Abstract
The human bacterial pathogen Streptococcus pyogenes has developed a broad variety of virulence mechanisms to evade the actions of the host immune defense. One of the best-characterized factors is the streptococcal cysteine protease SpeB, an important multifunctional protease that contributes to group A streptococcal pathogenesis in vivo. Among many suggested activities, SpeB has been described to degrade various human plasma proteins, including immunoglobulins (Igs). In this study, we show that SpeB has no Ig-cleaving activity under physiological conditions and that only Igs in a reduced state, i.e., semimonomeric molecules, are cleaved and degraded by SpeB. Since reducing conditions outside eukaryotic cells have to be considered nonphysiological and IgG in a reduced state lacks biological effector functions, we conclude that SpeB does not contribute to S. pyogenes virulence through the proteolytic degradation of Igs.
INTRODUCTION
Streptococcus pyogenes, also known as group A Streptococcus, is the causative agent of a wide variety of mild infections, like pharyngitis and impetigo, but also the cause of potentially life-threatening diseases, like streptococcal toxic shock syndrome and necrotizing fasciitis (1). S. pyogenes is a strictly human pathogen and has evolved a range of surface-attached and secreted virulence factors that allow the pathogen to survive and proliferate in its human host (1, 2). Among secreted proteins, the streptococcal cysteine proteinase SpeB is the most-prominent and probably best-characterized bacterial virulence factor. The vast amount of literature on SpeB transcription, secretion, maturation, and activation has recently been summarized in an excellent review (3). In an overview, SpeB is secreted as an inactive 40-kDa zymogen that is converted into a mature 28-kDa active protease either by autocatalytic cleavage or aided by host or streptococcal proteases (4–7). Regulation of speB transcription and SpeB expression is complex and involves environmental factors, transcriptional and posttranscriptional regulation, and posttranslational regulation by an endogenous inhibitor encoded on the streptococcal chromosome (for details, see reference 3 and references therein). Mature SpeB (mSpeB) exhibits promiscuous protease activity toward a large variety of both bacterial and human proteins, including vitronectin, fibronectin (8), fibrinogen (9), and immunoglobulins (10–12). Further examples include the activation of interleukin-1β (13), the matrix metalloproteinase MMP-2 (14), and the release of active proinflammatory kinins from H-kininogen (15). SpeB also targets numerous streptococcal proteins, like M1 protein, protein H, C5a peptidase (4, 16), and EndoS (17). Furthermore, analysis of the secreted streptococcal proteome suggests that SpeB can degrade the majority of abundant secreted streptococcal proteins in vitro (18). It has been suggested that hydrolysis of other streptococcal virulence factors by SpeB leads to less-severe disease progression (18, 19). In fact, SpeB expression is inversely correlated to severity of infection, i.e., the majority of streptococcal isolates from cases with severe invasive infections exhibit low or no SpeB expression (19). A comprehensive summary of target proteins for SpeB has recently been presented in another excellent review (20). Despite experimental variations, and partly conflicting data, there is no doubt that SpeB is an important virulence factor and contributes to group A streptococcal pathogenesis in vivo (20).
A strategy crucial for S. pyogenes survival and proliferation is the ability to evade adaptive immune responses, in particular, the recognition by specific, opsonizing antibodies. Specific antibodies mediate activation of phagocytic cells and the complement response. A common mechanism to avoid the detrimental effects of specific antibodies is to degrade immunoglobulins (Igs) by specific IgG or IgA proteases. S. pyogenes has been reported to secrete two IgG-degrading enzymes: IdeS (IgG-degrading enzyme of S. pyogenes), which is a highly efficient IgG-specific endopeptidase (21), and SpeB, which has been reported to degrade IgA, IgM, IgE, and IgD and to cleave IgG in the hinge region (10–12). However, a role for SpeB as part of the first line of defense against specific antibodies has been questioned, since SpeB is expressed during stationary growth (22), i.e., when infections are already established (23), and since, in contrast to IdeS, IgG proteolysis by SpeB appears to occur slowly over 24 to 48 h (10–12).
As a cysteine protease, SpeB activity is dependent on the reduction of the catalytic-site cysteine (4). In experimental settings, activation of SpeB is usually accomplished by incubation of SpeB with reducing compounds, e.g., dithiothreitol (DTT), β-mercaptoethanol, or l-cysteine. Furthermore, reducing compounds are commonly added in assay buffers or growth medium to maintain SpeB activity (5, 10–12, 17, 24–26). However, the inflammatory state that typically accompanies streptococcal infections leads to infiltration of activated neutrophils and the subsequent release of reactive oxygen species (ROS). Thus, an inflammatory environment has to be considered a rather oxidative environment, implying that in vitro analyses of SpeB activity in the presence of reducing agents might not be representative of physiological environments (20). In this study, the proteolytic cleavage of immunoglobulins by SpeB under nonreducing conditions was investigated. We demonstrate that in order to cleave and degrade the heavy chains of Ig, SpeB requires Ig in a reduced state, i.e., in a semimonomeric form in which the molecule lacks intact disulfide bonds and is held together only by noncovalent binding forces in the CH3 region (27). We therefore conclude that SpeB is not contributing to IgG cleavage under physiological conditions and that the contribution of SpeB to S. pyogenes virulence is not due to the proteolysis of immunoglobulins. Analyses of SpeB activity in physiological environments revealed that SpeB is not oxidized in the presence of human plasma, due to the antioxidant activity of human serum albumin, and therefore retains activity also in the presence of activated neutrophils.
MATERIALS AND METHODS
Proteins.
Fibrinogen, fibronectin, human serum albumin (HSA), immunoglobulins, and vitronectin were all purchased from Sigma-Aldrich.
Purification of SpeB.
For purification of mSpeB, the S. pyogenes strain 5448 was grown for approximately 16 h in Todd-Hewitt broth (BD Biosciences) in 5% (vol/vol) CO2 at 37°C. The bacteria were collected by centrifugation (3,800 × g for 10 min at 4°C), and culture supernatant was subjected to ammonium sulfate precipitation (50 to 80% [wt/vol]). Precipitated proteins were dissolved in 1× phosphate-buffered saline (PBS) buffer. After dialysis against 20 mM sodium acetate buffer (pH 5.0), protein samples were sterile filtrated, diluted in 20 mM sodium acetate buffer (pH 5.0), and applied to a HiTrap SP FF anion exchange column (GE Healthcare) equilibrated in the same buffer. Proteins were eluted in a gradient of 0 to 2 M NaCl over 20 column volumes at a flow rate of 1 ml/min, and SpeB starts to elute at 0.6 M NaCl. Eluted protein fractions were dialyzed overnight at 4°C against 1× PBS. Protein purity and identity were assayed by SDS-PAGE and Western blot. The amount of active SpeB was determined by active-site titration using various amounts of the cysteine protease inhibitor E-64 as previously described (28).
SpeB activity assays.
SpeB activity was measured as previously described (29). Briefly, purified SpeB (0.1 mg/ml) was incubated with 2 mM dithiothreitol (DTT) for 30 min at 37°C to reduce the active-site cysteine and activate the enzyme. DTT was removed by using Zeba spin desalting columns, with a 7-kDa molecular mass cutoff, according to the manufacturer's instructions (Thermo Scientific). Sixty microliters of the synthetic substrate n-benzoyl-proline-phenylalanine-arginine-p-nitroanilide hydrochloride (2.5 mM, pH 4.0) (BPFA) (Sigma) was mixed with 90 μl of 0.1 M phosphate buffer (pH 6.0) and added to activated SpeB. For determination of SpeB activity in human plasma or in the presence of human serum albumin (HSA), SpeB was incubated with plasma or increasing concentrations of HSA for 0 to 180 min prior to the addition of BPFA. Changes in absorbance were determined at an optical density of 405 nm (OD405) after 70 min of incubation at room temperature. All assays were performed in duplicate or triplicate.
SpeB cleavage of IgG.
For IgG cleavage assays with SpeB, 0.025 mg/ml activated SpeB (after removal of DTT) was incubated with 1 mg/ml IgG (reduced or nonreduced) at 37°C for various time periods. Reactions were stopped by addition of reducing loading buffer and incubation at 96°C for 10 min. To obtain reduced IgG substrate, 10 mg/ml IgG in PBS was incubated overnight at 37°C in the presence of 10 mM DTT. DTT was removed by buffer exchange using Zeba spin columns with a 7-kDa molecular mass cutoff.
SpeB cleavage of plasma proteins.
Purified SpeB (0.1 mg/ml) was activated by addition of 2 mM DTT. Different concentrations of activated, purified SpeB, with and without DTT, were incubated with 3 μg of fibrinogen, fibronectin, IgG, IgM, IgA, or vitronectin at 37°C in PBS for 1 h to 4 h. The reaction was terminated by the addition of an equal volume of SDS-PAGE sample buffer, followed by incubation at 95°C for 10 min. Proteins were separated and analyzed by standard SDS-PAGE and stained with Coomassie blue (R-250) (USB chemicals).
SpeB activity in plasma and whole human blood.
SpeB activity against IgG in human plasma or whole blood was determined by a Western blot. Purified SpeB (3.5 μg) was incubated in 1 ml human blood lacking specific antibodies against SpeB or IdeS at 37°C under rotation. As controls, human blood and human blood supplemented with recombinant IdeS (21) were used. Samples were diluted 1/100, separated by 8% SDS-PAGE, and transferred to an Immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore) using a semidry electrophoretic transfer cell (Bio-Rad). Goat anti-human IgG horseradish peroxidase (HRP)-conjugated antibodies (Bio-Rad) were used for detection of IgG. Immunoreactive proteins were detected using an ECL Plus Western blotting detection system (GE Amersham Biosciences) according to the manufacturer's instructions.
SpeB activity in the presence of human polymorphonuclear leukocytes and measurement of ROS.
Human polymorphonuclear leukocytes (PMNs) were isolated from heparinized blood using polymorphprep (Nycomed Pharma, Norway) as previously described (30). Briefly, whole blood was layered onto polymorphprep medium and centrifuged at 400 × g for 30 min. After centrifugation, the neutrophil layer was isolated and washed in PBS and residual erythrocytes were removed by hypotonic lysis in water. Neutrophils were collected by centrifugation, resuspended in 1× PBS, and counted using a counting chamber. For assaying SpeB activity in the presence of activated neutrophils, PMNs were stimulated with phorbol-12-myristate-13-acetate (PMA) at a final concentration of 0.8 μM in the presence of SpeB and BPFA. Generation of extracellular reactive oxygen species (ROS) was measured as chemiluminescence using isoluminol (0.04 mM) (Sigma) and horseradish peroxidase (2.4 units) (Sigma). Chemiluminescence was detected using an Infinite M 200 plate reader instrument (Tecan). SpeB activity against BPFA was measured simultaneously as described previously. For measurements of ROS and SpeB activity in the presence of HSA, increasing amounts of HSA (0 to 15 g/liter) were added to the reactions.
RESULTS
SpeB activity under nonreducing conditions.
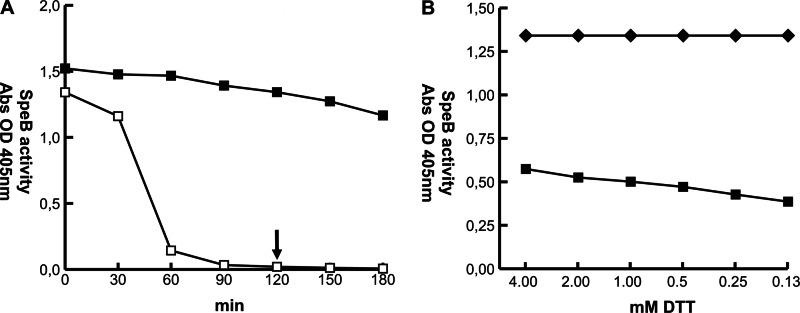
The proteolytic activity of activated SpeB in the absence of reducing agents was investigated. SpeB preparations were activated with 2 mM DTT at 37°C, DTT was removed by repeated buffer exchange, and SpeB activity against the synthetic substrate n-benzoyl-proline-phenylalanine-arginine-p-nitroanilide hydrochloride (BPFA) was monitored for up to 5 h in assay buffer and compared to that in similar reaction mixtures containing DTT. In a buffer system and in the absence of reducing compounds, SpeB activity decreases slowly until precipitously dropping after 30 min to baseline levels (Fig. 1A, open squares). In a reducing buffer, however, SpeB activity remains practically unchanged over at least 3 h (Fig. 1A, filled squares). Oxidation and inactivation of the cysteine protease occur much faster at elevated temperatures, as SpeB remains active for at least 3 h at room temperature, also in the absence of reducing compounds (data not shown). Under these experimental conditions, reincubation of oxidized SpeB in the presence of DTT results in less than half of the initial enzyme activity (Fig. 1B), indicating that the oxidation state of the enzyme is not easily reversed.
Fig 1.
SpeB activity under nonreducing conditions. (A) Activated SpeB was incubated in PBS with (■) or without (□) 2 mM DTT. Enzymatic activity against the chromogenic substrate BPFA was monitored for 180 min at 37°C. In the absence of DTT, SpeB activity begins to drop precipitously to baseline levels after 30 min of incubation. (B) SpeB preparations incubated for 120 min in nonreducing conditions (arrow in A) were resupplemented with various amounts of DTT. Approximately half of activity (■), compared to time point zero (◆), can be restored under reducing conditions in PBS.
SpeB activity in human plasma and in the presence of activated neutrophils.
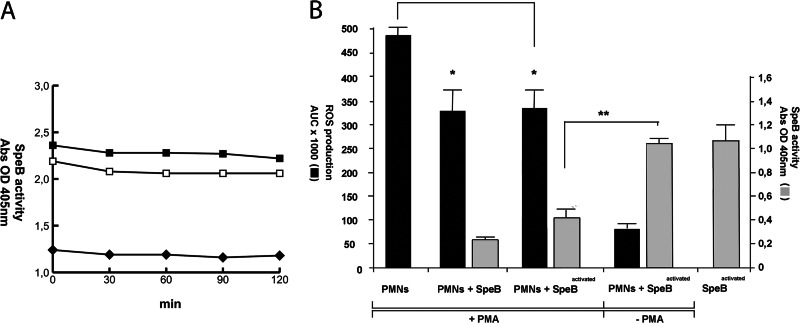
In the next set of experiments, assay buffer was replaced with human plasma. Activated SpeB preparations were incubated in human plasma with or without DTT at 37°C, and SpeB activity against BPFA was monitored for 4 h. Plasma contains endogenous BPFA-hydrolyzing activity, as evident by background absorbance readings, but SpeB-mediated BPFA hydrolysis in human plasma clearly remains stable over time independently of whether samples were supplemented with DTT or not (Fig. 2A).
Fig 2.
SpeB activities in human plasma and in the presence of activated neutrophils. (A) Activated SpeB was incubated in human plasma with (■) or without (□) 2 mM DTT for 0 to 120 min at 37°C. Enzymatic activity measurements were initiated by addition of the chromogenic substrate BPFA. SpeB activity remains stable in human plasma over time. The negative control is plasma only (◆). (B) SpeB activity in the presence of PMA-activated or nonactivated PMNs. PMN activation was monitored by measurements of extracellular ROS (black bars). SpeB activity (gray bars) against the chromogenic substrate BPFA is inhibited by ROS. **, P < 0.01 by Student's t test. Presence of activated and nonactivated SpeB inhibits PMA-induced extracellular ROS. *, P < 0.05 by Student's t test.
During streptococcal infection, migrating activated neutrophils will generate and degranulate reactive oxygen species (ROS) and create an oxidative environment at the infection site. SpeB activity in the presence of neutrophils was monitored, and SpeB protease activity is significantly inhibited in response to extracellular ROS, most likely due to rapid oxidation of the catalytic-site cysteine (Fig. 2B). However, ROS-associated cell and tissue damage is generally prevented by circulating antioxidants. Most free-radical-quenching activities in serum have been assigned to human serum albumin (HSA), one of the most abundant plasma proteins (31) with multiple antioxidant properties (32). HSA concentrations are elevated during inflammation (33), and therefore, the amount of extracellular ROS and SpeB activity in the presence of HSA was investigated (Fig. 3). HSA concentrations as low as approximately 10% of HSA serum concentrations were capable of efficiently trapping ROS (Fig. 3, open diamonds), and consequently, SpeB activity increased in correlation to the HSA concentration (Fig. 3, filled diamonds). Thus, in the presence of physiological HSA concentrations, SpeB remains active, also in an inflammatory environment, and there is no apparent requirement for reducing conditions to sustain enzymatic activity.
Fig 3.
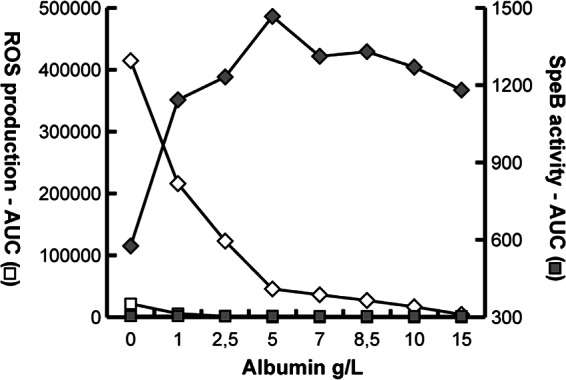
ROS production and SpeB activity in the presence of increasing concentrations of HSA. Detectable extracellular ROS (♢) are trapped by HSA, allowing SpeB activity (◆) to increase under the same conditions. Negative controls are PMNs alone (□) and PMNs and SpeB (■).
SpeB is not an immunoglobulin-degrading protease.
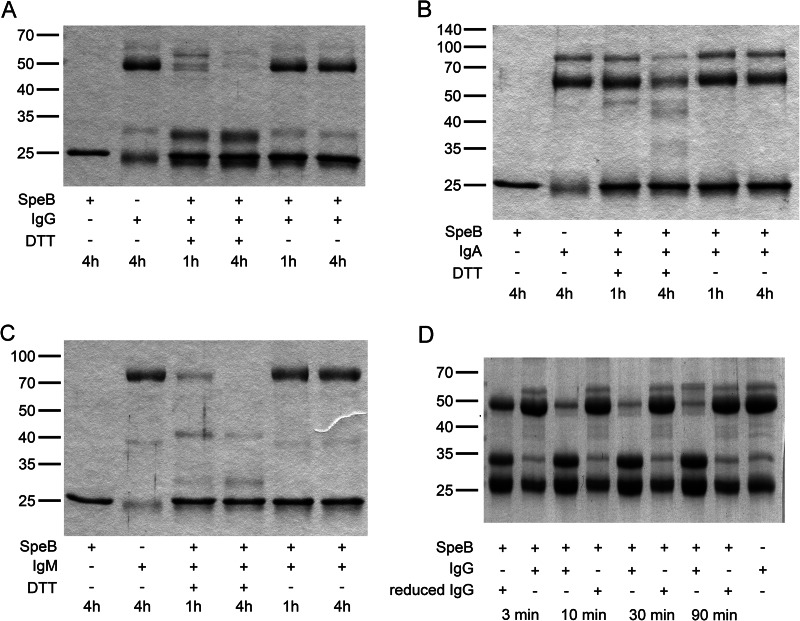
Immunoglobulins have previously been described as targets for SpeB proteolytic activity (10–12). In vitro treatment of specific IgG with SpeB was shown to interfere with immunoglobulin-mediated phagocytosis (25), and SpeB was suggested to contribute to survival of S. pyogenes in human blood by preferentially cleaving antigen-bound IgG (12). Common to all studies is that the proteolytic assay was performed under reducing conditions using incubation times ranging from 24 h to 48 h to accomplish cleavage of Igs. SpeB proteolytic activity against IgG, IgM, and IgA was reassessed with activated SpeB under reducing and nonreducing conditions. Clearly, while all three immunoglobulin types were efficiently cleaved under reducing conditions, incubation with SpeB under nonreducing conditions did not affect the integrity of the immunoglobulins (Fig. 4A to C). The lack of cleavage of IgG and IgM under nonreducing conditions was confirmed by Western blotting with IgG- and IgM-specific antibodies (data not shown). DTT present in experimental assay reactions not only ensures activation of SpeB but also reduces disulfide bridges in Igs, and we hypothesized that reduced, i.e., semimonomeric, IgG, but not intact IgG, is a substrate for SpeB. The IgG cleavage assay was repeated using either native IgG or IgG that had been reduced by DTT (followed by buffer exchange) as the substrate (Fig. 4D). SpeB readily and efficiently cleaved semimonomeric IgG already after 3 min of incubation, while nonreduced IgG remained intact also after 90 min of incubation at 37°C (Fig. 4D). In addition, no cleavage was detected when IgG was allowed to reoxidize prior to the proteolytic assay with activated SpeB, confirming that SpeB activity against IgG is dependent on separated Ig chains (data not shown).
Fig 4.
Immunoglobulin hydrolysis assay. (A to C) Activated SpeB was incubated with purified IgG (A), IgA (B), or IgM (C) under reducing or nonreducing conditions for 60 min or 240 min as indicated. Samples were analyzed by reducing SDS-PAGE. Ig hydrolysis is indicated by Ig heavy chain degradation and/or appearance of degradation products as previously described. Ig is degraded only under reducing conditions. (D) Immunoglobulin G hydrolysis assay with native or reduced IgG. IgG was reduced and DTT was removed from the samples prior to incubation with SpeB. Hydrolysis was monitored at 3, 10, 30, and 90 min. Enzymatic degradation was compared to that in identical reactions using native IgG. Ig hydrolysis is indicated by Ig heavy chain degradation and/or appearance of degradation products as previously described. Only reduced Ig is degraded by SpeB.
Several other plasma proteins, including fibrinogen (9), fibronectin, and vitronectin (8), have previously been shown to be substrates for SpeB. Degradation of fibrinogen has been demonstrated to occur under nonreducing conditions (9). Cleavage of fibronectin and vitronectin has also previously been assayed in the absence of reducing compounds in the assay buffer, but it was not evident whether reducing compounds had been removed from the SpeB preparation prior to incubation with the plasma proteins (8). However, fast and efficient cleavage of fibrinogen, vitronectin, and fibronectin under either reducing or nonreducing conditions was confirmed (data not shown).
SpeB has no immunoglobulin-degrading activity in human plasma or blood.
Initial experiments demonstrated that SpeB enzymatic activity in the absence of reducing compound dropped after 1 h of incubation in assay buffer to baseline levels (Fig. 1A), while activity in plasma samples was present for at least 4 h (Fig. 2A). Thus, it appears that the plasma environment preserves the protease in an active state, most likely due to the antioxidant activity of HSA. The potential cleavage of IgG by activated SpeB was assayed in plasma in the presence or absence of DTT. Cleavage of IgG was monitored by Western blotting using human IgG-specific HRP-conjugated antibodies. Again, in the absence of DTT, i.e., under nonreducing conditions, no IgG cleavage products were detected after 4 h of incubation (Fig. 5A), while IgG in reaction mixtures supplemented with DTT was cleaved during 4 h of incubation, albeit not to completion (Fig. 5A). Finally, SpeB activity against IgG was also assayed in whole human blood lacking specific antibodies against SpeB and IdeS. Human blood was incubated for 3 h at 37°C with or without SpeB. After 3 h of incubation, no degradation of IgG heavy chains was detected by Western blotting in samples with SpeB alone, while almost all IgG was cleaved in control samples that had been supplemented with IdeS after 2 h of the 3-h incubation period (Fig. 5B).
Fig 5.
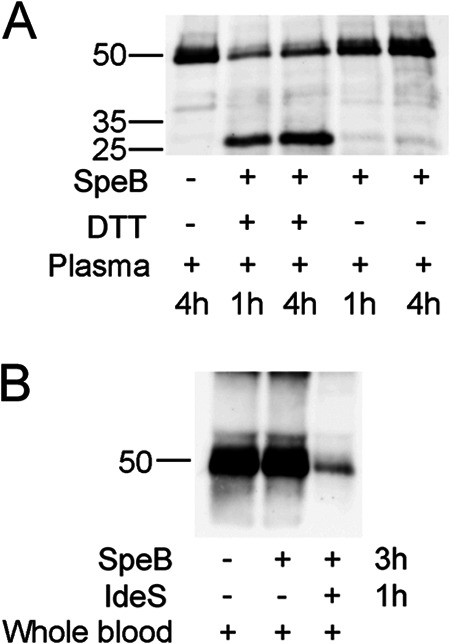
Western blot analyses of immunoglobulin hydrolysis in human plasma and human blood. (A) Activated SpeB was incubated in human plasma with or without resupplemented DTT for 60 min or 240 min at 37°C. Samples were analyzed by reducing SDS-PAGE, and IgG was detected by Western blotting using HRP-conjugated goat anti-human IgG as the primary antibody. SpeB does not hydrolyze IgG in plasma in the absence of DTT. (B) Human whole blood was incubated with activated SpeB without DTT for 3 h. No IgG cleavage was detectable in blood for SpeB alone, while supplemented IdeS efficiently degraded Ig heavy chains.
DISCUSSION
Streptococcus pyogenes has developed a significant number of virulence factors that manage many aspects of the host immune defense. One of the best-characterized factors is the streptococcal cysteine protease SpeB. The role of SpeB for streptococcal pathogenesis has been thoroughly investigated, and the list of reported substrates for this important virulence factor is growing constantly, reflecting the indiscriminant substrate recognition properties of the enzyme (24). However, does the fact that SpeB can hydrolyze a substrate in vitro necessarily mean that the enzyme will do so in vivo? Nelson and coworkers stated that a lot of controversy about SpeB function originates from the finding that reported activities would counteract each other (20). Historically, the identification of novel substrates has been a major objective in order to understand the role of SpeB for streptococcal virulence, and findings have not always been set in a physiological context. This is, in particular, evident for the reports of immunoglobulin-degrading activity of SpeB (10–12, 25). Immunoglobulins play a key role in adaptive immune responses, and S. pyogenes cells recognized by specific IgG antibodies are rapidly eliminated. Therefore, the identification of Ig proteolytic activity of SpeB has been seen as a matter of considerable interest. However, a role of SpeB as part of the first-line defense against specific antibodies can also be questioned. SpeB transcripts have certainly been detected at 24 h postinfection in a murine infection model (23), but for an Ig protease to contribute to protection against opsonizing IgG, it would be essential that the enzyme is instantly secreted to be able to act on circulating specific antibodies. Furthermore, proteases acting against IgG have to be highly effective and should be specific enough to avoid that other, redundant substrates occupy the enzyme and affect the cleavage of specific Ig.
In previous studies (10–12), IgG proteolysis by SpeB was carried out for 24 to 48 h to achieve cleavage or degradation of Ig. In the presence of opsonizing antibodies, IgG-mediated phagocytosis and bacterial killing occur within less than 15 min (34), and a time frame of 24 to 48 h to achieve inactivation of IgG does not represent a protective biological function. In addition, and most importantly, all proteolytic assays were performed under reducing conditions, and it has, in fact, been noted that such conditions are necessary to achieve cleavage of IgG (12). Although reducing conditions certainly sustain SpeB enzymatic activity, a reducing environment will also lead to disruption of disulfide bonds of immunoglobulins, thereby destroying Ig integrity and creating semimonomeric molecules. An infection site is considered an oxidative environment, and it is highly unlikely that SpeB will encounter semimonomeric IgG in vivo. The presence of reducing compounds in isolated microenvironments, e.g., sulfide in gingival pockets during periodontal disease, has been described (35). However, gingival pockets are not a natural habitat for S. pyogenes (36), and infiltrating neutrophils will rapidly oxidize and detoxify sulfide (35). In fact, even in the reducing environment in gingival pockets, sulfide concentrations are not sufficient to achieve reduction of the disulfide bridges of IgG (37).
In light of our findings of distinct differences in SpeB activity against immunoglobulins under reducing or nonreducing conditions, we also investigated SpeB activity against several plasma proteins that previously have been reported to be substrates for the streptococcal protease and that all contain inter- and intramolecular disulfide bonds. We corroborated the earlier findings that fibrinogen (9), fibronectin, and vitronectin (8) are substrates for SpeB under both reducing and nonreducing conditions (data not shown).
In the current study, we demonstrate that SpeB is not a natural Ig protease under physiological conditions. We also show that SpeB, once activated, retains its activity in a plasma environment in the absence of supplemented reducing compounds (Fig. 2A). In contrast, SpeB is oxidized and rapidly inactivated in the presence of activated neutrophils, but this mechanism can, at least initially, be counteracted by HSA. Notably, in the presence of SpeB protein, i.e., independently of enzymatic activity, the amount of extracellular ROS is clearly diminished compared to that of the control (Fig. 2B), an observation also reported for IdeS, another streptococcal cysteine protease (38).
Immunoglobulins are among the most abundant human plasma proteins. In light of the numerous important functions of SpeB and considering that Igs as redundant substrates are likely to occupy the enzyme and affect proteolytic activity against other substrates, the removal of Ig from the list of SpeB substrates somewhat eases the task to understand the role of SpeB during streptococcal infection. However, although SpeB is one of the most investigated prokaryotic virulence factors, investigations of SpeB function are far from being completed and future studies will certainly contribute to further understanding.
ACKNOWLEDGMENTS
This work was supported by the Swedish Research Council (project numbers 2006-4522 and 2009-4997) and Insamlingsstiftelsen at Umea University.
Footnotes
Published ahead of print 8 April 2013
REFERENCES
- 1. Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cole JN, Barnett TC, Nizet V, Walker MJ. 2011. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 9:724–736 [DOI] [PubMed] [Google Scholar]
- 3. Carroll RK, Musser JM. 2011. From transcription to activation: how group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production. Mol. Microbiol. 81:588–601 [DOI] [PubMed] [Google Scholar]
- 4. Elliott SD. 1945. A proteolytic enzyme produced by group A streptococci with special reference to its effect on the type-specific M antigen. J. Exp. Med. 81:573–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doran JD, Nomizu M, Takebe S, Menard R, Griffith D, Ziomek E. 1999. Autocatalytic processing of the streptococcal cysteine protease zymogen: processing mechanism and characterization of the autoproteolytic cleavage sites. Eur. J. Biochem. 263:145–151 [DOI] [PubMed] [Google Scholar]
- 6. Lyon WR, Caparon MG. 2003. Trigger factor-mediated prolyl isomerization influences maturation of the Streptococcus pyogenes cysteine protease. J. Bacteriol. 185:3661–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen CY, Luo CS, Kuo CF, Lin YS, Wu JJ, Lin MT, Liu CC, Jeng WY, Chuang WJ. 2003. Maturation processing and characterization of streptopain. J. Biol. Chem. 278:17336–17343 [DOI] [PubMed] [Google Scholar]
- 8. Kapur V, Topouzis M, Majesky W, Li LL, Hamrick MR, Hamill RJ, Patti JM, Musser JM. 1993. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb. Pathog. 15:327–346 [DOI] [PubMed] [Google Scholar]
- 9. Matsuka YV, Pillai S, Gubba S, Musser JM, Olmsted SB. 1999. Fibrinogen cleavage by the Streptococcus pyogenes extracellular cysteine protease and generation of antibodies that inhibit enzyme proteolytic activity. Infect. Immun. 67:4326–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collin M, Olsén A. 2001. EndoS, a novel secreted enzyme from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 20:3046–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collin M, Olsén A. 2001. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect. Immun. 69:7187–7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eriksson A, Norgren M. 2003. Cleavage of antigen-bound immunoglobulin G by SpeB contributes to streptococcal persistence in opsonizing blood. Infect. Immun. 71:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kapur VS, Majesky MW, Li LL, Black RA, Musser JM. 1993. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc. Natl. Acad. Sci. U. S. A. 90:7676–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burns EH, Marciel AM, Musser JM. 1996. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect. Immun. 64:4744–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herwald H, Collin M, Müller-Ester W, Björck L. 1996. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J. Exp. Med. 184:665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berge A, Björck L. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862–9867 [DOI] [PubMed] [Google Scholar]
- 17. Allhorn M, Olsén A, Collin M. 2008. EndoS from Streptococcus pyogenes is hydrolyzed by the cysteine proteinase SpeB and requires glutamic acid 235 and tryptophans for IgG glycan-hydrolyzing activity. BMC Microbiol. 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aziz RK, Pabst MJ, Jeng A, Kansal R, Low DE, Nizet V, Kotb M. 2004. Invasive M1T1 group A Streptococcus undergoes phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol. Microbiol. 51:123–134 [DOI] [PubMed] [Google Scholar]
- 19. Kansal RG, McGeer A, Low DE, Norrby-Teglund A, Kotb M. 2000. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect. Immun. 68:6362–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson DC, Garbe J, Collin M. 2011. Cysteine proteinase SpeB from Streptococcus pyogenes—a potent modifier of immunologically important host and bacterial proteins. Biol. Chem. 392:1077–1088 [DOI] [PubMed] [Google Scholar]
- 21. von Pawel-Rammingen U, Johansson BP, Björck L. 2002. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21:1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chaussee MS, Phillips ER, Ferretti JJ. 1997. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect. Immun. 65:1956–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loughman JA, Caparon M. 2006. Regulation of SpeB in Streptococcus pyogenes by pH and NaCl: a model for in vivo gene expression. J. Bacteriol. 188:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nomizu M, Pietrzynski G, Kato T, Lachance P, Menard R, Ziomek E. 2001. Substrate specificity of the streptococcal cysteine protease. J. Biol. Chem. 276:44551–44556 [DOI] [PubMed] [Google Scholar]
- 25. Collin M, Svensson MD, Sjöholm AG, Jensenius JC, Sjöbring U, Olsén A. 2002. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect. Immun. 70:6646–6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidtchen A, Frick Björck I-ML. 2001. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial α-defensin. Mol. Microbiol. 39:708–713 [DOI] [PubMed] [Google Scholar]
- 27. Vincents B, von Pawel-Rammingen U, Björck L, Abrahamson M. 2004. Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry 43:15540–15549 [DOI] [PubMed] [Google Scholar]
- 28. Nyberg P, Rasmussen M, von Pawel-Rammingen U, Björck L. 2004. SpeB modulates fibronectin-dependent internalization of Streptococcus pyogenes by efficient proteolysis of cell-wall-anchored protein F1. Microbiology 150:1559–1569 [DOI] [PubMed] [Google Scholar]
- 29. North MJ. 1994. Cysteine endopeptidases of parasitic protozoa. Methods Enzymol. 244:523–539 [DOI] [PubMed] [Google Scholar]
- 30. Söderberg JJ, von Pawel-Rammingen U. 2008. The streptococcal protease IdeS modulates bacterial IgGFc binding and generates 1/2Fc fragments with the ability to prime polymorphonuclear leucocytes. Mol. Immunol. 45:3347–3353 [DOI] [PubMed] [Google Scholar]
- 31. Bourdon E, Blache D. 2001. The importance of proteins in defense against oxidation. Antioxid. Redox Signal. 3:293–311 [DOI] [PubMed] [Google Scholar]
- 32. Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. 2008. The antioxidant properties of serum albumin. FEBS Lett. 582:1783–1787 [DOI] [PubMed] [Google Scholar]
- 33. Halliwell B. 1988. Albumin—an important extracellular antioxidant? Biochem. Pharmacol. 37:569–571 [DOI] [PubMed] [Google Scholar]
- 34. Staali L, Mörgelin M, Björck L, Tapper H. 2003. Streptococcus pyogenes expressing M and M-like surface proteins are phagocytosed but survive inside human neutrophils. Cell. Microbiol. 5:253–265 [DOI] [PubMed] [Google Scholar]
- 35. Claesson RM, Granlund-Edstedt M, Persson S, Carlsson J. 1989. Activity of polymorphonuclear leukocytes in the presence of sulfide. Infect. Immun. 57:2776–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wikström MB, Dahlén G, Linde A. 1983. Fibrinogenolytic and fibrinolytic activity in oral microorganisms. J. Clin. Microbiol. 17:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Granlund-Edstedt M, Johansson E, Claesson R, Carlsson J. 1993. Effect of anaerobiosis and sulfide on killing of bacteria by polymorphonuclear leukocytes. J. Periodontal Res. 28:346–353 [DOI] [PubMed] [Google Scholar]
- 38. Söderberg JJ, Engström P, von Pawel-Rammingen U. 2008. The intrinsic immunoglobulin G endopeptidase activity of streptococcal Mac-2 proteins implies a unique role for the enzymatically impaired Mac-2 protein of M28 serotype strains. Infect. Immun. 76:2183–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]