Abstract
Neisseria meningitidis serogroup B (MenB) is a major cause of bacterial sepsis and meningitis, with the highest disease burden in young children. Available vaccines are based on outer membrane vesicles (OMVs) obtained from wild-type strains. However, particularly in toddlers and infants, they confer protection mostly against strains expressing the homologous protein PorA, a major and variable outer membrane protein. In the quest for alternative vaccine antigens able to provide broad MenB strain coverage in younger populations, but potentially also across all age groups, ZnuD, a protein expressed under zinc-limiting conditions, may be considered a promising candidate. Here, we have investigated the potential value of ZnuD and show that it is a conserved antigen expressed by all MenB strains tested except for some strains of clonal complex ST-8. In mice and guinea pigs immunized with ZnuD-expressing OMVs, antibodies were elicited that were able to trigger complement-mediated killing of all the MenB strains and serogroup A, C, and Y strains tested when grown under conditions of zinc limitation. ZnuD is also expressed during infection, since anti-ZnuD antibodies were detected in sera from patients. In conclusion, we confirm the potential of ZnuD-bearing OMVs as a component of an effective MenB vaccine.
INTRODUCTION
Neisseria meningitidis is a Gram-negative, frequently encapsulated bacterium. It is a human-specific pathogen that asymptomatically colonizes the upper respiratory tract of around 10% of the adult population. Occasionally, it translocates to the bloodstream, resulting in bacteremia with possible progression to meningitis. N. meningitidis causes one of the most feared bacterial infections due to its rapid progression to death and its tendency to cause epidemics. The bacterium is classified into 12 serogroups on the basis of the immunochemical composition of the capsular polysaccharides. However, only serogroups A, B, C, Y, W, and, to a minor extent, X have been associated with disease. Conjugate polysaccharide vaccines that provide effective immunity in humans are becoming available for serogroups A, C, Y, and W. Unfortunately, the conjugate approach cannot be easily applied to serogroup B (MenB) because its capsular polysaccharide shares structural similarity with some polysialylated host glycoproteins (1, 2).
Until recently, MenB vaccines were based on outer membrane vesicles (OMVs) from wild-type strains, after their extraction with detergent to reduce the lipooligosaccharide (LOS) content (3). PorA is one of the most abundant outer membrane proteins (OMPs) and an immunodominant component in these OMVs (3). However, PorA displays high antigenic variability, which limits the efficacy of OMV-based vaccines, especially in the pediatric population (4), an age group that includes around 50% of MenB cases in Europe (5). To overcome this limitation, the use of conserved minor OMPs has been explored. Among them, the lipoprotein human factor H-binding protein (fHbp) is a promising vaccine candidate (6, 7), although this protein displays relative sequence and expression variability, which may significantly impact the ability of anti-fHbp antibodies to trigger complement-mediated killing of some strains.
Recently, the OMP ZnuD (for zinc uptake component D) was reported as a potential vaccine candidate (8). This protein is expressed under conditions of zinc limitation, and its expression was reported to be regulated by Zur (8). In Escherichia coli growing under zinc-replete conditions, Zur binds a Zur-binding element in the promoter of the znuABC operon and thereby blocks the transcription of this operon that encodes proteins involved in the transport of zinc from the periplasm to the cytoplasm (9). A putative znuCBA operon as well as putative Zur-binding sequences in regions upstream of the znuC and znuD genes were described for MenB (8). ZnuD has also been described as being regulated by iron in a Zur-independent way and to be involved in the meningococcal interaction with epithelial cells (10). Based on a limited number of sequences (n = 6), ZnuD appears to be potentially well conserved. In addition, it was demonstrated previously that ZnuD elicits the production of antibodies that are able to trigger complement-mediated killing of a homologous strain genetically modified to overproduce ZnuD (8). Because of the potential of this protein as a vaccine candidate, we have performed a systematic analysis of its variability, looked for the presence of anti-ZnuD antibodies in humans, and assessed its potential to induce cross-bactericidal antibodies, i.e., antibodies able to recognize ZnuD on heterologous MenB strains. Our analysis confirmed that ZnuD is very well conserved and expressed by all 223 N. meningitidis strains tested, independent of serogroup, with the exception of a few serogroup Y strains from the ST-23 clonal complex (cc) and two-thirds of the ST-8 cc/cluster A4 strains.
MATERIALS AND METHODS
Peptide arrays.
Custom peptide arrays were manufactured by JPT Technology. Each array comprised five subarrays with 84 individual peptides, each spotted in triplicate. The subarrays were divided in separated incubation chambers by adhesive multiwell gene frames (Abgene, United Kingdom). Peptides were 15 amino acids (aa) long with 8-aa overlaps and comprised aa 178 to 765 of ZnuD. Peptides corresponding to aa 1 to 177 were omitted because they represent the plug domain, which is not surface exposed. Control peptides for the reaction of the following secondary antibodies were spotted onto each slide: human immunoglobulin G (IgG), mouse IgG, and goat IgG. Human sera were diluted 1:100 in phosphate-buffered saline (PBS) supplemented with 5% fetal calf serum (FCS) and 0.5% Tween 20 and incubated on the arrays for 4 h at room temperature in a humid chamber with gentle movement. Secondary antibodies [Dy Light 649 anti-human IgG(H+L); KPL] were diluted 1:6,000 in PBS–5% FCS–0.5% Tween 20 and incubated for 1 h. Extensive washes with PBS–5% FCS–0.5% Tween 20 and deionized water after antibody incubations were used to achieve low background intensity. The peptide arrays were centrifuged for 5 min at 3,350 × g in 50-ml reaction tubes to remove liquid and scanned at a wavelength of 635 nm with a laser power of 100% at a photomultiplier tube level ranging from 450 to 500 and a resolution of 10 μm (Tecan scanner). Images were saved electronically in TIFF and JPG formats. Image analysis was performed by using GenePix Pro 6.0 software (Tecan Instruments) and GenePix Array List (GAL) files supplied by JPT Technology. For each spot, median foreground and background intensities at a wavelength of 635 nm were used for further analysis. Data are provided as the log2 ratio of foreground and background intensities. They are visualized by box plots summarizing the data for all sera for each peptide by demonstrating range, median, and 25th and 75th percentiles. For the values with a background intensity higher than the foreground, the log2 ratio (foreground/background) was set to zero.
Human sera.
Convalescent-phase sera collected between 2004 and 2006 were kindly provided by S. Heuberger (Graz, Austria). Patients were 2 months to 25 years old at the onset of invasive disease. There was no information available on the serogroup of the strains. Sera from healthy carriers were collected in 2007 at the Institute for Hygiene and Microbiology of the University of Würzburg. Carriers harbored diverse isolates, including serogroup B, C, Y, and E and capsule-null locus strains.
Strain collection.
The strain collection analyzed regarding ZnuD expression comprised a total of 223 invasive meningococcal isolates (see Table S1 in the supplemental material). Recent MenB isolates from the United Kingdom and Spain were kindly provided by Ray Borrow (Manchester, United Kingdom) and Julio Vásquez (Madrid, Spain), respectively. The majority of the isolates (n = 128) were isolated between 2000 and 2009 at the German National Reference Center for Meningococci (strains with the prefix DE). Most strains from healthy carriers used for sequence analyses were described previously (11) or were kindly provided by Oliver Kurzai (Jena, Germany). Furthermore, a few ST-8 cc/cluster A4 and ST-11 cc/ET-37c strains were kindly provided by Dominique Caugant (Oslo, Norway), Mark Achtman (Cork, Ireland), and Richard Moxon (Oxford, United Kingdom). The strains from Dominique Caugant and Mark Achtman had been typed by multilocus enzyme electrophoresis, providing the clonal complex but not the sequence type (PMID 3088568). Other strains were from an already established GlaxoSmithKline Vaccines collection.
Strain construction.
For complementation experiments, ZnuD was amplified from chromosomal DNA of type strain H44/76 by using primer pair KH31/KH32 (all oligonucleotides are listed in Table S2 in the supplemental material), and the PCR product was cloned into the neisserial replicative vector pAP2-1 (12), using SpeI and EcoRI restriction sites. This led to plasmid pIK6. MenY ST-23 cc wild-type strain DE8633 was transformed with either pIK6 or control vector pAP2-1. Overexpression or nonexpression of ZnuD was confirmed by Western blotting. The DE8633 variants were always grown in the presence of 100 μg/ml spectinomycin (Applichem, Germany) to prevent plasmid loss.
For OMV preparation, an H44/76 Δcps porA knockout (KO) strain expressing galactose-deficient LOS, also named galE LOS (ΔgalE strain), was constructed as described previously (13). OMVs enriched in ZnuD were prepared from this mutant strain transformed with a ZnuD-overexpressing plasmid described previously (8).
For the construction of fHbp KO mutants, the fHbp gene and adjacent regions were amplified by PCR with primers ND1870-5 and ND1870-7, using genomic DNA of strain H44/76 as the template, and cloned into pGEM-T (Promega). The resulting plasmid was then submitted to circle PCR with primers 5′fHBPcircBgl and 3′fHBPcircBgl and digested with the restriction enzyme BglII. A spectinomycin resistance gene was obtained after BamHI restriction of pCMS (14) and cloned into the BglII-digested circle PCR fragment generated as described above, resulting in plasmid pRIT16783. The plasmid was used to transform H44/76, NZ98/254, M05-240355, and SP17540 with a transformation procedure described previously (13). Integration at the expected genomic location and the absence of fHbp expression were confirmed by PCR and Western blot analysis.
The same procedure was used for the generation of znuD KO mutants with a plasmid described previously (8).
ZnuD induction for expression analysis.
ZnuD expression was induced either by growing meningococci on Columbia blood agar (bioMérieux, Germany) or by using the zinc-specific chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) (Fluka, Germany). For the former, bacteria were grown twice for 12 h on Columbia blood agar plates. For the latter, meningococci were grown overnight on Mueller-Hinton (MH) agar plates supplemented with 1% horse serum (Sigma, Germany) and 1% PolyVitex (bioMérieux), which was followed by additional growth for 4 h on either MH agar plates or MH agar plates supplemented with 20 μM TPEN. All growing steps were performed at 37°C in 5% CO2. After induction of ZnuD, meningococci were harvested, and bacterial pellets were resuspended in sample buffer for subsequent Western blot analysis.
Western blot analysis.
Proteins of 2 × 107 meningococci were separated by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Transfer of the proteins onto a nitrocellulose membrane (Protran, Germany) was achieved by using the wet transfer system from Bio-Rad (Germany) and a buffer containing 0.3% Tris-HCl, 1.44% glycine, and 20% methanol. After blocking for 1 h with 5% skim milk in PBS–0.1% Tween 20, the membrane was incubated with polyclonal rabbit anti-ZnuD 1041 serum (kindly provided by M. Bos, The Netherlands) diluted 1:10,000 in 1% skim milk overnight at 4°C. Antibody binding to ZnuD was visualized by using a peroxidase-labeled anti-rabbit IgG antibody diluted 1:5,000 in PBS–0.1% Tween 20 and enhanced chemiluminescence (Pierce, USA).
Growth curves.
Growth curves were determined largely as previously reported (8). Briefly, bacteria were grown on GC agar plates (Becton & Dickinson, France) for 12 h and for another 12 h on RPMI agar plates (Gibco, Germany). RPMI (Gibco) was supplemented with 100 μM ferric chloride (Roth, Germany) and, if appropriate, with 100 μg/ml spectinomycin and inoculated with bacteria to an optical density at 600 nm (OD600) of 0.2, which corresponds to approximately 2 × 108 CFU. TPEN was added to the medium in different concentrations to achieve zinc limitation. The cultures were incubated at 37°C with shaking at 200 rpm for 10 h (wild-type strains) or 24 h (DE8633 variants), and growth was examined by measuring the OD600 every 2 h during the first 10 h. Whole-cell lysates for Western blot analysis were prepared after 0, 4, and 10 h by resuspending bacterial pellets in sample buffer. Western blot analysis was performed as described above.
Sequencing and phylogenetic analysis of ZnuD.
ZnuD was amplified with Taq DNA polymerase (NEB) by using oligonucleotides KH15 and KH16. For 100-μl PCR mixtures, 2 μl of boiled bacterial suspensions in PBS (OD600 = 1) was used as the template. The whole PCR product was subsequently double-strand sequenced by employing oligonucleotides GSK1-F, GSK2-R, GSK3-F, GSK5-F, GSK6-R, GSK7-F, GSK9-F, GSK11-F, GSK13-F, GSK22, GSK27, GSK28, GSK29, GSK30, KH15, KH16, KH17, KH18, KH26, KH31, KH32, KH33, KH43, KH44, KH45, and KH47. The sequencing reaction took place in an Applied Biosystems 3180 sequencer, using the BigDye Terminator v1.1 cycle sequencing kit (Applied Biosystems, USA). The sequences were assembled by using SeqMan software and analyzed by using EditSeq and MegAlign (DNAStar, USA). The NRDB program (Warren Gish, Washington University) was accessed by using an interface written by Keith Jolley (University of Oxford; http://pubmlst.org/) to identify the number of unique ZnuD protein sequences. In addition, ZnuD sequences from 505 strains isolated in England, Wales, and Northern Ireland in the epidemiological year of 2010 to 2011 were analyzed. Sequences were retrieved from the Meningitis Research Foundation Meningococcus Genome Library (http://pubmlst.org/). Only complete coding sequences which could be translated into a mature protein were considered. The Mega 5.1 program (15) was used to construct an unrooted neighbor-joining tree (p-distance model, bootstrap method with 2,000 replications). The same program was also used to compute the overall mean distance (p-distance model, bootstrap method with 2,000 replications). Bioedit version 7.1.11 (16) was used to calculate the entropy at a given position of an amino acid sequence. Entropy is a measure of the degree of uncertainty regarding in this case the amino acid at a position of a sequence. Its value is zero if all sequences of the data set display the same amino acid. Its value is greater than zero if there is some degree of variability. Plotting the entropy values allows the visualization of the variable sites and the degree of variability.
Antigens and immunizations.
OMVs were prepared by using deoxycholate as described previously (17). Recombinant fHbp A and fHbp B were produced as described previously (18), and recombinant ZnuD was produced as described previously by Stork et al. (8).
Groups of 30 outbred OF1 mice (also known as CF1) (female, 6 to 8 weeks of age; Charles River, France) or 10 Hartley guinea pigs (female, 5 to 8 weeks of age; Charles River) received three injections of OMVs via the intramuscular route on days 0, 21, and 28 (mice) or days 0, 14, and 28 (guinea pigs). Each injection contained OMV antigen normalized to 5 μg (mice) or 10 μg (guinea pigs) of protein and formulated with aluminum phosphate in a volume of 50 or 100 μl. Blood samples were collected 14 days after the third injection.
Anti-fHbp sera were obtained from mice immunized three times by the intramuscular route on days 0, 21, and 28. Each injection contained 5 μg of monovalent fHbp vaccine (fHbp A or fHbp B) adsorbed onto aluminum hydroxide. On day 42, blood samples were taken for serum.
The animal experiments complied with the relevant national guidelines of Belgium and institutional policies of GlaxoSmithKline Vaccines.
Serum bactericidal assay (SBA).
The wild-type MenB strains used in this study were isolated either from cases during epidemics occurring in different regions around the world or from sporadic cases. The strains were grown overnight on MH agar (Difco) containing 1% (vol/vol) PolyVitex (bioMérieux) and 1% horse serum (Sigma) at 37°C in a 5% CO2 atmosphere. Bacteria from one plate were scraped with a sterile swab and streaked onto fresh agar plates without or with 20 μM TPEN. After 4 h of culture at 37°C in 5% CO2, the plates were swabbed, and bacteria were resuspended in PBS containing 0.5 mM MgCl2, 0.9 mM CaCl2, and 0.1% glucose (PBS-glucose) to an OD600 of 0.4 (bacterial suspension). Serum samples were inactivated (40 min at 56°C) and subsequently diluted in PBS-glucose. In wells of sterile flat-bottom microtiter plates (Nunc, Denmark), 25 μl of diluted test serum was mixed with 6.25 μl of baby rabbit complement (Cedarlane Laboratories) and 18.75 μl of bacterial suspension. Serial dilutions of test sera were treated similarly. Controls included bacteria plus complement, bacteria plus heat-inactivated complement, and serum plus bacteria plus heat-inactivated complement. The microtiter plates were sealed and incubated with shaking (220 rpm) for 75 min at 37°C without CO2. After incubation, 50 μl MH–0.9% agar was added to each well, followed by a second layer of 50 μl PBS–0.9% agar 30 min later. After overnight incubation at 33°C in 5% CO2, the colonies were counted. The average number of CFU of the controls corresponding to bacteria plus complement was set at 100%. Bactericidal titers were reported as the reciprocal value of the serum dilution yielding 50% killing of bacteria.
Enzyme-linked immunosorbent assay (ELISA).
Microtiter plates were coated overnight at 4°C with 5 μg/ml of purified recombinant fHbp B or purified ZnuD at 4°C. The plates were washed with 0.15 M NaCl–0.05% Tween 20 and blocked with PBS containing 1% bovine serum albumin. After the microwells were washed, serial dilutions of individual sera in PBS–0.2% bovine serum albumin–0.05% Tween 20 were added for 30 min at room temperature. The microwells were washed again, and peroxidase-labeled goat anti-mouse or goat anti-guinea pig IgG (Jackson) was added for 30 min at room temperature. The plates were then washed and developed by using o-phenylenediamine and H2O2 in citrate buffer. After 30 min, the reaction was stopped with HCl, and the absorbance was measured at 490 to 620 nm.
RESULTS
Prediction of the 3D structure of ZnuD.
The amino acid sequence of ZnuD was compared by BLAST (19) to the sequence of proteins in the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) (20), and only some weak sequence similarities with the plug domain of the TonB-dependent receptors were detected. Therefore, a PSI-BLAST (19) profile of ZnuD was computed from sequences from the nonredundant protein (NR) database of the NCBI (the hits collected by PSI-BLAST were in a large majority TonB-dependent receptors, which means that, according to Noinaj et al. [21], these proteins contain 22 transmembrane β-strands). The profile was then compared to the sequence of proteins in the data bank, and PSI-BLAST presented a very significant hit (expect = e−158; 12% identity; 104/803) between ZnuD and the PDB identification number 1FEP (outer membrane active transporter FepA from Escherichia coli) structure (22). Other significant hits were PDB identification numbers 3FHH (heme/hemoglobin outer membrane transporter ShuA from Shigella dysenteriae) (23), 2HDI (colicin I receptor bound to the R domain of colicin Ia from E. coli), and 2HDF (colicin I receptor bound to the R domain of colicin Ia from E. coli) (these are identical structures and sequences, reported previously by Buchanan et al. [24]). All these hits contain 22 transmembrane β-strands. The results of the Web servers PSIPRED (25), Pred-TMBB (26), and TMBpro (27) were used to establish a consensus prediction for the location of the 22 transmembrane β-strands of ZnuD. The potential template for ZnuD three-dimensional (3D) modeling, i.e., the structure reported under PDB identification number 1FEP, was compared with the Protein Data Bank data by using the Dali server (28). From the Dali output, a structural alignment of 11 protein structures, sharing less than 90% sequence identity, was computed.
The above-described results were used to prepare a first sequence alignment between ZnuD and the structures reported under PDB identification numbers 1FEP, 2HDF, and 3FHH. This alignment was carried out by using Modeler software (29) (part of the Discovery Studio package from Accelrys) to compute a first 3D model of ZnuD. The quality of this model was checked with the QMEAN server (30), and detected mistakes were corrected in the sequence alignment between ZnuD and templates. A new 3D model was then computed and checked again with QMEAN. This computing cycle was executed several times until good alignment and good structure quality were achieved for the 22 transmembrane β-strands. This 3D model (Fig. 1) does not contain the extracellular loops at residues 275 to 324 and 636 to 657 due to the lack of alignment with the templates in these two regions.
Fig 1.
Wired-frame representation of the ZnuD 3D model. In this model, computed by Modeler, the 3D structure of extracellular loops at residues 275 to 324 and 636 to 657 was not predicted.
ZnuD is immunogenic and expressed in humans.
As described previously (8), ZnuD is not expressed by N. meningitidis cells growing on classical agar culture media unless a zinc chelator such as TPEN is added, which may bring into question its expression in the host. An indirect way to demonstrate that ZnuD is expressed in vivo is to evaluate the presence of anti-ZnuD antibodies in sera from convalescent patients and/or healthy carriers. For this purpose, a peptide array approach was used, covering the C-terminal part (76%) of the mature protein, which does not include the plug domain of TonB-dependent receptors (31).
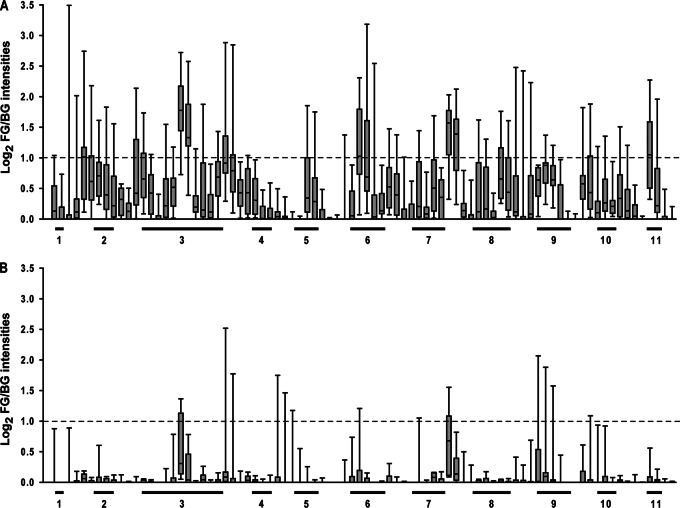
Sera from 12 convalescent subjects and from 11 carriers were tested 3 to 5 times on this peptide array. Figure 2 shows the responses of the two groups of subjects. Both the intensity of the response against the peptides and the number of peptides recognized by the sera were greater in the convalescent group than in the carrier group. There was a consistent reactivity of most convalescent patient sera with peptides localized in loop 3, loop 6, and loop 11, which comprise amino acids 233 to 309, amino acids 430 to 459, and amino acids 706 to 722 of the mature protein, respectively. There was also consistent reactivity of these sera with peptides derived from a predicted transmembrane region between loops 7 and 8. The recognition of the peptides by the convalescent human sera suggests that ZnuD is expressed during invasive disease.
Fig 2.
Responses of human sera obtained from convalescent patients (n = 12) (A) and healthy carriers (n = 11) (B) in a ZnuD peptide array. The localization of 11 putative exposed loops is indicated. Box plots indicate ranges, medians, and 25th and 75th percentiles. The dashed line indicates a foreground (FG) intensity-to-background (BG) intensity ratio of 2.
ZnuD is a well-conserved antigen, expressed by the majority of N. meningitidis strains investigated.
Since the transcription of znuD is repressed by Zur in the presence of Zn2+ ions (8), the use of specific culture conditions is necessary to reliably verify its expression in a large panel of meningococcal strains. Therefore, meningococci were either grown in the presence of the zinc-specific chelator TPEN (8) or cultivated for two passages on Columbia blood agar to induce the production of the protein.
Altogether, a total of 223 serogroup A, B, C, W, and Y strains (see Table S1 in the supplemental material) from individuals who developed invasive disease were examined for ZnuD expression by Western blotting (results not shown). Among the 159 MenB strains tested, only 7 strains (4%) did not express the protein. Furthermore, 13 of the 41 MenC strains and 3 of the 12 MenY strains showed no production of ZnuD upon induction. Expression of ZnuD was observed for all MenA and MenW strains tested (8 and 3 strains, respectively).
For 153 of the meningococcal isolates tested, the multilocus sequence type or the clonal complex determined by multilocus enzyme electrophoresis was available (see Table S1 in the supplemental material). All isolates belonging to the pathogenic meningococcal lineages ST-11 cc/ET-37c (n = 22), ST-32 cc (n = 19), ST-41/44 cc (n = 29), and ST-269 cc (n = 28) expressed ZnuD in response to zinc limitation. However, five out of the seven nonexpressing MenB and all nonexpressing MenC isolates belonged to the ST-8 cc/cluster A4 lineage. One ZnuD-negative MenB strain was assigned to ST-939, another was assigned to ST-162, and all three ZnuD-negative MenY strains were assigned to the ST-23 cc. Taken together, these results show that the ZnuD-negative strains are not scattered within the meningococcal population but are restricted to distinct clonal lineages.
For a deeper phylogenetic analysis of znuD, the gene was subsequently sequenced in a total of 54 meningococcal strains isolated from individuals with invasive disease. These isolates were supplemented with 28 isolates from healthy carriers to enhance the genetic diversity of the collection. During sequence analysis, it became obvious that in different clonal lineages, distinct mechanisms were responsible for the observed lack of ZnuD expression. In ST-8 cc/cluster A4 strains, a single cytosine insertion led to a premature stop at codon 26, and a point mutation in the ST-939 isolates introduces a stop at codon 278. In ST-23 cc strains from invasive disease, a single adenine deletion introduced a premature stop at codon 188 (Table 1). Of note, among 10 ST-23 cc strains analyzed from healthy carriers, 4 also harbored a stop codon in znuD, but it was derived from a point mutation, and it was located at codon 555 (Table 1).
Table 1.
Overview on the localization and mechanism of znuD stop mutations in meningococcal strains from different clonal lineages
| Clonal lineage | No. of strains analyzed | No. of strains with znuD stop | Stop at nucleotide positions | Stop at amino acid position | Mechanism leading to znuD stop |
|---|---|---|---|---|---|
| ST-8 cc/clusterA4 | 27 | 18 | 76–78 | 26 | Cytosine insertion |
| ST-23 cca | 11 | 3 | 562–564 | 188 | Adenine deletion |
| ST-939 | 1 | 1 | 832–834 | 278 | Point mutation |
| ST-23 ccb | 10 | 4 | 1663–1665 | 555 | Point mutation |
Invasive disease.
Carriage.
Sequence analysis of ZnuD.
Sixty-nine znuD sequences from strains with no premature stop codon were analyzed. Among the 69 sequences, there were 25 unique alleles. The deduced amino acid sequences displayed 75 variable sites out of a total of 758 aa (10%). The overall mean distance of unique amino acid sequences was low, 0.023. An unrooted neighbor-joining tree clustered ZnuD sequences from ST-8 cc, ST-32 cc, and ST-269 cc isolates into distinct clades, suggesting that znuD variants are associated with clonal lineages and not randomly distributed (Fig. 3). Nevertheless, there was one distinct group of ST-41/44 isolates with identical sequences, while two strains of this clonal cluster clustered with other lineages, suggesting that znuD, like many neisserial genes, is subject to horizontal gene transfer. There were two closely related groups of ZnuD sequences from ST-23 cc isolates, one consisting exclusively of carriage isolates (isolates a166, a237, a300, and a427). Accordingly, ST-11 isolates were split into two groups regarding ZnuD sequences. Taken together, despite the overall low antigenic diversity, phylogenetic signals could be observed according to the clonal complexes from which the strains were derived.
Fig 3.
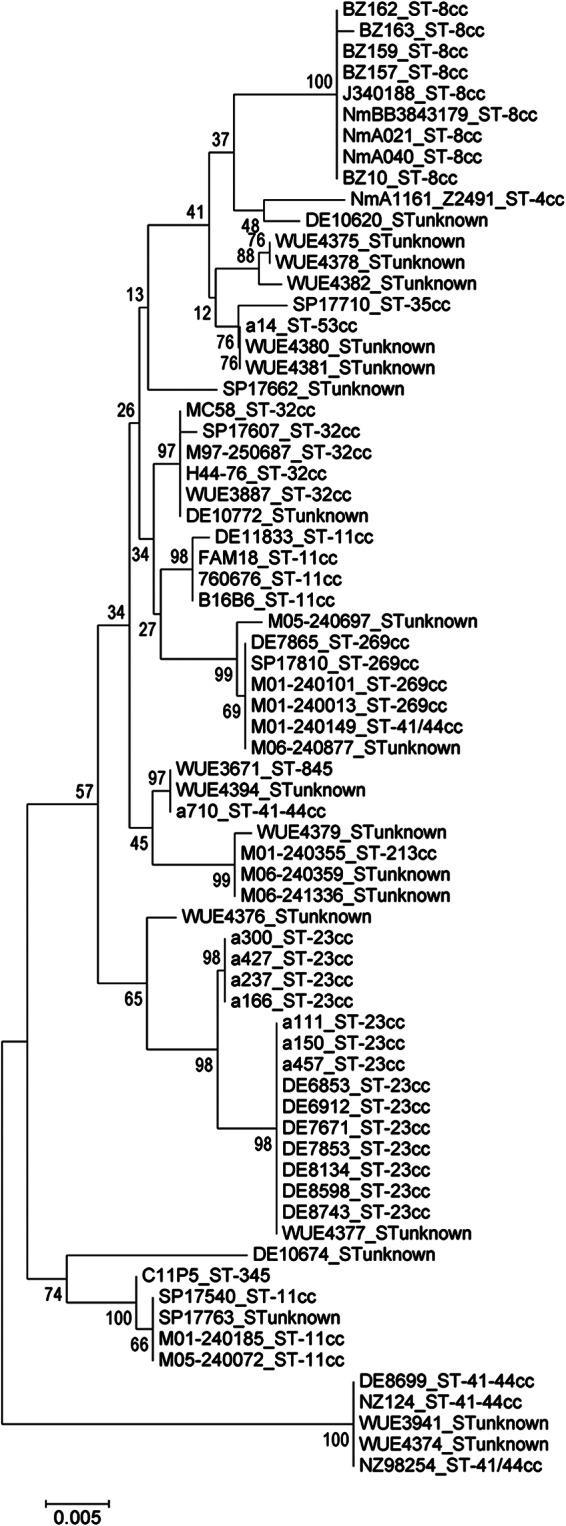
Neighbor-joining tree of ZnuD amino acid sequences analyzed in this study. A total of 765 amino acid positions were analyzed. An unrooted tree is shown. A total of 2,000 bootstrap replications were conducted. The strain designations and clonal complexes are provided as taxon information.
In addition to the 69 meningococcus B strains from various sources, we took advantage of the recent release of genome sequences from strains isolated in England, Wales, and Northern Ireland (the MRF Meningococcus Genome Library). A total of 505 ZnuD amino acid sequences were analyzed after exclusion of incomplete sequences or sequences with indels. A phylogenetic tree is depicted in Fig. 4. The neighbor-joining tree grouped together sequences retrieved from strains of the same lineage; i.e., groups were found, e.g., for the ST-269 cc, the ST-23 cc, and the ST-41/44 cc. The overall mean distance of unique amino acid sequences was low, 0.026. The number of polymorphic sites was almost identical to that of the above-mentioned set of sequences with 80 variable sites. In order to visualize the distribution of polymorphic sites in relation to the predicted extracellular loops of ZnuD, an entropy plot was generated (Fig. 5). The entropy at a position of a sequence reflects the degree of variability and uncertainty. An entropy value of zero demonstrates that there is no variability at all at a given position. Figure 5 demonstrates the distribution of variable sites. Variable sites were concentrated at the N terminus, where the plug domain is located. Furthermore, in the middle section of the protein, comprising loops 3 to 5, a larger number of variable sites was located. There were variable sites in other loops as well. However, their distribution appeared to be more or less random. The overall mean distance of the amino acid sequences of all loops was 0.025 and thus not higher than that of the whole protein. However, if only loops 3 and 5 were analyzed, the value went up to 0.053, supporting the entropy analysis.
Fig 4.

Phylogenetic relationships of ZnuD amino acid sequences of 505 strains isolated in England, Wales, and Northern Ireland within a period of 1 year. The neighbor-joining method was used to analyze the data. The p-distance method was applied. Bootstrapping was used with 2,000 replications.
Fig 5.

Entropy plot of ZnuD amino acid sequences of 505 strains isolated in the United Kingdom within a period of 1 year. A total of 758 positions were investigated. Entropy represents the variability or uncertainty at a given position of the sequence. The entropy is “0” if the site is not variable. The x axis provides the amino acid along with the predicted positions of 11 extracellular loops.
ZnuD is essential for growth in vitro under conditions of zinc depletion in ST-23 cc but not in ST-8 cc strains.
ZnuD was previously shown to be essential for the growth of ST-32 strain H44/76 under conditions of zinc limitation (8). To evaluate whether the growth of naturally occurring znuD mutants belonging to the ST-8 cc/cluster A4 and ST-23 cc was affected by zinc limitation, compared to ZnuD-expressing strains of the same clonal complex, growth curves using TPEN were determined. ST-8 cc strains expressing either serogroup B or serogroup C capsules were used; all ST-23 cc strains expressed the serogroup Y capsule. ZnuD expression was verified by Western blotting (data not shown). None of the ST-8 cc/cluster A4 and ST-23 cc strains, with a premature stop in znuD at codons 26 and 188, respectively, produced the protein in response to zinc starvation, while all others did.
The growth efficacy of ST-8 cc strains in the absence of TPEN was variable, in contrast to ST-23 cc strains, which invariably grew well over a period of 10 h (Fig. 6A and C). In the presence of 1 μM TPEN, when growth reduction of ST-8 cc strains was observed, this occurred independently of the expression of ZnuD (Fig. 6B). In contrast, all but one of the ST-23 cc isolates expressing ZnuD were able to grow despite zinc limitation, whereas three ST-23 cc strains without ZnuD failed to grow or showed impaired growth with 1 μM TPEN (Fig. 6D). This finding confirmed that in some but not all ST-8 cc or ST-23 cc strains, ZnuD is essential for growth under zinc-limited conditions, and this may depend on the clonal background.
Fig 6.
(A to D) Growth of wild-type ZnuD-expressing (black lines) and ZnuD-nonexpressing (gray lines) MenB/C ST-8 cc/cluster A4 (A and B) and MenY ST-23 cc (C and D) strains. Bacteria were grown in RPMI medium supplemented with 100 μM ferric chloride in the absence (A and C) or in the presence (B and D) of 1.0 μM TPEN. The OD600 of the bacterial culture was determined every 2 h. Each strain was tested in at least two independent experiments. (E) Growth of ZnuD-negative DE8633 complemented either with control vector pAP2-1 (black bars) or with pIK6 (gray bars) after 24 h in RPMI medium supplemented with 100 μM ferric chloride and different concentrations of TPEN. The graph shows the means of three independent experiments with standard deviations.
To evaluate whether the growth of ZnuD-negative MenY ST-23 cc strain DE8633 (Fig. 6D) under zinc-limited conditions could be restored by functional ZnuD, the strain was complemented in trans with znuD from type strain H44/76. Recombinant strains did not grow well at high TPEN concentrations. Therefore, the strain with an empty vector was compared with the ZnuD-expressing mutant at increasing concentrations of TPEN, from 0 to 0.75 μM. The ZnuD-expressing variant displayed a clear fitness benefit and grew better than the mock-transformed strain at increasing concentrations of TPEN (Fig. 6E). This finding confirmed that in the ST-23 cc, ZnuD is necessary for growth under conditions of zinc limitation.
ZnuD induces cross-bactericidal antibodies against serogroups A, B, C, and Y.
The demonstration of the immunogenicity of ZnuD in humans prompted us to evaluate the potential of ZnuD as a universal N. meningitidis vaccine antigen. Stork et al. demonstrated previously that anti-ZnuD antibodies are able to trigger the complement-mediated killing of a homologous strain genetically modified to overexpress ZnuD (8). Here, we evaluated the cross-bactericidal potential of ZnuD antibodies by using a large panel of strains under in vivo-like conditions via the use of zinc-restricted growth media.
OMVs were produced from two isogenic H44/76 strains differing by the level of ZnuD expression. To avoid the presence of residual capsular polysaccharide in OMV preparations and the induction of anti-LOS and anti-PorA bactericidal antibodies, galE and porA deletion mutations were engineered in both strains, leading to truncated LOS and absence of capsule, and lack of PorA expression, respectively. SDS-PAGE analysis suggested that ZnuD represents around 15% of the protein content of OMVs produced from the ZnuD-overexpressing strain (ZnuD OMVs), while ZnuD was not observed in the control OMV preparations (CTRL OMVs) (data not shown).
Mice and guinea pigs were immunized three times with OMVs (either ZnuD or CTRL OMVs) adsorbed on aluminum phosphate. Serum samples were obtained 2 weeks after the third immunization and were pooled in order to obtain three pools of sera per group. ELISA was first used to assess the response against ZnuD. Immunization with control OMVs induced low levels of ZnuD-specific antibodies in both mice and guinea pigs. As expected, high levels of ZnuD-specific antibodies were measured in the sera from animals immunized with ZnuD OMVs (Table 2). High anti-fHbp titers were also elicited by immunization. This protein, known to be immunogenic when presented in OMVs (32), has been measured in the OMVs by ELISA (data not shown). The amount of fHbp in ZnuD OMVs was approximately 4-fold higher than in the control OMVs. This is in line with the larger amount of anti-fHbp antibodies measured in the sera of animals immunized with ZnuD OMVs than in the sera of animals immunized with control OMVs. In our hands, a difference in fHbp content is frequently observed among different OMV lots and is attributed to variability in the small-scale ultracentrifugation process used to produce the vesicles, more particularly to the fact that the procedure with deoxycholate variably impacts lipoprotein content and, hence, fHbp content.
Table 2.
Level of anti-ZnuD antibodies produced in mice and guinea pigs after immunization with control OMVs and ZnuD OMVs
| OMV | Geometric mean titer (EU/ml)a |
|||
|---|---|---|---|---|
| Mouse sera |
Guinea pig sera |
|||
| Anti-ZnuD | Anti-fHbp | Anti-ZnuD | Anti-fHbp | |
| Control | 73 | 1,342 | <20 | 335 |
| ZnuD | 15,096 | 59,314 | 5,893 | 2,733 |
Geometric mean titers (expressed in endotoxin units [EU]/ml) from three pools of sera per group.
Pooled sera from either 30 mice or 10 guinea pigs were then tested in a serum bactericidal assay (SBA) against a panel of 14 MenB strains cultured with or without TPEN (Table 3). Six of these strains are routinely used by the vaccine evaluation unit of the Health Protection Agency (United Kingdom) for the evaluation of MenB vaccines. This panel included two epidemic strains (H44/76 and NZ98/254) as well as four strains of the four most prevalent United Kingdom serosubtypes, which were accountable for 59.3% of MenB disease in 2000 and 2001 (M01-240013, M01-240101, M01-240149, and M01-240355) (33). The expression of ZnuD in these 14 strains when grown under conditions of zinc limitation was confirmed by Western blotting (data not shown).
Table 3.
Serum bactericidal titers in the presence of baby rabbit complement on a panel of 14 serogroup B strains cultured with or without TPEN
| Strain | fHbp family | fHbp expressionc | ZnuD expressionc | Geometric mean titer (EU/ml)b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouse |
Guinea pig |
||||||||||||
| fHbp B or Aa |
Control OMVs |
ZnuD OMVs |
Control OMVs |
ZnuD OMVs |
|||||||||
| MH agar | MH-TPEN agar | MH agar | MH-TPEN agar | MH agar | MH-TPEN agar | MH agar | MH-TPEN agar | MH agar | MH-TPEN agar | ||||
| H44/76 | B | ++ | + | 3,383 | 3,539 | 103 | 265 | 3,605 | 7,903 | 55 | 115 | 331 | 1,751 |
| NZ98/254 | B | + | + | 110 | 388 | 50 | 112 | 69 | 1,854 | 50 | 50 | 50 | 2,592 |
| M01-240101 | B | + | + | 494 | 509 | 50 | 50 | 519 | 1,733 | 50 | 50 | 50 | 2,153 |
| M01-240355 | A | + | + | 50 | 50 | 50 | 50 | 50 | 3,310 | 50 | 50 | 50 | 2,497 |
| M01-240013 | A | + | + | 50 | 50 | 50 | 50 | 50 | 852 | 50 | 50 | 50 | 3,706 |
| M01-240149 | B | + | + | 2,293 | 2,104 | 71 | 50 | 3,116 | 3,374 | 50 | 50 | 94 | 2,961 |
| MC58 | B | ++ | + | 3,993 | 5,786 | 88 | 304 | 1,734 | 17,486 | 67 | 50 | 240 | 1,846 |
| 18025 | B | +/− | + | 3,804 | 4,373 | 50 | 182 | 50 | 4,872 | 67 | 50 | 234 | 752 |
| DE 10690-06 | B | +/− | + | 50 | 50 | 50 | 50 | 50 | 937 | 50 | 50 | 50 | 1,781 |
| M05-0240471 | B | +/− | + | 50 | 50 | 50 | 50 | 50 | 197 | 50 | 50 | 50 | 2,005 |
| 17540 | B | − | + | 50 | 50 | 50 | 50 | 50 | 2,130 | 50 | 50 | 50 | 8,877 |
| M05-0240072 | B | − | + | 50 | 50 | 50 | 50 | 50 | 3,400 | 50 | 50 | 94 | 6,315 |
| 760676 | A | − | + | 50 | 50 | 50 | 50 | 50 | 1,308 | 50 | 50 | 122 | 5,311 |
| M98-250771 | A | +/− | + | 50 | 50 | 50 | 50 | 50 | 143 | 50 | 50 | 50 | 2,021 |
Sera from mice immunized with fHbp A or fHbp B were used for SBA with strains expressing fHbp A or fHbp B, respectively.
Geometric mean titers (expressed in EU/ml) for 50% killing from three pools of sera per group. Geometric mean titers above the threshold for a positive result (titer of ≥128) are shown in boldface type.
++, high; +, medium; +/−, low; −, no expression.
In the absence of TPEN in the culture medium, all sera from mice and guinea pigs immunized with control OMVs were not able to induce complement-mediated killing of strains. When target bacteria were grown with TPEN, SBA was negative for all guinea pig sera and all but three mouse sera using control OMV antisera. After immunization with ZnuD OMVs, 3 and 4 strains out of 14 were killed by sera from guinea pigs and mice, respectively, in the absence of the zinc chelator. This “background” bactericidal activity could be due either to a basal expression of ZnuD in the presence of zinc (as previously observed for some strains [8]) or to the presence of minor proteins present in ZnuD OMV and absent in control OMV (see below). When using growth conditions inducing the expression of ZnuD (i.e., in the presence of 20 μM TPEN), the same anti-ZnuD OMV sera from both animal models were able to kill all 14 tested strains. In contrast, only 6 strains were killed by anti-fHbpA or anti-fHbpB mouse sera.
An additional set of three ZnuD-expressing strains from serogroups A, C, and Y was also tested (no serogroup W strain was tested). When cultured in the presence of TPEN, all three strains were killed by anti-ZnuD OMV antibodies and complement (data not shown).
Confirmation of ZnuD as a target of bactericidal antibodies.
Bactericidal activity of ZnuD OMV sera against most strains was observed only in the presence of TPEN, when ZnuD was expressed, strongly suggesting that ZnuD is the target of bactericidal antibodies. However, a significant bactericidal activity against some strains (notably H44/76 and MC58) (Table 3) was observed with anti-ZnuD OMV sera but not with anti-control OMV sera in the absence of the zinc chelator, suggesting differences in OMV composition (other than the presence or absence of ZnuD) between the control OMVs and the ZnuD OMVs. This implies that another protein(s) may be involved in the observed bactericidal activity, such as fHbp, as this protein is expressed at high levels in strains H44/76 and MC58 (Table 3), and anti-fHbp antibodies were elicited by the immunization.
To confirm that ZnuD was the main target of bactericidal antibodies and to evaluate the potential involvement of fHbp in the killing, ΔznuD, ΔfHbp, and double ΔznuD-ΔfHbp strains were constructed in four different genetic backgrounds by insertion of an antibiotic cassette as replacement of the ZnuD- and/or fHbp-coding sequences. Absence of ZnuD and/or fHbp expression under zinc-limited conditions was confirmed by Western blotting (data not shown). These strains were used for SBA with or without TPEN (Table 4).
Table 4.
Impact of culture conditions (ZnuD expression or not) on the bactericidal activity of sera on a panel of wild-type strains and on derived ΔfHbp, ΔznuD, or ΔznuD-fHbp strainsa
| Strain | Geometric mean titer (EU/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mouse |
Guinea pig |
|||||||
| MH agar |
MH-TPEN agar |
MH agar |
MH-TPEN agar |
|||||
| CTRL OMVs | ZnuD OMVs | CTRL OMVs | ZnuD OMVs | CTRL OMVs | ZnuD OMVs | CTRL OMVs | ZnuD OMVs | |
| H44/76 (fHbp B/++) strains | ||||||||
| WT | <200 | 2,930 | <200 | 3,446 | <200 | 546 | <200 | 1,055 |
| ΔfHbp | <200 | <200 | <200 | <200 | <200 | <200 | <200 | 322 |
| ΔznuD | <200 | 1,778 | <200 | 999 | <200 | 319 | <200 | 206 |
| ΔznuD-fHbp | <200 | <200 | <200 | <200 | <200 | <200 | <200 | <200 |
| NZ98/254 (fHbp B/+) strains | ||||||||
| WT | 3,332 | 8,646 | ||||||
| ΔfHbp | 1,899 | 4,462 | ||||||
| ΔznuD | 817 | <200 | ||||||
| ΔznuD-fHbp | <200 | <200 | ||||||
| M05-240355 (fHbp A/+) strains | ||||||||
| WT | 932 | 2,875 | ||||||
| ΔfHbp | 2,322 | 7,151 | ||||||
| ΔznuD | <200 | <200 | ||||||
| ΔznuD-fHbp | <200 | <200 | ||||||
| 17540 (fHbp B/−) strains | ||||||||
| WT | 658 | 2,525 | ||||||
| ΔfHbp | IR | 8,069 | ||||||
| ΔznuD | <200 | <200 | ||||||
| ΔznuD-fHbp | <200 | <200 | ||||||
IR, invalid result; WT, wild type.
Sera from animals immunized with control OMV were first tested on wild-type strain H44/76 and its mutant derivatives (Table 4). No bactericidal activity was observed with these pooled sera after growth of bacteria in culture medium with or without TPEN, suggesting that ZnuD and/or fHbp inactivation does not result in a significant increase in rabbit complement sensitivity.
Anti-ZnuD OMV sera were then analyzed by SBA without TPEN against wild-type strain H44/76 and its three derivatives (ΔznuD, ΔfHbp, and double ΔznuD-fHbp) (Table 4). The bactericidal activity observed previously was confirmed in the wild-type strain, was slightly decreased in the ΔznuD strain, and was completely abrogated in the ΔfHbp strain and the double mutants. These results confirm that in the absence of ZnuD expression, fHbp was the main target of bactericidal antibodies against H44/76, a strain known to express high levels of this protein. When wild-type strain H44/76 and its mutant derivatives were cultured in zinc-depleted medium and mouse sera were used, observations similar to those with complete medium were made. With guinea pig sera, however, ZnuD and fHbp seemed to contribute equally to the killing in the presence of TPEN, as demonstrated by the reduction of bactericidal titers in both single mutants. The reduced impact of anti-fHbp antibodies in guinea pig sera was in line with the total level of anti-ZnuD and anti-fHbp antibodies measured in the sera from mice and guinea pigs (Table 2).
In the three other genetic backgrounds tested, when zinc-depleted medium was used and with either serum source (mice or guinea pigs), inactivation of ZnuD resulted in a significant reduction or even a complete loss of the bactericidal titers (Table 4). These results suggested that under such culture conditions, ZnuD was the main or only target of the bactericidal antibodies against strains expressing either a heterologous fHbp (strain M05-240355) or low/nondetectable levels of a homologous fHbp (strains NZ98/254 and 17540).
Altogether, these data demonstrated that immunization with ZnuD OMVs induced anti-ZnuD bactericidal antibodies able to mediate the killing of genetically divergent strains.
DISCUSSION
To analyze the potential of ZnuD as a N. meningitidis vaccine candidate, we first aimed to study its occurrence and conservation. ZnuD is not expressed by meningococci when grown classically on MH or tryptic soy broth medium. These media were developed for the culture of fastidious microorganisms and, for this reason, are rich in dietary minerals and micronutrients. For example, in MH agar, the concentration of zinc ranges from 200 to 450 μg/liter, depending on the manufacturer. Under these conditions, free zinc is certainly available in large amounts so that N. meningitidis does not need to express an outer membrane receptor dedicated to zinc acquisition, as passive diffusion via the porins would probably satisfy growth requirements. For comparison, the concentration of free zinc in bovine serum is estimated to be approximately 0.01 μg/liter (0.15 nM), corresponding to around 0.0008% of the total amount of zinc in serum (800 μg/liter) (34). In human, the total zinc content in plasma is usually 1 mg/liter, varying as a function of age, sex, pregnancy, and time of day (35), and by extrapolation, the free zinc content should also be around 0.01 μg/liter. Under such conditions of zinc deprivation, bacteria need to express proteins dedicated to zinc acquisition, such as ZnuD. This has been illustrated in a recent study investigating gene expression in N. meningitidis in human whole blood and showing the induction of ZnuD in such environments (36). In our study, the in vivo expression of ZnuD was indirectly demonstrated by the detection of antibodies in sera from convalescent patients. In addition, peptide array experiment showed that anti-ZnuD antibodies are directed mostly against putative surface loops 3, 6, and 11, although there was also consistent reactivity with a predicted transmembrane region between loops 7 and 8. Based on the different intensities of the signals measured in the peptide array with convalescent and carrier sera, it was not possible to conclude whether the ZnuD expression level in the upper respiratory tract is lower than in blood, but it must be emphasized that the experiment was designed to detect serum IgG, which does not reflect the mucosal immune response induced by microorganism colonization.
ZnuD was originally discovered in N. meningitidis cultured in RPMI, a zinc-poor medium (8). Here, to induce the expression of ZnuD, the zinc chelator TPEN was added in a classical culture medium. A similar strategy was applied previously to evaluate the vaccine potential of iron-limitation-inducible proteins such as TbpA or TbpB, by using an iron chelator in the culture medium (37). The impact of the concentration of TPEN on the level of ZnuD expression was first investigated (data not shown) with the aim to select a concentration that allows the expression of ZnuD without an impact on bacterial growth, a concentration that could mimic ZnuD in vivo expression in a zinc-limited host environment. By using these experimental conditions, a total of 223 genetically diverse N. meningitidis invasive strains from different geographic origins and time periods were analyzed for the expression of ZnuD, and we found that the protein was expressed in 96% of these MenB strains. In all strains that did not express ZnuD, the lack of expression was due to mutations introducing a stop codon within the open reading frame. A total of 69 ZnuD amino acid sequences were analyzed, revealing 25 unique sequences. ZnuD appeared to be well conserved, with 10% variable sites or 90% amino acid site identity and an overall mean p-distance of 0.023. Similar results were obtained with ZnuD sequences of 505 strains from the MRF Meningococcus Genome library comprising an almost complete set of strains from England, Wales, and Northern Ireland isolated during the epidemiological year of 2010 to 2011. For comparison, a previously reported analysis of 107 fHbp sequences by Brehony et al. revealed 81% amino acid site identity for subfamily A/variant 2 and 87% amino acid site identity for subfamily B/variant 1 (38). The p-distances of the respective subfamilies were 0.052 and 0.038. Although the strain collections analyzed previously by Brehony et al. and in this study are not comparable, data suggest that the protein diversity of ZnuD is in the range of the within-group diversity of the major subfamilies of fHbp. These findings suggest that ZnuD is a well-conserved antigen. Phylogenic grouping revealed a conservation of amino acid sequences of the protein in clonal complexes. The only virulent clonal complex with an association to serogroup B in which most strains lacked ZnuD expression was the ST-8 cc. This globally distributed lineage causes serogroup C disease but has also been reported in relation to serogroup B, e.g., in Spain. The complex is related to the ST-11/ET-37c complex, which, at least in the past 2 decades, clearly dominated serogroup C disease worldwide. All ST-11 cc meningococci investigated in this study expressed ZnuD. Lack of ZnuD expression was restricted to strains of four clonal lineages (ST-23, serogroup Y; ST-8, serogroup B or C; ST-939, serogroup B; ST-162, serogroup B) and did not appear in other lineages. This gives rise to the hypothesis that in other clonal lineages, znuD mutation does not provide a fitness benefit but rather provides a fitness loss, given the frequency of horizontal gene transfer in meningococci. Furthermore, since the single-nucleotide mutations found were strictly associated with a given lineage, we assume that the genotypes are fixed in subpopulations of the lineages, which obviously cope well with the mutation. How this takes place remains obscure: investigation of ST-23 strains with a znuD mutation showed in vitro impaired growth under zinc-limited conditions, both in wild-type strains and in an isogenic recombinant pair. This finding suggests primarily that znuD mutation per se is negatively affecting fitness in the host, in which the bacterium must face a zinc-restricted environment. ST-8 cc strains, however, differed from this pattern, as their growth or impaired growth under conditions of zinc limitation seems to be independent of ZnuD expression. Mutations in znuD rendering ZnuD inactive were limited almost exclusively to the ST-23 and ST-8 cc. This restriction might be explained by the following hypotheses. (i) The mutations evolve in all meningococcal lineages at the same rate during transmission, carriage, or disease. However, the mutations are selected against in all lineages, with the exception of ST-8 and ST-23. (ii) ST-8 and ST-23 strains with mutated ZnuD evolved only a few times and were maintained in the population for unknown reasons, such as the presence of alternative Zn uptake systems or immune selection acting specifically on ST-8 and ST-23 strains.
Still, with the perspective of using ZnuD as a vaccine antigen, we also assessed its potential to induce cross-bactericidal antibodies. Because ZnuD is an integral OMP, we have selected OMVs for its presentation to the immune system, to ensure proper protein folding. These OMVs were extracted from a ΔgalE and ΔporA H44/76 strain with 0.5% deoxycholate, which reduces LOS content and avoids potential autoimmunity due to lactoneotetraose and immunodominance of the variable porins. We have previously demonstrated that such extraction conditions yield OMVs that do not induce the production of bactericidal antibodies directed against LOS (13, 17). In our study, mice and guinea pigs immunized with control OMVs produced no bactericidal antibodies against a panel of genetically diverse strains, independently of the presence of a zinc chelator in the medium. On the contrary, immunization of mice and guinea pigs with OMVs containing large amounts of ZnuD elicited antibodies that were bactericidal against all tested strains but only under the conditions of zinc restriction known to induce ZnuD expression. Furthermore, by using ΔznuD mutant strains, we confirmed that most of these antibodies specifically target ZnuD. In the absence of a zinc chelator, only a few strains were killed by the ZnuD-immunized sera. In at least one of them, this protective response was most probably related to the production of anti-fHbp antibodies, as demonstrated by their presence in ELISAs and by the diminution of SBA titers when using a ΔfHbp strain. Among the strains killed, two are of special interest because they were also used to assess the immune response in pediatric sera after immunization with an experimental vaccine based on three subunit proteins plus OMVs derived from strain NZ98/254 (39). Sera from subjects immunized with this complex vaccine failed to mediate the killing of strain M01-240355, and fewer than 50% of infants demonstrated serum bactericidal activity against strain M01-240101 after four immunizations. These two strains were isolated in the United Kingdom and are from the ST-213 cc and ST-269 cc, respectively. These clonal complexes represented 39% of United Kingdom MenB strains in 2008. In the present preclinical experiments, murine and guinea pig sera obtained after immunization with ZnuD OMVs demonstrated strong bactericidal activity against these two strains (SBA titers of >1,700). In addition, we have confirmed that strain M01-240101 is also killed by pooled anti-ZnuD OMV sera from guinea pigs in the presence of human complement when cultured in the presence of TPEN (SBA titers of <1/50 and 1/555 without and with TPEN, respectively). Although it is difficult to extrapolate preclinical data to the human situation, these observations and our results advocate the use of ZnuD OMV to improve MenB vaccine coverage.
In conclusion, ZnuD is a well-conserved protein of N. meningitidis, expressed by most strains of serogroups A, B, C, W, and Y under in vitro conditions aimed to mimic in vivo zinc limitation conditions. This antigen is able to mediate a cross-bactericidal response against most of the N. meningitidis strains from various serogroups. Similar to fHbp, ZnuD is one of the few antigens that could be the basis of a simple universal vaccine to protect against MenB infections. The major advantage of ZnuD over fHbp is that the former is a well-conserved protein for which only one family has yet been described. Nevertheless, we believe that an effective vaccine may need to combine both fHbp and ZnuD, as (i) it will improve vaccine coverage by allowing the killing of strains that do not express fHbp (like some meningococcal strains from the hypervirulent ST11 clonal complex [40]) or produce low levels of fHbp (poor or no killing of these strains by anti-fHbp was observed), as well as allowing the killing of strains that do not express ZnuD but fHbp (like some ST-8 strains), and (ii) enhance the bactericidal activity of the sera by targeting two antigens when expressed simultaneously by the bacteria.
Assessing the immunogenicity of ZnuD in humans will bring further insight into its potential as a broad MenB vaccine candidate.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sigrid Heuberger (Graz, Austria) for providing the convalescent-phase sera and Oliver Kurzai (formerly Würzburg, Germany) for carrier sera. We thank Isabell Kaluza for expert technical assistance. We gratefully acknowledge Dominique Caugant (Oslo, Norway), Mark Achtman (now Cork, Ireland), Ray Borrow (Health Protection Agency, Manchester, United Kingdom), Julio Vásquez (Madrid, Spain), and Richard Moxon (Oxford, United Kingdom) for kindly providing meningococcal strains. We also thank Pascal Cadot for editorial assistance and Ulrike Krause for coordinating the development of the manuscript. This publication made use of the Meningitis Research Foundation Meningococcus Genome Library (http://www.meningitis.org/research/genome), developed by the Health Protection Agency, the Wellcome Trust Sanger Institute, and the University of Oxford as a collaboration.
The project is funded by the Meningitis Research Foundation. This work was partly financed by GlaxoSmithKline Biologicals SA (GSK).
N.D., C.T., G.B., C.F., K.G., J.T.P., and V.W. are, or were at the time of the study, employees of the GlaxoSmithKline group of companies. The laboratory of J.T. has received grants for different studies, including this one, and fees for consultancy from GSK. The laboratory of K.H., I.M., and U.V. has received a grant from GSK for this study. U.V. has received fees from GSK for board membership and a grant from Novartis for a work not related to this one. N.D., C.F., G.B., J.T., J.T.P., and V.W. are designated inventors on patents owned by GSK. C.F., K.G., and V.W. own shares and options to shares in GSK.
Footnotes
Published ahead of print 18 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01312-12.
REFERENCES
- 1. Finne J, Leinonen M, Mäkelä PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 322:355–357 [DOI] [PubMed] [Google Scholar]
- 2. Nedelec J, Boucraut J, Garnier JM, Bernard D, Rougon G. 1990. Evidence for autoimmune antibodies directed against embryonic neural cell adhesion molecules (N-CAM) in patients with group B meningitis. J. Neuroimmunol. 29:49–56 [DOI] [PubMed] [Google Scholar]
- 3. Frasch CE, van Alphen L, Holst J, Poolman JT, Rosenqvist E. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p 81–107 In Pollard AJ, Maiden MCJ. (ed), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 4. Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27:B3–B12 doi:10.1016/j.vaccine.2009.04.071 [DOI] [PubMed] [Google Scholar]
- 5. Czumbel I. 2011. The epidemiology of invasive meningococcal disease in Europe, 2008 and 2009, p 3. Abstr. 11th Congr. Eur. Meningococcal Dis. Soc., Ljubljana, Slovenia, 18 to 20 May 2011 [Google Scholar]
- 6. Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 72:2088–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Aricò B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stork M, Bos MP, Jongerius I, de Kok N, Schilders I, Weynants VE, Poolman JT, Tommassen J. 2010. An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog. 6:e1000969 doi:10.1371/journal.ppat.1000969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patzer SI, Hantke K. 2000. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J. Biol. Chem. 275:24321–24332 [DOI] [PubMed] [Google Scholar]
- 10. Kumar P, Sannigrahi S, Tzeng Y-L. 2012. The Neisseria meningitidis ZnuD zinc receptor contributes to interactions with epithelial cells and supports heme utilization when expressed in Escherichia coli. Infect. Immun. 80:657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Claus H, Maiden MCJ, Wilson DJ, McCarthy ND, Jolley KA, Urwin R, Hessler F, Frosch M, Vogel U. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 191:1263–1271 [DOI] [PubMed] [Google Scholar]
- 12. Lappann M, Haagensen JAJ, Claus H, Vogel U, Molin S. 2006. Meningococcal biofilm formation: structure, development and phenotypes in a standardized continuous flow system. Mol. Microbiol. 62:1292–1309 [DOI] [PubMed] [Google Scholar]
- 13. Weynants V, Denoël P, Devos N, Janssens D, Feron C, Goraj K, Momin P, Monnom D, Tans C, Vandercammen A, Wauters F, Poolman JT. 2009. Genetically modified L3,7 and L2 lipooligosaccharides from Neisseria meningitidis serogroup B confer a broad cross-bactericidal response. Infect. Immun. 77:2084–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffiths NJ, Hill DJ, Borodina E, Sessions RB, Devos NI, Feron CM, Poolman JT, Virji M. 2011. Meningococcal surface fibril (Msf) binds to activated vitronectin and inhibits the terminal complement pathway to increase serum resistance. Mol. Microbiol. 82:1129–1149 [DOI] [PubMed] [Google Scholar]
- 15. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 17. Weynants VE, Feron CM, Goraj KK, Bos MP, Denoël PA, Verlant VG, Tommassen J, Peak IRA, Judd RC, Jennings MP, Poolman JT. 2007. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 75:5434–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Devos N, Tans C, Momin P, Plisnier M, Weynants V, Feron C, Poolman JT. 2012. Neisseria meningitidis serogroup B lipooligosaccharide genotyping reveals high prevalence of L2 strains in Spain and unexpected relationship with factor H-binding protein expression. Microbes Infect. 14:979–988 [DOI] [PubMed] [Google Scholar]
- 19. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rose PW, Beran B, Bi C, Bluhm WF, Dimitropoulos D, Goodsell DS, Prliæ A, Quesada M, Quinn GB, Westbrook JD, Young J, Yukich B, Zardecki C, Berman HM, Bourne PE. 2011. The RCSB Protein Data Bank: redesigned Web site and Web services. Nucleic Acids Res. 39:D392–D401 doi:10.1093/nar/gkq1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noinaj N, Guillier M, Barnard TJ, Buchanan SK. 2010. TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64:43–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56–63 [DOI] [PubMed] [Google Scholar]
- 23. Cobessi D, Meksem A, Brillet K. 2010. Structure of the heme/hemoglobin outer membrane receptor ShuA from Shigella dysenteriae: heme binding by an induced fit mechanism. Proteins 78:286–294 [DOI] [PubMed] [Google Scholar]
- 24. Buchanan SK, Lukacik P, Grizot S, Ghirlando R, Ali MMU, Barnard TJ, Jakes KS, Kienker PK, Esser L. 2007. Structure of colicin I receptor bound to the R-domain of colicin Ia: implications for protein import. EMBO J. 26:2594–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buchan DWA, Ward SM, Lobley AE, Nugent TCO, Bryson K, Jones DT. 2010. Protein annotation and modelling servers at University College London. Nucleic Acids Res. 38:W563–W568 doi:10.1093/nar/gkq427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bagos PG, Liakopoulos TD, Spyropoulos IC, Hamodrakas SJ. 2004. PRED-TMBB: a Web server for predicting the topology of β-barrel outer membrane proteins. Nucleic Acids Res. 32:W400–W404 doi:10.1093/nar/gkh417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Randall A, Cheng J, Sweredoski M, Baldi P. 2008. TMBpro: secondary structure, β-contact and tertiary structure prediction of transmembrane β-barrel proteins. Bioinformatics 24:513–520 [DOI] [PubMed] [Google Scholar]
- 28. Holm L, Rosenström P. 2010. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38:W545–W549 doi:10.1093/nar/gkq366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen M-Y, Pieper U, Sali A. 2006. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinformatics Chapter 5:Unit 5.6. doi:10.1002/0471250953.bi0506s15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benkert P, Künzli M, Schwede T. 2009. QMEAN server for protein model quality estimation. Nucleic Acids Res. 37:W510–W514 doi:10.1093/nar/gkp322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koebnik R. 2005. TonB-dependent trans-envelope signalling: the exception or the rule? Trends Microbiol. 13:343–347 [DOI] [PubMed] [Google Scholar]
- 32. Koeberling O, Welsch JA, Granoff DM. 2007. Improved immunogenicity of a H44/76 group B outer membrane vesicle vaccine with over-expressed genome-derived neisserial antigen 1870. Vaccine 25:1912–1920 [DOI] [PubMed] [Google Scholar]
- 33. Findlow J, Taylor S, Aase A, Horton R, Heyderman R, Southern J, Andrews N, Barchha R, Harrison E, Lowe A, Boxer E, Heaton C, Balmer P, Kaczmarski E, Oster P, Gorringe A, Borrow R, Miller E. 2006. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect. Immun. 74:4557–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang P, Allen JC. 1995. A novel dialysis procedure measuring free Zn2+ in bovine milk and plasma. J. Nutr. 125:1904–1910 [DOI] [PubMed] [Google Scholar]
- 35. Vallee BL, Falchuk KH. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 73:79–118 [DOI] [PubMed] [Google Scholar]
- 36. Echenique-Rivera H, Muzzi A, Del Tordello E, Seib KL, Francois P, Rappuoli R, Pizza M, Serruto D. 2011. Transcriptome analysis of Neisseria meningitidis in human whole blood and mutagenesis studies identify virulence factors involved in blood survival. PLoS Pathog. 7:e1002027 doi:10.1371/journal.ppat.1002027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rokbi B, Mignon M, Maitre-Wilmotte G, Lissolo L, Danve B, Caugant DA, Quentin-Millet MJ. 1997. Evaluation of recombinant transferrin-binding protein B variants from Neisseria meningitidis for their ability to induce cross-reactive and bactericidal antibodies against a genetically diverse collection of serogroup B strains. Infect. Immun. 65:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brehony C, Wilson DJ, Maiden MCJ. 2009. Variation of the factor H-binding protein of Neisseria meningitidis. Microbiology 155:4155–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, Oster P, Miller E, Pollard AJ. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin. Infect. Dis. 51:1127–1137 [DOI] [PubMed] [Google Scholar]
- 40. Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. 2011. Characterisation of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen, factor H binding protein. Clin. Vaccine Immunol. 18:1002–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.