Abstract
Clostridium difficile is a spore-forming bacillus that produces toxin-mediated enteric disease. C. difficile expresses two major virulence factors, toxin A (TcdA) and toxin B (TcdB). Human and animal studies demonstrate a clear association between humoral immunity to these toxins and protection against C. difficile infection (CDI). The receptor binding-domains (RBDs) of TcdA and TcdB are known to be immunogenic. Here, we tested the immunoadjuvant properties of Salmonella enterica serovar Typhimurium flagellin (FliC) subunit D1 as an innate immune agonist expressed as a recombinant fusion vaccine targeting the RBDs of TcdA and TcdB in mice. Intraperitoneally immunized mice developed prominent anti-TcdA and anti-TcdB immunoglobulin G in serum. The protective efficacy of the recombinant vaccines, with or without an adjuvant, was tested in a mouse model of CDI that closely represents the human disease. Following intraperitoneal immunization equivalent to two doses of toxoid A and toxoid B vaccine adjuvanted with alum and oral challenge with C. difficile VPI 10463, C57BL/6 mice were able to mount a protective immune response that prevented diarrhea and death compared to mice immunzed with alum alone. These results are significantly different from those for control mice (P < 0.001). These results provide evidence that a recombinant protein-based vaccine targeting the RBDs of the C. difficile toxins adjuvanted with S. Typhimurium flagellin can induce rapid, high-level protection in a mouse model of CDI when challenged with the homologous strain from which the vaccine antigens were derived and warrant further preclinical testing against clinically relevant C. difficile strains in the mouse and hamster models of CDI.
INTRODUCTION
Clostridium difficile is a spore-forming bacillus that produces toxin-mediated enteric disease. Broad-spectrum antibiotics and the emergence of a hypervirulent strain of C. difficile (BI/NAP1/027) have changed the epidemiology of C. difficile infection (CDI) (1). C. difficile expresses two major virulence factors, toxin A (TcdA) and toxin B (TcdB), which function as glucosyltransferases that inactivate RhoA, Rac, and Cdc42 within eukaryotic target cells, ultimately causing cell death (2, 3). The toxins have four functional domains: an enzymatically active glucosyltransferase N-terminal domain, an autocatalytic protease domain, a central translocation section, and a C-terminal receptor-binding domain (RBD), consisting of repeating units of 21, 30, or 50 amino acid residues (4, 5).
Antibodies against C. difficile are present in the sera of most adults and older children, although fewer than 5% of individuals are colonized (6, 7). Studies to date demonstrate a clear association between humoral immunity to these toxins and protection against CDI (7, 8, 9, 10). Protection against disease and relapse correlates predominantly with the presence of host IgG responses directed against TcdA and less strongly with response against TcdB. While antibody responses to TcdA demonstrate the strongest association with protection from disease in both animal models and human cohort studies, various lines of evidence suggest that an effective vaccine will likely need to target both TcdA and TcdB (11, 12). Epidemiologic data suggest that the development of prophylactic and therapeutic vaccines against C. difficile may be one of the ways to curtail the spread of this nosocomial disease, although elderly high-risk patients are likely to have a reduced response to active immunization at a critical time in their illness.
Protein as well as peptide vaccines targeting the RBDs, especially of TcdA, are immunogenic, capable of inducing neutralizing antibodies in the murine model, and are partially protective in the hamster challenge model although no human clinical trials have been done (13, 14, 15, 16, 17). Parenteral immunization with a recombinant protein expressing 33 of the 38 C-terminal repeats of TcdA can generate a TcdA-neutralizing systemic antibody response that partially protects against TcdA challenge (14). Another study has previously reported the use of an attenuated Salmonella enterica serovar Typhimurium aroA aroD vaccine strain, BRD509, expressing 14 C-terminal repeats of TcdA as a fusion to the immunogenic, nontoxic fragment C of tetanus toxin to target the mucosal immune system (16). Similar studies looking at the immunogenicity of TcdB have not been performed.
Flagellin is a structural component of certain bacteria which, in addition to conferring bacterial motility and pathogenicity, activates a number of host inflammatory signaling pathways that act as a defense against the pathogen (18). Purified flagellin proteins have adjuvant capabilities and can induce or boost adaptive immune responses. These immune-modulating effects of flagellin are mediated by the activation of antigen-presenting cells (APCs) through binding to toll-like receptor 5 (TLR5), its cognate receptor (19). TLRs are a family of pattern recognition receptors that recognize structural components shared by bacteria, fungi, and viruses. TLRs, when bound to their ligands such as FliC, both trigger innate immune responses and facilitate the development of adaptive immunity through various mechanisms, including activation and maturation of dendritic cells and expression of cytokines and other costimulatory proteins (20). S. Typhimurium FliC has previously been used with partial success as an adjuvant with experimental vaccines against pathogens such as influenza virus and Vibrio cholerae (18, 35). Recently it has been reported that S. Typhimurium flagellin-mediated stimulation of TLR5 in mice protects them from death during CDI by delaying C. difficile growth and toxin production in the gut (21).
In this study we demonstrate that a recombinant protein vaccine incorporating the RBDs of TcdA (TcdARBD) and TcdB (TcdBRBD) and the TLR5 agonist S. Typhimurium flagellin D1 is immunogenic and protective in a murine C. difficile bacterial challenge model, following a simple prime-boost regimen.
MATERIALS AND METHODS
Bacterial strains and media.
Escherichia coli DH5α was used for all subcloning steps, and E. coli BL21(DE3)* was used for protein expression (Invitrogen, Carlsbad, CA). S. Typhimurium LT2 (ATCC 15277) and E. coli cultures were grown at 37°C with aeration. All strains were maintained at −70°C in Luria-Bertani (LB) medium containing 15% glycerol. LB medium contained ampicillin (100 μg/ml), kanamycin (50 μg/ml), X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (80 μg/ml), or IPTG (isopropyl-β-d-thiogalactopyranoside) (0.1 mM) (Sigma-Aldrich, St. Louis, MO). C. difficile strain VPI 10463 (ATCC 43255) was grown anaerobically in Difco cooked-meat media (BD Diagnostic Systems, Sparks, MD) for 36 h at 37°C as previously reported (22).
Genetic methods.
Isolation of plasmid and bacterial chromosomal DNA, restriction enzyme digestion, and agarose gel electrophoresis were performed using standard biological techniques (23). DNA restriction endonucleases, T4 DNA ligase, and calf intestinal alkaline phosphatase were used according to manufacturer's specifications (New England BioLabs, Beverly, MA). Enzyme-digested products were separated on 1% agarose gel and extracted using the Qiaex II gel extraction kit (Qiagen,Valencia, CA).
Full-length protein sequences were obtained for TcdA (NCBI M30307) and TcdB (NCBI P18177) from C. difficile strain VPI 10463 (ATCC 43255). This isolate is toxinotype 0, which accounts for a majority of CDI episodes in the United States (24). The nontoxic carboxy-terminal RBDs for TcdA and TcdB were identified, and nucleotides were synthesized (Blue Heron Biotechnologies, Bothell, WA).
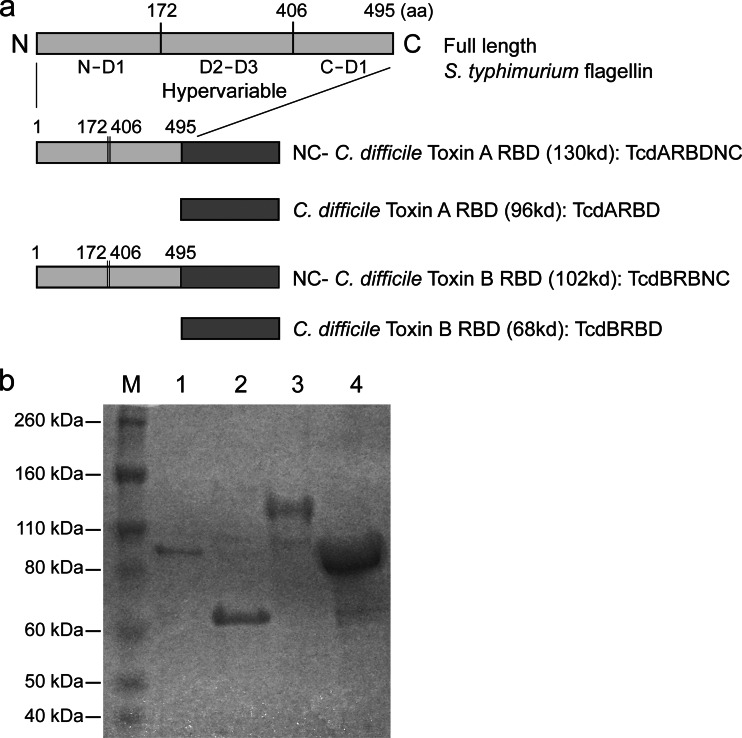
S. Typhimurium flagellin has a four-domain structure consisting of D0 and D1 domains, which are largely helical and well-conserved (19), and D2 and D3 domains, which are in the central portion of the protein and are highly variable (Fig. 1). FliC is a conformational protein and is formed when the N and C terminals of the D1 domain come together and the D2-D3 regions are surface exposed. The TLR5-activating residues lie in the conserved D1 domains. The coding sequences for the N terminus D1 domain and the C terminus D1 domain of S. Typhimurium flagellin (fliC NC) were amplified by overlapping PCR, thus allowing for the removal of the variable D2-D3 domains while retaining TLR5 binding activity.
Fig 1.
(a) Schematic diagram of Salmonella Typhimurium FliC and modified FliC and Clostridium difficile toxin receptor binding domain constructs. (b) Purified recombinant proteins expressed from recombinant E. coli strains were analyzed by SDS-PAGE on a gel stained with Coomassie blue. Lane 1, TcdARBD (96 kDa); lane 2, TcdBRBD (68 kDa); lane 3, TcdARBDNC (130 kDa); lane 4, TcdBRBDNC (102 kDa); lane M, markers.
Protein expression and purification.
Nucleotide coding sequences for TcdARBD and TcdBRBD were individually cloned into expression vector pET19b, as were fusion constructs with fliC NC (encoding TcdARBDNC and TcdBRBDNC) (Novagen) (Fig. 1). A histidine (His) tag was incorporated to facilitate purification. The expressed proteins were purified using Talon His tag purification resin according to the manufacturer's specifications (Clontech Laboratories Inc., Mountain View, CA). Protein was detected by immunoblotting using commercial polyclonal goat anti-toxin A (List Biological Laboratories, Campbell, CA) and anti-His tag antibody (Invitrogen). Endotoxin was removed by using Endotrap Blue columns according to the manufacturer's specifications (Hyglos GmbH, Bernried, Germany).
TLR5 bioassay.
HEK293 cells stably expressing TLR5 were transfected with a plasmid containing the NF-κB promoter driving the luciferase gene (Invivogen, San Diego, CA) to measure concentration-dependent stimulation of NF-κB production by flagellin. Reporter plasmid was cotransfected into HEK293 cells with bacterial flagellin protein from C. difficile, Bacillus subtilis, and S. Typhimurium and recombinant proteins TcdARBDNC, TcdBRBDNC, and S. Typhimurium FliC NC, and luciferase activity (relative light units [RLU]) was measured 24 h posttransfection. Recombinant C. difficile FliC, full-length S. Typhimurium FliC (Invivogen), and Bacillus subtilis FliC (Invivogen) were also tested for TLR5 activity. Experiments were done in duplicate.
Immunization regimen.
We immunized female, 3- to 5-week-old Swiss Webster mice (Charles River Laboratories, Wilmington, MA). Animal work was approved by the Institutional Animal Care and Use Committee at the Rockefeller University, New York, NY, or Beth Israel Deaconess Medical Center, Boston, MA. In the first study, we immunized five cohorts of 5 mice each by intraperitoneal (i.p.) injection with either 25 μg of TcdARBD or TcdBRBD, 25 μg of TcdARBD fused with the FliC NC (TcdARBDNC) or 25 μg of TcdBRBD fused with the FliC NC (TcdBRBDNC), 25 μg of TcdARBD or TcdBRBD adjuvanted with either 2.5 μg of LT(r192g), 2.5 μg of recombinant FliC (rFliC; Invivogen), or alum [Al(OH)3; 1:1 by volume] (Sigma-Aldrich). These mice were immunized on days 0 and 7; we collected, processed, and stored blood samples from mice on days 0, 7, and 14, as previously described (23).
In the second study, we immunized 3 cohorts of 5 mice each by i.p. injection with 25 μg of TcdARBDNC or TcdBRBDNC adjuvanted with either 2.5 μg of LT(r192g) or alum [Al(OH)3; 1:1 by volume], or both. These mice were immunized on days 0 and 7; we collected, processed, and stored blood samples from mice on days 0, 7, and 14.
To perform a direct comparison to the formalin-inactivated toxoid vaccine currently in human clinical trials, we immunized one cohort of mice with the toxoid preparation by the i.p. route using alum as the adjuvant. Toxoid A and toxoid B from C. difficile strain VPI 10463 were purchased from List Biological Laboratories.
Measurement of immune responses.
To detect antibody responses to TcdA or TcdB, we coated plates with 100 ng/well of purified TcdA or TcdB in 50 mM carbonate buffer, pH 9.6 (List Biological Laboratories). We blocked plates with phosphate-buffered saline (PBS)–1% bovine serum albumin (BSA) (Sigma-Aldrich). To detect anti-TcdA and anti-TcdB IgG or IgA in serum, we diluted sera 1:1,000 or 1:50 in PBS containing 0.05% Tween 20 (PBS-T) (Sigma-Aldrich), respectively, and incubated the plates at 37°C for 1 h. We detected bound antibodies using a 1:1,000 dilution in PBS-T of either goat anti-mouse IgG conjugated with horseradish peroxidase (HRP) (Southern Biotech, Birmingham, AL) or goat anti-mouse IgA conjugated with HRP (Southern Biotech), incubating plates for 1 h at 37°C. We developed the plates with 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (Sigma-Aldrich) and 0.03% H2O2 (Sigma-Aldrich) and determined optical density using a Vmax microplate reader (Molecular Devices Corp., Sunnyvale, CA) at 405 nm kinetically for 5 min at 14-s intervals as previously reported (23). To equilibrate, we divided readings of milliunits of optical density per minute for samples by those for plate controls comprising pooled blood or stool standards from unrelated experimental cohorts and reported the results as enzyme-linked immunosorbent assay (ELISA) units.
Serum neutralization assay.
To measure the neutralizing activity of sera, MRC-5 (ATCC CCL-171) cells were plated in 96-well tissue culture plates 24 h prior to the procedure at a density of 1 × 104 cells/well and incubated at 37°C in 5% CO2. We incubated 2-fold serial dilutions of sera from mice, starting at a 1:50 dilution, in minimal essential medium containing 10% fetal bovine serum at 37°C for 1 h with either TcdA at 60 ng/well or with TcdB at 20 pg/well as described previously. We used four times the minimal dosage of toxin A and toxin B required to cause 100% cell rounding after 48 h in the absence of serum for each well (0.6-μg/ml final concentration or 60 ng/well for toxin A or 200 pg/ml or 20 pg/well for toxin B) (23, 25). We added the toxin-serum mixtures to MRC-5 cells, incubated the plates overnight at 37°C in 5% CO2, and determined the proportion of cell rounding. We defined the neutralization antibody titer as the reciprocal of the highest serum dilution that inhibited cell rounding >50% (25). C. difficile toxins can induce cell rounding in virtually 100% of cells given adequate time and toxin concentrations. However, for quantification of toxin effect variation, the midpoint of the curve (i.e., 50% rounding) provides far greater accuracy of measurement than either the uppermost (100%) or lowermost (0%) absolute plateaus. We used commercially available goat anti-C. difficile toxin A (List Biological Laboratories, Campbell, CA), toxin A alone, and medium alone as controls. Experiments were performed in duplicate.
Mouse challenge model.
A murine model of antibiotic-associated CDI established recently in the Kelly laboratory was used to evaluate the protective efficacy of our recombinant vaccine constructs (22). Five cohorts of female, age-matched, 8- to 10-week-old C57BL/6 mice (Charles River Laboratories) were immunized on days 0 and 10. Each cohort was immunized with either TcdARBD and TcdBRDB, TcdARBDNC and TcdBRBDNC, TcdARBDNC and TcdBRBDNC adjuvanted with alum, or toxoid A and toxoid B adjuvanted with alum. Mice in each cohort were administered a cocktail of antibiotics (kanamycin at 40 mg/kg of body weight, gentamicin at 3.5 mg/kg, colistin at 4.2 mg/kg, metronidazole at 21.5 mg/kg, and vancomycin at 4.5 mg/kg) in their water daily for 3 days starting on day 14. Two days later, mice received one dose of clindamycin (10 mg/kg) given intraperitoneally. Twenty-four hours after intraperitoneal injection, mice were challenged with a dose of 105 CFU of C. difficile strain VPI 10463 (TcdA+ TcdB+ and a high toxin producer) and monitored daily for 14 days for mortality, morbidity, weight, and diarrhea. Additional serological assays were not performed in these mice.
Statistical analysis.
For normally distributed data, we used an unpaired Student t test analysis for comparison of means; for nonparametric data, we used the Mann-Whitney U test. We performed statistical analyses using Microsoft Excel 2002 and plotted graphs using Prism (GraphPad Software, San Diego, CA). A P value of less than 0.05 was considered to indicate statistical significance. Kaplan-Meier plots were used to analyze survival in the challenged mice. The Mantel-Cox test for pairwise comparisons was used to assess statistical significance between the cohorts.
RESULTS
Recombinant S. Typhimurium flagellin D1 subunit protein fused with C. difficile TcdARBD and TcdBRBD is a potent agonist of TLR5.
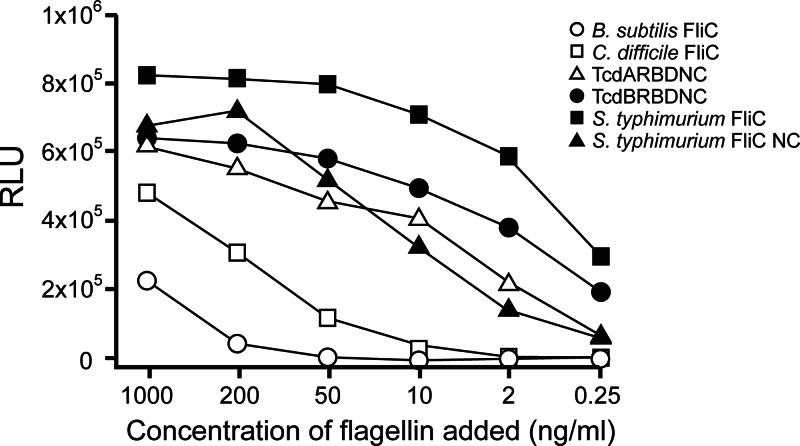
The S. Typhimurium FliC fusion constructs TcdARBDNC and TcdBRBDNC demonstrated TLR5 bioactivity in a cell-based TLR5-dependent NF-κB luciferase reporter assay and are comparable to S. Typhimurium FliC NC and full-length S. Typhimurium FliC (P not significant [NS]) (Fig. 2). Additionally, we found that C. difficile FliC also activated TLR5 in vitro (Fig. 2). C. difficile FliC consists of 290 amino acids and has the typical structure of bacterial flagellin, including its conserved D1 region, where the TLR5 activation domain resides (26).
Fig 2.
Concentration-dependent stimulation of NF-κB activity by flagellin. Reporter plasmids were cotransfected into cells with the indicated bacterial flagellin protein, and luciferase activity was measured 24 h posttransfection. RLU, relative light units.
Immunoadjuvant properties of TLR5 agonist S. Typhimurium flagellin D1 subunit fused with C. difficile TcdARBD and TcdBRBD in mice.
Mucosal immune responses are highly dependent upon the coadministration of mucosal adjuvants. Several toxins such as ADP-ribosylating cholera toxin (CT) and heat-labile enterotoxin (LT) have most commonly been used as mucosal immunoadjuvants in experimental animal models (27). Recently, several mutant derivatives of LT that retain the immunoadjuvant properties of LT but have decreased enterotoxic activity have been developed (28, 29). One such mutant, LT(r192g), has a single point mutation that changes the proteolytic cleavage site, thus rendering the toxin enzymatically inactive. We also tested the adjuvant properties of alum [Al(OH)3], which functions by absorbing and concentrating antigens for delivery to APCs, thereby increasing APC recruitment and activation (depot effect). Recently it has been reported to act through the Nalp3 inflammasome.
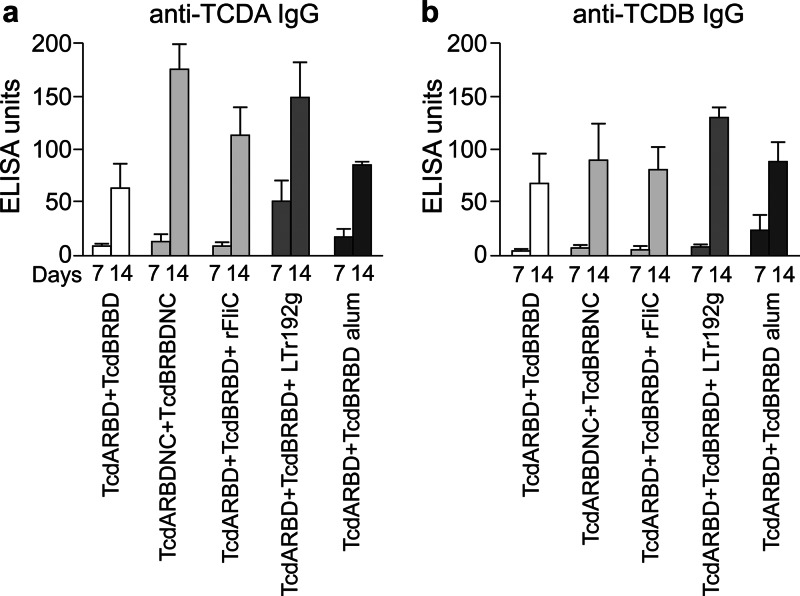
Immunization of mice with TcdARBDNC and TcdBRBDNC resulted in a significant anti-TcdA IgG response following the boost, compared to immunization with only TcdARBD and TcdBRBD, thus illustrating the adjuvant properties of FliC (P < 0.05) (Fig. 3a). This cohort of mice also had a level of anti-TcdA IgG responses comparable to the level for the cohorts of mice receiving LT(r192g) (P = 0.56) and rFliC (P = 0.15) but a higher anti-TCDA IgG response than that for the cohort receiving alum (P < 0.05) as the adjuvant. Immunization of mice with TcdARBDNC and TcdBRBDNC also resulted in a significant level of anti-TcdA IgA in stool following the boost, compared to levels for cohorts that received TcdARBD and TcdBRBD (P < 0.05) or levels for those that received TcdARBDNC and TcdBRBDNC coadministered with rFliC, alum, or LT(r192g) (P < 0.05) (Fig. 4a). All cohorts of mice produced comparable anti-TcdB IgG responses in serum and anti-TcdB IgA responses in stool (P NS). The cohort of mice that received LT(r192g) as an adjuvant showed a trend toward higher anti-TcdB IgG in serum and anti-TcdB IgA in stool, although the results are not statistically significant (Fig. 3b).
Fig 3.
Serum anti-TCDA IgG (a) and anti-TCDB IgG (b) responses in mice immunized on weeks 0 and 1. Cohorts of mice received TcdARBDNC and TcdBRBDNC, TcdARBDNC and TcdBRBDNC, or TcdARBDNC and TcdBRBDNC adjuvanted with rFliC, LT(r192g), or alum. Results were determined by kinetic ELISA; the geometric mean plus standard error of the mean for each cohort are shown.
Fig 4.
Stool anti-TcdA IgA (a) and anti-TcdB IgA (b) responses in mice immunized on weeks 0 and 1. Cohorts of mice received TcdARBDNC and TcdBRBDNC, TcdARBDNC and TcdBRBDNC, or TcdARBDNC and TcdBRBDNC adjuvanted with rFliC, LT(r192g), or alum. Results were determined by kinetic ELISA; the geometric mean plus standard error of the mean for each cohort are shown.
Immunization of mice with TcdARBDNC and TcdBRBDNC resulted in induction of C. difficile toxin A-neutralizing serum antibodies in comparison to the response seen in mice immunized with TcdARBD and TcdBRBD alone (P < 0.001) (Fig. 5a). This assay was performed using MRC-5 fibroblasts and was done in duplicate. Immunization with TcdARBD and TcdBRBD with immunoadjuvant LT(r192g) resulted in an increased toxin A-neutralizing response compared to immunizations adjuvanted with alum or rFliC and compared to immunizations with no additional adjuvants (P < 0.001). Immunization with TcdARBD and TcdBRBD comixed with rFliC resulted in the most prominent toxin B-neutralizing response compared to immunization of most of the other cohorts, which failed to induce even low levels of anti-toxin B antibodies in this assay (P < 0.001) (Fig. 5b). Immunization with TcdARBDNC and TcdBRBDNC resulted in moderate levels of toxin B-neutralizing antibodies.
Fig 5.
C. difficile toxin A (a) and toxin B (b) neutralizing antibody titers in day 14 sera collected from mice. The neutralizing titers were determined by a cell toxicity assay in MRC-5 cells. Cohorts of mice received TcdARBDNC and TcdBRBDNC, TcdARBDNC and TcdBRBDNC, or TcdARBDNC and TcdBRBDNC adjuvanted with rFliC, LT(r192g), or alum. Results are reported as the geometric mean plus standard error of the mean of the reciprocal titer for each cohort.
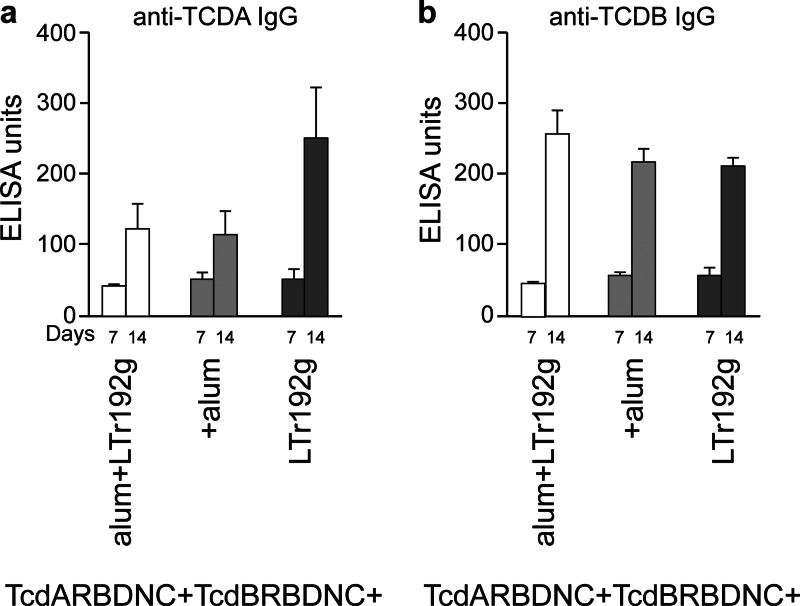
We also studied whether the combined effect of various adjuvants would significantly enhance the immunogenicity of the vaccines. We immunized mice with the TcdARBDNC and TcdBRBDNC proteins in the presence of one or two additional adjuvants. Cohorts of mice that received TcdARBDNC and TcdBRBDNC with LT(r192g) had the strongest anti-TcdA IgG response in serum (P < 0.05) and the strongest anti-TcdA IgA response in stool (P < 0.05) (Fig. 6a and 7a). Cohorts of mice receiving alum or alum plus LT(r192g) developed comparable anti-TcdA IgG and anti-TcdA IgA responses (P NS). In contrast, all cohorts of mice produced comparable anti-TcdB IgG responses in serum and anti-TcdB IgA responses in stool (P NS) (Fig. 6b and 7b).
Fig 6.
Serum anti-TcdA IgG (a) and anti-TcdB IgG (b) responses in mice immunized on weeks 0 and 1. Cohorts of mice received TcdARBDNC and TcdBRBDNC adjuvanted with LT(r192g) or alum or both. Results were determined by kinetic ELISA; the geometric mean plus standard error of the mean for each cohort are shown.
Fig 7.
Stool anti-TcdA IgA (a) and anti-TcdB IgA (b) responses in mice immunized on weeks 0 and 1. Cohorts of mice received TcdARBDNC and TcdBRBDNC adjuvanted with LT(r192g) or alum or both. Results were determined by kinetic ELISA; the geometric mean plus standard error of the mean for each cohort are shown.
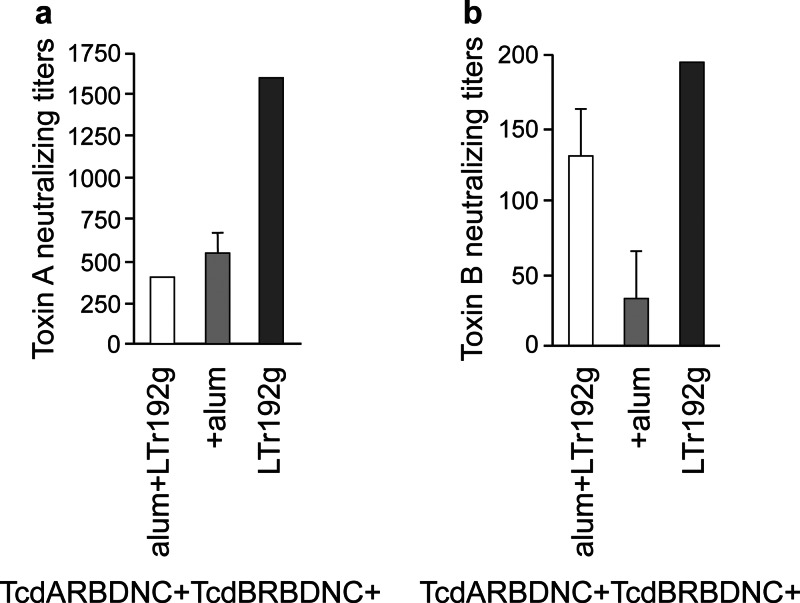
Immunization with TcdARBDNC and TcdBRBDNC adjuvanted with LT(r192g) resulted in the most prominent toxin A- as well as toxin B-neutralizing responses compared to immunization of the other cohorts (P < 0.05) although levels of anti-toxin B-neutralizing antibodies were present in very low levels in all cohorts tested (Fig. 8a and b).
Fig 8.
C. difficile toxin A (a) and toxin B (b) neutralizing antibody titers in day 14 sera collected from mice. The neutralizing titers were determined by a cell toxicity assay in MRC-5 cells. Cohorts of mice received TcdARBDNC and TcdBRBDNC adjuvanted with LT(r192g) or alum or both. Results are reported as the geometric mean plus standard error of the mean of the reciprocal titer for each cohort.
Interestingly we also find that, in almost all experiments, mice had a stronger immune response to TcdARBD than to TcdBRBD even though the proteins were administered in a 1:1 ratio. This difference is most significant in the antitoxin IgA responses in stool and in the antitoxin neutralization antibody levels (Fig. 7 and 8).
Protective efficacy of the recombinant protein vaccines containing C. difficile TcdARBD and TcdBRBD adjuvanted with the S. Typhimurium flagellin D1 subunit in a murine C. difficile challenge model.
One hundred percent of control animals receiving alum alone had symptomatic disease in the form of loose stool, and 7 out of 8 animals died within 3 days of vegetative bacterial cell challenge. By contrast, the cohorts of mice receiving TcdARBD and TcdBRBD with or without adjuvants such as alum or flagellin NC demonstrated 100% disease-free survival. Cohorts of mice receiving toxoid A and toxoid B adjuvanted with alum also showed 100% disease-free survival. These results indicate that immunization of mice with TcdARBD and TcdBRBD affords a robust protection comparable to alum-adjuvanted toxoids (P < 0.001).
DISCUSSION
Infection of humans with the spore-forming bacillus C. difficile can produce toxin-mediated enteric symptoms, ranging from mild diarrhea to life-threatening pseudomembranous colitis. Although a number of C. difficile vaccines are being developed, their utility has been limited by the need for repetitive dosing, the use of formalin-induced detoxification, and the stability of the vaccine constructs (30, 31). For a vaccine to warrant clinical development it must induce a strong protective immune response against autologous and heterologous strains, requiring the fewest immunizations with or without the added help of an adjuvant. Addressing these issues will ensure the identification of a clinical vaccine candidate of sufficient efficacy and potency such that there can be high confidence that the vaccine will be clinically and commercially feasible for patients susceptible to C. difficile in a public health setting, especially in older immunodeficient populations.
In our study we found that the recombinant fusion proteins representing the RBDs of TcdA and TcdB are immunogenic in mice, requiring a single prime-boost regimen, and that S. Typhimurium FliC has adjuvant properties when administered with these antigens. The induction of anti-C. difficile toxin A-neutralizing antibody responses following a prime and boost regimen occurred in all cohorts, and such responses were enhanced in the presence of adjuvant compared to the responses to TcdARBD alone. In contrast, we were unable to measure anti-C. difficile toxin B-neutralizing antibody responses in certain cohorts of mice that received TcdARBD and TcdBRBD either alone or adjuvanted. It is possible that these vaccine constructs were unable to induce anti-toxin B neutralization antibodies measurable by the in vitro cell-based assay but were able to induce measureable anti-toxin B binding antibodies as measured by ELISAs. It will be valuable to better characterize neutralizing antibodies in additional assays such as assays using IMR90 cells.
We also report that the induction of an immune response to the RBDs of the toxins from C. difficile strain VPI 10463 is protective against challenge using the homologous strain. This approach carries many substantial advantages over the toxoid-based C. difficile vaccine that is now in clinical trials and in active preclinical development (32). Specifically, we use recombinant RBDs and not holotoxins, which are difficult to purify and produce, require formalin inactivation, are unstable and degrade over time, and contain contaminating proteins and antigens. Recombinant RBDs may be as effective as protective antigens and are generally unencumbered by the limitations imposed by holotoxins but also may be less immunogenic than toxoids of whole toxin due to the limitations of antigen selection, in this case RBDs.
Historically, hamsters have been used to investigate disease pathogenesis and treatment in CDI but are not ideal models because of the lack of hamster-specific reagents and genetically modified animals (33). The newly established mouse model of antibiotic-induced CDI closely resembles some features of human disease, including antibiotic-associated diarrhea; a predominately colonic nature with minimal if any small intestinal disease; similar histologies, including pseudomembrane formation; and a variable disease severity depending on the challenge dose and strain of C. difficile used for infection (22). Our challenge data show that the RBDs of TcdA and TcdB not only are immunogenic but also afford complete diarrhea-free protection against C. difficile challenge using the toxin-producing homologous strain VPI 10463; these features are comparable to the toxoid vaccine adjuvanted with alum. These results suggest that the inherent immunoadjuvant properties of TcdA are able to induce a strong immune response in mice that is protective at the dose that we have used (34). Dose escalation studies and studies involving a shorter time to challenge and additional adjuvants such as LT(r192g/l211a) are needed to elucidate differences in the immunogenicity and protective efficacy between the various constructs. Additional experiments are warranted in CDI models in mice and hamsters using clinically relevant C. difficile strains as well as strains expressing binary toxin. This mouse model has yet to be correlated with CDI treatment and protection by antibody production.
Toll-like receptors (TLRs) detect microbial infection and play an essential role in linking innate and adaptive immunity (20). It has been reported that purified flagellin protein has adjuvant activity capable of inducing or boosting adaptive immune responses by specifically binding to TLR5 (18). Here we demonstrate that a recombinant fusion protein incorporating both antigens of interest and flagellin might have distinct advantages. Such a strategy would target the antigen to the APCs, couple stimulation of APC maturation with antigen uptake, and enhance processing and ultimately presentation. Various other studies have used S. Typhimurium flagellin as an adjuvant (18, 35).
Here we also demonstrate the important role of mucosal adjuvants such as LT(r192g), which works well as an adjuvant when combined with constructs expressing fusion FliC NC. LT(r192g) is a potent mucosal adjuvant that has been rendered nontoxic by mutating a proteolytic site and that has been shown to be associated with only mild self-limited diarrhea in adult volunteers receiving an oral inactivated Helicobacter pylori vaccine (36). This nontoxic derivative is different from LTK63, a synthetic LT molecule which lacks ADP-ribosylating activity but which was associated with Bell's palsy in a phase I trial of nasal subunit vaccines against tuberculosis (37). Additional experiments with a double mutant such as LT(r192g/l211a) may provide a safer alternative with an even greater reduction in enterotoxicity (38). In addition to alum, other mucosal adjuvants need to be studied in the context of a mucosal vaccine.
In conclusion, we have demonstrated that a recombinant protein-based vaccine targeting the RBDs of the C. difficile toxins adjuvanted with S. Typhimurium flagellin can induce rapid, high-level protection against both toxins and bacterial challenge using the homologous strain. That this vaccine strategy was able to induce complete protection in a mouse challenge model warrants further study in the hamster model of CDI using clinically relevant C. difficile strains such as BI/NAP1 strains expressing the binary toxin. In the past few years the emergence of a “new” hypervirulent strain has led to more-severe complications and an associated increase in mortality. The target population is also broadening to include a younger, community-based population. These challenges are even greater in the at-risk elderly population, who are unable to mount adequate protective immune responses to infection, or in patients with fulminant or refractory CDI. Therefore, there is a great need for a safe and immunogenic vaccine against C. difficile which can potentially stop the spread of the CDAD epidemic.
ACKNOWLEDGMENTS
We thank John Clements of Tulane University for LT(r192g). We thank Wendy Chen for help with figure production.
This research was supported in part by the NIH grant R01AI095256-01A1.
Footnotes
Published ahead of print 1 April 2013
REFERENCES
- 1. Bartlett JG. 2006. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145:758–764 [DOI] [PubMed] [Google Scholar]
- 2. Mylonakis E, Ryan ET, Calderwood SB. 2001. Clostridium difficile-associated diarrhea: a review. Arch. Intern. Med. 161:525–533 [DOI] [PubMed] [Google Scholar]
- 3. Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jank T, Giesemann T, Aktories K. 2007. Rho-glucosylating Clostridium difficile toxins A and B: new insights into structure and function. Glycobiology 17:15R–22R [DOI] [PubMed] [Google Scholar]
- 5. Jank T, Aktories K. 2008. Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends Microbiol. 16:222–229 [DOI] [PubMed] [Google Scholar]
- 6. Kyne L, Warny M, Qamar A, Fatimi A, Wei JY, Lamont JT, Kelly CP. 1999. Natural immunity against Clostridium difficile toxin A protects against diarrhea and pseudomembranous colitis. Gastroenterology 116:A895 [Google Scholar]
- 7. Kyne L, Warny M, Qamar A, Kelly CP. 2000. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342:390–397 [DOI] [PubMed] [Google Scholar]
- 8. Kyne L, Warny M, Qamar A, Kelly CP. 2000. High anti-toxin A antibody levels are associated with protection against recurrent Clostridium difficile diarrhea. Gastroenterology 118:A885 [Google Scholar]
- 9. Kyne L, Warny M, Qamar A, Kelly CP. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189–193 [DOI] [PubMed] [Google Scholar]
- 10. Leav BA, Blair B, Leney M, Knauber M, Reilly CLowy I, Gerding DN, Kelly CP, Katchar K, Baxter R, Ambrosino D, Molrine D. 2010. T Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine 28:965–969 [DOI] [PubMed] [Google Scholar]
- 11. Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713 [DOI] [PubMed] [Google Scholar]
- 13. Giannasca PJ, Warny M. 2004. Active and passive immunization against Clostridium difficile diarrhea and colitis. Vaccine 22:848–856 [DOI] [PubMed] [Google Scholar]
- 14. Ward SJ, Douce G, Dougan G, Wren BW. 1997. Delivery of non-toxic fragments of Clostridium difficile toxin A to the mucosal immune system. Rev. Med. Microbiol. 8:S34–S36 [Google Scholar]
- 15. Ward SJ, Douce G, Dougan G, Wren BW. 1999. Local and systemic neutralizing antibody responses induced by intranasal immunization with the nontoxic binding domain of toxin a from Clostridium difficile. Infect. Immun. 67:5124–5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ward SJ, Douce G, Figueiredo D, Dougan G, Wren BW. 1999. Immunogenicity of a Salmonella typhimurium aroA aroD vaccine expressing a nontoxic domain of Clostridium difficile toxin A. Infect. Immun. 67:2145–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gardiner DF, Rosenberg T, Zaharatos J, Franco D, Ho DD. 2009. A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A. Vaccine 27:3598–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Duin D, Medzhitov R, Shaw AC. 2006. Triggering TLR signaling in vaccination. Trends Immunol. 27:49–55 [DOI] [PubMed] [Google Scholar]
- 19. Jacchieri SG, Torquato R, Brentani RR. 2003. Structural study of binding of flagellin by Toll-like receptor 5. J. Bacteriol. 185:4243–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akira S, Takeda K, Kaisho T. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680 [DOI] [PubMed] [Google Scholar]
- 21. Jarchum I, Liu M, Lipuma L, Pamer EG. 2011. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infect. Immun. 79:1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen XH, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding D, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992 [DOI] [PubMed] [Google Scholar]
- 23. Ghose C, Kalsy A, Sheikh A, Rollenhagen J, John M, Young J, Rollins SM, Qadri F, Calderwood SB, Kelly CP, Ryan ET. 2007. Transcutaneous immunization with Clostridium difficile toxoid A induces systemic and mucosal immune responses and toxin A-neutralizing antibodies in mice. Infect. Immun. 75:2826–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geric B, Rupnik M, Gerding DN, Grabnar M, Johnson S. 2004. Distribution of Clostridium difficile variant toxinotypes and strains with binary toxin genes among clinical isolates in an American hospital. J. Med. Microbiol. 53:887–894 [DOI] [PubMed] [Google Scholar]
- 25. Torres JF, Lyerly DM, Hill JE, Monath TP. 1995. Evaluation of formalin-inactivated Clostridium difficile vaccines administered by parenteral and mucosal routes of immunization in hamsters. Infect. Immun. 63:4619–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tasteyre A, Barc MC, Karjalainen T, Dodson P, Hyde S, Bourlioux P, Borriello P. 2000. A Clostridium difficile gene encoding flagellin. Microbiology 146:957–966 [DOI] [PubMed] [Google Scholar]
- 27. Bowman CC, Clements JD. 2001. Differential biological and adjuvant activities of cholera toxin and Escherichia coli heat-labile enterotoxin hybrids. Infect. Immun. 69:1528–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dickinson BL, Clements JD. 1995. Dissociation of Escherichia-Coli heat-labile enterotoxin adjuvanticity from Adp-ribosyltransferase activity. Infect. Immun. 63:1617–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chong C, Friberg M, Clements JD. 1998. LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp. Vaccine 16:732–740 [DOI] [PubMed] [Google Scholar]
- 30. Salnikova MS, Joshi SB, Rytting JH, Warny M, Middaugh CR. 2008. Physical characterization of Clostridium difficile toxins and toxoids: effect of the formaldehyde crosslinking on thermal stability. J. Pharm. Sci. 97:3735–3752 [DOI] [PubMed] [Google Scholar]
- 31. Salnikova MS, Joshi SB, Rytting JH, Warny M, Middaugh CR. 2008. Preformulation studies of Clostridium difficile toxoids A and B. J. Pharm. Sci. 97:4194–4207 [DOI] [PubMed] [Google Scholar]
- 32. Foglia G, Shah S, Luxemburger C, Pietrobon PJ. 2012. Clostridium difficile: development of a novel candidate vaccine. Vaccine 30:4307–4309 [DOI] [PubMed] [Google Scholar]
- 33. Fekety R, Silva J, Toshniwal R, Allo M, Armstrong J, Browne R, Ebright J, Rifkin G. 1979. Antibiotic-associated colitis: effects of antibiotics on Clostridium difficile and the disease in hamsters. Rev. Infect. Dis. 1:386–397 [DOI] [PubMed] [Google Scholar]
- 34. Brun P, Scarpa M, Grillo A, Palu G, Mengoli C, Zecconi A, Spigaglia P, Mastrantonio P, Castagliuolo I. 2008. Clostridium difficile TxAC314 and SLP-36kDa enhance the immune response toward a co-administered antigen. J. Med. Microbiol. 57:725–731 [DOI] [PubMed] [Google Scholar]
- 35. Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, Kavita U, Stanberry L, Shaw A. 2011. Safety and immunogenicity of a recombinant M2e15 flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 29:5145–5152 [DOI] [PubMed] [Google Scholar]
- 36. Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 69:3581–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, Del Giudice G, Rappuoli R. 2009. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 4:e6999 doi:10.1371/journal.pone.0006999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 18:546–551 [DOI] [PMC free article] [PubMed] [Google Scholar]