D). Hence, we recovered at least two LF-specific MAbs that can neutralize LeTx in vitro. To determine whether MAb toxin neutralization was FcR dependent, a cell viability assay was performed using Fc Block (MAb 2.4G2). Cells were incubated with or without Fc Block prior to treatment with toxin and MAbs to LF. These results suggest that toxin neutralization mediated by MAbs 17F1 and 20C1 is partially dependent on FcR binding (Fig. 2E).
Since MAbs 12H11 and 20C1 were of the same isotype and had similar binding affinity and specificity to LF as well as similar toxin neutralization capability, we determined the VH and VL gene sequences of each MAb. Sequence analysis revealed that these MAbs shared identical variable gene sequences. Thus, we concluded that these hybridoma cell lines were derived from clonally related B cells that produced the same immunoglobulin and excluded 12H11 from further analysis.
Protective efficacy of anti-LF MAbs in vivo.
To determine whether these MAbs are protective in vivo, we carried out active and passive immunization to ascertain the level of protection in mice challenged with B. anthracis. Three types of experiments were done. First, we tested whether the polyclonal sera from LF-immunized mice conferred protection. LF-immunized mice (n = 10), along with the CFA (n = 10) and naïve (n = 5) mice, were each challenged with 105 cells/mouse of strain Sterne. LF immunization provided complete protection from B. anthracis challenge (Fig. 3A). This experiment was repeated twice with similar results. Next, we tested the efficacy of sera collected from the LF-immunized mice in a passive transfer experiment. Prior to infection, sera were collected and pooled from LF-immunized mice. Naïve C57BL/6 mice were administered 200 μl of undiluted sera 2 h prior to infection with strain Sterne. Passive transfer of sera from LF-immunized mice provided significantly (P = 0.0141) greater protection than sera from CFA-immunized or naïve sera (Fig. 3B).
Fig 3.
Survival of mice challenged with B. anthracis strain Sterne. (A) Challenge of LF-immunized mice with strain Sterne. Mice were given an inoculum of 105 cells/mouse (n = 10 mice for LF and CFA groups; n = 5 for the naïve group). (B) Passive transfer of polyclonal sera. Naïve C57BL/6 mice were administered 200 μl of polyclonal sera from the LF-immunized mice illustrated in panel A 2 h prior to infection with 105 cells of strain Sterne per mouse. n = 10 for mice given LF-immunized sera, n = 7 for CFA sera, n = 5 for naïve sera. (C) Characterization of MAbs in vivo: 100 μg of Ab was administered to 5 mice/group 2 h before challenge with 105 cells of strain Sterne per mouse. (D) MAbs 17F1 and 20C1 in combination. MAbs were given alone or in combination to mice 2 h before challenge with strain Sterne. n = 5 mice/group.
Lastly, we tested each MAb individually in passive protection experiments. For each MAb, five C57BL/6 mice were each given 100 μg of MAb 2 h prior to intravenous challenge with 105 cells of strain Sterne. MAb administration was not followed by increased survival for any of the MAbs tested relative to the control group (PBS) (Fig. 3C).
Analysis of neutralizing activity of MAb combinations to LF.
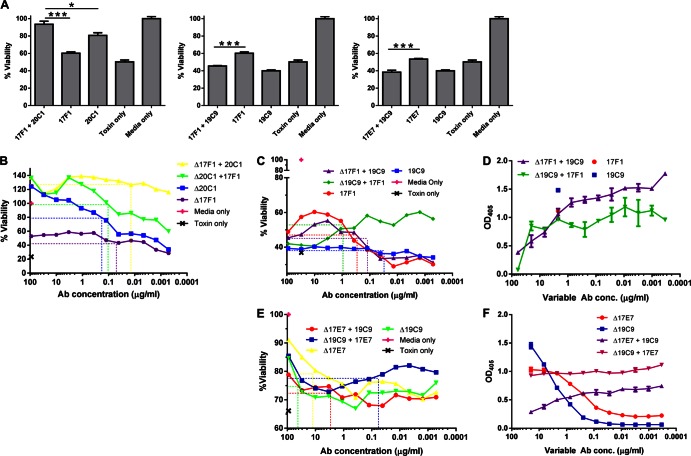
Given that under physiological conditions Abs function in combination with other Abs as part of a polyclonal response, we evaluated the neutralization capacity of MAbs in combination at a 1:1 ratio against LeTx by MTT assay. One striking Ab combination was 17F1 plus 20C1, which conferred a significant increase in cell viability compared to 17F1 or 20C1 alone (P < 0.0001 and P = 0.0111, respectively) (Fig. 4A), suggesting that 17F1 potentiated the toxin neutralization activity of 20C1. To further explore these observations, a subsequent MTT assay titrating 17F1 and 20C1 was performed. These results confirmed the previous results that 17F1 in combination with 20C1 was more effective at neutralizing LeTx than either MAb alone (Fig. 4B). The fractional inhibitory concentrations (FICs) were calculated by determining the antibody concentration at 50% of maximum cell survival for each curve. The FIC index is the sum of the FICs and is considered synergistic if the FIC index is <1, additive if it is equal to 1, indifferent if the value falls between 1 and 2, and antagonistic if it is >2. The FIC index for 17F1 and 20C1 was 0.89 (Table 2), suggesting a moderately synergistic interaction.
Fig 4.
Combinatory effects of LF MAbs on lethal toxin neutralization. (A) Macrophage viability assay of anti-LF MAbs in pairs. J774 cells were incubated with 0.1 μg/ml LeTx and 10 μg of each Ab. ***, P < 0.0001; **, P < 0.01; *, P < 0.05. (B) Cell survival assay titrating 17F1 in combination with 20C1. Cells were incubated with 0.1 μg/ml LeTx and 30 μg/ml of the constant MAb mixed with titrated amounts of the variable MAb. (C) Cell survival assay of 19C9 and 17F1 in combination. Cells were incubated with 0.1 μg/ml LeTx and 30 μg/ml of the constant MAb and titrated 1:3 with the variable Ab starting with 90 μg/ml. (D) Competition ELISA between 17F1 and 19C9. A 96-well plate was coated with 2 μg/ml LF and then incubated with 2 μg/ml of one MAb (constant concentration) and a variable concentration of the other. Binding of the constant MAb was detected with an isotype-specific AP-conjugated secondary Ab. (E) MTT assay with 17E7 and 19C9. The assay was performed as described for panels B and C. (F) Competition ELISA between 17E7 and 19C9. Dashed lines indicate the MAb concentration at 50% of maximum cell survival for each of the survival curves shown in panels B, C, and E.
Table 2.
FIC indices for multiple MAb interactions
| MAb(s)a | Survival (%) |
Ab concn (μg/ml) for 50% survival | FICb | FIC indexc | ||
|---|---|---|---|---|---|---|
| Maximum | Minimum | 50% | ||||
| Δ17F1 | 58.76 | 28.74 | 43.75 | 0.05 | ||
| Δ20C1 | 124.55 | 33.88 | 79.22 | 0.18 | ||
| Δ17F1 + 20C1 | 139.05 | 114.26 | 126.65 | 0.01 | 0.28 | |
| Δ20C1 + 17F1 | 137.07 | 59.79 | 98.43 | 0.11 | 0.61 | 0.89 |
| Δ17F1 | 60.35 | 28.96 | 44.65 | 0.40 | ||
| Δ19C9 | 40.28 | 34.10 | 37.19 | 0.05 | ||
| Δ17F1 + 19C9 | 55.28 | 31.18 | 43.23 | 0.18 | 0.45 | |
| Δ19C9 + 17F1 | 60.21 | 40.63 | 50.42 | 1.25 | 25.50 | 25.96 |
| Δ17E7 | 90.98 | 70.28 | 80.63 | 11.10 | ||
| Δ19C9 | 84.71 | 66.93 | 75.82 | 39.80 | ||
| Δ17E7 + 19C9 | 78.81 | 67.95 | 73.38 | 2.37 | 0.21 | |
| Δ19C9 + 17E7 | 85.44 | 72.83 | 79.14 | 0.04 | 1,130.68 | 1,130.90 |
| Δ20C1 | 54.30 | 17.00 | 35.65 | 2.95 | ||
| ΔN5C10 | 63.58 | 15.88 | 39.73 | 0.43 | ||
| Δ20C1 + N5C10 | 83.99 | 60.04 | 72.01 | 0.04 | 0.02 | |
| ΔN5C10 + 20C1 | 90.58 | 39.48 | 65.03 | 0.08 | 0.20 | 0.21 |
| Δ20C1 | 44.84 | 11.94 | 28.39 | 1.62 | ||
| Δ19D9 | 73.42 | 12.31 | 42.86 | 1.79 | ||
| Δ20C1 + 19D9 | 92.71 | 61.29 | 77.00 | 0.41 | 0.26 | |
| Δ19D9 + 20C1 | 96.48 | 22.79 | 59.63 | 0.27 | 0.15 | 0.41 |
MAbs preceded by a Greek capital delta (Δ) are “variable antibodies,” i.e., used at various concentrations (see Fig. 4 and 5).
FIC was calculated as follows: Ab concentration of the variable antibody A that yields 50% of maximum cell survival when in combination with a constant concentration of antibody B divided by the Ab concentration at 50% of maximum cell survival with antibody A alone.
We then studied whether these two MAbs showed a similar interaction in vivo. Thus, C57BL/6 mice were given either 17F1 or 20C1 alone or the two in combination at different ratios, and their survival was monitored for 10 days. Survival of mice given 100 μg 17F1 plus 10 μg 20C1 was significantly higher than that of mice given PBS alone (P = 0.037; Fig. 3D). Interestingly, the addition of 17F1 (10 or 100 μg) significantly (P = 0.042) increased mouse survival compared to use of 20C1 alone (Fig. 3D). These results support the FIC index value and further show that these MAbs demonstrate a synergistic interaction.
MAb 19C9 appeared to exhibit inhibitory effects in combination with other MAbs. There was a significant (P < 0. 0001) decrease in toxin neutralization with the combination of MAbs 17F1 and 19C9 in comparison to 17F1 alone; the same effect was observed when MAb 19C9 was combined with 17E7 (P < 0. 0001) (Fig. 4A). To better characterize this observation, the MTT assay titrating both MAbs in combination was repeated. Alone, MAb 19C9 showed no LeTx neutralization, while MAb 17F1 conferred ∼60% maximum cell survival (Fig. 4C). When MAb 17F1 was titrated with a constant concentration of MAb 19C9 (30 μg/ml), the curve mirrored that of MAb 17F1 alone. However, when a constant concentration of MAb 17F1 was mixed with various amounts of MAb 19C9, cell survival increased as the concentration of MAb 19C9 decreased, suggesting that MAb 19C9 interfered with MAb 17F1-mediated toxin neutralization, but MAb 17F1 was able to overcome this inhibition at high concentrations (Fig. 4C). FIC analysis revealed a strong antagonistic interaction (FIC index = 25.96; Table 2). We hypothesized that this could be due to these MAbs sharing the same epitope on LF; a competition ELISA between MAbs 17F1 and 19C9 was consistent with that premise (Fig. 4D). To determine whether these two MAbs also demonstrated an antagonistic interaction in vivo, mice were given 17F1 and 19C9 and challenged with strain Sterne. Although there was a trend toward increased killing with the two MAbs in combination, there was no significant decrease in survival over the course of several experiments (data not shown).
A very strong antagonistic reaction was observed between MAb 17E7 and MAb 19C9 (FIC = 1,130; Fig. 4E and Table 2). However, competition ELISA between 17E7 and 19C9 suggested that whereas 17E7 interfered with 19C9 binding to LF, 19C9 did not seem to interfere with 17E7 binding to LF (Fig. 4F).
In vitro characterization of multiple anti-LeTx Ab interactions.
Because we observed variation in toxin neutralization efficacy among different combinations of LF-specific MAbs, we also wanted to characterize multiple MAb interactions with MAbs to LF and PA. Thus, we took advantage of an array of MAbs to PA previously generated in our laboratory (24, 39). A cell viability screen was performed, combining the four unique IgG anti-LF MAbs generated in this study and 25 MAbs to PA: 3 protective, 6 toxin-enhancing, and 16 toxin-indifferent MAbs. Most of the antibody combinations did not show any changes in toxin neutralization levels (data not shown), with a few exceptions. Combining the MAb to PA N5C10 with MAb 20C1 resulted in robust toxin neutralization (Fig. 5A). This combination was deemed synergistic based on the FIC index (Table 2). MTT assays combining MAb 20C1 also showed strong synergistic interactions with another MAb to PA, 19D9, with an FIC index of 0.41 (Fig. 5B; Table 2).
Fig 5.
Combinatory effects of anti-LF and anti-PA MAbs in vitro. (A and B) MTT assays titrating LF MAb 20C1 with two MAbs to PA, N5C10 (A) and 19D9 (B). Mouse macrophage cells were incubated with 0.1 μg/ml LeTx, along with one MAb at a constant concentration of 5 μg/ml mixed with another MAb serially diluted 1:3 starting with 30 μg/ml. Dashed lines indicate the MAb concentration at 50% of maximum cell survival for each curve.
Given the experience with botulinum toxin, where the combination of 3 MAbs provided greater protection against botulinum toxin than one or two MAbs alone (31), we hypothesized that combinations of 3 MAbs to LF might also demonstrate synergistic effects. We tested all possible combinations of the 5 IgG MAbs by MTT assay, but there were no 3-MAb combinations that showed any distinct differences in cell survival compared to each MAb alone or in pairwise combinations (data not shown).
DISCUSSION
Over the past 2 decades, passive immunotherapeutics have become an increasingly attractive approach to treatment of infectious diseases (10), including that caused by the potential bioterrorism agent B. anthracis. Antimicrobial drugs can kill the bacteria but do not affect preformed toxins. Consequently, MAbs are an option for the development of new therapies. MAbs have the advantage over polyclonal reagents in that they are specific, have high activity per protein amount since all immunoglobulins bind to the intended target, and can be produced in virtually unlimited amounts. However, while many studies have described the generation of MAbs to B. anthracis virulence factors (as summarized in references 11 and 13), many are only partially effective. Most MAbs generated evaluated to date have been against PA; thus, we sought to produce LF-specific MAbs in a mouse strain (C57BL/6) that had not been used in previous, similar studies (14–18). Of the six MAbs generated in this study, two showed a moderate level of protection in vitro (Fig. 2D), but not in vivo (Fig. 3C). That the MAbs to LF were not protective in vivo is not surprising, as some other MAbs generated to LF have not been protective in mice (20). Further, while all the MAbs generated in this study reacted with LF, some of the MAbs also showed reactivity with PA and EF by ELISA, but not by Western analysis. This phenomenon is likely due to recognition of cross-reactive epitopes that are known to exist in anthrax toxin components (32). Interestingly, the MAb that did not show any cross-reactivity to other anthrax toxin components was 20C1, so perhaps this specificity contributed to its toxin neutralization capability.
Characterization of this MAb panel shows that the avidity and binding capacity of these MAbs does not determine their toxin neutralization capacity. All of the MAbs tested bound strongly to LF, yet only one appeared to show any effective toxin neutralization. This suggests that the epitope recognized by the MAb is the primary factor of protective efficacy. The same has been found for MAbs directed against other microbial virulence factors, such as MAbs targeting the capsule of Cryptococcus neoformans (33). However, a previous study generating Ab fragments to PA showed a strong correlation between antigen affinity and toxin neutralization (34). This raises the possibility that the correlation between binding affinity and neutralization efficiency is antigen-antibody specific.
None of the MAbs to LF generated in this study were protective in vivo when used as single agents. Although this result is disappointing, it is consistent with the recent finding that most toxin binding MAbs are not protective (13). In contrast, LF-immunized mice showed 100% survival when challenged with strain Sterne (Fig. 3A). That LF-immunized mice showed significantly greater survival than nonimmunized mice suggested that protection from LeTx intoxication requires a polyclonal response or that the MAbs with protective efficacy were not recovered by our screening technique. To establish that protection in LF-immunized mice was mediated by Ab, a passive transfer experiment was performed using sera from LF-immunized mice. The sera from these mice provided significant protection to naïve mice challenged with strain Sterne (Fig. 3B). A recent review summarizing the progress of anthrax vaccine development (7) argued that neutralization of PA is necessary for protection from anthrax intoxication, and studies in humans (6) have shown that Abs to LF or EF do not contribute to toxin neutralization. However, our results support early studies with rodents (35) in which LF alone was highly protective and suggest that sufficient protection can be achieved by immunization with LF protein and without the presence of PA as an immunogen.
A limitation of MAbs as prophylactic and therapeutic agents against infectious diseases is that pathogens are often antigenically variable and have redundant mechanisms of virulence. One option is to combine MAbs into therapeutic cocktails, but until recently this option was difficult to implement due to regulatory and cost concerns. However, a MAb cocktail has now been shown to be effective for the prophylaxis of rabies (36), providing an important precedent for future MAb development, and there is now great interest in the efficacy of combinations. While the use of individual MAbs to LF generated here is not promising as single therapeutic components, combinations of MAbs manifested different protective efficacies, some of which were synergistic. To that end, two LF MAbs, 17F1 and 20C1, showed synergy in vitro and in vivo. Adding 17F1 to 20C1 significantly increased protection from strain Sterne in mice, but the same effect was not observed when 20C1 was added to 17F1, despite 20C1 being the most protective MAb in vitro. Further, giving equal amounts of each MAb to mice did not increase survival compared to that provided by each MAb alone, suggesting that an optimal concentration of each MAb is necessary to maximize protection. Conversely, MAb 19C9 demonstrated antagonistic interactions with two other MAbs to LF, 17E7 and 17F1, in vitro. Again, these results indicate that there is a narrow range of Ab concentrations in which these synergistic or antagonistic interactions can be observed, emphasizing that combinations of MAbs must be carefully characterized if considered for therapeutic applications. Furthermore, the finding that blocking FcR reduced the neutralizing efficacy of some of these antibodies shows that their protective activity is partially dependent on this receptor, as recently described (24).
When we screened these MAbs to LF with a library of MAbs to PA, we discovered several MAb combinations that demonstrated synergy, resulting in increased cell protection in vitro. These results indicate that multiple MAbs could be a more effective form of therapeutics than individual MAbs, as has been observed with MAbs to LeTx (20, 21) and with MAbs to other bacterial toxins (31). Perhaps the combination of MAbs to multiple toxin components, as well as other virulence factors such as the capsule, would provide even greater protection (11).
The FIC index is used for evaluating drug combinations, but this approach has not been applied to antibodies. In this study, we pioneered the use of FIC to investigate the efficacy of MAb combinations. The applicability of the FIC formalism to antibody combinations was uncertain, since antibodies work through various mechanisms, including engagement of Fc receptors, and it was unclear whether the simple mathematics used for drug comparisons will hold with these biological agents. Calculation of FIC indices for MAb combinations that appeared to be synergistic or antagonistic subjected these interactions to a more rigorous analysis than previous studies claiming such MAb-MAb interactions (20, 21, 37, 38). However, FIC calculations must be carefully calculated from curves with a significant slope, otherwise the calculated value will not accurately reflect the nature of the interaction.
In summary, our results provide additional evidence that antibodies to LF can contribute to protection against anthrax and support the development of this antigen as a component of the next generation of vaccines against anthrax. Moreover, the synergy demonstrated between the LF and PA MAbs suggests that greater toxin-neutralizing efficacy can be achieved by combining antibodies with different toxin specificities. However, the protective efficacy of MAb combinations was unpredictable, suggesting that the outcome of mixing MAbs must be determined empirically. Our results illustrate a daunting complexity in Ab combinations and imply that these interactions should be carefully studied in order to generate the most effective monoclonal antibody-based therapeutics.
ACKNOWLEDGMENTS
We thank Karen Chave for purified toxin proteins, Susan Buhl and Manxia Fan at the Albert Einstein College of Medicine Hybridoma Core Facility for assistance with the hybridoma fusion assay, and Hui-Yong Cheng for assistance with SPR analyses.
This work was supported by the Northeast Biodefense Center Career Development Award (2 U54 AI057158).
Footnotes
Published ahead of print 18 March 2013
REFERENCES
- 1. Leppla SH. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 79:3162–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tonello F, Montecucco C. 2009. The anthrax lethal factor and its MAPK kinase-specific metalloprotease activity. Mol. Aspects Med. 30:431–438 [DOI] [PubMed] [Google Scholar]
- 3. Turnbull PC. 2000. Current status of immunization against anthrax: old vaccines may be here to stay for a while. Curr. Opin. Infect. Dis. 13:113–120 [DOI] [PubMed] [Google Scholar]
- 4. Ivins BE, Pitt ML, Fellows PF, Farchaus JW, Benner GE, Waag DM, Little SF, Anderson GW, Jr, Gibbs PH, Friedlander AM. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141–1148 [DOI] [PubMed] [Google Scholar]
- 5. Baillie L, Townend T, Walker N, Eriksson U, Williamson D. 2004. Characterization of the human immune response to the UK anthrax vaccine. FEMS Immunol. Med. Microbiol. 42:267–270 [DOI] [PubMed] [Google Scholar]
- 6. Taft SC, Weiss AA. 2008. Neutralizing activity of vaccine-induced antibodies to two Bacillus anthracis toxin components, lethal factor and edema factor. Clin. Vaccine Immunol. 15:71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chitlaru T, Altboum Z, Reuveny S, Shafferman A. 2011. Progress and novel strategies in vaccine development and treatment of anthrax. Immunol. Rev. 239:221–236 [DOI] [PubMed] [Google Scholar]
- 8. Cybulski RJ, Jr, Sanz P, O'Brien AD. 2009. Anthrax vaccination strategies. Mol. Aspects Med. 30:490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneemann A, Manchester M. 2009. Anti-toxin antibodies in prophylaxis and treatment of inhalation anthrax. Future Microbiol. 4:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casadevall A, Dadachova E, Pirofski LA. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695–703 [DOI] [PubMed] [Google Scholar]
- 11. Chen Z, Moayeri M, Purcell R. 2011. Monoclonal antibody therapies against anthrax. Toxins 3:1004–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saylor C, Dadachova E, Casadevall A. 2009. Monoclonal antibody-based therapies for microbial diseases. Vaccine 27(Suppl 6):G38–G46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chow SK, Casadevall A. 2012. Monoclonal antibodies and toxins—a perspective on function and isotype. Toxins 4:430–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim NK, Kim JH, Oh MS, Lee S, Kim SY, Kim KS, Kang HJ, Hong HJ, Inn KS. 2005. An anthrax lethal factor-neutralizing monoclonal antibody protects rats before and after challenge with anthrax toxin. Infect. Immun. 73:6547–6551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Little SF, Leppla SH, Friedlander AM. 1990. Production and characterization of monoclonal antibodies against the lethal factor component of Bacillus anthracis lethal toxin. Infect. Immun. 58:1606–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Staats HF, Alam SM, Scearce RM, Kirwan SM, Zhang JX, Gwinn WM, Haynes BF. 2007. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect. Immun. 75:5443–5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao P, Liang X, Kalbfleisch J, Koo HM, Cao B. 2003. Neutralizing monoclonal antibody against anthrax lethal factor inhibits intoxication in a mouse model. Hum. Antibodies 12:129–135 [PubMed] [Google Scholar]
- 18. Kulshreshtha P, Bhatnagar R. 2011. Inhibition of anthrax toxins with a bispecific monoclonal antibody that cross reacts with edema factor as well as lethal factor of Bacillus anthracis. Mol. Immunol. 48:1958–1965 [DOI] [PubMed] [Google Scholar]
- 19. Moayeri M, Leppla SH. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 30:439–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brossier F, Levy M, Landier A, Lafaye P, Mock M. 2004. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 72:6313–6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Z, Moayeri M, Crown D, Emerson S, Gorshkova I, Schuck P, Leppla SH, Purcell RH. 2009. Novel chimpanzee/human monoclonal antibodies that neutralize anthrax lethal factor, and evidence for possible synergy with anti-protective antigen antibody. Infect. Immun. 77:3902–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de StGroth SF. 1980. Production of monoclonal antibodies: strategy and tactics. J. Immunol. Methods 35:1–21 [DOI] [PubMed] [Google Scholar]
- 23. Rivera J, Nakouzi A, Abboud N, Revskaya E, Goldman D, Collier RJ, Dadachova E, Casadevall A. 2006. A monoclonal antibody to Bacillus anthracis protective antigen defines a neutralizing epitope in domain 1. Infect. Immun. 74:4149–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abboud N, Chow SK, Saylor C, Janda A, Ravetch J, Scharff M, Casadevall A. 2010. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J. Exp. Med. 207:2395–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torres M, Fernandez-Fuentes N, Fiser A, Casadevall A. 2007. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J. Biol. Chem. 282:13917–13927 [DOI] [PubMed] [Google Scholar]
- 26. Abboud N, De Jesus M, Nakouzi A, Cordero RJ, Pujato M, Fiser A, Rivera J, Casadevall A. 2009. Identification of linear epitopes in Bacillus anthracis protective antigen bound by neutralizing antibodies. J. Biol. Chem. 284:25077–25086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eliopoulos GM, Moellering RC. 1991. Antimicrobial combinations, 3rd ed The Williams & Wilkins Co., Baltimore, MD [Google Scholar]
- 28. Nguyen MH, Barchiesi F, McGough DA, Yu VL, Rinaldi MG. 1995. In vitro evaluation of combination of fluconazole and flucytosine against Cryptococcus neoformans var. neoformans. Antimicrob. Agents Chemother. 39:1691–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franzot SP, Casadevall A. 1997. Pneumocandin L-743,872 enhances the activities of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob. Agents Chemother. 41:331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bragg TS, Robertson DL. 1989. Nucleotide sequence and analysis of the lethal factor gene (lef) from Bacillus anthracis. Gene 81:45–54 [DOI] [PubMed] [Google Scholar]
- 31. Nowakowski A, Wang C, Powers DB, Amersdorfer P, Smith TJ, Montgomery VA, Sheridan R, Blake R, Smith LA, Marks JD. 2002. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. U. S. A. 99:11346–11350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen ML, Terzyan S, Ballard JD, James JA, Farris AD. 2009. The major neutralizing antibody responses to recombinant anthrax lethal and edema factors are directed to non-cross-reactive epitopes. Infect. Immun. 77:4714–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nussbaum G, Cleare W, Casadevall A, Scharff MD, Valadon P. 1997. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J. Exp. Med. 185:685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maynard JA, Maassen CB, Leppla SH, Brasky K, Patterson JL, Iverson BL, Georgiou G. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597–601 [DOI] [PubMed] [Google Scholar]
- 35. Mahlandt BG, Klein F, Lincoln RE, Haines BW, Jones WI, Jr, Friedman RH. 1966. Immunologic studies of anthrax. IV. Evaluation of the immunogenicity of three components of anthrax toxin. J. Immunol. 96:727–733 [DOI] [PubMed] [Google Scholar]
- 36. Muller T, Dietzschold B, Ertl H, Fooks AR, Freuling C, Fehlner-Gardiner C, Kliemt J, Meslin FX, Franka R, Rupprecht CE, Tordo N, Wanderler AI, Kieny MP. 2009. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. PLoS Negl. Trop. Dis. 3:e542 doi:10.1371/journal.pntd.0000542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Little SF, Webster WM, Fisher DE. 2011. Monoclonal antibodies directed against protective antigen of Bacillus anthracis enhance lethal toxin activity in vivo. FEMS Immunol. Med. Microbiol. 62:11–22 [DOI] [PubMed] [Google Scholar]
- 38. Ngundi MM, Meade BD, Little SF, Quinn CP, Corbett CR, Brady RA, Burns DL. 2012. Analysis of defined combinations of monoclonal antibodies in anthrax toxin neutralization assays and their synergistic action. Clin. Vaccine Immunol. 19:731–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chow SK, Smith C, MacCarthy T, Pohl MA, Bergman A, Casadevall A. Disease-enhancing antibodies improve the activity of toxin-neutralizing antibodies. Cell Host Microbe, in press [DOI] [PMC free article] [PubMed] [Google Scholar]