Abstract
Rtt109 is a lysine acetyltransferase that acetylates histone H3 at lysine 56 (H3K56) in fungi. This acetylation event is important for proper DNA replication and repair to occur. Efficient Rtt109 acetyltransferase activity also requires a histone chaperone, vacuolar protein sorting 75 (Vps75), as well as the major chaperone of the H3-H4 dimer, anti-silencing factor 1 (Asf1). Little is known about the role of these proteins in the opportunistic fungal pathogen Pneumocystis carinii. To investigate the functions of Asf1 and Vps75 in Pneumocystis carinii, we cloned and characterized both of these genes. Here, we demonstrate that both genes, P. carinii asf1 (Pcasf1) and Pcvps75, function in a fashion analogous to their Saccharomyces cerevisiae counterparts. We demonstrate that both P. carinii Asf1 (PcAsf1) and PcVps75 can bind histones. Furthermore, when Pcasf1 is expressed heterologously in S. cerevisiae asf1Δ cells, PcAsf1 can restore full H3 lysine acetylation. We further demonstrated that the Pcasf1 cDNA expressed in asf1Δ S. cerevisiae cells can restore growth to wild-type levels in the presence of genotoxic agents that block DNA replication. Lastly, we observed that purified PcAsf1 and PcVps75 proteins enhance the ability of PcRtt109 to acetylate histone H3-H4 tetramers. Together, our results indicate that the functions of the Rtt109-Asf1-Vps75 complex in the acetylation of histone H3 lysine 56 and in DNA damage response are present in P. carinii DNA and cell cycle progression.
INTRODUCTION
Infections from opportunistic fungal species, including Pneumocystis pneumonia, have increased significantly over the past decades. This reflects the increased numbers of patients with suppressed immune status, including patients with cancer, transplantation procedures, and HIV infection. Pneumocystis is one of the most medically prominent opportunistic fungi, representing a significant cause of pneumonia in immunocompromised hosts. The mortality associated with Pneumocystis pneumonia remains high despite the use of current treatments. Mortality rates range between 10 and 30% in patients with AIDS (1–4) and between 40 and 70% in patients who are immunosuppressed for conditions other than AIDS (2, 5, 6). Basic research on Pneumocystis has been limited by our poor understanding of its cell cycle and life cycle regulation and a persistent deficit in our ability to culture these organisms in vitro. These limitations render the development of new agents to treat this infection extremely challenging. In addition, many fungal genes and their cognate proteins are conserved in humans, rendering them less suitable as fungus-specific molecular targets for antimicrobial therapy without significant adverse effects on the human host. Evidence suggesting possible molecular resistance to currently available agents for Pneumocystis pneumonia and the presence of side effects of these agents support the need to identify new effective targets to treat Pneumocystis infection (7).
Our efforts to define the mechanisms of cell and life cycle control in Pneumocystis carinii have led us to investigate the organism's histone acetylation pathway, which represents a potentially attractive target for the development of new therapies for this important infection. Rtt109 histone acetyltransferases (HATs) are highly conserved among fungal species, yet in general they exhibit no obvious sequence homologies to mammalian HATs (8–11). In Saccharomyces cerevisiae and Schizosaccharomyces pombe, Rtt109 forms a complex with Vps75, which regulates the stability of Rtt109 (12–15). Rtt109 also forms a complex with Asf1 and histones H3 and H4 (15–18). Posttranslational acetylation of histones, which regulates a variety of cellular processes, requires Asf1 and Vps75. Therefore, Asf1 and Vps75 are active components of the histone acetylation pathway.
Previously, our group characterized P. carinii rtt109 (Pcrtt109), but the other components of this pathway have not yet been identified in P. carinii (19, 20). In these investigations, we have characterized the full-length sequences of Pcasf1 and Pcvps75, transformed them into S. cerevisiae, and expressed the Pneumocystis proteins in S. cerevisiae strains lacking ASF1 (asf1Δ) and VPS75 (vps75Δ), respectively. We observed that the essential histone acetyltransferase chaperone proteins P. carinii Asf1 (PcAsf1) and PcVps75 are conserved in Pneumocystis and function in a manner parallel to S. cerevisiae Asf1 (ScAsf1) and ScVps75.
The Pneumocystis gene and protein nomenclature conventions employed in this work are consistent with the standards adopted by the 11th International Workshop on Opportunistic Protists.
MATERIALS AND METHODS
Media and strains.
S. cerevisiae wild-type (BY4741), asf1Δ (YJL115W), and vps75Δ (YNL246W) strains and P. carinii inoculum organisms were obtained from the American Type Culture Collection (ATCC; Manassas, VA). All S. cerevisiae cultures were grown at 30°C in yeast extract-peptone-dextrose (YPD) medium or in minimal medium containing 2% glucose or 2% galactose and lacking uracil. Escherichia coli TOP10 chemically competent cells (Invitrogen, Grand Island, NY) were used for TOPO TA cloning and bacterial transformation according to the manufacturer's protocol. All E. coli cultures were grown at 37°C overnight. P. carinii organisms were cultivated and purified from corticosteroid-immunosuppressed rats as we previously reported (19).
Cloning and sequence analysis of Pcasf1 and Pcvps75.
Partial, though incomplete, DNA sequences of both Pcasf1 and Pcvps75 were obtained from The Pneumocystis Genome Project database (http://pgp.cchmc.org/). Primers for Pcasf1 and Pcvps75 were synthesized, and the rapid amplification of cDNA ends (RACE) technique (GeneRacer kit; Invitrogen, Carlsbad, CA) was used to generate full-length sequences of Pcasf1 and Pcvps75. The complete sequences of both genes were analyzed. The complete coding sequences for Pcasf1 and Pcvps75 have been deposited in GenBank under accession numbers KC169789 and KC169790, respectively. The predicted amino acid sequences of PcAsf1 and PcVps75 were compared with the amino acid sequences of ScAsf1 and ScVps75, respectively, using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) to derive sequence alignments.
Chromosomal localization and Southern hybridization of Pcasf1 and Pcvps75.
P. carinii genomic DNA was extracted from rats infected with P. carinii using TRIzol reagent (Invitrogen, Grand Island, NY). Genomic DNA was also extracted from the lungs of uninfected rats. Both rat DNA and P. carinii DNA were digested with BamHI, HindIII, and XbaI restriction endonucleases incubated at 37°C overnight. The digested products were separated by agarose gel electrophoresis and transferred to nitrocellulose membranes. P. carinii RNA was extracted from rat lungs using the RNeasy isolation kit (Qiagen, Inc., Valencia, CA). cDNA was synthesized from total P. carinii RNA using the SuperScript III first-strand synthesis system for reverse transcription-PCR (RT-PCR) (Invitrogen, Grand Island, NY) and was amplified by PCR. To make radiolabeled probes, amplicons from PCR were labeled with Amersham [α-32P]dATP (GE Healthcare, Piscataway, NJ) according to the RadPrime DNA labeling system protocol (Invitrogen, Grand Island, NY). Quick Spin columns (Roche, Indianapolis, IN) were used to remove unincorporated [α-32P]dATP. The radiolabeled probes and the nitrocellulose membrane were used for Southern hybridization. The probes were also hybridized to another nitrocellulose membrane containing P. carinii chromosomal DNA that was separated by contour-clamped homogenous electric field (CHEF) electrophoresis, kindly provided by Melanie Cushion at the University of Cincinnati. The ExpressHyb protocol (Clontech, Mountain View, CA) was followed for hybridization and wash conditions. The nitrocellulose membranes were air dried and exposed to X-ray films at −70°C for 48 h for Southern blots and 4 days for CHEF blots.
Expression and purification of PcAsf1 and PcVps75.
The plasmid pYES2.1/V5-His-TOPO (Invitrogen, Grand Island, NY) was used for cloning the relevant constructs into E. coli TOP10 chemically competent cells [E. coli BL21(DE3) cells] and into S. cerevisiae to express PcAsf1 and PcVps75 proteins. This plasmid contains the galactose 1 (GAL1) promoter to induce protein expression in the presence of galactose. Crude extracts from S. cerevisiae cells and E. coli were obtained using the French press technique as described in previous studies (8). The recombinant proteins contained a C-terminal V5 epitope for detection by anti-V5 antibody and a polyhistidine tag for purification using a nickel affinity column. We utilized S. cerevisiae-produced recombinant proteins in Pcasf1/Pcvps75 experiments on binding to core histones bound to agarose (see Fig. 4).
Fig 4.
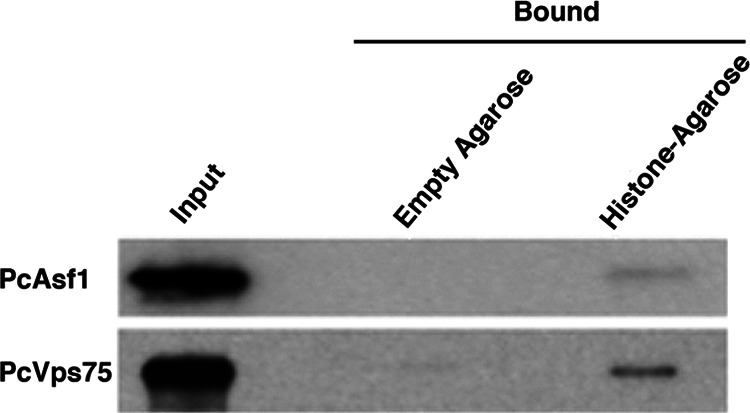
PcAsf1 and PcVps75 bind to histones in vitro. A histone-agarose binding assay was performed using purified PcAsf1 and PcVps75 to investigate binding to core histones immobilized on agarose resin. Empty agarose resin was used as a negative control. The same amount of recombinant protein without histones or resin was used as an input control.
The pCR4-TOPO (Invitrogen, Grand Island, NY) and pGEX-4T-1 (GE Healthcare, Piscataway, NJ) plasmids were used for TOPO TA cloning and E. coli BL21(DE3) transformation for PcAsf1 and PcVps75 expression, respectively. The recombinant proteins contained N-terminal glutathione S-transferase (GST) for detection by anti-GST antibody. Glutathione-Sepharose columns were used for GST pulldown in the purification process. Crude extracts from E. coli were obtained by the French press technique (19). The recombinant proteins were subsequently used in HAT assays. To our knowledge, as cytosolic proteins these molecules actually have minimal known posttranslational modifications, such as glycosylation. We were able to produce substantially greater quantities of these recombinant proteins using E. coli as required for functional assays. Orthologues of these proteins expressed in bacteria have been shown to be fully functional in other studies (8, 9, 21, 22). Hence, we utilized E. coli-produced recombinant proteins in the histone acetyltransferase assays (see Fig. 8 and 9).
Fig 8.

PcVps75 and PcAsf1 enhance the acetylation activity of PcRtt109 in vitro. Histone acetyltransferase (HAT) reactions were performed using tritium-labeled acetyl coenzyme A ([3H]acetyl-CoA). The radioactivity of 3H-acetylated histones was measured using a liquid scintillation counter (cpm). Equal amounts of purified PcRtt109 were incubated in the histone acetyltransferase reaction mixture either alone or, where indicated, with the addition of increasing amounts of purified PcVps75, with or without further addition of PcAsf1. An irrelevant protein (PcAce2) with no histone acetyltransferase activity was used as the negative control. Single asterisks denote P values of <0.05 in comparing levels of histone acetylation by PcRtt109 with and without PcVps75 alone, and double asterisks denote P values of <0.05 in comparing the levels of additional acetylation by PcRtt109/PcVps75 with and without the further addition of PcAsf1.
Fig 9.

Garcinol inhibits histone acetylation mediated by the PcRtt109, PcVps75, and PcAsf1 complex. Histone acetyltransferase (HAT) reactions were performed using the expressed PcRtt109, PcVps75, and PcAsf1 in the presence and absence of the histone acetyltransferase inhibitor garcinol (0 to 125 μM). Shown in the top panel is a Western blot for the acetylated H3K56 histone; the blotting was performed as previously reported (24). For a loading control, the lower panel shows the total H3/H4 chicken histone tetramers used in this assay. Lane 1 demonstrates H3K56 acetylation mediated by the PcRtt109/PcVps75/PcAsf1 complex in the absence of garcinol or dimethyl sulfoxide (DMSO) diluent (required to solubilize the garcinol). Lanes 2 to 5 represent the same amounts of DMSO diluent as those utilized in lanes 6 to 9. The DMSO diluent alone exerted no effect on histone acetylation. In contrast, lanes 6 to 9 indicate that increasing concentrations of garcinol (0 to 125 μM) exert substantial inhibition of histone acetylation mediated by the Pneumocystis PcRtt109/PcVps75/PcAsf1 complexes.
Histone-agarose binding assay.
Purified PcAsf1 or PcVps75 (5 μg each) generated in S. cerevisiae using the pYES2.1/V5-His-TOPO plasmid containing the relevant coding sequences was incubated with 60 μl of core histones immobilized on 4% agarose resin or 60 μl of empty 4% agarose resin in 500 μl of buffer A (20 mM Tris-HCl at pH 7.5, 200 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, 50 μg/ml bovine serum albumin, 10% glycerol, 0.1% Nonidet P-40) at 4°C for 2 h. The resins were washed 3 times in 500 μl of buffer A to remove unbound protein. Bound proteins were removed with Laemmli buffer and resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Western hybridization was performed using anti-V5 antibody diluted 1:5,000 in Tris-buffered saline (TBS) containing 1% milk and Tween 20 (1% milk–1× TBS-T) to detect histone-agarose-bound proteins.
Detection of histone H3 lysine 56 (H3K56) acetylation.
Next, asf1Δ and asf1Δ + Pcasf1/pYES2.1 S. cerevisiae strains were cultured overnight in a liquid minimal medium lacking uracil and containing 2% galactose to induce the expression of PcAsf1. Wild-type S. cerevisiae cultures transformed with pYES2.1/V5-His/lacZ were used as controls. H3K56 acetylation was investigated in whole-cell lysates by Western blotting using an antibody recognizing acetylated H3K56 (anti-H3K56Ac) diluted 1:1,000 in 1% milk–1× TBS-T. In parallel, an antibody recognizing the C terminus of histone H3 (anti-H3C) diluted 1:2,000 in 1% milk–1× TBS-T was used to verify equal total histone loading. In addition, histone acetylation reactions were performed in vitro (the details of the HAT assay are described below) using 6 pmol of nonradiolabeled acetyl coenzyme A. The reaction mixtures were resolved using SDS-PAGE, and Western hybridization used anti-H3K56Ac antibody diluted 1:1,000 in 1% milk–1× TBS-T and anti-H3C antibody diluted 1:2,000 in 1% milk–1× TBS-T.
Growth in the presence of DNA-damaging agents or acidic environments.
To test for growth in the presence of DNA-damaging agents, the asf1Δ and asf1Δ + Pcasf/YES2.1 S. cerevisiae strains were grown overnight in liquid minimal medium lacking uracil and containing 2% galactose and were serially diluted to 1 × 106 cells/ml. Wild-type S. cerevisiae transformed with pYES2.1/V5-His/lacZ was used as a control. We plated 10 μl of 10-fold serial dilutions of the indicated yeast strains onto solid minimal medium lacking uracil and containing 2% galactose and onto solid minimal medium lacking uracil and containing 2% galactose plus one of the following DNA-damaging agents: 1 μg/ml camptothecin, 50 mM hydroxyurea, and 0.005% methyl methanesulfonate. Cells were grown for 72 h at 30°C.
To test the ability of these strains to grow under weakly acidic conditions, the vps75Δ and vps75Δ + Pcvps75/pYES2.1 S. cerevisiae strains were grown overnight in liquid minimal medium lacking uracil and containing 2% galactose and were serially diluted to 1 × 107 cells/ml. Wild-type S. cerevisiae transformed with pYES2.1/V5-His/lacZ was also tested as a control. We plated 10 μl of 10-fold serial dilutions of the indicated yeast strains onto solid minimal medium lacking uracil and containing 2% galactose and onto solid minimal medium lacking uracil and containing 2% galactose and 40 mM acetic acid. Cells were grown for 72 h at 30°C.
HAT assays.
HAT assays were performed as previously reported with slight modifications (19). PcRtt109 (1 μg) or PcRtt109-PcVps75 complex (1 μg of PcRtt109 incubated on ice for 1 h with various amounts of PcVps75) was further incubated on ice for 1 h in the presence of chicken erythrocyte histone H3-H4 tetramers (1 μg; Abcam, Cambridge, MA) or H3-H4-PcAsf1 complex (1 μg of chicken erythrocyte histones incubated on ice for 1 h with various amounts of PcAsf1). A mixture lacking PcRtt109 but including an irrelevant protein (PcAce2) with no histone acetyltransferase activity was used as a negative control. The mixtures were next incubated for 1 h at 30°C with buffer A (50 mM Tris-HCl, pH 8.0, 0.1 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium butyrate, 50 mM potassium chloride, 5% glycerol) and 6 pmol of [3H]acetyl coenzyme A (MP Biomedicals, Santa Ana, CA). The reaction mixtures (5 μl) were then spotted onto P-81 phosphocellulose filter papers. The filter papers were air dried, washed 5 times for 5 min with 100 ml of 50 mM NaHCO3, pH 9.0, and washed once with 50 ml of acetone. The radioactivity of 3H-acetylated histones was measured using liquid scintillation counting. In addition, we separated 5 μl of the reaction mixtures using SDS-PAGE. The polyacrylamide gel was soaked in Amersham Amplify fluorographic reagent (GE Healthcare, Piscataway, NJ) for 30 min before being dried in a vacuum at 80°C for 2 h and exposed to X-ray films at −70°C for 24 h to demonstrate the autoradiography of the 3H-acetylated histone proteins. In further experiments, HAT assays were performed in the presence of garcinol (0 to 125 μM), an agent that inhibits histone acetyltransferase reactions (23).
Statistical analysis.
All experimental conditions were repeated at least 3 times. Data are expressed as the means ± standard errors of the means (SEM). Differences between data groups were first determined using analysis of variance (ANOVA) and subsequently by Student's paired t tests as indicated. Statistical testing was performed using the GraphPad Prism version 5.0b software program, and statistical differences were considered significant if P was <0.05.
Nucleotide sequence accession numbers.
The complete coding sequences for Pcasf1 and Pcvps75 have been deposited in GenBank under accession numbers KC169789 and KC169790, respectively.
RESULTS
PcAsf1 and PcVps75 predicted amino acid sequences share significant homology with ScAsf1 and ScVps75.
Completion cloning and sequencing were performed on the full-length Pcasf1 and Pcvps75 cDNAs obtained using the RACE technique. Pcasf1 is composed of 642 bp, and Pcvps75 consists of 753 bp. The amino acid sequences of PcAsf1 and PcVps75 were compared with the amino acid sequences of ScAsf1 and ScVps75. As shown in Fig. 1A and B, the amino acid sequences of PcAsf1 and PcVps75 are homologous to ScAsf1 and ScVps75 (BLASTX homology of PcAsf1 to ScAsf1 was 68%; BLASTX homology of PcVps75 to ScVps75 was 46%). In particular, the amino acids at positions 47 (Ser), 48 (Ala), 50 (Ser), 54 (Asp), 91 (Gly), and 92 (Val) of Asf1 known to interact with histone H3α3 are conserved in Pneumocystis (16, 17). These amino acids are conserved in multiple yeasts, parasites, and human (16). We made similar observations about the homology of PcAsf1. The X-ray crystal structure of ScVps75 revealed dimerization and the formation of a homodimeric, headphone-like architecture with two globular earmuff domains at opposite ends (10, 12, 24–28). The amino acids involved in this conformation and orientation are located at positions 15 (Phe), 18 (Leu), 22 (Glu), 44 (Tyr), 47 (Arg), and 51 (Ile) (26). Additionally, it has been reported that Vps75 forms a complex with Rtt109 in a 2:1 (25) or 2:2 (24) stoichiometry. Notably, we observed significant amino acid conservation between ScVps75 and PcVps75 in these positions involved in the Vps75 homodimeric structure and the interfaces between Vps75 and Rtt109. This strongly indicates that the PcAsf1 and PcVps75 structures and functions are similar to those of ScAsf1 and ScVps75, respectively.
Fig 1.
PcAsf1 and PcVps75 are homologous to ScAsf1 and ScVps75. (A) Alignment of the PcAsf1 and ScAsf1 amino acid sequences. (B) Alignment of the PcVps75 and ScVps75 amino acid sequences. The alignments were generated by ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The asterisks indicate positions that have a single, fully conserved residue. The colons indicate conservation between groups of strongly similar properties with scores of >0.5 in the Gonnet PAM 250 matrix. The periods indicate conservation between groups of weakly similar properties with scores of ≤0.5 in the Gonnet PAM 250 matrix.
Pcasf1 and Pcvps75 sequences are specific to P. carinii and are located on the same chromosome.
Southern hybridization was next used to verify that the predicted P. carinii genes were present in the P. carinii genome and were not identified as a result of amplification of rat lung genomic DNA that may have been present as a low-level contaminant in the RACE reaction mixtures. However, as shown in Fig. 2A and B, 32P-labeled Pcasf1 and Pcvps75 probes did not hybridize to rat lung DNA that was digested with restriction endonucleases. Instead, these probes hybridized specifically to the digested P. carinii genomic DNA. In addition, a CHEF blot was used to demonstrate the chromosomal localization of Pcasf1 and Pcvps75. According to the Saccharomyces Genome Database (http://www.yeastgenome.org/), ScASF1 is located on chromosome 10, while ScVPS75 is located on chromosome 14. In contrast, our CHEF-Southern blot demonstrated that the 32P-labeled Pcasf1 and Pcvps75 probes hybridized to a single P. carinii chromosome (Fig. 3), suggesting that Pcasf1 and Pcvps75 are actually located on the same Pneumocystis chromosome.
Fig 2.

Pcasf1 and Pcvps75 gene fragments specifically bind P. carinii genomic DNA. Southern hybridization of rat lung DNA and P. carinii DNA digested with BamHI, HindIII, and XbaI restriction endonucleases, using [α-32P]dATP-labeled Pcasf1 (A) and Pcvps75 (B) probes. The probes failed to hybridize to rat genomic DNA but did specifically bind to P. carinii-related nucleic acids.
Fig 3.

Chromosomal localization of Pcasf1 and Pcvps75. Contour-clamped homogenous electric field (CHEF)-Southern blotting using [α-32P]dATP-labeled Pcasf1 and Pcvps75 probes demonstrated the chromosomal localization of Pcasf1 and Pcvps75. Lanes 1 and 2, P. carinii chromosomes stained with ethidium bromide; lanes 3 and 4, P. carinii chromosomes hybridized with the [32P]dATP-labeled Pcasf1 probe; lanes 5 and 6, P. carinii chromosomes hybridized with the [32P]dATP-labeled Pcvps75 probe.
PcAsf1 and PcVps75 bind to total histones in vitro.
To demonstrate the histone-binding capacity of these P. carinii proteins, we incubated PcAsf1-V5 and PcVps75-V5 recombinant proteins with core histones immobilized onto agarose resin. The recombinant proteins were also incubated with empty agarose resin, which was used as a negative control, and the same amount of recombinant protein without histones or resin was used as the total input (positive control). Figure 4 showed that both PcAsf1 and PcVps75 bound core histones. The intensity of the detected bands was substantially lower than that of the input because we used total core histones in our experiment, whereas Asf1 (29) and Vps75 are specific for histones H3 and H4. Specifically, Asf1 interacts with histone H3-H4 heterodimers mostly with a 1:1:1 or 1:2:2 stoichiometry (Asf1:H3:H4) (18), while Vps75 promotes Rtt109 acetylation of (H3-H4)2 tetramers (30). It remains possible that the V5 tag could affect protein structure and function; hence, it could conceivably reduce the binding capacity of recombinant P. carinii proteins.
PcAsf1 is required for histone H3 lysine 56 acetylation.
Both Rtt109 and Asf1 are required for H3K56 acetylation in S. pombe (9) and S. cerevisiae (8, 31). Previous studies demonstrated that Asf1 stimulates Rtt109 acetylation of H3K56 by presenting H3-H4 to the Rtt109-Vps75 complex (9). Recently, the complete sequencing and characterization of PcRtt109 were performed by our group (19). Similar to the case in S. pombe, we found that PcRtt109 is required to acetylate H3K56 (19). In this study, we demonstrated that PcAsf1 is also required for H3K56 acetylation. As shown in the Western blot analysis of whole-yeast-cell extracts from wild-type, asf1Δ, and asf1Δ + PcAsf1/pYES2.1 S. cerevisiae strains, H3K56 acetylation was not detected in the asf1Δ strain, while H3K56 acetylation was detected in the wild-type S. cerevisiae and asf1Δ + PcAsf1/pYES2.1 strains (Fig. 5A). Thus, PcAsf1 can restore H3K56 acetylation in S. cerevisiae cells lacking ScAsf1. The results of the in vitro HAT reactions using nonradiolabeled acetyl coenzyme A (Fig. 5B) further confirmed that PcAsf1 is required for acetylation of H3K56. H3K56 acetylation was not detected in HAT reactions without PcAsf1 (PcRtt109 only and PcRtt109+PcVps75).
Fig 5.

PcAsf1 is required for acetylation of lysine 56 of histone H3. (A) Acetylated H3K56 (H3K56ac) was detected in whole-yeast-cell lysates of the indicated strains by Western blot analysis. The detection of total histone H3 (anti-H3C) served as a loading control. (B) Histone acetyltransferase (HAT) reactions using unlabeled acetyl coenzyme A and detection by Western blotting using anti-H3K56ac and anti-H3C (loading control).
PcAsf1 participates in DNA repair.
Asf1 has been implicated in the DNA damage response in yeast species (22). Data in previous publications revealed that the growth rates of H3K56R and asf1Δ mutants were similar (31, 32). This suggests that a loss of H3K56 acetylation causes sensitivity to DNA-damaging agents. One of the functions of H3K56 is to promote a favorable environment for DNA repair of replication-associated lesions in S phase (33). Asf1 triggers the derepression of RNR3 and HUG1, DNA damage response genes that are repressed under normal conditions (34). We next used three DNA-damaging agents that have different mechanisms of DNA damage in this study. Camptothecin binds to topoisomerase I and arrests the replication fork (35). Hydroxyurea inhibits ribonucleoside diphosphate reductase, which prevents the conversion of ribonucleotides to deoxyribonucleotides and leads to defects in replication fork progression in S phase of the cell division cycle (36). Finally, methyl methanesulfonate is an alkylating agent that causes DNA breakage, resulting in incomplete replication of the alkylated template (37). These agents have been shown to have effects on Asf1-deficient yeast strains (38–40). Consistent with this, Fig. 6 demonstrated that the asf1Δ S. cerevisiae strain, which exhibited growth defects, was hypersensitive to all of the DNA-damaging agents tested. In contrast, the asf1Δ + Pcasf1/pYES2.1 S. cerevisiae strain exhibited growth similar to that of the wild-type control, indicating functional activity of the PcAsf1 protein.
Fig 6.

PcAsf1 is involved in the DNA repair process. The indicated S. cerevisiae strains were serially diluted 10-fold and were plated onto solid medium containing one of the following DNA-damaging agents: 1 μg/ml camptothecin, 50 mM hydroxyurea, and 0.005% methyl methanesulfonate. Both the wild-type and asf1Δ strains were transformed with a control vector (pYES2.1/V5-His/lacZ).
PcVps75 participates in the weak acid response.
Data from studies in S. cerevisiae support the concept that Vps75 is involved in regulating cellular growth responses under weakly acidic conditions. Previous studies in S. cerevisiae indicated that the growth phenotype of the haa1Δ and vps75Δ double mutant was similar to that of wild-type S. cerevisiae in a weakly acidic medium containing acetic acid (41). These observations suggest that Vps75 has an opposite function from that of Haa1, a transcriptional activator that is involved in the adaptation to weak acid stress, by controlling the expression of acid-responsive genes (37). Vps75 has a negative effect on these genes, causing the haa1Δ strain to be more sensitive to acetic acid than the wild-type strain, while the vps75Δ strain was resistant to weak acetic acid treatment (41, 42). Interestingly, we observed a similar growth phenotype in the wild-type control and vps75Δ + PcVps75/pYES2.1 strains, which were sensitive to acetic acid, while the vps75Δ strain had a growth advantage in medium containing 40 mM acetic acid (Fig. 7). These observations support a role for PcVps75 in mediating growth responses under weakly acidic conditions.
Fig 7.

PcVps75 is involved in the weak acid response. The indicated S. cerevisiae strains were serially diluted 10-fold and were plated onto medium containing 40 mM acetic acid. Both the wild-type and vps75Δ strains were transformed with a control vector (pYES2.1/V5-His/lacZ).
PcAsf1 and PcVps75 enhance acetylation by PcRtt109 in vitro.
Our previous investigations revealed that the HAT activities of PcRtt109 and S. pombe Rtt109 (SpRtt109) were comparable. Specifically, SpAsf1 and SpVps75 enhanced the HAT activity of PcRtt109 in vitro (19). As illustrated in Fig. 8, PcVps75 enhanced the formation of 3H-acetylated H3 histone mediated by PcRtt109. In addition, we observed some further enhancement of histone acetylation when PcAsf1 was added to the reaction mixtures already containing PcRtt109/PcVps75. Thus, the chaperone proteins PcAsf1 and PcVps75 enhance histone acetylation mediated by PcRtt109.
Finally, we tested the ability of garcinol, an agent that is known to inhibit histone acetyltransferase reactions, to suppress the activity of Pneumocystis PcRtt109-PcVps75-PcAsf1 complexes (Fig. 9). We observed that increasing concentrations of garcinol (0 to 125 μM) caused substantial inhibition of H3 histone acetylation mediated by the Pneumocystis PcRtt109-PcVps75-PcAsf1 acetyltransferase complex.
DISCUSSION
Recently, we published the full sequence of Pcrtt109 and characterized the activity of the PcRtt109 histone acetyltransferase. We found that this Pneumocystis acetyltransferase exhibited features that were similar to those of S. cerevisiae and S. pombe Rtt109 (19). Asf1 and Vps75 are important components that function in concert with Rtt109 in the histone acetylation pathway of fungi. To understand this pathway in P. carinii, an opportunistic fungus that causes infection with significant mortality in immunosuppressed hosts, we need to understand the roles of these two important chaperone components. In this study, we first sought to generate and amplify full-length cDNA sequences of Pcasf1 and Pcvps75 from partial DNA sequences that were obtained from the Pneumocystis Genome Project Database. The predicted amino acid sequences shared significant homology with ScAsf1 and ScVps75. This evidence further convinced us to characterize PcAsf1 and PcVps75 using S. cerevisiae as a model organism that supports the heterologous expression of Pneumocystis genes. To accomplish this, we transformed Pcasf1 and Pcvps75 into S. cerevisiae lacking ScASF1 and ScVPS75 and characterized these recombinant proteins both in vitro and in vivo.
In vitro, PcAsf1 and PcVps75 were able to bind core histones. PcAsf1 is required for the acetylation of H3K56. The results of our HAT assays demonstrated that PcVps75 enhanced the histone acetylation activity of PcRtt109 in a dose-dependent fashion. The HAT activity further increased when PcAsf1 was added to the reaction mixtures. In contrast to our previous report that used ScAsf1 and ScVps75 as components of the reaction mixtures (19), we were able to demonstrate only low-level enhancement of PcRtt109 activity with the further addition of PcAsf1, in contrast to the dramatic enhancement observed with ScAsf1. Several factors may contribute to the different magnitudes of the responses to Asf1 in these two studies. First, the relative affinities of PcAsf1 and PcVps75 to histones and PcRtt109 are likely somewhat different from those of ScAsf1 and ScVps75, even though they share significant sequence homology. In addition, we employed chicken embryo H3-H4 tetramers in this study instead of Drosophila melanogaster histone tetramers because of availability and technical difficulties related to purification of adequate quantities of Drosophila histones for these repeated assays. Histones are known to be highly conserved proteins across the eukaryotes (43, 44). However, the equilibrium of H3-H4 heterodimers and H3-H4 heterotetramers could differ between chicken embryo and Drosophila histones, particularly under the in vitro conditions used in this study.
Asf1 has been proposed to present histones H3 and H4 to the Rtt109-Vps75 complex for histone acetylation (9). However, over several attempts we did not observe any significantly enhanced binding of PcVps75 following preincubation of PcAsf1 with histones. Others have shown that Asf1 may also interact with the histone H3-H4 heterodimers and prevent tetramerization, while Vps75 binds to (H3-H4)2 tetramers (15, 17, 18, 30). While we postulate that both PcVps75 and PcAsf1 function as coordinate chaperones facilitating activity of the active PcRtt109 acetyltransferase complex, it still remains possible that PcAsf1 and PcVps75 could actually be unrelated histone chaperones that enhance the catalytic activity of PcRtt109 through independent mechanisms. In addition, other chaperone proteins beyond PcAsf1 and PcVps75 may also be involved in histone acetylation by PcRtt109.
In addition, the current study provides characterization of functional activity of both PcVps75 and PcAsf1 following heterologous expression in S. cerevisiae. While such evidence is indirect, in light of the inability to directly manipulate genes in Pneumocystis, it remains the best available data supporting a similar function in Pneumocystis. Specifically, our in vivo observations revealed that both PcAsf1 and PcVps75 restored protein function in asf1Δ and vps75Δ S. cerevisiae strains. The investigations indicate that PcVps75 is involved in the weak acid response and PcAsf1 is required for H3K56 acetylation, which participates in DNA damage repair. These data further support the concept that the Rtt109 histone acetylation pathway of fungi, while sufficiently diverged from mammalian hosts to represent a viable therapeutic target, may also be applicable for the treatment of the unique fungal opportunist Pneumocystis.
Taken together, our studies support a central role for Pneumocystis PcRtt109-PcVps75-PcAsf1 in histone acetylation in this medically important fungus. While some difference in the molecular regulatory pathways of cell cycle control may yet be revealed in Pneumocystis in contrast to other fungi, the current observations support the idea that PcRtt109 and its related known chaperones function in a conserved manner parallel to many other fungal organisms. We also observed substantial suppression of histone acetylation by the active PcRtt109/PcAsf1/PcVps75 complex in the presence of garcinol. While garcinol is known to widely inhibit eukaryotic histone acetyltransferases, such observations do suggest that selective low-toxicity agents that specifically inhibit histone acetyltransferase activity in fungi may eventually be identified that may prove useful in treating Pneumocystis pneumonia and other fungal infections. The cloning and characterization of the complete PcRtt109/PcAsf1/PcVps75 are an important first step in the process of eventually identifying such agents. Since these complexes are conserved across fungi, but diverged from mammals, such agents may potentially have activity against a variety of fungal infections.
ACKNOWLEDGMENTS
These studies were funded by Mayo Foundation and NIH grants R01-HL62150 and R01-HL55934 to A.H.L. This research is also supported by the Walter and Leonore Annenberg Foundation.
We greatly appreciate the efforts of Deanne Hebrink in the generation of the P. carinii organisms used in these studies. We further appreciate the kind gift of the CHEF blot membrane containing separated P. carinii chromosomes, which was the kind gift of Melanie Cushion, University of Cincinnati.
None of the authors have any financial or other conflicts of interest with any of the research findings reported in the manuscript.
Footnotes
Published ahead of print 8 April 2013
REFERENCES
- 1. Ewig S. 1996. The effect of prophylaxis on the outcome of HIV-associated Pneumocystis carinii pneumonia. Chest 109:586–587 [DOI] [PubMed] [Google Scholar]
- 2. Monnet X, Vidal-Petiot E, Osman D, Hamzaoui O, Durrbach A, Goujard C, Miceli C, Bouree P, Richard C. 2008. Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit. Care 12:R28 doi:10.1186/cc6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fei MW, Kim EJ, Sant CA, Jarlsberg LG, Davis JL, Swartzman A, Huang L. 2009. Predicting mortality from HIV-associated Pneumocystis pneumonia at illness presentation: an observational cohort study. Thorax 64:1070–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisk M, Sage EK, Edwards SG, Cartledge JD, Miller RF. 2009. Outcome from treatment of Pneumocystis jirovecii pneumonia with co-trimoxazole. Int. J. STD AIDS 20:652–653 [DOI] [PubMed] [Google Scholar]
- 5. Mansharamani NG, Garland R, Delaney D, Koziel H. 2000. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 118:704–711 [DOI] [PubMed] [Google Scholar]
- 6. Festic E, Gajic O, Limper AH, Aksamit TR. 2005. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest 128:573–579 [DOI] [PubMed] [Google Scholar]
- 7. Huang L, Crothers K, Atzori C, Benfield T, Miller R, Rabodonirina M, Helweg-Larsen J. 2004. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg. Infect. Dis. 10:1721–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han J, Zhou H, Horazdovsky B, Zhang K, Xu RM, Zhang Z. 2007. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science 315:653–655 [DOI] [PubMed] [Google Scholar]
- 9. Han J, Zhou H, Li Z, Xu RM, Zhang Z. 2007. Acetylation of lysine 56 of histone H3 catalyzed by RTT109 and regulated by ASF1 is required for replisome integrity. J. Biol. Chem. 282:28587–28596 [DOI] [PubMed] [Google Scholar]
- 10. Tsubota T, Berndsen CE, Erkmann JA, Smith CL, Yang L, Freitas MA, Denu JM, Kaufman PD. 2007. Histone H3-K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol. Cell 25:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Driscoll R, Hudson A, Jackson SP. 2007. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science 315:649–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berndsen CE, Tsubota T, Lindner SE, Lee S, Holton JM, Kaufman PD, Keck JL, Denu JM. 2008. Molecular functions of the histone acetyltransferase chaperone complex Rtt109-Vps75. Nat. Struct. Mol. Biol. 15:948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jessulat M, Alamgir M, Salsali H, Greenblatt J, Xu J, Golshani A. 2008. Interacting proteins Rtt109 and Vps75 affect the efficiency of non-homologous end-joining in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 469:157–164 [DOI] [PubMed] [Google Scholar]
- 14. Selth L, Svejstrup JQ. 2007. Vps75, a new yeast member of the NAP histone chaperone family. J. Biol. Chem. 282:12358–12362 [DOI] [PubMed] [Google Scholar]
- 15. D'Arcy S, Luger K. 2011. Understanding histone acetyltransferase Rtt109 structure and function: how many chaperones does it take? Curr. Opin. Struct. Biol. 21:728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antczak AJ, Tsubota T, Kaufman PD, Berger JM. 2006. Structure of the yeast histone H3-ASF1 interaction: implications for chaperone mechanism, species-specific interactions, and epigenetics. BMC Struct. Biol. 6:26 doi:10.1186/1472-6807-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK. 2006. Structural basis for the histone chaperone activity of Asf1. Cell 127:495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. English CM, Maluf NK, Tripet B, Churchill ME, Tyler JK. 2005. ASF1 binds to a heterodimer of histones H3 and H4: a two-step mechanism for the assembly of the H3-H4 heterotetramer on DNA. Biochemistry 44:13673–13682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kottom TJ, Han J, Zhang Z, Limper AH. 2011. Pneumocystis carinii expresses an active Rtt109 histone acetyltransferase. Am. J. Respir. Cell Mol. Biol. 44:768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Limper AH, Weiss LM. 2011. Guidelines for the naming of genes, gene products, and mutants in the opportunistic protists. J. Eukaryot. Microbiol. 58:537–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han J, Zhou H, Li Z, Xu RM, Zhang Z. 2007. The Rtt109-Vps75 histone acetyltransferase complex acetylates non-nucleosomal histone H3. J. Biol. Chem. 282:14158–14164 [DOI] [PubMed] [Google Scholar]
- 22. Kim JA, Haber JE. 2009. Chromatin assembly factors Asf1 and CAF-1 have overlapping roles in deactivating the DNA damage checkpoint when DNA repair is complete. Proc. Natl. Acad. Sci. U. S. A. 106:1151–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, Kundu TK. 2004. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J. Biol. Chem. 279:33716–33726 [DOI] [PubMed] [Google Scholar]
- 24. Su D, Hu Q, Zhou H, Thompson JR, Xu RM, Zhang Z, Mer G. 2011. Structure and histone binding properties of the Vps75-Rtt109 chaperone-lysine acetyltransferase complex. J. Biol. Chem. 286:15625–15629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang Y, Holbert MA, Delgoshaie N, Wurtele H, Guillemette B, Meeth K, Yuan H, Drogaris P, Lee EH, Durette C, Thibault P, Verreault A, Cole PA, Marmorstein R. 2011. Structure of the Rtt109-AcCoA/Vps75 complex and implications for chaperone-mediated histone acetylation. Structure 19:221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang Y, Meeth K, Jiang E, Luo C, Marmorstein R. 2008. Structure of Vps75 and implications for histone chaperone function. Proc. Natl. Acad. Sci. U. S. A. 105:12206–12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park YJ, Luger K. 2006. The structure of nucleosome assembly protein 1. Proc. Natl. Acad. Sci. U. S. A. 103:1248–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park YJ, Sudhoff KB, Andrews AJ, Stargell LA, Luger K. 2008. Histone chaperone specificity in Rtt109 activation. Nat. Struct. Mol. Biol. 15:957–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donham DC, II, Scorgie JK, Churchill ME. 2011. The activity of the histone chaperone yeast Asf1 in the assembly and disassembly of histone H3/H4-DNA complexes. Nucleic Acids Res. 39:5449–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowman A, Ward R, Wiechens N, Singh V, El-Mkami H, Norman DG, Owen-Hughes T. 2011. The histone chaperones Nap1 and Vps75 bind histones H3 and H4 in a tetrameric conformation. Mol. Cell 41:398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Recht J, Tsubota T, Tanny JC, Diaz RL, Berger JM, Zhang X, Garcia BA, Shabanowitz J, Burlingame AL, Hunt DF, Kaufman PD, Allis CD. 2006. Histone chaperone Asf1 is required for histone H3 lysine 56 acetylation, a modification associated with S phase in mitosis and meiosis. Proc. Natl. Acad. Sci. U. S. A. 103:6988–6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fillingham J, Recht J, Silva AC, Suter B, Emili A, Stagljar I, Krogan NJ, Allis CD, Keogh MC, Greenblatt JF. 2008. Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109. Mol. Cell. Biol. 28:4342–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masumoto H, Hawke D, Kobayashi R, Verreault A. 2005. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 436:294–298 [DOI] [PubMed] [Google Scholar]
- 34. Minard LV, Williams JS, Walker AC, Schultz MC. 2011. Transcriptional regulation by Asf1: new mechanistic insights from studies of the DNA damage response to replication stress. J. Biol. Chem. 286:7082–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hawkins MJ. 1992. New anticancer agents: taxol, camptothecin analogs, and anthrapyrazoles. Oncology (Williston Park) 6:17–23 [PubMed] [Google Scholar]
- 36. Krakoff IH, Brown NC, Reichard P. 1968. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res. 28:1559–1565 [PubMed] [Google Scholar]
- 37. Schwartz JL. 1989. Monofunctional alkylating agent-induced S-phase-dependent DNA damage. Mutat. Res. 216:111–118 [DOI] [PubMed] [Google Scholar]
- 38. Emili A, Schieltz DM, Yates JR, III, Hartwell LH. 2001. Dynamic interaction of DNA damage checkpoint protein Rad53 with chromatin assembly factor Asf1. Mol. Cell 7:13–20 [DOI] [PubMed] [Google Scholar]
- 39. Sutton A, Bucaria J, Osley MA, Sternglanz R. 2001. Yeast ASF1 protein is required for cell cycle regulation of histone gene transcription. Genetics 158:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller A, Yang B, Foster T, Kirchmaier AL. 2008. Proliferating cell nuclear antigen and ASF1 modulate silent chromatin in Saccharomyces cerevisiae via lysine 56 on histone H3. Genetics 179:793–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Selth LA, Lorch Y, Ocampo-Hafalla MT, Mitter R, Shales M, Krogan NJ, Kornberg RD, Svejstrup JQ. 2009. An rtt109-independent role for vps75 in transcription-associated nucleosome dynamics. Mol. Cell. Biol. 29:4220–4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keck KM, Pemberton LF. 2011. Interaction with the histone chaperone Vps75 promotes nuclear localization and HAT activity of Rtt109 in vivo. Traffic 12:826–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bernhard D, Schlegel M. 1998. Evolution of histone H4 and H3 genes in different ciliate lineages. J. Mol. Evol. 46:344–354 [DOI] [PubMed] [Google Scholar]
- 44. Waterborg JH. 2012. Evolution of histone H3: emergence of variants and conservation of post-translational modification sites. Biochem. Cell Biol. 90:79–95 [DOI] [PubMed] [Google Scholar]



