Abstract
Molecular mimicry between Campylobacter jejuni sialylated lipooligosaccharides (LOS) and human nerve gangliosides can trigger the production of cross-reactive antibodies which induce Guillain-Barré syndrome (GBS). To better understand the immune events leading to GBS, it is essential to know how sialylated LOS are recognized by the immune system. Here, we show that GBS-associated C. jejuni strains bind to human sialoadhesin (hSn), a conserved, mainly macrophage-restricted I-type lectin. Using hSn-transduced THP-1 cells, we observed that C. jejuni strains with α(2,3)-sialylated LOS, including strains expressing GM1a- and GD1a-like epitopes, bind to hSn. This observation is of importance, as these epitopes are frequently the targets of the cross-reactive antibodies detected in GBS patients. Interestingly, the Sn binding domains were not constitutively exposed on the surface of C. jejuni. Heat inactivation and the environmental conditions which food-borne C. jejuni encounters during its passage through the intestinal tract, such as low pH and contact with bile constituents, exposed LOS and facilitated Sn binding. Sn binding enhanced bacterial uptake and increased the production of interleukin-6 (IL-6) by primary human Sn-expressing monocyte-derived macrophages compared to control conditions, where Sn was blocked using neutralizing antibodies or when nonsialylated C. jejuni was used. Sn-mediated uptake has been reported to enhance humoral immune responses. As C. jejuni strains expressing ganglioside mimics GD1a and GM1a are closely associated with GBS, Sn binding may be a determining event in the production of cross-reactive antibodies and the development of GBS.
INTRODUCTION
Guillain-Barré syndrome (GBS) is an acute, rapidly progressing, postinfectious neuropathy which results in severe muscle paresis. In the acute phase of the development of GBS, autoantibodies with specificity for gangliosides are frequently detected in patient serum (1, 2). These antibodies bind to ganglioside structures which are enriched on the peripheral nerves, resulting in immune-mediated damage and subsequent paralysis (3). Autoantibodies against α(2,3)-sialylated carbohydrate epitopes, present in gangliosides GM1a and GD1a, are especially detected in GBS patients (3, 4). Although it is accepted that antecedent infection by microorganisms carrying surface-exposed ganglioside-like structures can lead to production of anti-ganglioside antibodies (5–7), the precise immune events leading to anti-ganglioside antibody production are unclear. Infection with Campylobacter jejuni, an intestinal pathogen, most commonly precedes the production of anti-ganglioside antibodies and the development of GBS (7). Lipooligosaccharides (LOS) are major C. jejuni surface antigens that may contain sialylated carbohydrate moieties which are structurally identical to the carbohydrate moieties on human gangliosides (8, 9). Depending on gene content, phase variation, and mutations in the LOS biosynthesis loci, C. jejuni can express various ganglioside-like structures (10). The presence of genes involved in sialic acid biosynthesis and transfer is essential for the production of these mimics (11).
Recent studies have demonstrated that sialylation of LOS enhances the infectivity of bacteria, elicits enhanced immune responses, and induces the production of anti-ganglioside antibodies, leading to GBS (12–15). In particular, sialylated C. jejuni strains are more invasive in intestinal epithelial cells than nonsialylated strains (12), and in patients, sialylated strains are associated with an increased severity of gastroenteritis (13). In addition, sialylation induces a stronger IgM antibody response in the human host (13). By generating a Campylobacter sialyltransferase (cst-II) knockout mutant, we were able to demonstrate that sialylation of LOS modulates dendritic cell (DC)-mediated T helper cell differentiation and enhances DC-driven B-cell proliferation (14, 15). Most importantly, the presence of cst-II in C. jejuni is crucial for the induction of anti-ganglioside antibodies (16), which have the capacity to induce peripheral nerve damage and paralysis in rabbits and mice (17, 18).
Specific recognition of sialylated LOS versus nonsialylated LOS by the host immune system can be considered a crucial step in anti-ganglioside antibody formation.
Toll-like receptor 4 (TLR-4) interacts with the lipid A component of LOS; however, sialylation of the LOS outer core appears to influence TLR-4 signaling, as neuraminidase-desialylated LOS and cst-II mutant LOS activate DCs less efficiently, leading to reduced B-cell proliferation compared to that of the wild-type strains (14). We hypothesize that other receptors, which specifically bind to sialylated carbohydrates, determine sialylated C. jejuni LOS recognition. Two members of the sialic acid-binding immunoglobulin-like lectin (Siglecs) family have been demonstrated to specifically recognize sialylated C. jejuni LOS. A sialic acid-specific interaction with Siglec-7 was demonstrated previously (19), and we have recently shown that sialoadhesin (Sn; also called Siglec-1 and CD-169) from mice is able to bind to C. jejuni LOS in a sialic acid-dependent manner. Interestingly, GBS-associated C. jejuni strains, in particular, bound murine Sn (mSn) (20). Sn is a conserved Siglec found in both rodents and humans, and it is mainly expressed on macrophages (21). Therefore, in the current study, we aimed to identify whether C. jejuni binds to human Sn (hSn) expressed on macrophages and we assessed the consequences of hSn binding on bacterial uptake, bacterial survival, and macrophage activation.
MATERIALS AND METHODS
Bacterial strains.
A panel of 11 well-characterized C. jejuni strains with known ganglioside-like structures was used in this study (see Table S1 in the supplemental material) (11, 16). Eight strains isolated from GBS patients were selected, based on their properties of ganglioside mimic-specific binding to mSn, as previously demonstrated using enzyme-linked immunosorbent assay (ELISA) (20). To verify sialic acid-specific binding, two sialic acid transferase (cst-II) knockout mutants, GB2Δcst-II and GB11Δcst-II (16), and the reference C. jejuni strain NCTC 11186 (22), were included. C. jejuni strains were routinely grown from −80°C stocks and cultured on Colombia blood agar (BA) plates (BD Biosciences, Alphen aan den Rijn, The Netherlands), as previously described (20). For the cryoelectron microscopy (cryo-EM) experiments and gentamicin exclusion assays, bacteria were grown for 1 day on Campylobacter blood-free, charcoal-based, selective medium agar (CSM) plates (BD Biosciences) which contain 0.1% deoxycholate (DOC).
FITC labeling of C. jejuni strains.
C. jejuni cultures were grown for 2 days, harvested, washed with phosphate-buffered saline (PBS), and incubated for 1 h with 5 μl/ml fluorescein isothiocyanate (FITC; 100 mg/ml stock solution in dimethylsulfoxide [DMSO]) with shaking. Unbound FITC was removed by washing extensively with PBS, the bacteria were heat inactivated for 45 min at 56°C in PBS containing 2 mM MgCl2, and then the bacteria were stored in 10% glycerol broth at −80°C. Before use in binding experiments, the bacteria were thawed and washed, and the optical density at 600 nm (OD600) was adjusted to 1 in PBS (for C. jejuni, an OD600 of 1 equals ∼2.5 × 109 CFU/ml). In some experiments, the bacteria were used directly after FITC labeling or incubated for 1 h in PBS (pH 3.0) or in PBS (pH 7.0) containing 0.1% DOC (Sigma). The fluorescence intensities of FITC-labeled C. jejuni were assessed using a FACSCalibur flow cytometer (BD Biosciences).
Culture of THP-1 cells and preparation of human Sn-expressing monocyte-derived macrophages.
THP-1 (a human monocytic leukemia cell line) and THP-1 cells transduced with full-length human Sn cDNA (TSn or THP-1-Sn) were maintained as previously described (21). To prepare Sn-expressing monocyte-derived macrophages (Sn+MDMs), human peripheral blood mononuclear cells were isolated from buffy coats by density gradient centrifugation using Lymphoprep (Axis Shield, Oslo, Norway), and CD14+ monocytes were isolated by positive selection using CD14 microbeads (Miltenyi Biotec, Utrecht, The Netherlands) according to the manufacturer's protocol. To obtain MDMs, the cells were grown on low-attachment flasks in RPMI 1640 containing 10% human AB serum (HS), penicillin-streptomycin, 2 mM l-glutamine (growing medium), and 25 ng/ml human macrophage colony-stimulating factor (M-CSF; Invitrogen, Breda, The Netherlands) for 5 days. To obtain Sn+MDMs, the media were refreshed with growing medium containing 500 U/ml alpha 2a-interferon (IFN-α2a) (PBL, Piscataway, NJ), and the cells were cultured for 2 days. Sn was also induced by culturing MDMs for 2 days in media containing either E. coli lipopolysaccharide (LPS; 10 ng/ml; Sigma-Aldrich, Zwijndrecht, Netherlands) or LOS isolated from C. jejuni GB11 or GB11Δcst-II (10 ng/ml), which was purified as previously described (14).
Expression of Sn on THP-1 cells and MDMs.
To determine the expression of Sn on THP-1, THP-1-Sn, MDMs, or Sn+MDMs, the cells were incubated with PE-labeled mouse IgG1 κ anti-human CD169 monoclonal antibody (anti-hSn-PE; BioLegend, Uithoorn, The Netherlands) for 45 min at 4°C, fixed in paraformaldehyde (PFA), and analyzed using the FACSCalibur flow cytometer. A PE-labeled mouse IgG1 κ isotype antibody (BioLegend) was used as a control for background staining.
Binding of C. jejuni to Sn-transfected and wild-type THP-1 cells.
THP-1 and THP-1-Sn cells were harvested, washed, and resuspended in RPMI 1640 media containing 1% fetal calf serum (FCS). FITC-labeled C. jejuni was added at a cell/bacterium ratio of 1:100 and incubated for 2 h at 37°C in 5% CO2. The cells were washed to remove unbound bacteria, fixed in 2% paraformaldehyde (PFA), and analyzed using a FACSCalibur flow cytometer. To discriminate between living and dead cells, the nuclear stain 7-amino-actinomycin D (7-AAD; BD Biosciences) was added to the cells immediately before fluorescence-activated cell sorter (FACS) analysis.
For the Sn-blocking experiments, the cells were incubated with anti-hSn-PE antibody for 15 min prior to the addition of bacteria; phycoerythrin (PE)-labeled mouse IgG1, κ isotype control antibody (BioLegend) was used as a control for Sn-specific blocking. For immunofluorescence microscopy, the cells were incubated with FITC-labeled bacteria and stained with anti-hSn-PE, cytospin preparations were made, and the expression of Sn was evaluated using an Olympus IX51 microscope and CellF imaging software (Olympus, Zoeterwoude, Netherlands).
Ganglioside mimics exposure on C. jejuni.
C. jejuni strains GB11 and GB11Δcst-II were grown on BA plates either left untreated or heat inactivated and incubated with biotinylated cholera toxin (CT-biotin) (Sigma-Aldrich) diluted 1:100. The cells were subsequently incubated with streptavidin-FITC diluted 1:100, washed, fixed using 2% PFA, and analyzed with a FACSCalibur flow cytometer.
Cryoelectron microscopy.
C. jejuni strain GB11 was either grown on BA plates or on DOC-containing CSM plates. Bacteria were left untreated or were heat inactivated and incubated with CT-biotin diluted 1:100. Subsequently, they were labeled with streptavidin-conjugated quantum dots 525 (1:50, vol/vol) (Invitrogen). The samples were vitrified using a Vitrobot Mark IV (FEI) at room temperature and 100% humidity, blotted for 2 s at blot force 10 using filter paper, and then plunged into an ∼2:1 mixture of liquid ethane and propane, which was cooled using liquid nitrogen. The vitrified samples were mounted in a Gatan 626 high-tilt cryo holder and imaged using a Tecnai F20 transmission electron microscope (TEM) at 200 kV (FEI). The images were recorded with 2k × 2k (pixels) charge-coupled-device (CCD) cameras (GIF 2002; Gatan GmbH) with postcolumn energy filters in zero-loss mode using a slit width of 20 eV.
Binding and uptake of C. jejuni by Sn-expressing MDMs.
Sn+MDMs were harvested from low-attachment culture plates using cell dissociation buffer (Invitrogen), washed, and resuspended in RPMI 1640 containing 1% HS, and the binding of C. jejuni to Sn+MDMs was determined as described for THP-1 cells. To distinguish between internalized and external bacteria, the external bacteria were quenched with 0.2% trypan blue (MP Biomedicals, Illkirch, France) for 20 min prior to flow-cytometric analysis.
Gentamicin exclusion assay.
Human CD14+ monocytes were grown on 24-well plates in growing medium containing 25 ng/ml M-CSF. After 5 days, media were changed to that lacking penicillin-streptomycin but containing 500 U/ml IFN-α2a (PBL), and cells were grown for another 2 days. Cells were either left untreated or were preincubated for 15 min with anti-hSn-PE antibody. C. jejuni organisms harvested from overnight cultures on CSM plates were added at a multiplicity of infection (MOI) of 50 and incubated for 3 h, after which the cells were washed and the media replaced with growth media containing 200 μg/ml gentamicin. After 2 h, cells were washed and lysed in Hanks balanced salt solution (HBSS) containing 0.2% Triton X-100. Serial dilutions were prepared and plated on BA plates. After overnight culture under microaerophilic conditions, the number of colonies was counted.
Cytokine measurements.
Sn+MDMs cultured in 96-well plates were either left untreated or pretreated with anti-hSn-PE or an isotype control antibody for 15 min, and then they were incubated with heat-inactivated C. jejuni strains at a cell/bacterium ratio of 1:100 in growing medium lacking penicillin-streptomycin. The supernatants were harvested after 6 h, and cytokine levels were measured using a cytometric bead array (CBA) human inflammatory cytokine kit (BD Biosciences) according to the manufacturer's instructions.
Statistical analysis.
One-way analysis of variance (ANOVA) (SPSS software) and two-tailed t tests (GraphPad Prism software) were used for statistical analysis, as indicated.
RESULTS
C. jejuni binds to human Sn expressed on Sn-transduced THP-1 cells.
THP-1 cells transduced with full-length hSn cDNA (THP-1-Sn) were used to identify whether C. jejuni interacts with hSn (21). Flow-cytometric analysis confirmed high levels of Sn membrane expression on THP-1-Sn cells (mean fluorescence intensity [MFI], 1,526.3 ± 24.7; n = 3), whereas Sn expression was low on untransduced THP-1 cells (MFI, 6.7 ± 0.2; n = 3) (Fig. 1A). To assess whether C. jejuni binds to hSn, THP-1-Sn cells and control THP-1 cells were incubated with heat-inactivated FITC-labeled C. jejuni strain GB11, which was previously shown to bind mSn (20). Flow-cytometric analysis revealed a strong association of GB11-FITC with THP-1-Sn cells (MFI, 81.2 ± 7.3; n = 3), while virtually no FITC signal was detected on THP-1 cells (MFI, 3.5 ± 0.3; n = 3) (Fig. 1B). To visualize the binding of C. jejuni strain GB11 to hSn, THP-1-Sn and control THP-1 cells incubated with C. jejuni-FITC were cytospun onto glass slides, fixed, and stained with anti-hSn-PE. Fluorescence microscopy revealed a clear binding of C. jejuni strain GB11 with Sn on THP-1-Sn cells (Fig. 1C, upper), whereas almost no interaction between GB11 and control THP-1 cells was observed (Fig. 1C, lower). A difference in cellular morphology was observed between the parental THP-1 cells and the THP-1-Sn cells. This difference is probably due to aspecific priming of the cells by the transduction method.
Fig 1.
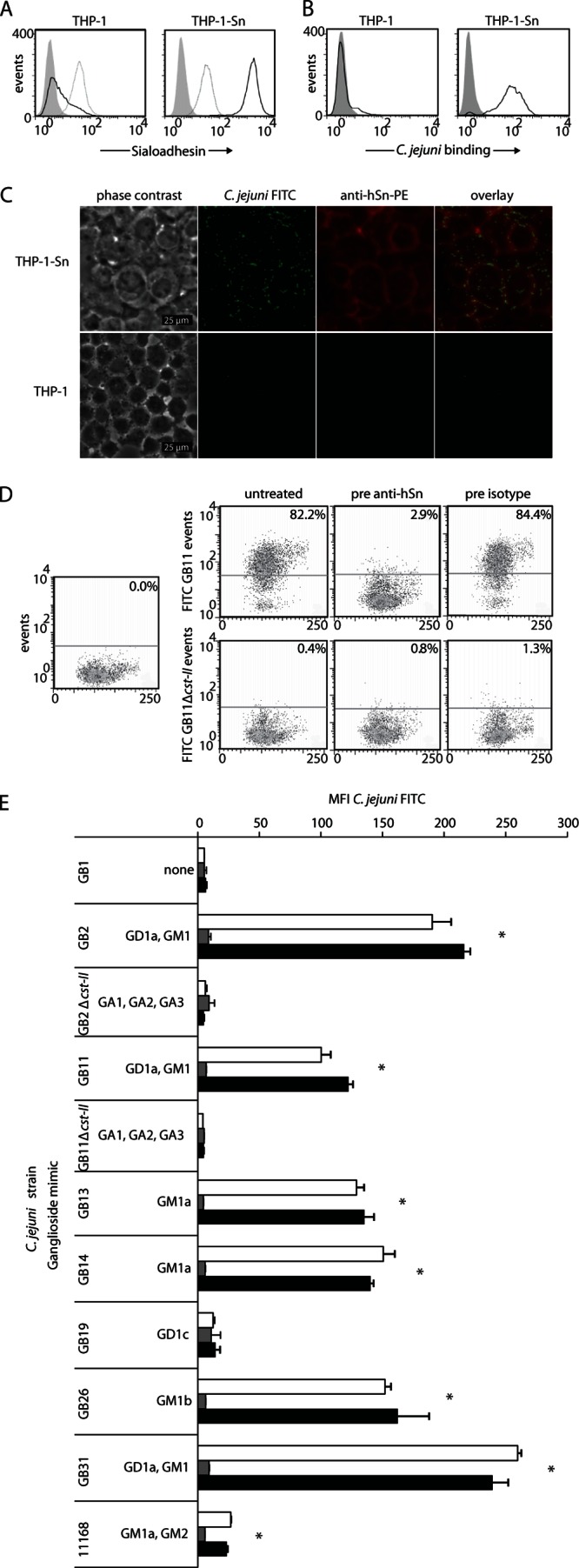
C. jejuni interacts with human Sn expressed on THP-1-Sn cells in a sialic acid- and Sn-dependent manner. (A) Confirmation of Sn expression on THP-1-Sn cells. Flow-cytometric analysis of Sn expression on THP-1 and THP-1-Sn cells, using an anti-hSn-PE antibody or isotype control antibody. Events indicate the FL-2 signal within the live cell population of unstained cells (filled gray curves), isotype control-stained cells (dotted-line curves), and anti-hSn-PE-stained cells (solid-line curve). (B) Flow-cytometric analysis of the interaction of heat-inactivated FITC-labeled C. jejuni strain GB11 incubated with THP-1 or THP-1-Sn cells for 2 h. Events indicate the FL-1 signal within the living cell population in the absence (filled gray curves) or presence (solid-line curves) of FITC-labeled GB11. (C) Immunofluorescent staining of hSn on THP-1 and THP-1-Sn cells incubated with heat-inactivated FITC-labeled C. jejuni strain GB11 for 2 h, using an anti-hSn-PE antibody. C. jejuni is shown in green, hSn in red. (D) Flow-cytometric analysis of the interaction of FITC-labeled C. jejuni strains GB11 and GB11Δcst-II with THP-1-Sn cells in the presence or absence of hSn blocking using an anti-hSn antibody or isotype control antibody, respectively. The percentages of cells binding C. jejuni within the living cell populations are indicated. (E) Binding of a panel of FITC-labeled, heat-inactivated C. jejuni strains with known ganglioside-mimicking structures to THP-1-Sn cells. The cells were either untreated (white bars) or pretreated with an antibody against hSn (gray bars) or an isotype control antibody (black bars). Living cells were gated and used for analysis. Results represent the data from one experiment that was repeated at least two times, shown as means ± SD of triplicate measurements. *, P < 0.05 (by one-way ANOVA) for the comparison of untreated (white bars) and isotype control treatment (black bars) versus anti-hSn treatment (gray bars).
Binding of C. jejuni to THP-1-Sn cells is sialic acid and Sn dependent.
To determine whether the binding of C. jejuni to hSn was sialic acid specific, we incubated THP-1-Sn cells with heat-inactivated, FITC-labeled wild-type C. jejuni strain GB11 or FITC-labeled bacteria from a previously generated sialic acid GB11 knockout mutant (denoted GB11Δcst-II) (16). Flow-cytometric analysis demonstrated that 82.2% of THP-1-Sn cells bound GB11, whereas only 0.4% bound GB11Δcst-II cells, indicating that the presence of sialic acid was crucial for hSn binding (Fig. 1D). To confirm that GB11 bound to Sn and not to other cell surface proteins, THP-1-Sn cells were treated for 15 min with an antibody against hSn before addition of the bacteria. Pretreatment with the anti-hSn antibody almost completely neutralized the binding of GB11 to THP-1-Sn cells, reducing the proportion of positive cells to 2.9% (Fig. 1D). Preincubation with an isotype control antibody had no effect on Sn binding (Fig. 1D).
Ganglioside mimic-specific interaction of C. jejuni with human Sn.
Using an ELISA, we previously demonstrated that mSn binds to C. jejuni strains which express terminal α(2, 3)-linked sialic acid residues, as found in gangliosides such as GM3, GD1a, GM1b, and GT1b (20, 23). To identify whether hSn has a binding profile similar to that of mSn, the binding of C. jejuni strains with known ganglioside-mimicking structures to hSn was tested using THP-1-Sn cells. In agreement with the mSn binding capacities, C. jejuni strains GB2, GB11, GB26, and GB31 positively bound hSn, and C. jejuni strains GB1, GB11Δcst-II, and GB19 did not bind hSn (Fig. 1E) (20). Unexpectedly, hSn bound the C. jejuni strains GB13 and GB14, which were not recognized by mSn (Fig. 1E). GB13 and GB14 both express GM1a-like LOS (11). The GM1a structure does not contain a terminally linked sialic acid residue but instead contains an α(2,3)-linked sialic acid attached to an internal galactose of the LOS outer core, and hSn apparently can interact with this internal sialic acid. The enteritis-associated C. jejuni reference strain 11168 also has GM1a-ganglioside mimicry. Investigation of the hSn binding capacity of this strain indicated that 11168 could bind to THP-1-Sn cells at a relatively low level compared to strains GM13 and GM14 (Fig. 1E). Similar to GB11Δcst-II, GB2Δcst-II did not bind to hSn, again demonstrating that an absence of sialic acid in the LOS prevents hSn binding (Fig. 1E). It should be noted that THP-1-Sn cells were also incubated with an antibody against human Sn for 15 min prior to addition of the bacteria as a control for Sn-specific binding. Preincubation with anti-hSn antibody significantly reduced the Sn binding capacity of all strains, as illustrated by a reduction in cell-associated fluorescence intensity to background levels (P < 0.05 by one-way ANOVA); however, an isotype control antibody did not affect cell-associated fluorescence (Fig. 1E). To determine if the strains were adequately labeled, the FITC-labeled bacteria were analyzed by flow cytometry. Although there was slight variation in the labeling intensities, the MFI was generally high (MFI, 1,053 ± 170) (see Fig. S1 in the supplemental material).
Growth conditions determine ganglioside mimic exposure and Sn binding.
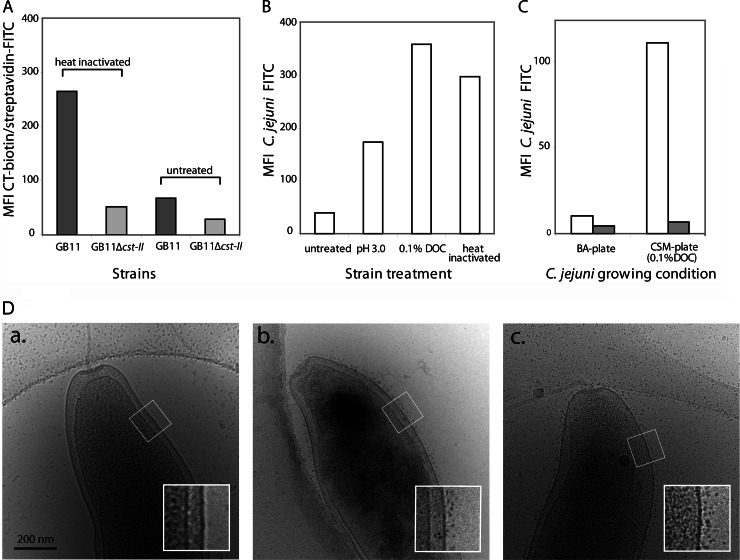
In the experiments described above, heat-inactivated bacteria grown on BA plates were used to determine Sn-dependent binding. The binding of untreated bacteria grown on BA plates to THP-1-Sn cells was also assessed, and surprisingly, no binding was observed (data not shown). Cholera toxin (CT) strongly binds to ganglioside GM1 (24). To determine whether the ganglioside mimics were adequately exposed on the bacterial surface, living and heat-inactivated bacteria from the GB11 (GM1 positive) and GB11Δcst-II (GM1 negative) strains were incubated with CT-biotin and streptavidin-FITC and analyzed by flow cytometry. Indeed, CT-biotin was able to bind to strain GB11 when the bacteria were heat inactivated; however, the binding was severely reduced when the strain was left untreated (Fig. 2A). As expected, CT-biotin did not bind to heat-inactivated or untreated GB11Δcst-II, which lacks a GM1-like structure (Fig. 2A). We investigated whether the exposure of bacteria to environmental conditions similar to those they would encounter after ingestion, such as low pH or bile salts in the stomach and intestine, respectively, would influence the exposure of ganglioside mimics on the bacterial cell surface. Therefore, C. jejuni strain GB11 was cultured on BA plates, FITC labeled, and incubated for 1 h in PBS (pH 3.0) or in PBS (pH 7.0) containing 0.1% DOC, and then the binding to THP-1-Sn cells was analyzed using FACS. Compared to untreated bacteria, incubation at pH 3.0 and, to an even greater extent, incubation in 0.1% DOC enhanced the bacterial ability to bind THP-1-Sn cells (Fig. 2B). Furthermore, when the bacteria were freshly cultured on commercial CSM plates which contain 0.1% DOC, Sn-specific binding of the bacteria to THP-1-Sn cells was also observed (Fig. 2C). In order to localize the exposure of ganglioside mimics on the bacterial cell surface, untreated GB11 cultured on BA plates, GB11 cultured on BA plates and heat inactivated, and CSM plate-cultured GB11 were incubated with CT-biotin and subsequently with streptavidin-conjugated quantum dots and visualized by cryo-EM. Quantum dots were visible on the heat-inactivated and CSM plate-grown bacteria and covered the surface of the bacteria (Fig. 2D). Interestingly, no quantum dots were present on the surface of most of the BA plate-grown, non-heat-inactivated bacteria.
Fig 2.
Exposure of ganglioside mimics on the surface of C. jejuni. (A) Flow-cytometric analysis of the expression of ganglioside mimics on the surface of C. jejuni. Bacteria were either heat inactivated or left untreated and then subsequently incubated with CT-biotin and streptavidin-FITC. (B) Flow-cytometric analysis of the binding of FITC-labeled C. jejuni strain GB11 to THP-1-Sn cells. Bacteria grown on BA plates were left untreated, incubated for 1 h in PBS (pH 3.0) or in PBS (pH 7.0) containing 0.1% DOC, or heat inactivated. (C) Flow-cytometric analysis of the binding of FITC-labeled C. jejuni strain GB11 to THP-1-Sn cells. Bacteria were grown on either BA plates or CSM plates. THP-1-Sn cells were left untreated (white bars) or were pretreated to block Sn binding using an anti-hSn antibody (gray bars). (D) Cryo-EM visualization of C. jejuni strain GB11 grown on BA plates and left untreated (a), grown on BA plates and heat inactivated (b), or grown on CSM plates and left untreated (c). The bacteria were incubated with CT-biotin followed by streptavidin-conjugated quantum dots.
IFN-α and C. jejuni LOS induce the expression of Sn by monocyte-derived macrophages.
To study Sn-dependent binding and internalization and the functional consequences of Sn binding in a more biologically relevant setting, we used Sn-expressing primary human macrophages. As it has been demonstrated that Sn expression can be induced on CD14+ monocytes by stimulation with IFN-α (21), we investigated whether IFN-α induced expression of Sn on human MDM. After 2 days of culture with IFN-α, MDMs exhibited expression of Sn (MFI, 68.4 ± 4.3; n = 3), whereas non-IFN-α-stimulated MDMs remained negative for Sn (MFI, 3.9 ± 0.5; n = 3) (Fig. 3A). We also tested if C. jejuni LOS could directly induce expression of Sn by MDMs and, therefore, whether LOS could modulate its own recognition. Flow-cytometric analysis revealed that GB11 LOS potently induced the expression of Sn by MDMs at a level similar to that of IFN-α treatment (Fig. 3B). The induction of Sn by C. jejuni LOS was not sialic acid dependent, as treatment with nonsialylated LOS isolated from strain GB11Δcst-II or treatment with purified E. coli LPS resulted in similar levels of Sn expression in MDMs (Fig. 3B).
Fig 3.
Sn-specific binding of sialylated C. jejuni strains results in enhanced uptake by Sn+MDMs. Human monocyte-derived macrophages were either left untreated (MDMs) or treated with IFN-α to induce expression of Sn (Sn+MDMs), and then Sn expression and the capacity to bind and internalize C. jejuni were quantified. FITC-labeled, heat-inactivated C. jejuni stains were incubated with the cells for 2 h prior to flow-cytometric analysis. Living cells were gated and used for analysis. (A) Flow-cytometric analysis of the expression of Sn on human MDMs and Sn+MDMs in unstained cells (filled gray curve) and cells stained with anti-hSn-PE (solid-line curve) or a PE-labeled isotype control antibody (dotted-line curve). (B) Flow-cytometric analysis of the expression of Sn on untreated human MDMs or MDMs incubated for 48 h with either IFN-α (500 U/ml), E. coli LPS (10 ng/ml), C. jejuni GB11, or GB11Δcst-II LOS (10 ng/ml) and subsequently stained with anti-hSn-PE. (C) Interaction of FITC-labeled C. jejuni strains containing known ganglioside-mimicking structures with Sn+MDMs which had been untreated (white bars) or pretreated with an antibody against hSn (gray bars) or with an isotype control antibody (black bars). Results represent data from one experiment that was repeated at least one time. Means ± SD from triplicate measurements are shown. (D) Internalization of C. jejuni strains by Sn+MDMs. Sn+MDMs were incubated with FITC-labeled bacteria as described for panel C and then treated with trypan blue prior to flow-cytometric analysis to discriminate between external and internalized bacteria. For panels C and D, *, P < 0.05 (one-way ANOVA) for comparison of untreated (white bar) and isotype control treatment (black bar) versus anti-hSn treatment (gray bar). **, P < 0.05 (t test) for GB11 versus GB11Δcst-II (the combined MFI values of the untreated [white bar] and isotype control-treated [black bar] conditions were compared).
Binding to Sn enhances the uptake of C. jejuni by MDMs.
As primary monocyte-derived macrophages express many different pattern recognition molecules, which may not all be present on THP-1 cells, we assessed whether the binding of C. jejuni to hSn on IFN-α-induced primary MDMs (Sn+MDMs) was similar to that of THP-1-Sn cells. The Sn-specific binding of a selection of C. jejuni strains to Sn+MDMs was determined. In particular, four strains (GB11, GB13, GB26, and GB31) that positively bound THP-1-Sn cells and two strains (GB1 and GB11Δcst-II) that did not bind THP-1-Sn cells were included in the flow-cytometric binding assay. All of the C. jejuni strains tested showed an interaction with Sn+MDMs. However, the fluorescence intensities of Sn+MDMs incubated with strains GB11, GB26, and GB31 were higher than those of strains GB1 and GB11Δcst-II (Fig. 3C), indicating that increased numbers of GB11, GB26, and GB31 were associated with each individual Sn+MDM cell. Pretreatment of Sn+MDMs with an antibody against hSn significantly reduced the binding of strains GB11, GB26, and GB31 (P < 0.05 by one-way ANOVA) to levels similar to those of Sn+MDMs incubated with strains GB1 and GB11Δcst-II. Pretreatment with the hSn antibody had no significant effect on the interaction of Sn+MDMs with strains GB1 and GB11Δcst-II. Additionally, pretreatment with an isotype control antibody did not influence the interaction of Sn+MDMs with any C. jejuni strain, indicating that the enhanced interactions of strains GB11, GB26, and GB31 with Sn+MDMs, unlike those of strains GB1 and GB11Δcst-II, were hSn specific. Overall low binding to Sn+MDMs was observed for strain GB13. Pretreatment of Sn+MDMs with an antibody against hSn, however, did result in a significant reduction in binding, whereas pretreatment with an isotype control antibody had no effect. This indicates that the enhanced binding of C. jejuni strain GB13 to Sn+MDMs was Sn specific and that the internal α(2,3)-linked sialic acid, as present in the GM1a-like structure on strain GB13, indeed binds to hSn.
To determine whether Sn binding is a prerequisite to bacterial endocytosis, the extracellular bacteria in the experiment described above were quenched using trypan blue, and the cells were reanalyzed using flow cytometry. The MFI of Sn+MDMs incubated with hSn binding strains GB11, GB26, and GB31 was higher than that of Sn+MDMs incubated with the non-hSn-binding strains GB1 and GB11Δcst-II (Fig. 3D), indicating that Sn binding leads to increased uptake of bacteria. In agreement with this hypothesis, preincubation with anti-Sn significantly decreased bacterial uptake (P < 0.05 by one-way ANOVA). As could be expected from the previously described binding experiment, the MFI of Sn+MDMs incubated with strain GB13 again were relatively low. Preincubation with anti-Sn antibody, however, significantly reduced the uptake of strain GB13 (P < 0.05 by one-way ANOVA). There was no difference in the interaction of untreated and isotype control antibody-treated cells, confirming that the increased internalization of strains GB11, GB13, GB26, and GB31 was Sn dependent. To assess the quenching efficiency of trypan blue, FITC-labeled C. jejuni strains were analyzed by flow cytometry before and after incubation with trypan blue. Trypan blue severely reduced the fluorescent signal of all strains (see Fig. S2 in the supplemental material).
Sn-specific binding enhances phagocytosis of living C. jejuni by Sn+MDMs.
To further assess phagocytosis using live, unstained bacteria, we performed gentamicin exclusion assays using Sn+MDMs which had been incubated for 3 h with fresh, CSM plate-grown GB11 or GB11Δcst-II. Sn-specific binding significantly increased the uptake of C. jejuni by untreated cells compared to that of cells pretreated with anti-Sn antibody (P < 0.05 by two-tailed t test) (Fig. 4). The survival of C. jejuni in Sn+MDMs was also assessed, and we observed that all of the bacteria were dead after 24 h (data not shown), indicating that Sn binding does not result in altered intracellular trafficking or escape of C. jejuni from lysosomal degradation.
Fig 4.
Sn mediates the phagocytosis of C. jejuni. Sn+MDMs were left untreated or were pretreated with an antibody against hSn and then incubated with C. jejuni strain GB11 or GB11Δcst-II for 3 h. The gentamicin exclusion assay was used to quantify the number of internalized bacteria. Results represent data from one experiment that was repeated at least two times. Means ± standard errors of the means from triplicate measurements are shown. *, P < 0.05 (t test).
Sn binding elevates production of the cytokine IL-6 by Sn+MDMs.
To assess if the interaction of C. jejuni with Sn affected cytokine production, Sn+MDMs were incubated for 6 h with wild-type GB11 or GB11Δcst-II, and then the levels of six cytokines were measured in the cell culture supernatants. The cytokine interleukin-6 (IL-6) was specifically elevated in an Sn- and sialic acid-dependent manner (Fig. 5A). IL-6 levels were significantly higher when Sn+MDMs were incubated with wild-type GB11 than when Sn+MDMs were incubated with GB11Δcst-II (P < 0.05 by t test). High levels of IL-8 and tumor necrosis factor alpha (TNF-α) were also induced by C. jejuni (19,867 ± 3,683 and 19,704 ± 4,709 pg/ml, respectively); however, the induction of these cytokines was not Sn dependent (Fig. 5B and C). Other cytokines, such as IL-1b, IL-10, and IL-12p70, were also induced in an Sn-independent manner to levels between ∼100 and 400 pg/ml (data not shown).
Fig 5.

Production of the cytokine IL-6 is elevated in C. jejuni-treated Sn+MDMs. Sn+MDMs were left untreated or were pretreated with an anti-hSn antibody or an isotype control antibody, incubated for 6 h with C. jejuni strain GB11 or GB11Δcst-II, and then IL-6 (A), IL-8 (B), or TNF-α (C) production was measured. The cytokine levels in the cell supernatants were quantified using a cytometric bead array human inflammatory cytokine kit. The plots display the mean values from triplicate measurements (indicated by the line) and the range values from one representative experiment that was repeated at least two times. *, P < 0.05 (t test) for GB11 versus GB11Δcst-II; the combined values from the untreated and isotype control-treated conditions were compared.
DISCUSSION
In this study, we demonstrated that C. jejuni strains isolated from patients with GBS can bind to Sn expressed on primary human macrophages via the presence of sialic acid residues in the bacterial LOS. Using Sn-transduced THP-1 cells and primary human macrophages, we showed that C. jejuni strains, in particular those containing α(2,3)-linked sialic acid residues, as present in the gangliosides GM1a, GD1a, GM1b, and GM3, bind to hSn. Ganglioside-like structures were not constitutively exposed on the bacterial surface but required particular growth conditions or treatments. Direct growth of the bacteria on media containing DOC or treatment of C. jejuni initially grown on BA plates by heat inactivation, incubation at pH 3.0, or exposure to DOC was necessary for Sn binding. In primary human macrophages, Sn binding resulted in enhanced bacterial uptake and increased release of cytokine IL-6. Regardless of Sn binding, internalized bacteria were killed within 24 h, suggesting that Sn binding does not enable C. jejuni to escape lysosomal degradation.
It has previously been reported that mSn has a preference for binding terminal α(2,3)-linked sialic acid, which is present on purified gangliosides, such as GT1b, GD1a, and GM3 (23). We recently demonstrated that the ganglioside-like structures present in the LOS on the surface of C. jejuni have binding properties similar to those of purified gangliosides (20). Using hSn in this study, we showed that internal α(2,3)-linked sialic acids, as present in GM1a-like LOS, as well as terminal α(2,3)-linked sialic acids can bind to hSn. This is of interest, as C. jejuni strains carrying α(2,3)-sialylated LOS structures, especially the strains expressing GM1a- and GD1a-like LOS, are associated with GBS. It should be noted that the enteritis-associated C. jejuni reference strain 11168 could also bind Sn; however, it did so at relatively low levels, despite the presence of GM1a-ganglioside mimicry. Besides a GM1a-like structure, C. jejuni strain 11168 also has GM2 mimicry (see Table S1 in the supplemental material). The relatively low level of hSn binding for C. jejuni strain 11168 may be due to complex formation between the GM1a and the GM2 mimic, as it has been demonstrated that ganglioside complexes can attenuate Siglec binding (25). Low expression of ganglioside mimics on strain 11168 may also account for the relatively low Sn binding that was observed. Alternatively, other bacterial structures than terminal or internal α(2,3)-linked sialic acids may inhibit or enhance binding to hSn.
Our finding that specific microenvironments are crucial for the binding of C. jejuni to Sn is of particular importance. In this study, both heat-inactivated and living bacteria were used to assess Sn-dependent bacterial binding and uptake. An association with Sn could not be detected when living bacteria grown on standard BA plates were tested, whereas heat inactivation resulted in Sn binding. This discrepancy was explained by exploring LOS exposure on living and heat-inactivated C. jejuni using biotinylated CT, which has a high binding affinity for surface-exposed GM1. In agreement with the observed Sn binding capacity, FACS analysis and cryo-EM revealed that GM1 was extensively exposed when C. jejuni was heat inactivated. The fact that GBS patients often have antibodies against C. jejuni LOS indicates that these structures are exposed during the course of infection. Therefore, we reasoned that the outer surface of C. jejuni is altered during the passage of food-borne C. jejuni through the stomach and intestine by, for example, modification of its glycosylation composition, a reduction or loss of the capsular layer, differential expression of membrane proteins, or conformational changes of surface proteins (26, 27). Indeed, when living bacteria were incubated at low pH or in buffer containing the bile constituent DOC, or when the bacteria were grown on culture plates containing DOC, the LOS were exposed and able to bind Sn. Culture of C. jejuni in the presence of DOC may also enhance LOS expression; however, microarray-based analysis has indicated that the expression of genes involved in LOS biosynthesis or modification are not upregulated in the presence of DOC (28). In future experiments, we plan to assess the role of the capsule in Sn binding.
We further explored the possible role of Sn in GBS by studying primary human macrophages. Using CHO cells transfected with mSn, we previously demonstrated that Sn is sufficient for the binding of ganglioside-like structures without the need for additional coreceptors (20). However, unlike most Siglecs, Sn lacks intracellular signaling motifs; thus, it most likely cooperates with other surface molecules. Our data clearly indicate that Sn binding leads to enhanced phagocytosis of C. jejuni. This is in line with a previously described role for Sn in microbial uptake. Neisseria meningitides, HIV, and porcine reproductive and respiratory syndrome virus (PRRSV) are internalized upon Sn binding in an Sn-specific manner (21, 29, 30). These observations raise questions regarding the consequences of Sn-mediated phagocytosis. Sn binding may play a role in redirecting C. jejuni or the LOS to specific intracellular compartments. For example, Sn accompanies PRRSV from the cell surface to inside the cell just beneath the plasma membrane, after which the virus can be detected in early endosomes (30). Regardless of Sn dependency, the uptake of C. jejuni by macrophages resulted in bacterial death within 24 h, indicating that Sn-mediated uptake of C. jejuni does not enable the bacteria to escape lysosomal degradation in macrophages.
Alternatively, Sn-mediated uptake of C. jejuni may affect the macrophage activation state and cytokine release, leading to altered innate immunity. Indeed, Sn-mediated C. jejuni uptake enhanced the release of IL-6 by primary macrophages. Moreover, in collaboration, we have recently shown that sialylation of LOS increased the production of type I interferons in mice (31). These data imply that Sn-mediated uptake determines the quality of the innate immune response to C. jejuni.
Sn may also indirectly affect antigen presentation to other cells of the immune system via its selective expression pattern on specific immune cells. Sn is mainly expressed on tissue-resident macrophages found in the intestine, marginal zone of the spleen, and subcapsular sinus of lymph nodes (32). One function of these macrophages is the presentation of antigenic debris to follicular DCs and B cells (33). As such, it can be envisaged that increased Sn-mediated binding and/or phagocytosis may lead to the presentation of more C. jejuni fragments, resulting in increased immune activation. Evidence for a direct role of Sn-mediated uptake in antibody production is provided by a recent immunization study of pigs, which demonstrated that direct targeting of the immunizing protein to Sn resulted in a more rapid and robust induction of specific IgM and IgG immune responses compared to immunization with the protein alone (34). Further study is required to confirm whether Sn-dependent uptake leads to C. jejuni antigen trapping and presentation, resulting in cross-reactive anti-ganglioside antibody production.
In conclusion, this study demonstrates that GBS-associated sialylated C. jejuni strains are able to bind to hSn, which results in enhanced production of the cytokine IL-6 and increased uptake of the bacteria by monocyte-derived macrophages. Sn-mediated differentiation between C. jejuni strains on the basis of ganglioside mimic expression may be an important initial event in the production of anti-ganglioside antibodies and the development of GBS.
Supplementary Material
ACKNOWLEDGMENT
We thank Maria A. J. Ridder, Department of Biostatistics, Erasmus MC, Rotterdam, The Netherlands, for statistical advice.
Footnotes
Published ahead of print 25 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01437-12.
REFERENCES
- 1. Jacobs BC, Hazenberg MP, van Doorn PA, Endtz HP, van der Meché FGA. 1997. Cross-reactive antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in patients with Guillain-Barré or Miller Fisher syndrome. J. Infect. Dis. 175:729–733 [DOI] [PubMed] [Google Scholar]
- 2. Yuki N, Taki T, Handa S. 1996. Antibody to GalNAc-GD1a and GalNAc-GM1b in Guillain-Barré syndrome subsequent to Campylobacter jejuni enteritis. J. Neuroimmunol. 71:155–161 [DOI] [PubMed] [Google Scholar]
- 3. Hughes RAC, Hadden RD, Gregson NA, Smith KJ. 1999. Pathogenesis of Guillain-Barré syndrome. J. Neuroimmunol. 100:74–97 [DOI] [PubMed] [Google Scholar]
- 4. Carpo M, Pedotti R, Allaria S, Lolli F, Mata S, Cavaletti G, Protti A, Pomati S, Scarlato G, Nobile-Orazio E. 1999. Clinical presentation and outcome of Guillain-Barré and related syndromes in relation to anti-ganglioside antibodies. J. Neurol. Sci. 168:78–84 [DOI] [PubMed] [Google Scholar]
- 5. Jacobs BC, Van Doorn PA, Schmitz PIM, Tio-Gillen AP, Herbrink P, Visser LH, Hooijkaas H, Van der Meché FGA. 1996. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barré syndrome. Ann. Neurol. 40:181–187 [DOI] [PubMed] [Google Scholar]
- 6. Yuki N. 1997. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barré syndrome and Miller Fisher syndrome. J. Infect. Dis. 176:S150–153 [DOI] [PubMed] [Google Scholar]
- 7. Jacobs BC, Rothbarth PH, van der Meché Herbrink FGAP, Schmitz PIM, De Klerk MA, van Doorn PA. 1998. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology 51:1110–1115 [DOI] [PubMed] [Google Scholar]
- 8. Aspinall GO, McDonald AG, Raju TS, Pang H, Kurjanczyk LA, Penner JL, Moran AP. 1993. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur. J. Biochem. 213:1029–1037 [DOI] [PubMed] [Google Scholar]
- 9. Moran AP, Rietschel ET, Kosunen TU, Zahringer U. 1991. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-D-glucose. J. Bacteriol. 173:618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilbert M, Karwaski Bernatchez M-FS, Young NM, Taboada E, Michniewicz J, Cunningham Wakarchuk A-MWW. 2002. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277:327–337 [DOI] [PubMed] [Google Scholar]
- 11. Godschalk PC, Kuijf ML, Li J, St. Michael F, Ang CW, Jacobs BC, Karwaski MF, Brochu D, Moterassed A, Endtz HP, van Belkum A, Gilbert M. 2007. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barre and Miller Fisher syndromes. Infect. Immun. 75:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louwen R, Heikema A, van Belkum A, Ott A, Gilbert M, Ang W, Endtz HP, Bergman MP, Nieuwenhuis EE. 2008. The sialylated lipooligosaccharide outer core in Campylobacter jejuni is an important determinant for epithelial cell invasion. Infect. Immun. 76:4431–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mortensen NP, Kuijf ML, Ang CW, Schiellerup P, Krogfelt KA, Jacobs BC, van Belkum A, Endtz HP, Bergman MP. 2009. Sialylation of Campylobacter jejuni lipooligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 11:988–994 [DOI] [PubMed] [Google Scholar]
- 14. Kuijf ML, Samsom JN, van Rijs W, Bax M, Huizinga R, Heikema AP, van Doorn PA, van Belkum A, van Kooyk Y, Burgers PC, Luider TM, Endtz HP, Nieuwenhuis EE, Jacobs BC. 2010. TLR4-mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J. Immunol. 185:748–755 [DOI] [PubMed] [Google Scholar]
- 15. Bax M, Kuijf ML, Heikema AP, van Rijs W, Bruijns SC, Garcia-Vallejo JJ, Crocker PR, Jacobs BC, van Vliet SJ, van Kooyk Y. 2011. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect. Immun. 79:2681–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Godschalk PCR, Heikema AP, Gilbert M, Komagamine T, Ang CW, Glerum J, Brochu D, Li J, Yuki N, Jacobs BC, van Belkum A, Endtz HP. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barre syndrome. J. Clin. Investig. 114:1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, Yamada M. 2004. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barré syndrome. Proc. Natl. Acad. Sci. U. S. A. 101:11404–11409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halstead SK, Zitman FM, Humphreys PD, Greenshields K, Verschuuren JJ, Jacobs BC, Rother RP, Plomp JJ, Willison HJ. 2008. Eculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine model. Brain 131:1197–1208 [DOI] [PubMed] [Google Scholar]
- 19. Avril T, Wagner ER, Willison HJ, Crocker PR. 2006. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect. Immun. 74:4133–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heikema AP, Bergman MP, Richards H, Crocker PR, Gilbert M, Samsom JN, van Wamel WJ, Endtz HP, van Belkum A. 2010. Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect. Immun. 78:3237–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rempel H, Calosing C, Sun B, Pulliam L. 2008. Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS One 3:e1967 doi:10.1371/journal.pone.0001967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668 [DOI] [PubMed] [Google Scholar]
- 23. Crocker PR, Kelm S, Dubois C, Martin B, McWilliam AS, Shotton DM, Paulson JC, Gordon S. 1991. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 10:1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heyningen SV. 1974. Cholera toxin: interaction of subunits with ganglioside GM1. Science 183:656–657 [DOI] [PubMed] [Google Scholar]
- 25. Rinaldi S, Brennan KM, Goodyear CS, O'Leary C, Schiavo G, Crocker PR, Willison HJ. 2009. Analysis of lectin binding to glycolipid complexes using combinatorial glycoarrays. Glycobiology 19:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stintzi A, Marlow D, Palyada K, Naikare H, Panciera R, Whitworth L, Clarke C. 2005. Use of genome-wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni. Infect. Immun. 73:1797–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corcionivoschi N, Clyne M, Lyons A, Elmi A, Gundogdu O, Wren BW, Dorrell N, Karlyshev AV, Bourke B. 2009. Campylobacter jejuni cocultured with epithelial cells reduces surface capsular polysaccharide expression. Infect. Immun. 77:1959–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malik-Kale P, Parker CT, Konkel ME. 2008. Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression. J. Bacteriol. 190:2286–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones C, Virji M, Crocker PR. 2003. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 49:1213–1225 [DOI] [PubMed] [Google Scholar]
- 30. Delputte PL, Van Breedam W, Barbe F, Van Reeth K, Nauwynck HJ. 2007. IFN-alpha treatment enhances porcine arterivirus infection of monocytes via upregulation of the porcine arterivirus receptor sialoadhesin. J. Interferon Cytokine Res. 27:757–766 [DOI] [PubMed] [Google Scholar]
- 31. Huizinga R, Easton AS, Donachie AM, Guthrie J, van Rijs W, Heikema A, Boon L, Samsom JN, Jacobs BC, Willison HJ, Goodyear CS. 2012. Sialylation of Campylobacter jejuni lipo-oligosaccharides: impact on phagocytosis and cytokine production in mice. PLoS One 7:e34416 doi:10.1371/journal.pone.0034416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crocker PR, Paulson JC, Varki A. 2007. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7:255–266 [DOI] [PubMed] [Google Scholar]
- 33. You Y, Myers RC, Freeberg L, Foote J, Kearney JF, Justement LB, Carter RH. 2011. Marginal zone B cells regulate antigen capture by marginal zone macrophages. J. Immunol. 186:2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delputte PL, Van Gorp H, Favoreel HW, Hoebeke I, Delrue I, Dewerchin H, Verdonck F, Verhasselt B, Cox E, Nauwynck HJ. 2011. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PLoS One 6:e16827 doi:10.1371/journal.pone.0016827 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.