Abstract
Severe sepsis and septic shock caused mainly by bacterial infections are life-threatening conditions that urge the development of novel therapies. However, host responses to and pathophysiology of sepsis have not been clearly understood, which remains a major obstacle for the development of effective therapeutics. Recently, we have shown that stimulation of a costimulatory molecule, CD137, enhanced survival of mice infected with the Gram-positive (G+) intracellular bacterium Listeria monocytogenes but decreased survival in a polymicrobial sepsis model. Herein, we report that CD137 deficiency or blocking of CD137 signaling decreased antibacterial responses of mice infected with G+ bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis) but increased these responses in mice infected with Gram-negative (G−) bacteria (Escherichia coli, Pseudomonas aeruginosa, and Salmonella enterica serovar Typhimurium). Consistent with these findings, stimulation of CD137 by administration of agonistic antibody enhanced responses against G+ bacteria, whereas it decreased these responses against G− bacteria. Neutrophils were responsible for CD137-mediated opposite roles in control of G+ and G− bacterial infections. Stimulation of CD137 enhanced activities of neutrophils against S. aureus but decreased these activities against E. coli, while CD137 blocking produced opposite results with the stimulation of CD137 in vivo and in vitro. Furthermore, we found that combined signaling of CD137 and Toll-like receptor 2 (TLR2) induced synergistic production of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) by neutrophils, but combined signaling of CD137 and TLR4 did not. Our data strongly suggest that CD137 may play a dual role in sepsis in association with TLRs.
INTRODUCTION
Sepsis caused by bacterial infection produces high mortality rates in intensive care units (ICUs). It has been believed that death from sepsis is due to the host's uncontrolled hyperinflammatory responses, which induce cell death and organ injury. However, a number of clinical trials of anti-inflammatory agents, including tumor necrosis factor alpha (TNF-α)- and interleukin-1 (IL-1)-specific therapeutics, failed to improve survival in patients with sepsis. These trials indicate that further understanding of the pathophysiologic mechanisms in sepsis is required to reduce sepsis-associated mortality. It has been recognized that most septic patients in ICUs survive the initial hyperinflammatory phase, but this is followed by a hypoinflammatory state resulting in sepsis-induced multiorgan dysfunction and death, suggesting that sepsis-induced immunosuppression is the major factor contributing to these deaths (1). Therefore, enhancement of immune responses to pathogens in these septic patients may improve survival. Immunotherapy, a clinical modulation of lymphocyte function by manipulating costimulatory or coinhibitory molecules, is rapidly being applied to therapeutic regimens for a number of incurable diseases such as cancer and autoimmune diseases. Immunotherapy can also be successfully applied to sepsis cases. For example, a coinhibitory molecule, programmed death PD-1, is upregulated in immune cells of septic patients (2), and its levels correlate with increased mortality and immune dysfunction (3). Blockade of PD-1 signaling may help some septic patients, as demonstrated in experimental murine sepsis (4–6).
CD137 is a potent costimulatory molecule and member of the tumor necrosis factor receptor family (7). CD137 is expressed on a variety of immune cells, including T cells, neutrophils (8), dendritic cells (9), natural killer cells (10), mast cells (11), and eosinophils (12). CD137 activation by binding of its ligand, CD137L, induces a number of immune-enhancing responses, such as proliferation and inhibition of apoptosis in T cells; tumor rejection by natural killer cells (13); prevention of cancer recurrence (14); production of cytokines by dendritic cells; proliferation, survival, and cytokine production in monocytes (15); and abrogation of the granulocyte-macrophage colony-stimulating factor (GM-CSF)-mediated antiapoptotic action of human neutrophils (16). Previously, our research group showed that CD137-deficient (CD137−/−) mice are very susceptible to infection by Listeria monocytogenes, a Gram-positive (G+) intracellular bacterium, because the antilisteria activity of these neutrophils is defective (8). Furthermore, CD137 activation by administration of agonistic anti-CD137 antibodies completely protected mice from sepsis-induced death caused by L. monocytogenes infection (17). These studies indicated that CD137 protects mice from L. monocytogenes infection. Recently, we also found that CD137 aggravates sepsis induced by cecal ligation and puncture (CLP), which is a polymicrobial sepsis model including various G+ and G− bacteria (18). CD137 deficiency or blocking of CD137 signaling significantly improved survival of CLP mice, whereas stimulation of CD137 decreased it. Based on our previous findings, we hypothesized that CD137 may differentially function depending on the infecting bacterial species. In this study, we compared the effects of CD137 deficiency and stimulation and blocking of CD137 signaling on six different G+ and G− bacterial infections in mice and found that CD137 is detrimental in G− but beneficial in G+ bacterial infections.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free male BALB/c, C57BL/6J mice, 7 to 9 weeks old, were purchased from Orient Bio-Charles River (Seoul, Republic of Korea). CD137−/− and littermate mice were established as previously described (8), and TLR2−/− and MyD88−/− mice (19) developed by Shizuo Akira (Osaka, Japan) were bred into a C57BL/6J background in our laboratory. All experiments were conducted according to the regulations of the Animal Committee of the University of Ulsan. Staphylococcus aureus (ATCC 25923), Streptococcus pneumoniae (ATCC 6303), Enterococcus faecalis (ATCC 51299), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and Salmonella enterica serovar Typhimurium (ATCC 14028) were obtained from the American Type Culture Collection. Mice were inoculated intraperitoneally (i.p.) with 200 μg of rat IgG, 3E1, or TKS-1. After 24 h, they were i.p. injected with bacteria at the doses indicated below, and survival was recorded every 12 h for 7 days. To deplete neutrophils, anti-Gr-1 antibody RB6-8C5 (500 μg/mouse) was i.p. injected into mice 2 days before infection.
Antibodies and reagents.
TKS-1 and 3E1 hybridoma cells were donated by H. Yagita (Juntendo University, Japan) and R. Mittler (Emory University, United States), respectively (18). Monoclonal antibodies (MAbs) were produced from ascites of nude mice and were further purified on a protein G column. The agonistic anti-human CD137 MAb (4B4) was purchased from BD Pharmingen (San Diego, CA). Pam3CysSerLys4 (Pam3), poly(I·C) (poly IC), and lipopolysaccharide (LPS) from E. coli O111:B4, S. Typhimurium (ATCC 7824), and P. aeruginosa (ATCC 27316) were purchased from EMC Microcollections (Tübingen, Germany), InvivoGen (San Diego), and Sigma-Aldrich, respectively. All other antibodies were purchased from BD Pharmingen.
Cell preparation and flow cytometry.
Mice were sacrificed and peritoneal cavities were washed with 3 ml of sterile phosphate-buffered saline (PBS). Lavage fluid was centrifuged at 400 × g for 5 min at 4°C. Cell pellets were treated with red blood cell lysing buffer, washed, and resuspended in PBS containing 3 mM EDTA. For flow cytometry, cells were incubated with 1 μg/ml of anti-mouse Fcγ MAb (clone 2.4G2) to block nonspecific binding of MAb and then stained with 1 μg/ml of fluorescence-labeled antibodies.
Determination of viable bacteria in peritoneum and liver.
Mice were anesthetized and their livers were perfused with sterile RPMI 1640 medium containing 10% fetal bovine serum (FBS) to wash bacteria out of their blood vessels. Liver was homogenized in 2 ml of sterile PBS containing 0.1% Triton X-100. CFU in livers and peritoneal lavage fluid were determined by plating serial dilutions in PBS containing 0.1% bovine serum albumin (BSA) and counting the colonies. CFU were calculated as CFU/ml of peritoneal lavage fluid or CFU/liver.
Isolation of neutrophils and culture.
Mouse neutrophils were isolated from bone marrow using the Anti-Ly6G MicroBead kit (Miltenyi Biotec) according to the manufacturer's protocols. Isolated neutrophils were 98% pure, as assessed by flow cytometry. Human neutrophils were purified from adult peripheral blood by centrifugation on a Polymorphprep (Axis-Shield) gradient. Human and murine neutrophils (5 × 105 cells/well) in RPMI 1640 medium containing 10% FBS were cultured with rat IgG (5 μg/ml), 3E1 (5 μg/ml), Pam3 (200 ng/ml), 4B4 (10 μg/ml), or LPS (100 ng/ml).
Phagocytosis assays.
Phagocytosis assays were performed as previously described (20, 21), with minor modifications. Bacteria were heat killed (HK) at 95°C for 20 min and stained in 0.1% fluorescein isothiocyanate (FITC; 0.1 M carbonate buffer, pH 9.5). For in vivo phagocytosis assay, mice were i.p. injected with 5 × 108 FITC-labeled HK S. aureus or 1 × 107 FITC-labeled HK E. coli. After 1 h, peritoneal cells were collected, stained with phycoerythrin (PE)-anti-Ly6G and Cy-anti-CD11b, and analyzed by FACS. For in vitro phagocytosis assay, neutrophils were incubated with FITC-labeled HK bacteria, which were opsonized in 40% serum (37°C, 30 min) at a multiplicity of infection (MOI) of 10:1 at 37°C for 1 h. Phagocytosis was stopped by transfer of the cells to 4°C. Extracellular fluorescence was quenched by the addition of 200 μl of PBS containing 0.04% trypan blue and 1% formaldehyde, pH 5.5, and neutrophils were analyzed by fluorescence-activated cell sorting (FACS). Results are expressed as mean fluorescence intensity (MFI) of cells.
Bacterial killing assays.
Bacterial killing was measured as described previously (21). Briefly, cells were mixed with opsonized bacteria at an MOI of 10:1 and incubated at 37°C for 10 min with continuous rotation. Noningested bacteria were discarded by centrifugation, and cells were cultured for the next 60 min with slow rotation. Killing was stopped by spinning the cells onto ice after addition of 1 ml of distilled water containing 0.01% bovine serum albumin, and the number of viable bacteria was determined by plating 10-fold serial dilutions. The percent killing was calculated as follows: [1 − (number of viable bacteria at 60 min/number of viable bacteria at 0 min)] × 100.
Measurements of reactive oxygen species (ROS) generation.
Purified neutrophils were stained with 2 μM 2′,7′-dichlorodihydrofluorescein diacetate (Molecular Probes, MO) for 20 min at 37°C in the dark. After incubation, they were washed twice with PBS, incubated with bacteria at 37°C for 1 h, and analyzed by FACS.
Cytokine analysis by CBA.
Cytokines in peritoneal exudates and cell culture supernatants were quantified using a cytometric bead array (CBA) kit (BD Biosciences) with a FACSCaliber cytometer equipped with CellQuestPro and CBA software (Becton, Dickinson) according to the manufacturer's instructions. For measuring cytokine production from cultured murine neutrophils, purified neutrophils were seeded at 5 × 105 cells/well in RPMI 1640 medium containing 10% FBS and antibiotics and cultured with reagents as indicated below. After 20 h, culture supernatants were collected and cytokines in the supernatants were quantified using a CBA mouse inflammatory cytokine kit (R&D). For determining cytokine production from cultures of human neutrophils, cells were seeded at 5 × 105 cells/well in RPMI 1640 medium containing 10% FBS and antibiotics and cultured with the indicated reagents at the concentration of mouse IgG1, 4B4 (10 μg/ml), Pam3 (200 ng/ml), and LPS (100 ng/ml). After 4 h, cytokines in supernatants were determined by the human CBA inflammatory cytokine kit, as described above.
Statistical analysis.
All data were analyzed using GraphPad Prism5. Survival and paired data were analyzed by log rank test and t test, respectively. Data are expressed as means ± standard errors (SE). A P value of <0.05 was considered statistically significant.
RESULTS
CD137-deficient mice are susceptible to G+ but resistant to G− bacterial infections.
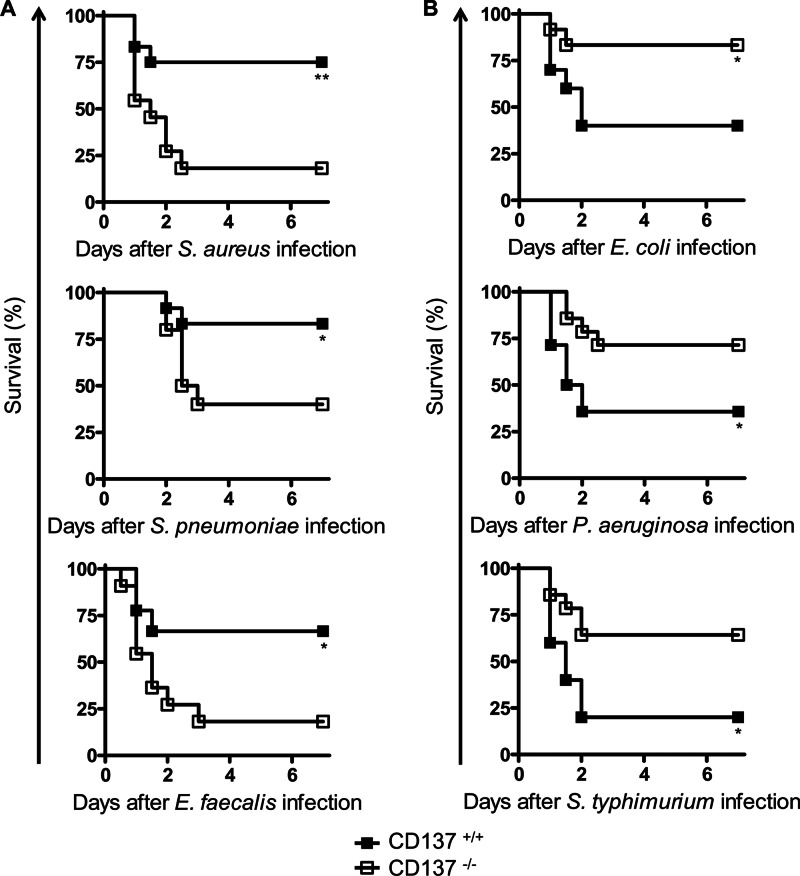
To explore the role of CD137 in bacterial infection, survival rates of CD137+/+ and CD137−/− mice inoculated i.p. with G+ (S. aureus, S. pneumoniae, or E. faecalis) or G− (E. coli, P. aeruginosa, or S. Typhimurium) bacteria were compared. As shown in Fig. 1A, for all G+ bacteria tested, survival rates of CD137−/− mice were significantly lower than those of CD137+/+ mice. On postinfection (p.i.) day 4, survival rates of CD137+/+ mice infected with S. aureus, S. pneumoniae, or E. faecalis were 75, 83, and 67%, respectively, but those of CD137−/− mice were only 18, 40, and 18%, respectively. In sharp contrast, in all G− bacterial infections, survival rates of CD137−/− mice were higher than those of CD137+/+ mice (Fig. 1B). On p.i. day 4, survival rates of CD137+/+ mice infected with E. coli, P. aeruginosa, or S. Typhimurium were 40, 36, and 20%, respectively, but those of CD137−/− mice were 83, 71, and 64%, respectively. Previously, it has been shown that CD137−/− mice are also more susceptible to infection with another G+ bacterium, L. monocytogenes (8), but more resistant to polymicrobial sepsis than are CD137+/+ mice (18).
Fig 1.
CD137-deficient mice are resistant to infection with G− bacteria but susceptible to infection with G+ bacteria. BALB/c CD137−/− and CD137+/+ littermates were inoculated i.p. with either G+ bacteria (5 × 108 S. aureus, 5 × 103 S. pneumoniae, or 8 × 107 E. faecalis organisms) (A) or G− bacteria (1 × 107 E. coli, 6 × 108 P. aeruginosa, or 2 × 106 S. Typhimurium organisms) (B), and mouse survival was monitored for 7 days. Each group contained 10 to 15 mice, and the results of 2 or 3 different experiments were pooled. *, P < 0.05; **, P < 0.01 (comparison with CD137+/+ mice, as determined by a log rank test).
Modulation of CD137 signaling oppositely regulates antibacterial responses to G+ and G− bacterial infections.
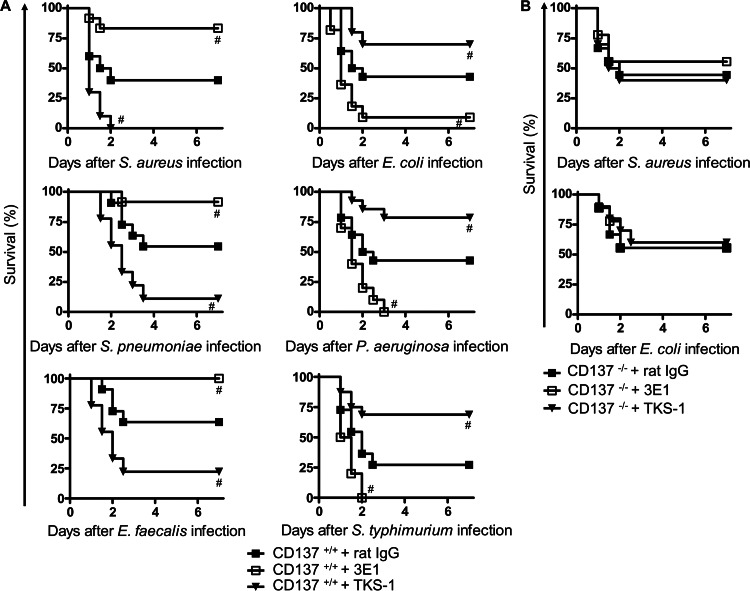
The role of CD137 in G+ or G− bacterium-induced sepsis was investigated by blocking or stimulating CD137 signaling using antibodies. Anti-CD137 MAb (3E1) has been used as an agonistic antibody that stimulates CD137 signaling in a variety of immune cells, including neutrophils (17). In contrast, anti-CD137L MAb (TKS-1) blocks CD137 signaling by binding to CD137L and inhibiting CD137-CD137L interactions (22). It was previously shown that 3E1 and TKS-1 can be used for stimulation and blocking of CD137 signaling, respectively, in CLP-induced sepsis (18). Mice were pretreated with 3E1, TKS-1, or control antibody, and after 24 h, mice were infected with G+ or G− bacteria. As shown in Fig. 2A, 3E1 pretreatment significantly enhanced survival of mice infected with each of the three strains of G+ bacteria, while TKS-1 pretreatment decreased survival. For example, on p.i. day 2 of S. aureus infection, survival rates of control antibody (rat IgG)-, 3E1-, and TKS-1-pretreated mice were 40, 83, and 0%, respectively. Surprisingly, opposite results were observed in G− bacterial infection experiments. 3E1 pretreatment significantly decreased survival of mice infected with each of the three G− bacteria, while TKS-1 treatments enhanced survival. On p.i. day 2, survival rates of E. coli-infected mice were 9, 43, and 70% for 3E1-, rat IgG-, and TKS-1-pretreated groups, respectively. In P. aeruginosa and S. Typhimurium infection groups, 3E1 pretreatment killed all mice (0 survivors/10 mice tested), while TKS-1 pretreatment enhanced survival up to 80%. Survival rates of CD137−/− mice infected with S. aureus or E. coli were not changed by 3E1 or TKS-1 pretreatment (Fig. 2B), indicating that 3E1 and TKS-1 exerted their effects through CD137 in our experiments. Another clone of agonistic anti-CD137 MAb, 3H3, had the same effect, and survival of S. aureus-infected C57BL/6 mice was also enhanced as much as that of S. aureus-infected BALB/c mice by 3E1 administration, indicating that CD137 effects on bacterially infected mice were not antibody isotype or mouse strain specific (data not shown).
Fig 2.
Effects of CD137 signaling modulation on survival rates of septic mice induced by G+ or G− bacteria. (A) CD137+/+ mice were injected with 200 μg of 3E1, TKS-1, or rat IgG. After 24 h, they were inoculated i.p. with either G+ bacteria (7.5 × 108 S. aureus, 1 × 104 S. pneumoniae, or 8 × 107 E. faecalis organisms) (left graphs) or G− bacteria (1 × 107 E. coli, 6 × 108 P. aeruginosa, or 2 × 106 S. Typhimurium organisms) (right graphs). (B) CD137−/− mice were administered 200 μg of 3E1, TKS-1, or rat IgG. After 24 h, they were inoculated i.p. with either 3.5 × 108 S. aureus or 2 × 107 E. coli organisms. Mouse survival was determined for 7 days. Each group contained 10 to 15 mice, and results of 2 or 3 different experiments were pooled. #, P < 0.05 (compared with control rat IgG-treated mice, as determined by log rank test).
In S. aureus (G+ bacterium) infection, CD137 signaling enhances antibacterial responses of mice, but in E. coli (G− bacterium) infection, CD137 signaling decreases antibacterial responses.
S. aureus and E. coli are the most common causes of G+ and G− bacterium-induced sepsis, respectively (20, 23). Therefore, these two bacteria were used to study underlying mechanisms of CD137 signaling in G+ and G− bacterial infections. It was first determined whether modulation of CD137 signaling affected bacterial clearance in S. aureus- or E. coli-infected mice. CD137−/− and CD137+/+ mice were infected, and the number of viable bacteria in the peritoneum and liver was determined. Figure 3A shows that CD137−/− mice had more S. aureus organisms and fewer E. coli organisms in their organs than did CD137+/+ mice. These results demonstrate that the presence of CD137 enhances bacterial clearance in S. aureus infection but inhibits this process in E. coli, which may influence mouse survival. The same result was observed in the infection study of wild-type mice using 3E1 or TKS-1. Pretreatment of mice with 3E1 decreased the numbers of bacteria in organs of S. aureus-infected mice but increased those in E. coli-infected mice. However, pretreatment of TKS-1 produced results that were opposite those obtained using 3E1, indicating that CD137 signaling helps mice eradicate S. aureus but inhibits this process in E. coli infection.
Fig 3.
CD137 stimulation enhances antibacterial activities of mice infected with S. aureus but suppresses those of mice infected with E. coli. CD137+/+ and CD137−/− mice were inoculated i.p. with 5 × 108 S. aureus or 1 × 107 E. coli organisms (top graphs), or CD137+/+ mice were preinjected with 200 μg of 3E1, TKS-1, or rat IgG and then inoculated with 7.5 × 108 S. aureus or 1 × 107 E. coli organisms (bottom graphs). Bacterial load (A), leukocyte recruitment to peritoneum (B), and cytokine levels in peritoneal fluid (C) and serum (D) were determined at 20 h after bacterial infection. Data are expressed as pg of cytokine/ml of peritoneal wash fluid or serum. Results are means ± SE. Results of 3 independent experiments including 3 to 6 mice per group were similar and pooled. *, P < 0.05; **, P < 0.01 (comparison with CD137+/+ mice). #, P < 0.05; ##, P < 0.01 (compared with control rat IgG-pretreated mice).
In bacterial infection, mouse survival is closely correlated with the numbers of infiltrated neutrophils and macrophages (20). Therefore, it was determined whether CD137 signaling in bacterium-infected mice affects leukocyte infiltration into infection sites. In S. aureus infection, CD137+/+ mice had higher numbers of peritoneal neutrophils and macrophages than did CD137−/− mice. In contrast, in E. coli infection, CD137+/+ mice had significantly lower numbers of peritoneal leukocytes than CD137−/− mice (Fig. 3B, top graphs). The opposite roles of CD137 in infiltration of leukocytes in S. aureus- and E. coli-infected mice were also confirmed in a study of stimulating or blocking CD137 by antibodies. As shown in Fig. 3B (bottom graphs), pretreatment with 3E1 increased leukocyte infiltration into the peritonea of S. aureus-infected mice but decreased those in E. coli-infected mice. In contrast, treatment with TKS-1 enhanced the number of leukocytes in the peritonea of E. coli-infected mice but decreased leukocyte numbers in S. aureus-infected mice. Taken together, these results suggest that stimulation of CD137 signaling induces rapid recruitment of leukocytes in mice infected with S. aureus but suppresses this process in mice infected with E. coli.
There has been a well-established correlation between mortality and enhanced production of proinflammatory cytokines such as TNF-α and IL-6 (24). Therefore, it was investigated whether modulation of CD137 signaling affected cytokine levels in mice infected with S. aureus or E. coli. As shown in Fig. 3C and D, S. aureus-infected CD137−/− mice had higher levels of TNF-α, IL-6, and IL-10 in their peritonea and sera than did CD137+/+ mice infected with S. aureus. However, E. coli-infected CD137−/− mice had lower levels of these cytokines than did CD137+/+ mice infected with E. coli. Blocking of CD137 signaling in wild-type mice by TKS-1 produced the same results as in CD137-deficient mice in S. aureus and E. coli infection experiments. However, stimulation of CD137 signaling by 3E1 produced opposite results with blocking experiments, that is, downregulation of TNF-α, IL-10, and IL-6 in S. aureus infection and upregulation of these cytokines in E. coli infection. Monocyte chemoattractant protein 1 (MCP-1), gamma interferon (IFN-γ), and IL-12 levels in mice infected with S. aureus or E. coli were not affected by CD137 signaling (data not shown).
CD137 signaling in vivo oppositely regulates antibacterial activities of neutrophils against S. aureus and E. coli.
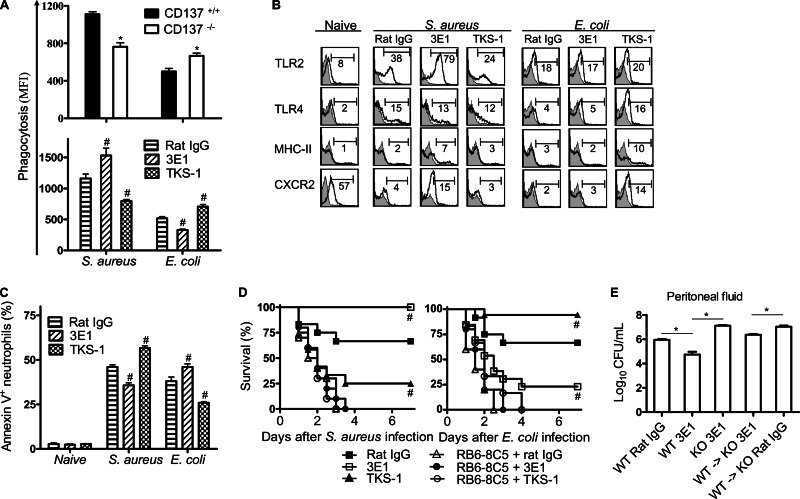
Neutrophils play a central role in host defense in the early stages of bacterial infection. It has been previously shown that murine neutrophils, but not monocytes or macrophages, constitutively express CD137, and stimulation of CD137 by 3E1 enhances activities of neutrophils against L. monocytogenes (8, 17). Neutrophils isolated from mice infected with S. aureus or E. coli were characterized. As shown in Fig. 4A, neutrophils of either CD137−/− mice or TKS-1-pretreated mice showed lower phagocytic activity against S. aureus, but higher phagocytic activity against E. coli, than did controls. In sharp contrast to TKS-1, 3E1 pretreatment increased neutrophil phagocytosis against S. aureus but decreased it against E. coli.
Fig 4.
CD137 signaling differentially modulates antibacterial activities of neutrophils in S. aureus and E. coli infections. (A) In vivo phagocytosis by neutrophils was determined by injection of FITC-labeled S. aureus or E. coli, isolation of neutrophils 1 h after infection, and flow cytometric analysis of neutrophils as described in Materials and Methods. (B) Flow cytometric analysis of neutrophil surface marker expression. CD137+/+ mice were preinjected with 200 μg of 3E1, TKS-1, or rat IgG and then with 7.5 × 108 S. aureus or 1 × 107 E. coli organisms, and peritoneal cells were isolated at 6 h after bacterial infection. Cells were gated on CD11b+ Ly6G+ and analyzed for each expression marker. Histograms are representative of at least 3 independent experiments in which each group contained 3 to 6 mice. Values in histograms depict percentage of cells with the indicated gate. Gray histograms represent staining with appropriate isotype control MAb. (C) CD137+/+ mice were preinjected with 200 μg of 3E1, TKS-1, or rat IgG and then with 7.5 × 108 S. aureus or 1 × 107 E. coli organisms, and peritoneal cells were harvested 20 h after bacterial infection, gated on CD11b+ Ly6G+, and analyzed for annexin V-positive cells. Data are shown as percentage of annexin V-positive neutrophils. (D) CD137+/+ mice were injected i.p. with 200 μg of RB6-8C5 MAb on day −2 and 3E1, TKS-1, or rat IgG on day −1. On day 0, mice were inoculated i.p. with 5 × 108 S. aureus or 8 × 106 E. coli organisms. Survival was monitored every 12 h for 7 days. Each group contained 10 to 15 mice, and results of 3 different experiments were pooled. #, P < 0.05 (compared with control rat IgG-pretreated mice). (E) 3E1 treatment of CD137−/− mice adoptively transferred with CD137+/+ neutrophils have enhanced clearance rates against S. aureus. Neutrophils were isolated from bone marrow of wild-type (WT) mice by MACS column with anti-Ly6G beads. CD137−/− (KO) mice were adoptively transferred with 5 × 106 purified neutrophils via the tail vein. Immediately after adoptive transfer, mice were i.p. injected with 200 μg of rat IgG or 3E1, and then after 4 h, mice were i.p. infected with 5 × 108 S. aureus organisms. Mice were sacrificed at 20 h p.i., and the numbers of viable bacteria in peritonea were determined as described in Materials and Methods. Results are means ± SE, and each group contained 4 mice. *, P < 0.05 (for comparison with wild-type mice). #, P < 0.05 (compared with control rat IgG-treated group).
Several studies have shown that Toll-like receptor 2 (TLR2) and TLR4 play crucial roles in host defense against S. aureus and E. coli infection, respectively (25, 26). CXCR2 mediates recruitment of neutrophils from the circulation into inflammation sites (27). It has been shown that impaired neutrophil migration during sepsis is correlated with downregulation of CXCR2 expression on neutrophils. It was therefore evaluated whether modulation of CD137 signaling affected surface marker expression on neutrophils in S. aureus and E. coli infection. Mice were treated with 3E1 or TKS-1 and infected with bacteria. After 6 h, neutrophils were isolated from peritonea and analyzed for TLR2, TLR4, major histocompatibility complex class II (MHC-II), and CXCR2. As shown in Fig. 4B, 3E1 treatment in mice increased expression of TLR2, MHC-II, and CXCR2 in S. aureus infection, but there was no change in these protein levels in E. coli infection, compared to the control group. In contrast, TKS-1 treatment increased expression of TLR4, MHC-II, and CXCR2 in E. coli infection, but there was little change in these levels in S. aureus infection. It is noteworthy that TLR2 levels on neutrophils were downregulated by TKS-1 treatment in S. aureus infection, which was not observed in E. coli infection.
Apoptosis of lymphocytes and neutrophils in sepsis induces defects in immunity and has been considered a critical factor determining sepsis-induced mortality. It was found that expression of annexin V, which is an indicator of early apoptotic cells, was also differentially regulated by modulation of CD137 signaling in S. aureus- and E. coli-infected mice. As shown in Fig. 4C, 3E1 treatment reduced annexin V+ neutrophils in S. aureus-infected mice but enhanced these cells in E. coli-infected mice. The opposite results were observed during TKS-1 pretreatment.
It was confirmed that neutrophils are essential to produce modulatory effects of 3E1 and TKS-1 on antibacterial responses of mice. Mice were depleted of neutrophils by injecting them with anti-Gr-1 MAb (RB6-8C5) before treatment with 3E1 or TKS-1. As expected, neutrophil depletion completely abolished 3E1 and TKS-1 effects on survival rates in S. aureus- and E. coli-infected mice (Fig. 4D). Furthermore, adoptive-transfer experiments also confirmed that neutrophils are responsible for CD137-mediated modulation of antibacterial responses. CD137−/− mice were adoptively transferred with CD137+/+ neutrophils and treated with 3E1. Infection of those mice with S. aureus resulted in enhanced clearance of bacteria compared to that in CD137−/− mice adoptively transferred but not treated with 3E1 (Fig. 4E). These results clearly indicate that CD137 on neutrophils modulates antibacterial responses in mice.
In vitro stimulation of CD137 on isolated neutrophils enhances antibacterial responses against S. aureus but decreases those against E. coli.
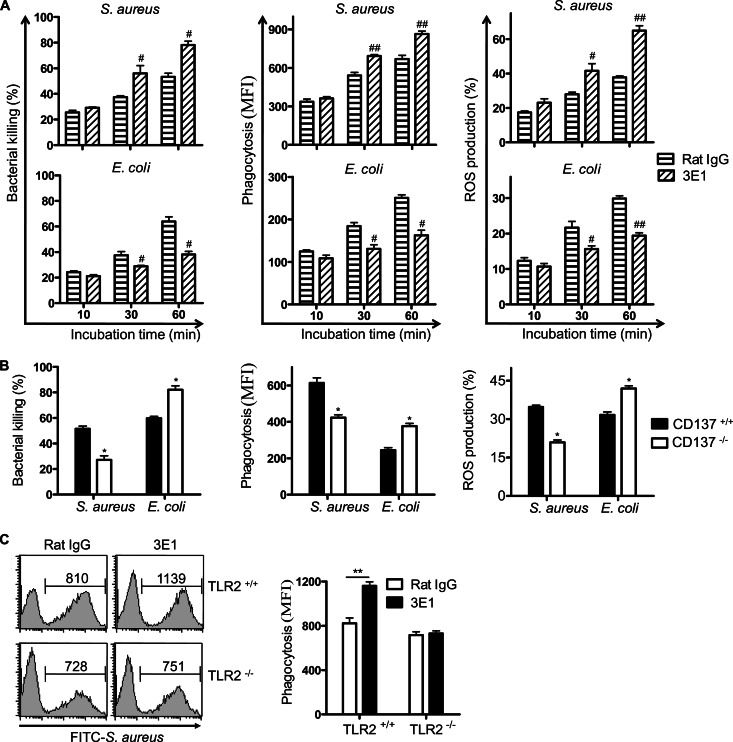
To directly demonstrate that CD137 on neutrophils differentially regulates antibacterial activities of neutrophils in G+ and G− bacterial infections, in vitro experiments were performed using purified neutrophils. Neutrophils were incubated with 3E1 for 2 h, and bacterial killing activities, bacterial phagocytosis, and ROS productions in response to S. aureus or E. coli were determined. Surprisingly, it was found that 3E1 treatment increased all antibacterial responses of neutrophils against S. aureus but decreased those against E. coli (Fig. 5A). Experiments using neutrophils isolated from CD137−/− mice also confirmed that CD137 enhanced antibacterial responses against S. aureus but decreased those against E. coli (Fig. 5B). When neutrophils from TLR2−/− mice were stimulated with 3E1, there was no increase of phagocytic activities against S. aureus, indicating that TLR2 is involved in the CD137-mediated increase of antibacterial responses against S. aureus (Fig. 5C). In addition, it was found that stimulation of CD137 on neutrophils oppositely modulated killing activities against other G+ (L. monocytogenes) and G− (P. aeruginosa) bacteria (data not shown).
Fig 5.
CD137 signaling oppositely regulates antibacterial activity of neutrophils against S. aureus and E. coli. Neutrophils were isolated from bone marrow of wild-type mice and cultured with rat IgG or 3E1 (5 μg/ml) at 37°C for 2 h (A), or neutrophils were isolated from bone marrow of CD137+/+ and CD137−/− mice (B). Bacterial killing activities, phagocytic activities, and ROS production by neutrophils against S. aureus or E. coli were determined as described in Materials and Methods. (C) Neutrophils were isolated from bone marrow of TLR2+/+ and TLR2−/− mice, cultured with rat IgG or 3E1 (5 μg/ml) at 37°C for 2 h, and incubated with FITC-labeled HK S. aureus (bacterium/cell ratio of 10:1) for 1 h. Cells were harvested and analyzed by FACS. Shown are a representative histogram (left side) and results of 3 experiments expressed as means plus standard deviations (right side). **, P < 0.01 between indicated groups.
Combined signaling of CD137 and TLR regulates antibacterial activities and cytokine production in neutrophils.
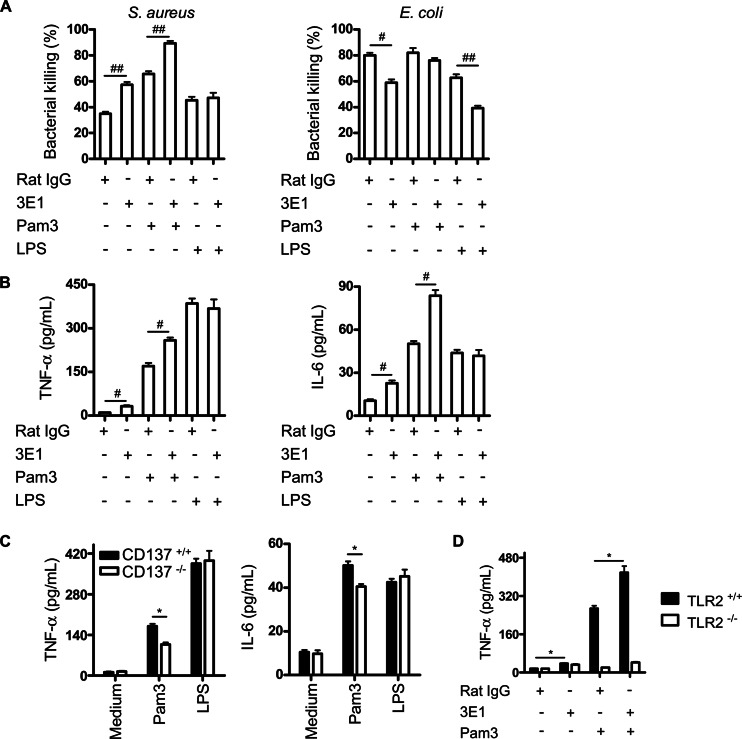
In bacterial infections, TLR2 and TLR4 expressed on immune cells are principal receptors for recognizing G+ and G− bacterial cell wall components. It was hypothesized that CD137 on neutrophils plays dual roles in G+ and G− bacterial infections with TLR2 and TLR4. To test this, it was determined whether combined stimulation of CD137 and TLR2 or TLR4 differentially affects antibacterial activities of neutrophils against S. aureus and E. coli infection. Pam3 and LPS were used to stimulate TLR2 and TLR4, respectively. As shown in Fig. 6A, treatment of cells with Pam3 and 3E1 synergistically increased killing activities of neutrophils against S. aureus but not against E. coli. In addition, combined treatment of neutrophils with 3E1 with Pam3 but not with LPS synergistically enhanced production of TNF-α and IL-6 (Fig. 6B). CD137−/− mouse neutrophils produced smaller amounts of TNF-α and IL-6 in response to Pam3 treatments than CD137+/+ mouse neutrophils did (Fig. 6C). As expected, the synergy of Pam3 and 3E1 on production of cytokines was not observed when neutrophils from TLR2−/− mice were used in experiments (Fig. 6D). These results suggest that CD137 signaling modulates antibacterial activities of neutrophils depending on TLR2 and TLR4 signaling.
Fig 6.
Combined stimulation of CD137 and TLR modulates antibacterial activities of neutrophils. (A) Neutrophils from wild-type mice were cultured with combinations of rat IgG, 3E1 (5 μg/ml), Pam3 (1 μg/ml), and LPS (1 μg/ml) for 2 h at 37°C. Cells were mixed with opsonized bacteria at an MOI of 10:1 and incubated for 10 min, and noningested bacteria were discarded by centrifugation. Cells containing ingested bacteria were cultured for 30 min at 37°C. After incubation, cells were harvested and the number of viable bacteria was determined by plating 10-fold serial dilutions. (B) Neutrophils from CD137+/+ mice were cultured with combinations of rat IgG, 3E1 (5 μg/ml), Pam3 (200 ng/ml), and LPS (100 ng/ml) as indicated. After 20 h, supernatants were harvested and cytokine levels were measured with a CBA kit. Data are presented as means of triplicate cultures. (C) Neutrophils from CD137+/+ and CD137−/− mice were cultured with Pam3 (200 ng/ml) or LPS (100 ng/ml) for 20 h, and concentrations of each cytokine in culture supernatants were determined. (D) Neutrophils from TLR2+/+ and TLR2−/− mice were cultured with 3E1 (5 μg/ml) and/or Pam3 (200 ng/ml) for 20 h, and concentrations of TNF-α in culture supernatants were determined. Results are representative of two independent experiments and are means of triplicate cultures.
DISCUSSION
In this study, we demonstrated that CD137 plays dual roles in control of G+ and G− bacterium-induced sepsis. First, CD137 gene deficiency or blocking of CD137 signaling increased murine susceptibility to G+ bacterial infection but made mice more resistant to G− bacterial infection (Fig. 1). Second, in G+ bacterial infection, stimulation of CD137 by agonistic antibody increased mouse survival, whereas in G− bacterial infection, it diminished mouse survival (Fig. 2). CD137 stimulation in S. aureus-infected mice lowered bacterial load, induced early leukocyte recruitment, and augmented phagocytic activity and activation markers in neutrophils. In contrast, CD137 stimulation in E. coli-infected mice increased bacterial outgrowth and neutrophil apoptosis and reduced neutrophil phagocytic capacity. Third, we demonstrated that CD137 stimulation increased phagocytosis, killing, and ROS production in purified neutrophils against S. aureus but decreased these antibacterial parameters in neutrophils against E. coli (Fig. 5). Our data strongly suggest that CD137 on neutrophils plays opposing roles against G− and G+ bacterial infections. Because we also established these dual roles of CD137 in human neutrophils (data not shown), our findings could be applicable in understanding the role of CD137 in human bacterial sepsis.
G+ bacterium-induced sepsis has been less studied than G− bacterial sepsis because of earlier general conceptions that G+ bacteria were less frequently involved in clinical human sepsis and that host responses to G+ bacteria were similar to those to G− bacteria. However, our study and others contradict these views and indicate that there might be fundamental differences in host responses to G+ and G− bacterial infections. First, the prevalence of G+ bacterial pathogens as a cause of sepsis has been increasing in relation to G− pathogens in recent years (28). In modern ICUs, there are more cases of G+ bacterial sepsis than G− bacterial sepsis, accounting for up to 50% of all severe sepsis cases. Second, G+ and G− bacterial infections can cause different pathophysiologies in hosts. Yu et al. reported that of 6,144 murine genes examined, 17 of them showed differential expression profiles between E. coli- and S. aureus-induced septic mice at particular time points (29). Differential gene expression in G+ and G− bacterial infections was also observed in human patients (30). In addition, it has been reported that G+ and G− bacteria differentially induced dysfunction of microcirculation in blood vessels (31). Our previous and present data showing that CD137 plays opposite roles in G+ and G− bacterial infections also indicate that hosts may differentially respond to infections with G+ and G− bacteria (17, 18).
TLRs are pattern recognition receptors that play critical roles in recognizing pathogens by binding to microbial pathogen-associated molecular patterns (PAMPs) and initiating immune responses to invading pathogens. Of all identified TLRs, it is known that TLR2 and TLR4 differentially recognize G+ and G− bacteria. TLR4 recognizes LPS, the most potent immunostimulant of G− bacteria, whereas TLR2 plays a major role in detecting G+ bacteria by recognizing lipoproteins and lipoteichoic acid (32). After binding their agonists, TLRs transduce intracellular activation signals by recruiting adaptor molecules at their intracellular domains. Both TLR4 and TLR2 use TIRAP to recruit MyD88 followed by IRAK and TRAF6, ultimately leading to NF-κB activation. CD137 is activated by binding of the CD137L, which is present on antigen-presenting cells and other cells (33). The CD137 signaling pathway involves TRAF1 and TRAF2 recruitment in cytoplasmic domains, which results in NF-κB and mitogen-activated protein kinase (MAPK) activation (34). Because both TRAF2 and TRAF6 downstream signaling pathways have been known to involve TAK1 and MAPK activation (35), it is reasonable to hypothesize that when both TLR and CD137 are stimulated, there may be cross talk between TLR and CD137 signaling, resulting in alteration of NF-κB activation. Although molecular mechanisms of TLR and CD137 cross talk have not been defined in this study, our results show that signaling of TLR2 and CD137 augments antibacterial activities of neutrophils, while that of TLR4-CD137 diminishes them.
There have been several reports suggesting that CD137 signaling with TLR stimulation results in modulation of immune cell activities and subsequent host responses to pathogens or antigens. It has been reported that CD137-deficient mice are very resistant to LPS-induced shock and that blocking of CD137-CD137L interaction by anti-CD137L antibody rescued wild-type mice from LPS-induced mouse death (36). This study correlated with our findings that CD137 deficiency in mice and blocking of CD137 signaling protects mice against G− bacterial infections in which major pathogenic molecules are LPS (Fig. 1 and 2). Shin et al. also observed that CD137-deficient mice have higher neutrophil and macrophage infiltration into LPS-injected peritonea, indicating that CD137 signaling modulated TLR4-induced inflammation (22). It has been shown that CD137 stimulation also modulates LPS-mediated CD8 T-cell clonal expansion and dendritic cell survival (19, 37). Therefore, it is evident that there are interactions between TLR4 and CD137 signaling in several different immune cells and that this signaling affects immune responses to pathogens or antigens. Although there have been a few reports implicating interactions of TLR4 (or LPS) and CD137 signaling, our study is the first observation of TLR2 and CD137 signaling interactions that synergistically activate antibacterial activities of neutrophils (Fig. 5). Because we also found TLR2 and CD137 interactions in antigen-primed CD8 T cells (data not shown), we believe that cross talk of CD137 and TLR signaling may be a general phenomenon in various immune cells.
In conclusion, our results indicate that CD137 activation improves G+ bacterial sepsis, whereas blockade of it has the same effect in G− bacterial sepsis. We found that neutrophils are responsible for these dual effects of CD137. Using two representative G+ and G− bacteria, we determined that stimulation of CD137 on neutrophils oppositely modulates antibacterial activities of neutrophils. Further understanding the role of CD137 in bacterial infections and enhancement of antibacterial activities by CD137 modulation may help to develop therapeutic regimens against bacterial sepsis, a major cause of high mortality and morbidity in ICUs.
ACKNOWLEDGMENTS
We thank Ki-Up Lee (University of Ulsan College of Medicine, Republic of Korea), R. Mittler (Emory University, United States), and H. Yagita (Juntendo University, Japan) for their kind gift of antibodies and for helpful comments and discussion.
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2010-0012408, 2009-0094050, and BRL-2011-0087350).
We have no conflicts of interest.
Footnotes
Published ahead of print 1 April 2013
REFERENCES
- 1. Hotchkiss RS, Opal S. 2010. Immunotherapy for sepsis—a new approach against an ancient foe. N. Engl. J. Med. 363:87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, Zhu K, Wan X, Cai Z, Deng X. 2011. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit. Care 15:R70 doi:10.1186/cc10059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Chéron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, Venet F. 2011. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit. Care 15:R99 doi:10.1186/cc10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. 2010. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J. Leukoc. Biol. 88:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. 2009. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. U. S. A. 106:6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. 2011. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock 36:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho HR, Kwon B, Yagita H, La S, Lee EA, Kim JE, Akiba H, Kim J, Suh JH, Vinay DS, Ju SA, Kim BS, Mittler RS, Okumura K, Kwon BS. 2004. Blockade of 4-1BB (CD137)/4-1BB ligand interactions increases allograft survival. Transpl. Int. 17:351–361 [DOI] [PubMed] [Google Scholar]
- 8. Lee SC, Ju SA, Pack HN, Heo SK, Suh JH, Park SMB, Choi K, Kwon BS, Kim BS. 2005. 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect. Immun. 73:5144–5151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. 2002. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 14:275–286 [DOI] [PubMed] [Google Scholar]
- 10. Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. 1998. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell. Immunol. 190:167–172 [DOI] [PubMed] [Google Scholar]
- 11. Nishimoto H, Lee SW, Hong H, Potter KG, Maeda-Yamamoto M, Kinoshita T, Kawakami Y, Mittler RS, Kwon BS, Ware CF, Croft M, Kawakami T. 2005. Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood 106:4241–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukushima A, Yamaguchi T, Ishida W, Fukata K, Mittler RS, Yagita H, Ueno H. 2005. Engagement of 4-1BB inhibits the development of experimental allergic conjunctivitis in mice. J. Immunol. 175:4897–4903 [DOI] [PubMed] [Google Scholar]
- 13. Choi BK, Kim YH, Kim CH, Kim MS, Kim KH, Oh HS, Lee MJ, Lee DK, Vinay DS, Kwon BS. 2010. Peripheral 4-1BB signaling negatively regulates NK cell development through IFN-γ. J. Immunol. 185:1404–1411 [DOI] [PubMed] [Google Scholar]
- 14. Narazaki H, Zhu Y, Luo L, Zhu G, Chen L. 2010. CD137 agonist antibody prevents cancer recurrence: contribution of CD137 on both hematopoietic and nonhematopoietic cells. Blood 115:1941–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kienzle G, von Kempis J. 2000. CD137 (ILA/4-1BB), expressed by primary human monocytes, induces monocyte activation and apoptosis of B lymphocytes. Int. Immunol. 12:73–82 [DOI] [PubMed] [Google Scholar]
- 16. Heinisch IV, Daigle I, Knöpfli B, Simon HU. 2000. CD137 activation abrogates granulocyte-macrophage colony-stimulating factor-mediated anti-apoptosis in neutrophils. Eur. J. Immunol. 30:3441–3446 [DOI] [PubMed] [Google Scholar]
- 17. Lee SC, Ju SA, Sung BH, Heo SK, Cho HR, Lee EA, Kim JD, Lee IH, Park SM, Nguyen QT, Suh JH, Kim BS. 2009. Stimulation of the molecule 4-1BB enhances host defense against Listeria monocytogenes infection in mice by inducing rapid infiltration and activation of neutrophils and monocytes. Infect. Immun. 77:2168–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen QT, Ju SA, Park SM, Lee SC, Yagita H, Lee IH, Kim BS. 2009. Blockade of CD137 signaling counteracts polymicrobial sepsis induced by cecal ligation and puncture. Infect. Immun. 77:3932–3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takahashi C, Mittler RS, Vella AT. 2001. Differential clonal expansion of CD4 and CD8 T cells in response to 4-1BB ligation: contribution of 4-1BB during inflammatory responses. Immunol. Lett. 76:183–191 [DOI] [PubMed] [Google Scholar]
- 20. Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, Gad F, Hamblin MR, Sastry KN, Ezekowitz RA. 2004. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 199:1379–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heo SK, Ju SA, Lee SC, Park SM, Choe SY, Kwon B, Kwon BS, Kim BS. 2006. LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. J. Leukoc. Biol. 79:330–338 [DOI] [PubMed] [Google Scholar]
- 22. Shin HH, Lee JE, Choi HS. 2007. Absence of 4-1BB increases cell influx into the peritoneal cavity in response to LPS stimulation by decreasing macrophage IL-10 levels. FEBS Lett. 581:4355–4360 [DOI] [PubMed] [Google Scholar]
- 23. Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. 2009. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 106:2348–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takahashi K, Shi L, Gowda LD, Ezekowitz RA. 2005. Relative roles of complement factor 3 and mannose-binding lectin in host defense against infection. Infect. Immun. 73:8188–8193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takeuchi O, Hoshino K, Akira S. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392–5396 [DOI] [PubMed] [Google Scholar]
- 26. van Westerloo DJ, Weijer S, Bruno MJ, de Vos AF, Van't Veer C, van der Poll T. 2005. Toll-like receptor 4 deficiency and acute pancreatitis act similarly in reducing host defense during murine Escherichia coli peritonitis. Crit. Care Med. 33:1036–1043 [DOI] [PubMed] [Google Scholar]
- 27. Alves-Filho JC, Freitas A, Souto FO, Spiller F, Paula-Neto H, Silva JS, Gazzinelli RT, Teixeira MM, Ferreira SH, Cunha FQ. 2009. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. U. S. A. 106:4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 29. Yu SL, Chen HW, Yang PC, Peck K, Tsai MH, Chen JJ, Lin FY. 2004. Differential gene expression in gram-negative and gram-positive sepsis. Am. J. Respir. Crit. Care Med. 169:1135–1143 [DOI] [PubMed] [Google Scholar]
- 30. Ramilo O, Allman W, Chung W, Mejias A, Ardura M, Glaser C, Wittkowski KM, Piqueras B, Banchereau J, Palucka AK, Chaussabel D. 2007. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood 109:2066–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Legrand M, Klijn E, Payen D, Ince C. 2010. The response of the host microcirculation to bacterial sepsis: does the pathogen matter? J. Mol. Med. (Berl.) 88:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650 [DOI] [PubMed] [Google Scholar]
- 33. Asai T, Choi BK, Kwon PM, Kim WY, Kim JD, Vinay DS, Gebhardt BM, Kwon BS. 2007. Blockade of the 4-1BB (CD137)/4-1BBL and/or CD28/CD80/CD86 costimulatory pathways promotes corneal allograft survival in mice. Immunology 121:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watts TH. 2005. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23:23–68 [DOI] [PubMed] [Google Scholar]
- 35. Oeckinghaus A, Hayden MS, Ghosh S. 2011. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12:695–708 [DOI] [PubMed] [Google Scholar]
- 36. Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS. 2004. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J. Immunol. 173:4218–4229 [DOI] [PubMed] [Google Scholar]
- 37. Choi BK, Kim YH, Kwon PM, Lee SC, Kang SW, Kim MS, Lee MJ, Kwon BS. 2009. 4-1BB functions as a survival factor in dendritic cells. J. Immunol. 182:4107–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]