Abstract
Toxoplasma gondii infects both hematopoietic and nonhematopoietic cells and can cause cerebral and ocular toxoplasmosis, as a result of either congenital or postnatally acquired infections. Host protection likely acts at both cellular levels to control the parasite. CD40 is a key factor for protection against cerebral and ocular toxoplasmosis. We determined if CD40 induces anti-T. gondii activity at the level of nonhematopoietic cells. Engagement of CD40 on various endothelial cells including human microvascular brain endothelial cells, human umbilical vein endothelial cells, and a mouse endothelial cell line as well as human and mouse retinal pigment epithelial cells resulted in killing of T. gondii. CD40 stimulation increased expression of the autophagy proteins Beclin 1 and LC3 II, enhanced autophagy flux, and led to recruitment of LC3 around the parasite. The late endosomal/lysosomal marker LAMP-1 accumulated around the parasite in CD40-stimulated cells. This was accompanied by killing of T. gondii dependent on lysosomal enzymes. Accumulation of LAMP-1 and killing of T. gondii were dependent on the autophagy proteins Beclin 1 and Atg7. Together, these studies revealed that CD40 induces toxoplasmacidal activity in various nonhematopoietic cells dependent on proteins of the autophagy machinery.
INTRODUCTION
CD40 is a type I transmembrane glycoprotein and a member of the tumor necrosis factor (TNF) receptor superfamily. CD40 is constitutively expressed on antigen-presenting cells and on various nonhematopoietic cells such as endothelial cells, epithelial cells, fibroblasts, vascular smooth muscle cells, keratinocytes, and certain neurons (1–4). Its natural ligand, CD154, is expressed primarily on activated CD4+ T cells but also in activated platelets as well as in plasma as a soluble protein (5–8). The finding that the congenital immunodeficiency X-linked Hyper IgM syndrome (X-HIM) is caused by the lack of functional CD154 provided evidence of the clinical relevance of the CD40-CD154 pathway (9). Studies in patients with X-HIM and in mice revealed that the interaction between CD40 and CD154 is important for resistance against a variety of pathogens including Mycobacterium tuberculosis, Mycobacterium avium, Cryptosporidium parvum, Leishmania major, Leishmania amazonensis, Toxoplasma gondii, Cryptococcus neoformans, Pneumocystis jirovecii, and Salmonella (10–19). One of the mechanisms by which CD40 confers protection against pathogens is likely by inducing antimicrobial activity. Indeed, CD40 ligation in macrophages and microglia induces antimicrobial activity (15–17, 20).
Many pathogens infect both hematopoietic and nonhematopoietic cells. Thus, effective control of these pathogens likely requires activation of mechanisms of resistance at both cellular compartments. Much work has been done investigating the hematopoietic cellular response to infection, yet much less is known about the specific contribution of nonhematopoietic cells during infection. Activated nonhematopoietic cells are reported to contribute to the immune response against M. tuberculosis, Escherichia coli, or Listeria monocytogenes infection through cytokine regulation and recruitment of cellular infiltrate (21–23). However, whether other mechanisms of host protection are active in nonhematopoietic cells in vivo is less understood.
T. gondii is an obligate intracellular parasite that is capable of infecting virtually any nucleated cell through active invasion. T. gondii resides within the host cell in a specialized parasitophorous vacuole that resists acidification and lysosomal fusion, allowing the parasite to replicate within host cells (24–26). CD40 and CD154 play a key role in protection against cerebral and ocular toxoplasmosis since CD40−/− and CD154−/− mice display marked susceptibility to these manifestations of toxoplasmosis (16, 17). The CD40-CD154 pathway regulates interleukin-12 (IL-12) production (16, 27) and elicits degradation of T. gondii in macrophage and microglial cells through the autophagy pathway (17, 20). However, it is unclear whether CD40 ligation could directly activate antimicrobial activity in nonhematopoietic cells. In this regard, in a model of C. parvum infection, CD40 expression in hematopoietic but not in nonhematopoietic cellular compartment was necessary for protection against the pathogen (28).
Using various nonhematopoietic cells of relevance to the pathogenesis of toxoplasmosis (endothelial cells and retinal pigment epithelial cells [RPE]), we report that CD40 engagement induced sequestration of the parasite by structures that express the autophagy protein LC3, fusion of the parasitophorous vacuole with late endosomes/lysosomes, and killing of the parasite that was dependent on the autophagy proteins Beclin 1 and Atg7. These studies uncovered CD40 as an activator of toxoplasmacidal activity in nonhematopoietic cells.
MATERIALS AND METHODS
Cells.
Primary human brain microvascular endothelial cells (BMVEC; ScienCell Research Laboratories, Carlsbad, CA) were cultured in fibronectin-coated tissue culture flasks and basal medium supplemented with endothelial cell growth supplement (ECGS) and 5% fetal bovine serum (FBS), all from ScienCell. Human umbilical endothelial cells (HUVEC; Lonza, Allendale, NJ) were cultured in endothelial basal medium (EBM) supplemented with epidermal growth factor (EGF), bovine brain extract, and 2% FBS (Lonza). The mouse endothelial cell line mHEVc (29) (a gift from Joan Cook-Mills, Northwestern University, Chicago, IL) was cultured in Dulbecco's modified Eagle medium (DMEM) plus 10% FBS (HyClone; Logan, UT). Primary mouse retinal pigment epithelial cells (RPE) were isolated as described previously (30). Briefly, eyecups from 10-day-old C57BL/6 mice were sequentially treated with 0.5 mg/ml bovine hyaluronidase (Sigma Chemical, St. Louis, MO), 0.05 mg/ml of collagenase (Sigma-Aldrich), and 0.1% trypsin (Difco-BD Biosciences, Sparks, MD) for 60 min for each incubation to allow the peeling off of the neural retina and expose the RPE. Patches of RPE were peeled off manually from Bruch's membrane. To further dissociate the RPE patches, purified RPE were incubated with 0.05% trypsin–0.53 mM EDTA for 2 min at 37°C. A human RPE cell line (ARPE-19; American Type Culture Collection, Manassas, VA) was cultured in DMEM plus 10% FBS.
Transfections.
mHEVc are CD40− and thus were transfected with linearized pRSV.5 (neo-) plasmid encoding the extracellular domain of human CD40 with the intracellular domain of mouse CD40 (hmCD40) or with pRSV.5 alone (gifts from Gail Bishop, University of Iowa) (31). While primary human RPE can express CD40, ARPE-19 cells are CD40− (32). Therefore, similar to mHEVc, ARPE-19 cells were stably transfected with a plasmid that encodes human CD40 (gift from Gail Bishop) (33). Cells were transiently transfected with a plasmid encoding LC3-enhanced green fluorescent protein (EGFP) or a plasmid encoding tandem monomeric red fluorescent protein-(RFP)-GFP-tagged LC3 (tfLC3) (34) (gifts from T. Yoshimori, National Institute for Basic Biology, Okazaki, Japan). In addition, cells were transiently transfected with either control small interfering RNA (siRNA), Beclin 1 siRNA (35), or Atg7 siRNA (35) (all from Dharmacon Inc., Lafayette, CO). Transfections of endothelial cells were performed using Lipofectamine 2000 (Invitrogen, Life Technologies, Grand Island, NY), while RPE were transfected using an Amaxa nucleofector per the manufacturer's protocol (Lonza).
T. gondii and infection.
The type I strain (RH) and the type II strain (PTG-ME49) of T. gondii were maintained in human foreskin fibroblasts. Parasites expressing cytoplasmic-yellow fluorescent protein (YFP), cytoplasmic-DsRed (RFP), or secreted-DsRed (Sec-RFP) have been described (36–38). Endothelial or epithelial cells were cultured on eight-chamber tissue culture glass slides (Falcon; Becton, Dickinson Labware, Franklin Lakes, NJ) in their respective culture medium without growth supplements followed by challenge for 1 h with T. gondii tachyzoites. Monolayers were washed to remove extracellular parasites. At the indicated time points, monolayers were either fixed and stained with Diff-Quick (Dade Diagnostics; Aguada, Puerto Rico) or fixed with 4% paraformaldehyde and used for immunofluorescence. The number of parasites per 100 cells in triplicate monolayers was determined by light microscopy by counting at least 200 cells per monolayer. Changes in the percentages of infected cells were not due to differences in cell detachment during the processing of samples. In addition, cell densities as determined with an eyepiece grid were similar in all experimental groups. To induce CD40 stimulation, cells were treated with human CD154 (3 μg/ml; a gift from William Fanslow, Amgen, Thousand Oaks, CA; or cell-free supernatants containing multimeric human CD154, obtained from Richard Kornbluth, University of California, San Diego, currently at Multimeric Biotherapeutics Inc., La Jolla, CA) for 18 h at 37°C as previously described (39, 40). The two preparations of human CD154 gave similar results, and specificity of CD154 was confirmed by detecting >95% neutralization in response to coincubation with anti-human CD154 monoclonal antibody (MAb) (Ancell Corporation, Bayport, MN). As negative controls, cells were incubated in culture medium alone without CD154 or with a nonfunctional mutant of CD154 (41) (T147N; obtained from Richard Kornbluth). Primary mouse RPE were incubated with mouse CD154 (cell-free supernatants containing multimeric CD154, obtained from Richard Kornbluth). Cells were also treated with human or mouse gamma interferon (IFN-γ; 100 U/ml) or human or mouse tumor necrosis factor alpha (TNF-α; 1 ng/ml) for 18 h prior to infection. In certain experiments, mammalian cells were incubated with leupeptin (10 μM; EMD Millipore Corporation, Billerica, MA), pepstatin (10 μM; EMD Millipore), NG-monomethyl-l-arginine (NMA, 100 μM; EMD Millipore), l-tryptophan (100 μg/ml; Sigma Chemical), or diphenyleneiodonium chloride (DPI, 5 μM; Sigma Chemical) 1 h prior to infection. Mammalian cells were also treated with 3-methyl adenine (3-MA) (10 mM; Sigma Chemical) 2 h after infection.
Immunofluorescence microscopy.
Endothelial or epithelial cells expressing LC3-eGFP or tfLC3 were fixed with 4% paraformaldehyde, and slides were mounted with Fluoromount G (Southern Biotech; Birmingham, AL) and analyzed by fluorescence microscopy for distinct structures that measured at least 1 μm in diameter. LC3-EGFP-expressing endothelial or epithelial cells were stimulated with or without CD154, infected with T. gondii, and then fixed with 4% paraformaldehyde at designated time points postinfection and analyzed by fluorescence microscopy. In certain experiments, endothelial or epithelial cells were fixed with 4% paraformaldehyde at designated time points postinfection and permeabilized with ice-cold methanol followed by incubation in blocking buffer. Monolayers were incubated overnight at 4°C with either mouse anti-human LAMP-1 or rat anti-mouse LAMP-1 (all from Developmental Studies Hybridoma Bank, Iowa City, IA). Monolayers were washed with phosphate-buffered saline (PBS) plus 1% bovine serum albumin (BSA) and then incubated for 1 h at room temperature with Alexa 568-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Slides were mounted using Fluoromount G. Specificity of staining was determined by incubating monolayers with secondary antibody alone. Monolayers were analyzed using a Leica DMI 6000 B automated microscope equipped for epifluorescence microscopy. Accumulation of LC3 or LAMP-1 was deemed to have taken place if there was a ring of staining around the parasite (25). Experimental groups had triplicate samples, and at least 100 cells per sample were counted.
Immunoblotting.
Endothelial or epithelial cells were lysed at designated time points after CD154 treatment. Cells transfected with control siRNA, Beclin 1 siRNA, or Atg7 siRNA were lysed after 48 h. Proteins were resolved on 10% or 15% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes. Antibodies were directed against LC3 (MBL International; Woburn, MA), Beclin 1 (BD Bioscience; San Jose, CA), Atg7 (Cell Signaling Technology, Danvers, MA), or actin (Santa Cruz Biotechnologies; Santa Cruz, CA).
Statistics.
Statistical significance was assessed by 2-tailed Student's t test and analysis of variance (ANOVA) using InStat version 3.0 (GraphPad, La Jolla, CA). Differences were considered statistically significant when P was <0.05.
RESULTS
Role of CD40 engagement in killing of T. gondii within nonhematopoietic cells.
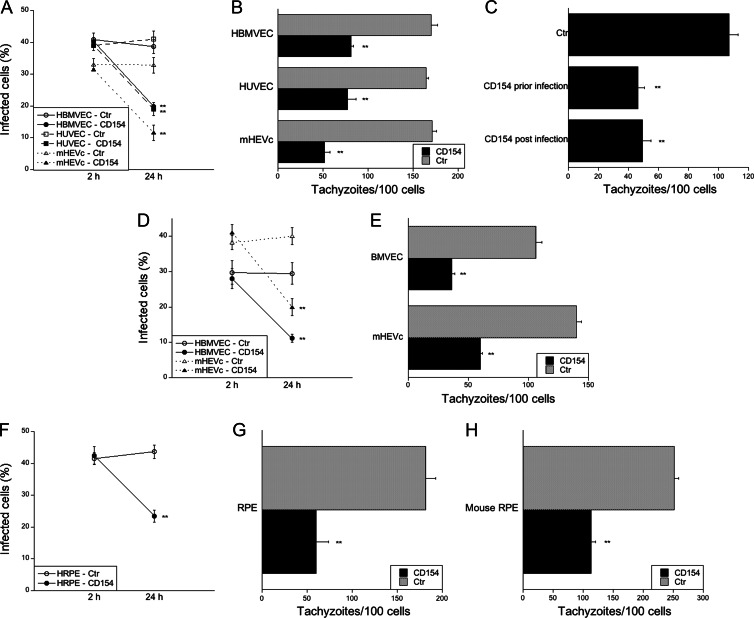
We investigated if CD40 stimulation of nonhematopoietic cells induces antimicrobial activity against T. gondii. These studies were conducted using human brain microvascular endothelial cells (HBMVEC) and human umbilical vein endothelial cells (HUVEC). These cells are likely involved in the invasion of the brain and fetus, respectively, during parasite dissemination via the bloodstream (42, 43). Primary HBMVEC and HUVEC express CD40 (3, 44). These cells were treated with or without CD154 followed by challenge with a type I (RH) strain of T. gondii and determination of the percentage of cells infected at 2 h or 24 h postinfection (Fig. 1A). In response to CD40 stimulation, there was a reduction in the percentage of infected cells by 24 h, indicative of parasite killing (Fig. 1A). Furthermore, killing of T. gondii was also observed in a mouse endothelial cell line. Mouse high endothelial venule cells that express a human-mouse CD40 chimera (hmCD40-mHEVc) exhibited a reduction in the percentage of infected cells in response to CD40 stimulation (Fig. 1A). Parasite load was quantitated at 24 h postinfection by determining the number of parasites per 100 host cells. CD40 stimulation induced a significant reduction in the parasite load in all types of endothelial cells (Fig. 1B). Anti-T. gondii activity was observed not only in endothelial cells incubated with CD154 prior to infection but also in cells that were exposed to CD154 after challenge with T. gondii (Fig. 1C). In addition, CD40 stimulation caused antimicrobial activity against not only a type I but also a type II strain of T. gondii. CD40 stimulation of HBMVEC and mHEVc infected with the ME49 (type II) strain of T. gondii reduced the percentage of infected cells at 24 h and decreased the parasite load (Fig. 1D and E).
Fig 1.
CD40 stimulation of nonhematopoietic cells induces killing of T. gondii. (A to C) Human brain microvascular endothelial cells (HBMVEC), human umbilical cord endothelial cells (HUVEC), and a mouse high endothelial venule cell line that expresses a human-mouse CD40 chimera (hmCD40-mHEVc) were incubated with or without CD154 prior to infection with tachyzoites of a type I strain of T. gondii (RH). (A) The percentages of infected cells were assessed 2 h or 24 h after challenge with T. gondii. (B) The numbers of tachyzoites per 100 cells were assessed at 24 h. (C) hmCD40-mHEVc were incubated with CD154 either 18 h before challenge with RH T. gondii or 1 h after challenge. The numbers of tachyzoites per 100 cells were assessed at 24 h. (D, E) HBMVEC and hmCD40-mHEVc were stimulated with or without CD154 prior to infection with tachyzoites of a type II strain of T. gondii (ME49). The percentages of infected cells (D) and the numbers of tachyzoites per 100 cells (E) were assessed as described above. (F to H) A human retinal pigment epithelial cell line that expresses CD40 (hCD40-HRPE) (F, G) or primary mouse RPE cells (H) were stimulated with or without CD154 prior to infection with tachyzoites of a type I strain of T. gondii (RH). The percentages of infected cells (F) and the numbers of tachyzoites per 100 cells (G, H) were assessed as described above. Results are shown as means ± standard errors of the means (SEM) and are representative of 3 or 5 independent experiments. **, P ≤ 0.01.
Retinal pigment epithelial cells (RPE) are another type of nonhematopoietic cell that is relevant to toxoplasmosis since RPE are thought to be effectors of resistance against ocular toxoplasmosis (45). We used primary mouse RPE and a human RPE (HRPE) cell line (ARPE-19) to examine the effects of CD40 ligation on the induction of anti-T. gondii activity. Primary mouse RPE expressed CD40 as assessed by fluorescence-activated cell sorter (FACS) (not shown). While primary HRPE can express CD40, the cell line ARPE-19 is CD40− (32). Thus, we used this cell line, which was stably transfected to express human CD40 (hCD40-HRPE). CD40 stimulation of hCD40-HRPE and primary mouse RPE elicited anti-T. gondii activity (Fig. 1F to H). Taken together, CD40 stimulation activates toxoplasmacidal activity against type I and type II T. gondii strains in various nonhematopoietic cells.
Role of CD40 as a regulator of autophagy in nonhematopoietic cells.
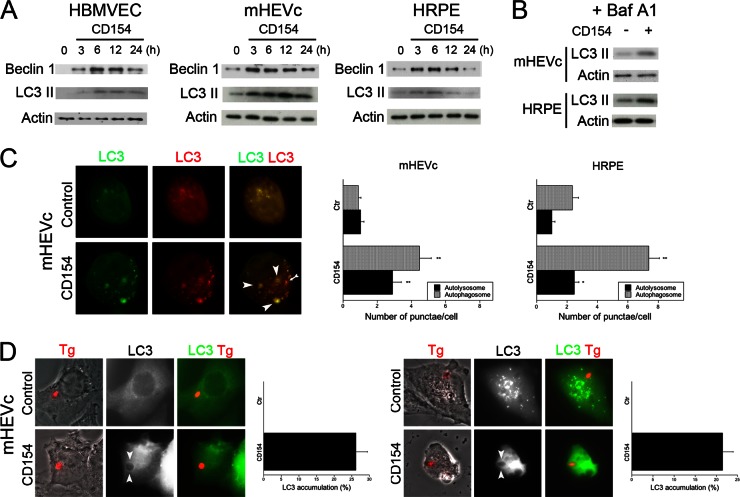
Treatment with an oxidative pathway inhibitor (DPI), tryptophan, or an NOS2 inhibitor (NMA) did not affect anti-T. gondii activity induced by CD40 ligation in nonhematopoietic cells (data not shown), indicating that anti-T. gondii activity induced by CD40 in these cells was unlikely to be dependent on the oxidative pathway, tryptophan starvation, or nitric oxide production. We previously reported that CD40 stimulates autophagy in macrophages/microglia and leads to killing of T. gondii dependent on autophagy proteins (17, 20). Thus, we sought to examine the role of autophagy in the anti-T. gondii activity induced by CD40 in nonhematopoietic cells. First, we assessed if CD40 stimulation alters expression of Beclin 1, a critical regulator of autophagy (46). HBMVEC, hmCD40-mHEVc, and hCD40-HRPE were stimulated with or without CD154 and lysed to monitor for expression of Beclin 1. All cell types exhibited increased expression of Beclin 1 at 3 h postincubation with CD154 (Fig. 2A). This enhanced expression persisted over the course of 12 to 24 h of stimulation. Next, we examined the effect of CD40 ligation on the expression of LC3 II, a molecule associated with the autophagosome membrane (47). Incubation with CD154 increased the levels of LC3II in HBMVEC, hmCD40-mHEVc, and hCD40-HRPE (Fig. 2A). To determine whether increased expression of LC3 II was due to enhanced autophagy or rather inhibition of autophagosome turnover, we treated cells with bafilomycin A1, an inhibitor of the vacuolar ATPase, which prevents autophagosome degradation (48). Incubation with CD154 in the presence of bafilomycin A1 also resulted in an increase in LC3 II expression in hmCD40-mHEVc and hCD40-HRPE, supporting a role for CD40 in stimulating the induction of autophagy (Fig. 2B). To further determine whether CD40 ligation stimulates autophagy, cells were transfected with a plasmid that encodes tandem fluorescently labeled LC3 (tfLC3). This plasmid allows detection of autophagosomes and autolysosomes (34). hmCD40-mHEVc and hCD40-HRPE transfected with a plasmid encoding tfLC3 exhibited an increase in both autophagosome and autolysosome punctae after incubation with CD154 (Fig. 2C). Thus, CD40 ligation upregulates expression of Beclin 1 and enhances autophagy in nonhematopoietic cells.
Fig 2.
CD40 stimulation enhances autophagic activity in nonhematopoietic cells. (A) HBMVEC, hmCD40-mHEVc, or hCD40-HRPE cells stimulated with CD154 for indicated times were lysed and analyzed by immunoblotting for expression of Beclin 1, LC3 II, or actin. (B) hmCD40-mHEVc or hCD40-HRPE cells stimulated with or without CD154 in the presence of bafilomycin A1 (Baf A1; 100 nM) for 6 h were lysed and analyzed by immunoblotting for expression of LC3 II or actin. (C) hmCD40-mHEVc or hCD40-HRPE transfected with tfLC3 were stimulated with or without CD154 for 5 to 6 h and monitored by fluorescence microscopy for the number of autophagosomes (yellow; arrowheads) or autolysosomes (red; arrow). (D) hmCD40-mHEVc or hCD40-HRPE cells transfected with LC3-EGFP were stimulated with or without CD154 and infected with T. gondii expressing RFP. At 5 h postinfection, cells were analyzed and quantitated by fluorescence microscopy for accumulation of LC3 around the parasite (arrowheads). Results are shown as means ± SEM and are representative of 3 or 4 independent experiments. *, P ≤ 0.05; **, P ≤ 0.01.
Next, we examined the distribution of LC3 in T. gondii-infected cells after CD40 stimulation. hmCD40-mHEVc and hCD40-RPE transfected with a plasmid encoding LC3-EGFP were treated with or without CD154 and infected with T. gondii organisms that express RFP in their cytoplasm (T. gondii-RFP). CD40 stimulation led to accumulation of LC3 around the parasite in both hmCD40-mHEVc and hCD40-RPE (Fig. 2D). These results indicate that CD40 ligation stimulates autophagy in various nonhematopoietic cells and suggest that CD40 stimulation induces the formation of an autophagosome that encases the parasite.
Role of autophagy in T. gondii delivery to late endosomes/lysosomes in CD40-stimulated nonhematopoietic cells.
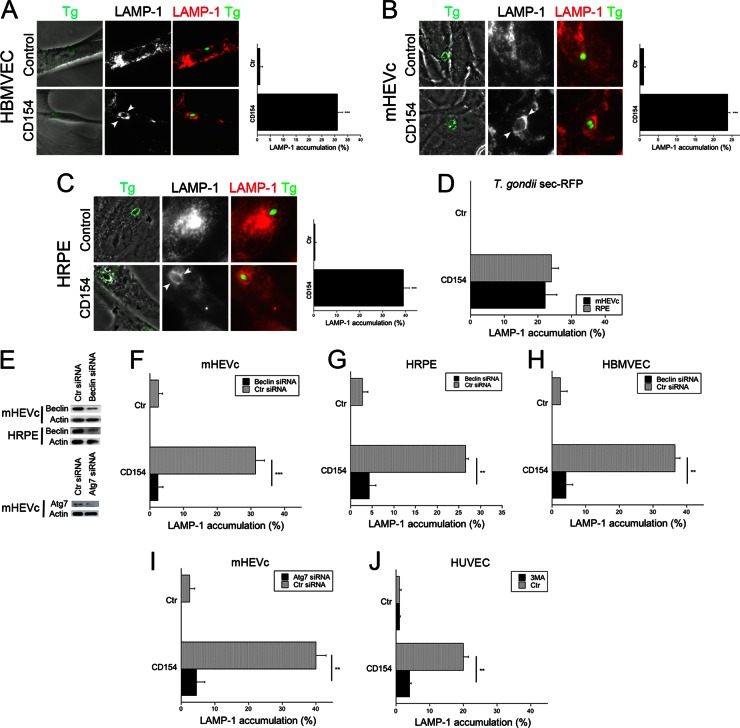
A key aspect of autophagy is the fusion of autophagosomes with lysosomes for cargo degradation. To this end, we examined the localization of the late endosomal/lysosomal marker, LAMP-1. HBMVEC, hmCD40-mHEVc, or hCD40-RPE were treated with or without CD154 and infected with T. gondii-YFP. CD40 stimulation resulted in enhanced accumulation of LAMP-1 around the parasite in all these cells (Fig. 3A to C). Secretion of dense granule contents by the parasite into the parasitophorous vacuole is a key aspect of the formation of these vacuoles after active invasion of a host cell by T. gondii (49). We utilized parasites that express fluorescence targeted to dense granules (T. gondii sec-RFP) to determine whether CD40 stimulation caused accumulation of LAMP-1 around parasitophorous vacuoles as opposed to phagosomes. Incubation with CD154 caused accumulation of LAMP-1 around vacuoles that associate with RFP (parasitophorous vacuoles), indicating that vacuole-lysosome fusion had occurred (Fig. 3C). Next, we examined whether delivery of T. gondii to the lysosomal pathway was dependent on autophagy proteins. Figure 3E shows that transfection of endothelial cells or retinal pigment epithelial cells with Beclin 1 siRNA or Atg7 siRNA diminished expression of these autophagy molecules. Moreover, gene knockdown impaired CD40-induced enhancement in autophagy flux as assessed by expression of autophagosomes and autolysosomes in cells transfected with tfLC3 plasmid (not shown). Silencing of Beclin 1 in hmCD40-mHEVc and hCD40-RPE ablated accumulation of LAMP-1 around the parasite in cells treated with CD154 (Fig. 3F and G). These findings also applied to primary cells since HBMVEC transfected with Beclin 1 siRNA exhibited diminished LAMP-1 accumulation around T. gondii after incubation with CD154 (Fig. 3H). The effect of Beclin 1 silencing was specific since it did not affect LAMP-1 accumulation around latex beads in hCD40-RPE (not shown). To further explore the role of the autophagy machinery, we examined the effects of knockdown of another autophagy protein. Similar to the results obtained with Beclin 1 silencing, knockdown of Atg7 inhibited accumulation of LAMP-1 around the parasites in hmCD40 mHEVc incubated with CD154 (Fig. 3I). In addition, HUVEC stimulated with CD154 and infected with T. gondii exhibited accumulation of LAMP-1 around the parasite, which was abrogated when cells were treated with 3-MA, an inhibitor of autophagy (Fig. 3J). Taken together, these results indicate that delivery of T. gondii to the late endosomal/lysosomal compartment is dependent on autophagy proteins.
Fig 3.
CD40 induces vacuole-lysosome fusion in T. gondii-infected nonhematopoietic cells, dependent on the autophagy machinery. (A to C) HBMVEC (A), hmCD40-mHEVc (B), and hCD40-HRPE (C) were incubated with or without CD154 and infected with RH T. gondii-YFP. At 8 h postinfection, LAMP-1 expression was assessed by immunofluorescence, and the percentages of cells that exhibited accumulation of LAMP-1 around the parasites (arrowheads) were quantitated. (D) hmCD40-mHEVc were stimulated with or without CD154 and infected with T. gondii expressing Sec-RFP. Percentages of cells that exhibited accumulation of LAMP-1 around vacuoles that associate with RFP (parasitophorous vacuoles) were quantitated. (E) hmCD40-mHEVc or hCD40-HRPE were transfected with control siRNA, Beclin 1 siRNA, or Atg7 siRNA. Expression of Beclin 1, Atg7, and actin was assessed by immunoblotting. (F to H) hmCD40-mHEVc (F), hCD40-HRPE (G), or HBMVEC (H) transfected with control or Beclin 1 siRNA were incubated with or without CD154 and infected with T. gondii expressing YFP. Cells were immunostained for LAMP-1 and analyzed by fluorescence microscopy for accumulation of LAMP-1 around the parasite. (I) hmCD40-mHEVc were transfected with either control siRNA or Atg7 siRNA. Cells were incubated with or without CD154 and infected with T. gondii expressing YFP. Cells were immunostained for LAMP-1 expression and then analyzed by fluorescence microscopy for accumulation of LAMP-1 around the parasite. (J) HUVEC were incubated with or without CD154, infected with T. gondii-YFP, and treated with or without 3-MA. LAMP-1 expression was assessed by immunofluorescence. Results are shown as means ± SEM and are representative of 3 independent experiments. **, P ≤ 0.01; ***, P ≤ 0.001.
Role of autophagy in CD40-induced killing of T. gondii in nonhematopoietic cells.
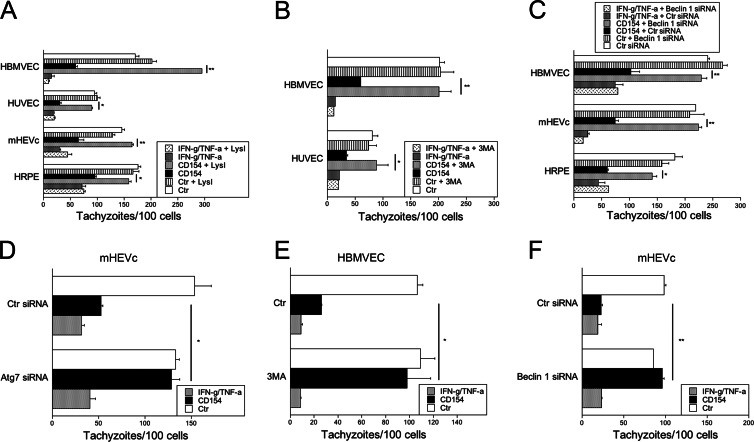
We next investigated if CD40-induced killing of T. gondii in nonhematopoietic cells was dependent on the autophagy machinery. CD40-activated cells were compared to cells treated with IFN-γ, since studies in astrocytes indicated that IFN-γ-induced anti-T. gondii activity was not accompanied by detectable encasing of the parasite or parasitophorous vacuoles by LC3 (50). Given that autophagosomes deliver their contents to lysosomes for degradation, we initially addressed the role of lysosomal degradation in killing of T. gondii utilizing the lysosomal protease inhibitors leupeptin and pepstatin. HBMVEC, HUVEC, hmCD40-mHEVc, and hCD40-RPE were stimulated with or without CD154 or IFN-γ/TNF-α and infected with T. gondii. At 1 h postinfection, cells were treated with or without leupeptin plus pepstatin. Lysosomal protease inhibitors ablated anti-T. gondii activity in all CD40-stimulated cell types (Fig. 4A). In contrast, these inhibitors did not affect killing of T. gondii in response to IFN-γ/TNF-α (Fig. 4A). Next, we assessed if CD40-mediated killing of T. gondii was dependent on the autophagy machinery. Treatment with the autophagy inhibitor 3-MA impaired anti-T. gondii activity induced by CD40 ligation in HBMVEC and HUVEC, while it had no effect on the anti-T. gondii activity induced by incubation with IFN-γ/TNF-α (Fig. 4B). In addition, silencing of Beclin 1 in HBMVEC, hmCD40-mHEVc, and hCD40-RPE ablated anti-T. gondii activity induced by CD40 ligation but not that induced by IFN-γ/TNF-α (Fig. 4C). Similar results were observed after silencing of Atg7 in hmCD40-mHEVc (Fig. 4D). Finally, 3-MA or silencing of Beclin 1 inhibited the antimicrobial activity against a type II T. gondii strain (ME49) in CD40-stimulated HBMVEC or hmCD40-mHEVc, respectively, but had no effect on cells treated with IFN-γ/TNF-α (Fig. 4E and F). Taken together, these studies indicate that in various nonhematopoietic cells CD40 triggers a toxoplasmacidal activity that is dependent on autophagy proteins.
Fig 4.
CD40 induces toxoplasmacidal activity in nonhematopoietic cells dependent on the autophagy machinery. (A) BMVEC, HUVEC, hmCD40-mHEVc, or hCD40-HRPE were stimulated with or without CD154 or IFN-γ/TNF-α, infected with T. gondii, and treated 1 h postinfection with or without lysosomal protease inhibitors (LysI) leupeptin and pepstatin. Cells were assessed 24 h postinfection for control of T. gondii by enumeration of tachyzoites per 100 cells. (B) BMVEC or HUVEC stimulated with or without CD154 or IFN-γ/TNF-α and infected with T. gondii. Cells were treated with the pharmacological inhibitor of autophagy 3-MA, and the numbers of tachyzoites per 100 cells were assessed at 24 h. (C) HBMVEC, hmCD40-mHEVc, or hCD40-HRPE transfected with either control siRNA or Beclin 1 siRNA were stimulated with or without CD154 or IFN-γ/TNF-α. Cells were infected with T. gondii, and the numbers of tachyzoites per 100 cells were examined at 24 h. (D) hmCD40-mHEVc transfected with either control siRNA or Atg7 siRNA were stimulated with or without CD154 or IFN-γ/TNF-α. Cells were infected with T. gondii, and the numbers of tachyzoites per 100 cells were examined at 24 h. (E) BMVEC were stimulated with or without CD154 or IFN-γ/TNF-α prior to infection with type II T. gondii. Cells were treated with or without 3-MA, and the numbers of tachyzoites per 100 cells were assessed at 24 h. (F) hmCD40-mHEVc transfected with either control siRNA or Beclin 1 siRNA were stimulated with or without CD154 or IFN-γ/TNF-α. Cells were infected with type II T. gondii and assessed 24 h postinfection for control of T. gondii by enumeration of tachyzoites per 100 cells. Results are shown as means ± SEM and are representative of 3 to 5 independent experiments. *, P ≤ 0.05; **, P ≤ 0.01.
DISCUSSION
Herein, we report that ligation of CD40 induced toxoplasmacidal activity in endothelial cells and retinal pigment epithelial cells. CD40 stimulation enhanced expression of Beclin 1 and enhanced autophagy flux in these cells. While evaluation for the potential role of classical autophagy in immunity needs to consider that LC3 can target structures other than autophagosomes (for example, phagosomes) and certain autophagy genes can have autophagy-independent function, several lines of evidence support that CD40 stimulation of endothelial cells and retinal pigment epithelial cells resulted in sequestration and degradation of intracellular T. gondii through classical autophagy: (i) activation of these cells through CD40 not only enhanced autophagy flux but also resulted in accumulation of LC3 around the parasite, which was followed by accumulation of LAMP-1, an event that took place around parasitophorous vacuoles and not phagosomes; (ii) CD40-induced killing of the parasite was dependent on the lysosomal protease activity; (iii) knockdown of either of 2 autophagy genes (Beclin 1 and Atg7) as well as the use of an autophagy inhibitor (3-MA) inhibited vacuole-lysosome fusion and toxoplasmacidal activity induced by CD40 in nonhematopoietic cells.
CD40 is a mediator of resistance against numerous pathogens. One of the mechanisms by which CD40 likely confers resistance is by activating antimicrobial activity in macrophages/microglia. Much less is known about the antimicrobial effects of CD40 ligation in nonhematopoietic cells. It has been reported that incubation of hepatocytes, a hepatocellular carcinoma cell line, and fibroblasts with CD154 diminished replication of hepatitis C virus, cytomegalovirus (CMV), and herpes simplex virus (HSV), respectively, through mechanism(s) that remain to be fully elucidated (51–53). In addition, CD154 stimulation of a hepatocellular carcinoma cell line impaired growth of C. parvum apparently by inducing apoptosis of host cells (18). Here, we report that CD40 stimulation of various endothelial cells and of RPE induces toxoplasmacidal activity that is dependent on lysosomal degradation of T. gondii.
While some pathogens appear to utilize autophagy to promote their intracellular growth (54), nonhematopoietic cells have been reported to utilize autophagy as an innate mechanism for degradation of various pathogens. Autophagy can be a cell-autonomous innate mechanism of defense against bacteria that colonize the cytosol. Epithelial cells exhibit transient clearance of Streptococcus pyogenes cells that invade the cytosol by targeting the bacteria with autophagosome-like compartments, leading to lysosomal degradation, a process that is dependent on Atg5 (55). Shigella flexneri cells that do not express the virulence factor IcsB cannot prevent recognition of the bacterial protein VirG by Atg5 (56) and avoid binding to the ubiquitin adaptor molecules p62 or NDP52 (57). As a result, the bacteria become susceptible to killing via autophagy within epithelial cells (56). Nonmotile Listeria monocytogenes actA mutant strains can no longer camouflage themselves by binding to host proteins of the Arp2/3 complex and Ena/VASP (58). Consequently, they become ubiquitinated and are surrounded by p62 and LC3, leading to targeting by autophagic clearance (58). Similarly, Salmonella enterica serovar Typhimurium cells that escape the salmonella-containing vacuoles and invade the cytosol from epithelial cells become coated with ubiquitin (59, 60). This results in recognition of the bacteria by NDP52, recruitment of LC3, and directing of the bacteria to autophagosomes and restriction in their growth (60). Autophagy has also been implicated in the degradation of viruses in nonhematopoietic cells. Protein kinase R (PKR)- and α subunit of eukaryotic initiation factor 2 (eIF2α)-dependent autophagy mediates degradation of herpes simplex virus 1 (HSV-1) (61). This virus produces ICP34.5, a neurovirulence factor that inhibits autophagy by reversal of PKR-mediated eIF2α phosphorylation and by antagonizing Beclin 1 (62, 63). As discussed above, our studies strongly suggest that CD40 triggers autophagy-dependent killing of T. gondii in various nonhematopoietic cells. This work indicates that, in addition to exhibiting the intrinsic ability to restrict certain pathogens via autophagy, nonhematopoietic cells can be activated by CD40 to acquire antimicrobial activity, likely via the autophagy pathway.
IFN-γ and TNF-α are key mediators of resistance against T. gondii. Studies using bone marrow chimeras indicated that these cytokines likely act in both the hematopoietic and nonhematopoietic compartments to elicit protection against cerebral toxoplasmosis (64). While studies in an animal model of C. parvum infection suggested that CD40 expression in hematopoietic but not in nonhematopoietic cells was required for protection (28), our findings raise the possibility that CD40 signaling in nonhematopoietic cells may play a role in protection against toxoplasmosis. Relevant to this possibility is the notion that endothelial cells and retinal pigment epithelial cells are thought to be important in the pathogenesis of toxoplasmosis. T. gondii disseminates throughout the bloodstream within either infected monocytes or dendritic cells, as well as via free tachyzoites (65, 66), indicating that organs like the brain, eye, and placenta would be invaded when the parasite traverses endothelial layers. Although the mechanisms of invasion are not well established, one of them appears to entail the migration of the parasite into the brain and presumably the eye within infected leukocytes (65–67). Extracellular tachyzoites could potentially enter organs, including the placenta if the parasite infects endothelial cells (transcellular traversal) or if the parasite migrates between endothelial cells (paracellular traversal) (67). It has been proposed that even when the parasite disseminates in the blood within infected leukocytes, T. gondii may actually traverse endothelial layers as an extracellular parasite after egress from infected leukocytes (67). Thus, the induction of antimicrobial activity in endothelial cells could potentially diminish organ invasion when T. gondii invades organs through transcellular traversal. Retinal pigment epithelial cells are thought to play an important role in ocular toxoplasmosis. These cells become hypertrophic, migrate to the retina, and appear to contain parasites (45, 68, 69). It has been suggested that this may represent an attempt at parasite eradication by retinal pigment epithelial cells (45). Our studies raise the possibility that the autophagy protein-dependent antimicrobial activity induced by CD40 ligation in endothelial cells and retinal pigment epithelial cells may play a role in protection against T. gondii. Of potential relevance, mice with Atg7 deficiency targeted to epithelial cells exhibited reduced clearance of Citrobacter rodentium and were more susceptible to disease, although these studies did not examine whether autophagy in epithelial cells promoted killing of the pathogen (70).
Several studies have reported that cytokines induce anti-T. gondii activity of various nonhematopoietic cells. HUVEC acquire toxoplasmastatic activity (inhibit T. gondii replication) in response to IFN-γ or the combination of TNF-α and IL-1β (43, 71). Incubation of immortalized human microvascular brain endothelial cells with IFN-γ causes toxoplasmastatic activity that is mediated by upregulation of the enzyme indoleamine 2,3-dioxygenase (IDO) (72), an enzyme that starves the parasite of tryptophan and was previously shown to mediate toxoplasmastasis induced by IFN-γ in human fibroblasts (73). IFN-γ also utilizes IDO upregulation to inhibit the growth of the parasite within astrocytoma cells without reducing the number of infected cells (74, 75). In contrast, IFN-γ induces anti-T. gondii activity in primary mouse astrocytes by recruiting the p47 GTPase IGTP to the parasitophorous vacuole and causing vacuolar disruption (76). Epithelial cells have been reported to inhibit T. gondii replication in response to cytokines (77, 78). Human retinal pigment epithelial cells inhibit T. gondii replication in response to IFN-γ and, to a lesser extent, in response to TNF-α and IFN-α/β (78). Similar to other human cells, the effect of IFN-γ is mediated by IDO (78). Our studies indicate that, similar to what occurs with macrophages/microglia (17, 20), the mechanism of anti-T. gondii activity induced by CD40 ligation in nonhematopoietic cells is different from those triggered by IFN-γ since knockdown of Beclin 1 or Atg7 or the use of 3-MA or lysosomal inhibitors blocked CD40-induced killing of T. gondii but had no appreciable effect on the anti-T. gondii activity induced by IFN-γ. Of relevance, autophagosomes did not engulf parasites or disrupted vacuoles in astrocytes treated with IFN-γ (50, 79). Moreover, while IFN-γ requires Atg5 to induce recruitment of p47 GTPase to the membrane of the parasitophorous vacuole in professional phagocytes, the effect of Atg5 occurs in the absence of autophagosome formation (80). Indeed, this finding represents an example of autophagosome-independent function of Atg5. The dichotomy in the mechanisms of anti-T. gondii activity-induced CD40 and IFN-γ likely explains the cooperation between these two pathways in the restriction of T. gondii growth (39). Another example of this cooperation may lie in the reports of strong CD40 upregulation in cells treated with IFN-γ (81).
In summary, we report that CD40-activated endothelial and epithelial cells control T. gondii likely through the autophagy machinery. These findings expand the knowledge on the antimicrobial effects triggered by CD40 ligation and on immune-mediated activation of the autophagy machinery in nonhematopoietic cells. This work may provide insight into mechanisms of host protection garnered by nonhematopoietic cells against other pathogens that infect these cells and that can be controlled by CD40.
ACKNOWLEDGMENTS
This work was supported by NIH Grants EY018341 (C.S.S.) and P30 EY11373. J.V.G. and L.M.-F were supported by the Visual Sciences Training Program grant from the National Institutes of Health (T32 EY007157). J.V.G. is a recipient of a predoctoral fellowship from Prevent Blindness Ohio. J.-A.C.P. is a recipient of a postdoctoral fellowship from the Ohio Lions Eye Research Foundation.
We thank William Fanslow (Amgen, Thousand Oaks, CA) and Richard Kornbluth (Multimeric Biotherapeutics Inc., La Jolla, CA) for providing reagents. We thank Scott Howell for assistance in image collection.
Footnotes
Published ahead of print 18 March 2013
REFERENCES
- 1. Clark EA, Ledbetter J. 1986. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc. Natl. Acad. Sci. U. S. A. 83:4494–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. 1993. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J. Exp. Med. 178:669–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, Aruffo A. 1995. Expression of functional CD40 by vascular endothelial cells. J. Exp. Med. 182:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schriever F, Freedman AS, Freeman G, Messner Lee G, Daley J, Nadler LM. 1989. Isolated human follicular dendritic cells display a unique antigenic phenotype. J. Exp. Med. 169:2043–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Aderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, Clark EA, Smith CA, Grabstein KH, Cosman D, Spriggs MK. 1992. Molecular and biological characterization of a murine ligand for CD40. Nature 357:80–82 [DOI] [PubMed] [Google Scholar]
- 6. Hollenbaugh D, Grosmaire LS, Kullas CD, Chalupny NJ, Braesch-Andersen S, Noelle RJ, Stamenkovic I, Ledbetter JA, Aruffo A. 1992. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell costimulatory activity. EMBO J. 11:4313–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. 1998. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391:591–594 [DOI] [PubMed] [Google Scholar]
- 8. Graf D, Muller S, Korthauer U, van Kooten C, Weise C, Kroczek RA. 1995. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur. J. Immunol. 25:1749–1754 [DOI] [PubMed] [Google Scholar]
- 9. Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S. 1993. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell 72:291–300 [DOI] [PubMed] [Google Scholar]
- 10. Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders EAM, Tabone MD, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrahamsen T, Jones A, Finn A, Klemola T, de Vries E, Sanal O, Peitsch MC, Notarangelo LD. 1997. Clinical spectrum of X-linked hyper-IgM syndrome. J. Pediatr. 131:47–54 [DOI] [PubMed] [Google Scholar]
- 11. Winkelstein JA, Marino MC, Ochs HD, Fuleihan R, Scholl PR, Geha R, Stiehm ER, Conley ME. 2003. The X-linked Hyper-IgM syndrome. Clinical and immunologic features of 79 patients. Medicine 82:373–384 [DOI] [PubMed] [Google Scholar]
- 12. Lazarevic V, Myers AJ, Scanga CA, Flynn JL. 2003. CD40, but not CD40L, is required for optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity 19:823–835 [DOI] [PubMed] [Google Scholar]
- 13. Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. 1996. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4:283–289 [DOI] [PubMed] [Google Scholar]
- 14. Soong L, Xu JC, Grewal IS, Kima P, Sun J, Longley BJ, Ruddle NH, McMahon-Pratt D, Flavell RA. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263–273 [DOI] [PubMed] [Google Scholar]
- 15. Kamanaka M, Yu P, Yasui T, Yoshida Kawabe T, Horii T, Kishimoto T, Kikutani H. 1996. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity 4:275–281 [DOI] [PubMed] [Google Scholar]
- 16. Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA. 2000. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 68:1312–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Portillo J-AC, Okenka G, Reed E, Subauste A, Van Grol J, Gentil K, Komatsu M, Tanaka K, Landreth G, Levine B, Subauste CS. 2010. The CD40-autophagy pathway is needed for host protection despite IFN-γ-dependent immunity and CD40 induces autophagy via control of p21 levels. PLoS One 5(2):e14472 doi:10.1371/journal.pone.0014472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cosyns M, Tsirkin S, Jones M, Flavell R, Kikutani H, Hayward AR. 1998. Requirement for CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect. Immun. 66:603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marriott I, Thomas EK, Bost KL. 1999. CD40-CD40 ligand interactions augment survival of normal mice, but not CD40 ligand knockout mice, challenged orally with Salmonella dublin. Infect. Immun. 67:5253–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. 2006. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Invest. 116:2366–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desvignes L, Ernst JD. 2009. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 31:974–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mosa A, Trumstedt C, Eriksson E, Soehnlein O, Heuts F, Janik K, Klos A, Dittrich-Breiholz O, Kracht M, Hidmark A, Wigzell H, Rottenberg ME. 2009. Nonhematopoietic cells control the outcome of infection with Listeria monocytogenes in a nucleotide oligomerization domain 1-dependent manner. Infect. Immun. 77:2908–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, Long EM, Robbins SM, Kubes P. 2009. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J. Clin. Invest. 119:1921–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joiner KA, Fuhrman SA, Mietinnen H, Kasper LH, Mellman I. 1990. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor transfected fibroblasts. Science 249:641–646 [DOI] [PubMed] [Google Scholar]
- 25. Mordue DG, Sibley LD. 1997. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanisms of entry. J. Immunol. 159:4452–4459 [PubMed] [Google Scholar]
- 26. Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, Boothroyd JC, Joiner KA. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125:261–274 [DOI] [PubMed] [Google Scholar]
- 27. Subauste CS, Wessendarp M, Sorensen RU, Leiva L. 1999. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type-1 immune response which can be restored by soluble CD40L trimer. J. Immunol. 162:6690–6700 [PubMed] [Google Scholar]
- 28. Hayward AR, Cosyns M, Jones M, Ponnuraj EM. 2001. Marrow-derived CD40 positive cells are required for mice to clear a Cryptosporidium parvum infection. Infect. Immun. 69:1630–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cook-Mills JM, Gallagher JS, Feldbush TL. 1996. Isolation and characterization of high endothelial cell lines derived from mouse lymph nodes. In vitro Cell. Dev. Biol. Anim. 32:167–177 [DOI] [PubMed] [Google Scholar]
- 30. Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. 2004. Loss of synchromized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J. Exp. Med. 200:1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrade RM, Wessendarp M, Portillo Yang J-ACJQ, Gomez FJ, Durbin JE, Bishop GA, Subauste CS. 2005. TRAF6 signaling downstream of CD40 primes macrophages to acquire anti-microbial activity in response to TNF-α. J. Immunol. 175:6014–6021 [DOI] [PubMed] [Google Scholar]
- 32. Kanuga N, Winton HL, Beauchene L, Koman A, Zerbib A, Halford S, Couraud PO, Keegan D, Coffey P, Lund RD, Adamson P, Greenwood J. 2002. Characterization of genetically modified human retinal pigment epithelial cells developed for in vitro and transplantation studies. Invest. Ophthalmol. Vis. Sci. 43:546–555 [PubMed] [Google Scholar]
- 33. Hsing Y, Hostager BS, Bishop GA. 1997. Characterization of CD40 signaling determinants regulating nuclear factor-kappa B activation in B lymphocytes. J. Immunol. 159:4898–4906 [PubMed] [Google Scholar]
- 34. Kimura S, Noda T, Yoshimori T. 2007. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3:452–460 [DOI] [PubMed] [Google Scholar]
- 35. Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. 2004. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304:1500–1502 [DOI] [PubMed] [Google Scholar]
- 36. Gubbels MJ, Li C, Striepen B. 2003. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob. Agents Chemother. 47:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. 2004. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J. Immunol. 173:2632–2640 [DOI] [PubMed] [Google Scholar]
- 38. Gubbels MJ, Striepen B, Shastri N, Turkoz M, Robey EA. 2005. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect. Immun. 73:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andrade RM, Wessendarp M, Subauste CS. 2003. CD154 activates macrophage anti-microbial activity in the absence of IFN-γ through a TNF-α-dependent mechanism. J. Immunol. 171:6750–6756 [DOI] [PubMed] [Google Scholar]
- 40. Portillo J-AC, Van Grol J, Zheng L, Okenka G, Gentil K, Garland A, Carson EC, Kern TS, Subauste CS. 2008. CD40 mediates retinal inflammation and neuro-vascular degeneration. J. Immunol. 181:8719–8726 [DOI] [PubMed] [Google Scholar]
- 41. Bajorath J, Seyama K, Nonoyama S, Ochs HD, Aruffo A. 1996. Classification of mutations in the human CD40 ligand, gp39, that are associated with X-linked hyper IgM syndrome. Protein Sci. 5:531–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lachenmaier SM, Deli MA, Meissner M, Liesenfeld O. 2011. Intracellular transport of Toxoplasma gondii through the blood-brain barrier. J. Neuroimmunol. 232:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dimier JH, Bout DT. 1993. Co-operation of interleukin-1β and tumour necrosis factor-α in the activation of human umbilical vein endothelial cells to inhibit Toxoplasma gondii replication. Immunology 79:336–338 [PMC free article] [PubMed] [Google Scholar]
- 44. Omari KM, Dorovini-Zis K. 2003. CD40 expressed by human brain endothelial cells regulates CD4+ T cell adhesion to endothelium. J. Neuroimmunol. 134:166–178 [DOI] [PubMed] [Google Scholar]
- 45. Tedesco RC, Smith RL, Corte-Real S, Calabrese KS. 2004. Ocular toxoplasmosis: the role of retinal pigment epithelium migration in infection. Parasitol. Res. 92:467–472 [DOI] [PubMed] [Google Scholar]
- 46. Liang XH, Jackson S, Seaman M, Brown KD, Kempkes B, Hibshoosh H, Levine B. 1999. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672–676 [DOI] [PubMed] [Google Scholar]
- 47. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19:5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. 1998. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 23:33–42 [DOI] [PubMed] [Google Scholar]
- 49. Carruthers VB, Sibley D. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114–123 [PubMed] [Google Scholar]
- 50. Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. 2005. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 1:187–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rau SJ, Hildt E, Himmelsbasch K, Thimme R, Wakita T, Blum HE, Fischer R. 2013. CD40 inhibits replication of hepatitis C virus in primary human hepatocytes by JNK activation independent from the interferon pathway. Hepatology 57:23–26 [DOI] [PubMed] [Google Scholar]
- 52. Hayward AR, Levy J, Facchetti F, Notarangelo L, Ochs HD, Etzioni A, Bonnefoy JY, Cosyns M, Weinberg A. 1997. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J. Immunol. 158:977–983 [PubMed] [Google Scholar]
- 53. Ruby J, Bluethmann H, Aguet M, Ramshaw IA. 1995. CD40 ligand has potent antiviral activity. Nat. Med. 1:437–441 [DOI] [PubMed] [Google Scholar]
- 54. Fine KL, Metcalfe MG, White ES, Virji M, Karls RK, Quinn FD. 2012. Involvement of the autophagy pathway in trafficiking of Mycobacterium tuberculosis bacilli through cultured human type II epithelial cells. Cell. Microbiol. 14:1402–1414 [DOI] [PubMed] [Google Scholar]
- 55. Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. 2004. Autophagy defends cells against invading Group A Streptococcus. Science 306:1037–1040 [DOI] [PubMed] [Google Scholar]
- 56. Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. 2005. Escape of intracellular Shigella from autophagy. Science 307:727–731 [DOI] [PubMed] [Google Scholar]
- 57. Mostowy S, Sancho-Shimizu V, Hamon MA, Simeone R, Brosch R, Johansen T, Cossart P. 2011. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 286:26987–26995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M, Mimuro H, Nakagawa I, Yanagawa T, Ishii T, Kakizuka A, Sztul E, Chakraborty T, Sasakawa C. 2009. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat. Cell Biol. 11:1233–1240 [DOI] [PubMed] [Google Scholar]
- 59. Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. 2006. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281:11374–11383 [DOI] [PubMed] [Google Scholar]
- 60. Thurston TLM, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. 2009. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 10:1215–1221 [DOI] [PubMed] [Google Scholar]
- 61. Talloczy Z, Virgin HW, Levine B. 2006. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2:24–29 [DOI] [PubMed] [Google Scholar]
- 62. Talloczy Z, Jiang W, Virgin HW, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. 2002. Regulation of starvation- and virus-induced autophagy by the eIF2α signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 99:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23–35 [DOI] [PubMed] [Google Scholar]
- 64. Yap G, Sher A. 1999. Effector cells of both nonhematopoietic and hematopoietic origin are required for interferon (IFN)-γ- and tumor necrosis factor (TNF)-α-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 189:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. 2006. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. 2006. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell. Microbiol. 8:1611–1623 [DOI] [PubMed] [Google Scholar]
- 67. Lambert H, Barragan A. 2010. Modelling parasite dissemination: host cell subversion and immune evasion by Toxoplasma gondii. Cell. Microbiol. 12:292–300 [DOI] [PubMed] [Google Scholar]
- 68. Gazzinelli RT, Brezin A, Li Q, Nussenblatt RB, Chan CC. 1994. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-α and IFN-γ. Exp. Parasitol. 78:217–229 [DOI] [PubMed] [Google Scholar]
- 69. Nicholson DH, Wolchok EB. 1976. Ocular toxoplasmosis in an adult receiving long-term corticosteroid therapy. Arch. Ophthalmol. 94:248–254 [DOI] [PubMed] [Google Scholar]
- 70. Inoue J, Nishiumi S, Fujishima Y, Masuda A, Shiomi H, Yamamoto K, Nishida M, Azuma T, Yoshida M. 2012. Autophagy in the intestinal epithelium regulates Citrobacter rodentium infection. Arch. Biochem. Biophys. 521:95–101 [DOI] [PubMed] [Google Scholar]
- 71. Woodman JP, Dimier IH, Bout DT. 1991. Human endothelial cells are activated by IFN-γ to inhibit Toxoplasma gondii replication. J. Immunol. 147:2019–2023 [PubMed] [Google Scholar]
- 72. Daubener W, Spors B, Hucke C, Adam R, Stins M, Kim KS, Schroten H. 2001. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect. Immun. 69:6527–6531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pfefferkorn ER. 1984. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cell to degrade tryptophan. Proc. Natl. Acad. Sci. U. S. A. 81:908–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Daubener W, Pilz K, Seghrouchni-Zennati S, Bilzer T, Fischer HG, Hadding U. 1993. Induction of toxoplasmostasis in a human glioblastoma by interferon γ. J. Neuroimmunol. 43:31–38 [DOI] [PubMed] [Google Scholar]
- 75. Daubener W, Remscheid C, Nockemann S, Pilz K, Seghouchni S, Mackenzie C, Hadding U. 1996. Anti-parasitic effector mechanisms in human brain tumor cells: role of interferon-gamma and tumor necrosis factor-alpha. Eur. J. Immunol. 26:487–492 [DOI] [PubMed] [Google Scholar]
- 76. Melzer T, Duffy A, Weiss LM, Halonen SK. 2008. The gamma interferon (IFN-γ)-inducible GTP-binding protein IGTP is necessary for Toxoplasma vacuolar disruption and induces parasite egression in IFN-γ-stimulated astrocytes. Infect. Immun. 76:4883–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dimier I, Bout D. 1993. Rat intestinal epithelial cell line IEC-6 is activated by recombinant interferon-γ to inhibit replication of the coccidian Toxoplasma gondii. Eur. J. Immunol. 23:981–983 [DOI] [PubMed] [Google Scholar]
- 78. Nagineni CN, Pardhasaradhi K, Martins MC, Detrick B, Hooks JJ. 1996. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect. Immun. 64:4188–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Halonen SK. 2009. Role of autophagy in the host defense against Toxoplasma gondii in astrocytes. Autophagy 5:268–269 [DOI] [PubMed] [Google Scholar]
- 80. Zhao Z, Fux B, Goodwin M, Dunay R, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, Schmidt RE, Mizushima N, Deretic V, Sibley LD, Virgin HW. 2008. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 4:458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Willermain F, Caspers-Velu L, Baudson N, Dubois C, Hamdane M, Willems F, Velu T, Bruyns C. 2000. Role and expression of CD40 on human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 41:3485–3491 [PubMed] [Google Scholar]