Abstract
Arthritis is one of the most common complications of human active brucellosis, but its pathogenic mechanisms have not been completely elucidated. In this paper, we describe the role of synoviocytes in the pathogenesis of brucellar arthritis. Our results indicate that Brucella abortus infection inhibited synoviocyte apoptosis through the upregulation of antiapoptotic factors (cIAP-2, clusterin, livin, and P21/CIP/CDNK1A). In contrast, infection did not change the expression of proteins that have been involved in apoptosis induction such as Bad, Bax, cleaved procaspase 3, CytC, and TRAIL, among others; or their expression was reduced, as occurs in the case of P-p53(S15). In addition, B. abortus infection induced upregulation of adhesion molecules (CD54 and CD106), and the adhesion of monocytes and neutrophils to infected synoviocytes was significantly higher than to uninfected cells. Despite this increased adhesion, B. abortus-infected synoviocytes were able to inhibit apoptosis induced by supernatants from B. abortus-infected monocytes and neutrophils. Moreover, B. abortus infection increased soluble and membrane RANKL expression in synoviocytes that further induced monocytes to undergo osteoclastogenesis. The results presented here shed light on how the interactions of B. abortus with synovial fibroblasts may have an important role in the pathogenesis of brucellar arthritis.
INTRODUCTION
Brucellae are Gram-negative intracellular pathogens that can survive and multiply within phagocytic cells (1, 2). Humans become infected by ingesting unpasteurized dairy products, being in direct contact with infected animals, or inhaling infectious aerosols (3). The distribution of this disease is worldwide, and areas of high endemicity include the Mediterranean, the Middle East, Latin America, and Asia (4–6).
Osteoarticular brucellosis is the most common presentation of the active disease in humans, affecting up to 85% of patients (7–9). The three most frequent forms of osteoarticular involvement are sacroileitis, spondylitis, and peripheral arthritis (7, 10–13). Arthritis is one of the most common presentations of localized disease in human brucellosis and may be caused by different Brucella species (7–9). Osteoarticular involvement may be observed in acute or chronic cases of human brucellosis (7–9) and may affect patients of any age (7–9, 14). Imaging studies have revealed cartilage loss and bone erosion in brucellar arthritis affecting different joints (12, 14). These lesions may eventually lead to permanent joint dysfunction. Brucella spp. are isolated from synovial fluid samples in about 50% of the cases (8, 11). The synovial membrane of the affected joint may present a lymphomononuclear infiltrate in the chronic phase of the disease but usually presents a polymorphonuclear infiltrate in acute cases (8, 11). Modulation of adhesion molecules by B. abortus might be central in this process.
Since Brucella spp. are intracellular pathogens, they may survive and multiply despite the hostile environment generated by the inflammatory immune response induced. Successful strategies for intracellular survival include a panoply of mechanisms, such as the ability to survive in membrane-bound vesicles (15–17), alteration of macrophage apoptosis (18, 19), and the inhibition of membrane expression of major histocompatibility complex (MHC) class II and I (20, 21), among others.
We have recently partially deciphered potential mechanisms that involved synoviocytes in bone damage caused by Brucella. We demonstrated that Brucella spp. can infect and survive within human synoviocytes and that this infection elicits the secretion of matrix metalloproteases (MMPs) that might be involved in the osteoarticular manifestations of brucellosis (22). Notwithstanding, at present it has not been investigated whether Brucella-infected synoviocytes are involved in the pathological increase in numbers of osteoclasts (cells involved in bone resorption) and whether Brucella infection alters synoviocyte survival. In addition, considering the relevance of macrophages and neutrophils as infiltrating cells in inflammatory tissues and taking into account that synoviocytes secrete monocyte chemoattractant protein 1 (MCP-1) and interleukin-8 (IL-8) in response to Brucella infection (22), we also decided to investigate the role of these cells as modulators of synoviocyte survival, which has been associated with osteoarticular damage (23).
In the present study, we demonstrated that B. abortus infection inhibited synoviocyte apoptosis. In addition, infection induced the upregulation of adhesion molecules (CD54 and CD106), which led to the adhesion of monocytes and neutrophils to synoviocytes. Despite this increased adhesion, B. abortus-infected synoviocytes were able to inhibit apoptosis induced by supernatants from B. abortus-infected monocytes and neutrophils. Moreover, B. abortus infection increased soluble and membrane RANKL expression in synoviocytes, which further induced monocytes to undergo osteoclastogenesis.
MATERIALS AND METHODS
Bacterial cultures.
Brucella abortus S2308 was grown overnight in 10 ml of tryptic soy broth (Merck, Buenos Aires, Argentina) with constant agitation at 37°C. Bacteria were harvested by centrifugation for 15 min at 6,000 × g at 4°C and washed twice in 10 ml of phosphate-buffered saline (PBS). The numbers of bacteria in stationary-phase cultures were determined by comparing the optical densities at 600 nm (OD600) with a standard curve obtained in our laboratory. To obtain the standard curve, the spectrophotometer was calibrated using tryptic soy broth as the blank reference. A single colony of B. abortus was selected and inoculated into 1 ml of tryptic soy broth and then incubated at 37°C under agitation at 200 rpm for 16 h. Then, the bacterial solution was diluted in tryptic soy broth to an OD600 of 0.100 using the spectrophotometer reader. At each 30-min interval, an aliquot of the sample was used to determine the OD600, and another aliquot was used to determine the CFU by plating cells onto tryptic soy agar. This procedure was followed during 48 h.
To prepare the inocula, cultures were diluted in sterile PBS to the desired bacterial concentration on the basis of the optical density readings, but the precise concentrations of inocula were determined by plating cells onto tryptic soy agar. All live Brucella manipulations were performed in biosafety level 3 facilities located at the Instituto de Investigaciones Biomédicas en Retrovirus y SIDA (INBIRS).
Cell cultures.
Primary synovial fibroblasts (synoviocytes) were isolated from synovial tissues obtained from patients undergoing total knee replacement surgery in accordance with the guidelines of the Ethical Committee of the INIGEM Institute. A written consent was obtained from all patients. The number of patients included in this study was 11. The tissue was finally minced and subjected to collagenase digestion at 1 mg/ml (Invitrogen, Carlsbad, CA) in Dulbecco's minimal essential medium (DMEM). Digested cells were resuspended in DMEM containing 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 mg/ml of streptomycin (complete medium). When the cells were confluent, they were passaged by gentle trypsinization (0.25% trypsin in PBS) and were used between passages 4 and 8 (24).
Human neutrophils were isolated from venous blood from healthy human volunteers by a Ficoll-Paque (GE Healthcare, Uppsala, Sweden) gradient, followed by the sedimentation of erythrocytes in 6% dextran and hypotonic lysis (25, 26). Neutrophils were harvested, washed twice with sterile PBS, and resuspended at a concentration of 1 × 106 cells/ml in RPMI 1640 medium supplemented with 5% FBS, 1 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell viability was 98%, as determined by trypan blue exclusion. The purity of the final neutrophil preparation was 95%, as assessed by morphological examination after Giemsa staining and flow cytometry light scatter patterns as described earlier (27–32).
Human monocytes isolated from peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll-Hypaque using a monocyte-negative isolation kit (BD Biosciences, San Diego, CA). The human monocytic cell line THP-1 was cultured in a 5% CO2 atmosphere at 37°C in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 2 mM l-glutamine, 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. To induce THP-1 maturation, cells were cultured in the presence of 0.05 μM 1,25-dihydroxyvitamin D3 (Calbiochem-Nova Biochem International, La Jolla, CA) for 72 h, as described previously (33). All cells were seeded at 5 × 105 cells/well in 24-well plates.
Cellular infection.
Synoviocytes were infected with B. abortus at different multiplicities of infection (MOI), and neutrophils and THP-1 cells were infected at an MOI of 100. After the bacterial suspension was dispensed, the plates were centrifuged for 10 min at 2,000 rpm and then incubated for 2 h at 37°C under a 5% CO2 atmosphere. Cells were extensively washed with DMEM to remove extracellular bacteria and incubated in medium supplemented with FBS (10%), 100 μg/ml gentamicin, and 50 μg/ml streptomycin to kill extracellular bacteria. Synoviocytes were harvested at 24 h postinfection. Supernatants from THP-1 cells or neutrophils were harvested at 24 h postinfection (p.i.) to be used as conditioned medium.
CD54, CD106, CD62L, and RANKL expression on synoviocytes.
Synoviocytes were infected at different MOI with B. abortus. As a positive control, some cells were stimulated with 200 mM phorbol 12–myristate 13–acetate (PMA). At the end of culture, cells were washed and incubated with a fluorescein isothiocyanate (FITC)-labeled anti-human CD54, anti-human CD106, and anti-human CD62L monoclonal antibody (BD Pharmingen, San Jose, CA), or FITC-labeled anti-human receptor activator for nuclear factor κB ligand (anti-RANKL) (BioLegend, San Diego, USA), or the appropriate isotype-matched control antibody for 30 min on ice. Cells were then washed and analyzed with a FACScan flow cytometer using CellQuest software (both from Becton-Dickinson, Franklin Lakes, NJ). The results were expressed as mean fluorescence intensity (MFI). In addition, RANKL expression was assessed using a fluorescence plate reader (Victor3; PerkinElmer, Waltham, MA).
Adhesion of neutrophils and monocytes to synoviocytes.
Synoviocytes grown in 96-well plates were infected with B. abortus S2308 at different MOI as described previously (34) or stimulated with PMA as a positive control for 24 h. Uninfected/untreated cells were used as a negative control. Then, synoviocytes were washed to eliminate residual bacteria or stimulant before the addition of 1 × 106 human neutrophils or monocytes (THP-1 cells or monocytes purified from venous blood) previously labeled with calcein acetoxymethyl ester fluorescent dye (BD Biosciences). After a 1-h incubation at 37°C, the nonadherent cells were washed away and plates were read in a fluorescence plate reader (Victor3; PerkinElmer) using 485/530-nm excitation/emission filters. Average percent adhesion was calculated according to the following formula: (RFU after wash/RFU before wash) × 100, where RFU represents relative fluorescence units.
Apoptosis assays.
Sinoviocytes were infected at different MOI and 24 h after infection were stimulated with 1 μM staurosporine (STS) or a 1/2 dilution of supernatants from B. abortus-infected monocytes or neutrophils at an MOI of 100. As a positive control, cells were treated with 4% paraformaldehyde (PFA) or STS. Twenty-four hours later, cells were washed, and the percentage of apoptotic cells was assessed by the annexin V-FITC (Sigma-Aldrich de Argentina S.A.) assay with fluorescence-activated cell sorter (FACS) analysis. The percentage of apoptotic cells was also assessed by fluorescence microscopy after the cells were labeled by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay or by staining with the Hoechst 33342 dye.
Osteoclast formation assay.
THP-1 monocytes were induced to undergo osteoclastogenesis as described previously (35). Briefly, THP-1 cells were cultured with 30 ng/ml M-CSF in 24-well plates for 48 h. No adherent cells were washed out, and adherent cells were collected and seeded onto glass coverslips in 24-well plates for 6 days and cultured in complete medium containing 30 ng/ml of macrophage colony-stimulating factor (M-CSF; R&D Systems, Minneapolis, MN, USA) and 0.2 ml of supernatants from B. abortus-infected synoviocytes. As positive controls of osteoclast formation, THP-1cultures received 50 ng/ml of human receptor activator for nuclear factor κB ligand (RANKL; R&D Systems). On day 3, the culture media and all reagents were replaced. To identify osteoclasts, cells were fixed in 4% paraformaldehyde and stained for tartrate-resistant acid phosphatase (TRAP) (Sigma-Aldrich). TRAP-positive, multinucleated (i.e., containing more than three nuclei) cells were defined as osteoclasts, and the number was determined by microscopic counts. In addition, vitronectin receptor (CD51) expression was determined using phycoerythrin (PE)-labeled anti-human CD51 (BD Biosciences). TRAP- or CD51-positive multinucleated cells were defined as osteoclasts, and their number was determined by microscopic counts.
Pit formation assay.
THP-1 cells (2 × 104 cells/0.25-ml well), used as osteoclasts precursors, were plated on dentine disks (BD BioCoat Osteologic) in 96-well culture dishes and cultured in complete medium containing stimulated synoviocytes with 30 ng/ml of M-CSF for 6 days. Medium and all reagents were replaced every day to avoid acidification. After culture with cells, dentine disks were washed with 1 M NH4OH to remove adherent cells. After rinsing with water, dentine disks were visualized by light microscopy to determine and enumerate resorption lacunae.
Neutralization experiments.
Neutralization experiments were performed using 20 μg/ml of osteoprotegerin (OPG; R&D Systems), RANKL's decoy receptor. Supernatants from Brucella-infected synoviocytes were preincubated with the decoy receptor for 1 h at 37°C before being used to stimulate THP-1.
Antibody array.
Determination of the relative levels of apoptosis-related proteins was determined using the human apoptosis array kit (R&D Systems) according to the manufacturer's instructions. Briefly, array membranes were incubated with 200 μg of lysates from B. abortus-infected synoviocytes or uninfected cells as a control, diluted in 250 μl of array buffer provided by the customer, and incubated during 18 h at 4°C. Then, membranes were washed with wash buffer and incubated with a detection antibody cocktail conjugated to peroxidase. Spots were visualized using Hyperfilm ECL (GE, Little Chalfont, United Kingdom) enhanced chemiluminescence.
Western blotting.
Infected synoviocytes were lysed in ice-cold lysis buffer consisting of 20 mM HEPES (pH 8), 5 mM EDTA, 10 mM EGTA, 5 mM NaF, 10% glycerol, 1 mM dithiothreitol, 400 mM KCl, 0.4% Triton X-100, 20 mM sodium β-glycerophosphate, and a protease inhibitor cocktail (Sigma-Aldrich). Lysates were incubated on ice for 10 min and cleared by centrifugation at 13,000 × g for 10 min. Protein concentrations were measured in the supernatants by the Bradford method (36), and equal amounts of proteins were loaded onto electrophoresis gels. After separation, proteins were transferred to a nitrocellulose membrane (GE Healthcare) and blocked for 1 h with 5% milk protein–0.1% Tween 20. Then, membranes were incubated with primary goat polyclonal IgG anti-human clusterin (R&D Systems) overnight at 4°C. After washing, the membrane was incubated with peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (1:2,000 dilution), for 1 h. Protein bands were visualized on Hyperfilm ECL (GE Healthcare) by chemiluminescence. Equal loading was checked by Ponceau S staining and by incubation of the blots with an anti-β-actin (clone C4; Santa Cruz Biotechnology).
ELISA.
Clusterin was quantified by enzyme-linked immunosorbent assay (ELISA) (R&D Systems). The protocol was performed according to the manufacturer's instructions.
RANKL was detected in culture supernatants using an ELISA kit (R&D Systems) according to the manufacturer's instructions.
Statistical analysis.
Statistical analysis was performed with one-way analysis of variance (ANOVA), followed by post hoc Tukey's test using GraphPad Prism 4.0 software. Data are represented as means ± standard deviations (SD).
RESULTS
Brucella abortus invades and multiplies in primary human synoviocytes.
We have demonstrated that Brucella spp. can infect and replicate within a human fibroblast-like synoviocytic cell line and that this infection may be involved in the osteoarticular manifestations of brucellosis (22). Yet the bacterium's capacity to infect and multiply in primary human synoviocytes has not been elucidated. Infection experiments showed that B. abortus is internalized by human primary synoviocytes in vitro. A follow-up of infected cultures revealed that B. abortus can replicate inside human primary synoviocytes (Fig. 1C) and that at 24 h postinfection intracellular CFU counts increased by about 3 log and then increased slightly during the next 24 h. Since in macrophages the establishment of a persistent infection relies on the ability of the bacterium to form a Brucella-containing vacuole (BCV), which traffics from the endocytic compartment to the endoplasmic reticulum (ER) (17, 37, 38), we decided to investigate the intracellular fate of the bacterium in human synoviocytes. By using green fluorescent protein (GFP)-expressing B. abortus and primary antibodies specific for subcellular compartments followed by Alexa 546-labeled (red) secondary antibody, we observed that after 24 h of infection B. abortus was enclosed within calnexin-positive vacuoles and did not colocalize with the early endosomal antigen 1 (EEA1), the lysosome-associated membrane protein 2 (LAMP-2), or the Golgi matrix protein GM130, indicating that the bacterium replicates in the endoplasmic reticulum of primary human synoviocytes (Fig. 1A and B).
Fig 1.
B. abortus replicates within LAMP-positive vacuoles in human primary synoviocytes. (A) Confocal micrographs of human synoviocytes infected with GFP-expressing B. abortus at an MOI of 100 and stained at 24 h after infection with the subcellular localization markers EEA1, LAMP-2, GM130, and calnexin followed by Alexa 546-labeled secondary antibody (red). Results are representative of 3 independent experiments. (B) Magnification of the inset in panel A showing B. abortus enclosed within calnexin-positive vacuoles. (C) Infection and replication of synoviocytes. After infection with B. abortus (MOI, 100), cells were incubated with antibiotics to kill extracellular bacteria. Cells were lysed at different times p.i. and plated on agar to determine intracellular CFU. Values are means ± standard errors of the means (SEM) of triplicates from one experiment, which was repeated twice with similar results.
B. abortus inhibits staurosporine-induced apoptosis of synoviocytes.
Because apoptotic and antiapoptotic effects have been observed in Brucella species infections (18, 19, 34, 39–41) and these phenomena were dependent on the cell type, we decided to investigate the effect of B. abortus infection on synoviocytes viability. For this, these cells were infected with B. abortus at different MOI, and after 24 h, cells were stained with annexin V-phosphatidylinositol (PI) and analyzed by flow cytometry to evaluate apoptosis. Paraformaldehyde (PFA; 4%) was used as a positive control. B. abortus infection did not induce synoviocyte apoptosis at any MOI tested. This result was confirmed by Hoechst 33342 staining (Fig. 2A and C) and TUNEL (Fig. 2D and F) assay. In contrast, B. abortus infection was able to inhibit synoviocyte apoptosis induced by staurosporine (STS) in an MOI-dependent fashion as was determined by Hoechst 33342 staining (Fig. 2B and C) and corroborated by TUNEL (Fig. 2E and F). These results indicate that B. abortus infection prevents STS-induced synoviocyte apoptosis.
Fig 2.
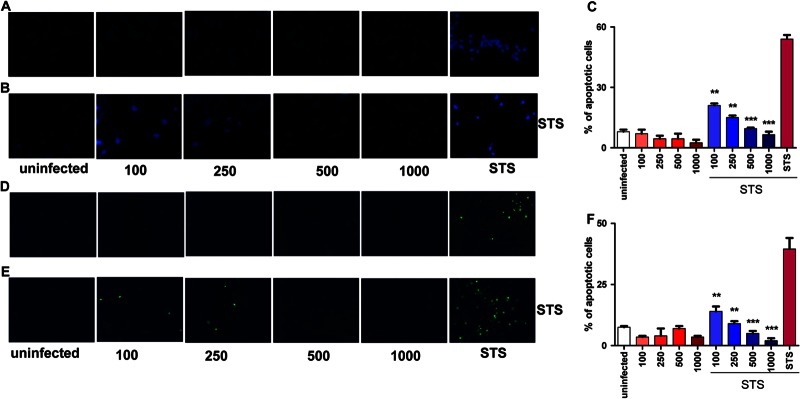
B. abortus inhibits STS-induced apoptosis of synoviocytes. Cells were infected or not at different MOI (100 to 1,000). After 24 h, some infected cells were treated with staurosporine (STS). Uninfected cells treated with STS were used as a positive control. Apoptosis was evaluated by the Hoechst 33342 and TUNEL techniques, using fluorescence microscopy. (A) Apoptosis of B. abortus-infected cells evaluated by Hoechst 33342. (B) Apoptosis of B. abortus-infected cells treated with STS 24 h after infection, by Hoechst 33342. (C) Quantitative analysis of experiments presented in panels A and B. (D) Apoptosis of B. abortus-infected cells, analyzed by TUNEL assay. (E) Apoptosis of B. abortus-infected cells treated with STS 24 h after infection, analyzed by TUNEL assay. (F) Quantitative analysis of experiments presented in panels D and E. Bars express the means ± SEM of duplicates. Data shown are from a representative experiment of five performed. **, P < 0.01; ***, P < 0.001 for STS-treated and uninfected cells.
B. abortus infection of synoviocytes upregulates the expression of antiapoptotic factors.
To further investigate the mechanism involved in the inhibition of synoviocyte apoptosis, differential expression of apoptotic and antiapoptotic proteins was determined in synovial fibroblast cells infected with B. abortus, and these results were compared to those obtained with uninfected cells. Protein array analysis revealed the upregulation of antiapoptotic factors such as cIAP-2 apoptotic suppressor (fold increase, 1.39), clusterin (fold increase, 1.33), livin (fold increase, 1.54), and P21/CIP/CDNK1A (fold increase, 1.16). In contrast, the expression of proteins that are involved in apoptosis induction [Bad, Bax, cleaved procaspase 3, CytC, TRAIL R1/DR4, FADD, Fas/TNFRSF6, HTRA2/Omi, p27/Kip1, P-Rad17(S635), SMAC/Diablo, HIF-1a, and TRAIL R2/DR5] was not changed; or their expression was reduced, as in the case of P-p53(S15) (fold decrease, 1.48) and tumor necrosis factor (TNF) RI/TNFRSF1A (fold decrease, 2.44) (Table 1).
Table 1.
Protein array of pro- and antiapoptotic factors
| Factor | Fold increase over uninfected cells |
|---|---|
| Antiapoptotic | |
| Bcl-2 | 1 |
| Bcl-x | 1 |
| cIAP-1 | 1 |
| cIAP-2 | 1.39 |
| Claspin | 1 |
| Clusterin | 1.33 |
| HO-1/HMOX1/HSP32 | 1 |
| HO-2/HMOX2 | 1 |
| HSP27 | 1 |
| HSP60 | 1 |
| HSP70 | 1 |
| Livin | 1.54 |
| PON2 | 1 |
| P21/CIP/CDNK1A | 1.16 |
| Survivin | 1 |
| XIAP | 1 |
| Proapoptotic | |
| Bad | 1 |
| Bax | 1 |
| Procaspase 3 | 1 |
| Cleaved | 1 |
| CytC | 1 |
| TRAIL R2/DR5 | 1 |
| TRAIL R1/DR4 | 1 |
| FADD | 1 |
| Fas/TNFRSF6 | 1 |
| HTRA2/Omi | 1 |
| p27/Kip1 | 1 |
| P-p53 (S15) | 0.67 |
| P-p53 (S46) | 1 |
| P-p53 (S392) | 1 |
| P-Rad17 (S635) | 1 |
| SMAC/Diablo | 1 |
| TNF RI/TNFRSF1A | 0.40 |
| HIF-1a | 1 |
To further validate the protein array results, we corroborated the upregulation of two antiapoptotic factors, clusterin and p21; the downregulation of one of the apoptotic factors, TNFR1; and the unmodified regulation of two nonmodulated molecules including a proapoptotic factor, TRAIL R1DR4 and the antiapoptotic factor survivin. Clusterin expression was determined by Western blotting using the eukaryotic housekeeping β-actin protein as a reference for gel loadings. Upon infection of synoviocytes, B. abortus induced significant (P < 0.001) upregulation of the expression of clusterin when these cells were infected with MOI of 100 to 1,000 (Fig. 3A and B). Quantitative analysis of clusterin expression by ELISA in synovial fibroblast lysates and culture supernatants corroborated that B. abortus was able to induce the upregulation of clusterin expression with respect to the level of unstimulated cells in an MOI-dependent fashion (Fig. 3C and D). In addition, B. abortus infection induced the upregulation of p21 (Fig. 3E) and the downregulation of TNFR1 (Fig. 3F) in an MOI-dependent manner, as assessed by ELISA. Also, in concordance with the array, the expression of survivin and TRAIL R1DR4 did not change in response to B. abortus infection, as was determined by ELISA. Altogether, these results indicate that infection by B. abortus inhibits apoptosis of synoviocytes by upregulating the expression of antiapoptotic factors.
Fig 3.
B. abortus infection induces clusterin and p21 expression but inhibits TNFR1 expression. Synoviocytes were infected at MOI of 100 to 1,000. (A) Cell lysates obtained at 24 h postinfection were used to determine clusterin expression by Western immunoblotting. (B) Densitometric analysis of results from two independent experiments performed as described for panel A. Clusterin production by B. abortus-infected synoviocytes was measured at 24 h after infection with B. abortus by ELISA in cell lysates (C) and culture supernatants (D). (E and F) P21 (E) and TNFR1 (F) production by B. abortus-infected synoviocytes was assessed by ELISA in cell lysates. Bars express the means ± SEM of duplicates. Data shown are from a representative experiment of three performed. **, P < 0.01; ***, P < 0.001 versus uninfected cells.
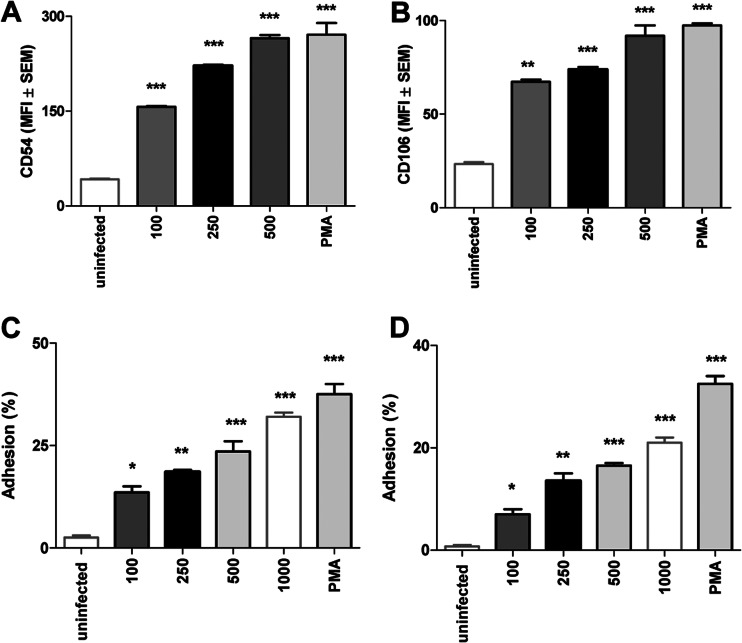
B. abortus infection upregulates CD54 and CD106 but not CD62L expression.
Adhesion molecules are involved in cellular recruitment during immune responses, and intercellular adhesion molecule-1 (ICAM-1, CD54), vascular cell adhesion protein-1 (VCAM-1, CD106), or CD62L (L-Selectin) are expressed by several cell types, including synoviocytes (42, 43). As lympho-polymorphonuclear infiltrates are present in the synovia of infected patients (8, 11), we speculate that modulation of adhesion molecules in synoviocytes by B. abortus might be central in this process, and also this modulation of expression might lead to a harmful response induced by infiltrating cells. B. abortus infection upregulated the CD54 and CD106 expression on synoviocytes in an MOI-dependent fashion (Fig. 4A and B). In contrast, CD62L expression was not affected (not shown). PMA stimulation was used as a positive control. These results indicate that B. abortus infection may induce the upregulation adhesion molecules, and this upregulation could lead to an increase in the adhesion of cell from the innate immunity.
Fig 4.
Monocyte and neutrophil adhesion to B. abortus-infected synoviocytes. Flow cytometry analysis of CD54 (A) and CD106 (B) expression in B. abortus-infected synoviocytes and uninfected cells. Adhesion of calcein-labeled neutrophils (C) and purified human monocytes (D) to synoviocytes infected with B. abortus at different MOI, uninfected cells, or PMA-treated cells after coincubation for 1 h at 37°C. Nonadherent cells were washed away, and plates were read in a fluorescence plate reader. The percentage of adhesion was calculated as follows: (RFU after wash/RFU before wash) × 100. Data shown are from a representative experiment of five performed. *, P < 0.1; **, P < 0.01; ***, P < 0.001 versus uninfected cells (uninfected).
Neutrophils and monocytes adhere to B. abortus-infected synoviocytes.
In previous studies, we demonstrated that B. abortus-infected synoviocytes secrete IL-8 and MCP-1, inducing neutrophil and monocyte migration (22). Cell-to-cell adherence is a mandatory step in the interaction between cells in the immune response, and neutrophilic and monocytic infiltrates have been found in histological studies on osteoarticular involvement in human brucellosis (42, 43). Since CD54 and CD106 expression was upregulated in B. abortus-infected synoviocytes, we investigated if neutrophils and monocytes can adhere to such cells. To this end, synoviocytes were infected at different MOI, and calcein-labeled uninfected neutrophils or monocytes (THP-1 cells or monocytes purified from human venous blood) were added to the culture. As shown in Fig. 4C and D, the percentage of adhered purified neutrophils and monocytes increased significantly when synoviocytes were infected with B. abortus, and this increase was MOI dependent. The same result was obtained with THP-1 cells (not shown). This indicates that B. abortus-infected synoviocytes may induce the adhesion of immune cells that could lead to a potential harmful response.
B. abortus infection inhibits apoptosis induced by supernatants from B. abortus-infected monocytes and neutrophils.
Neutrophils and monocytes have been shown to induce apoptosis of cells from infiltrated tissues due to the release of MMPs, reactive oxygen species, and TNF-α (34, 44, 45). However, our results indicate that B. abortus infection inhibits apoptosis of synoviocytes induced by STS. Thus, experiments were conducted to determine whether B. abortus infection may also inhibit apoptosis induced by neutrophils' and monocytes' secreted factors. For this, supernatants from B. abortus-infected purified monocytes (or neutrophils) were used to stimulate synoviocytes previously infected with B. abortus for 24 h. Supernatants from monocytes and neutrophils infected with B. abortus induced apoptosis of uninfected synoviocytes. Yet upon infection of synoviocytes, apoptosis was inhibited in an MOI-dependent manner (Fig. 5A and B). These results indicate that supernatant from B. abortus-infected monocytes or neutrophils induces synoviocyte apoptosis but this phenomenon could be inhibited when synoviocytes were previously infected with B. abortus. Also, they indicate that despite the ability of B. abortus-infected synoviocytes to recruit and attach monocytes and neutrophils, infection protects them from the potential harm induced by these cells.
Fig 5.
Effects of B. abortus-infected monocytes and neutrophils on the viability of infected and uninfected synoviocytes. Fluorescence microscopy analysis of apoptosis using Hoechst 33342 techniques of B. abortus-infected synoviocytes treated with supernatants added at 1/2 proportion from noninfected purified human monocytes (A) and neutrophils (D) or with supernatants added at 1/2 proportion from B. abortus-infected purified human monocytes (B) and neutrophils (E). Data shown are from a representative experiment of five performed. *, P < 0.1; **, P < 0.01 versus uninfected cells (uninfected). Bars express the means ± SEM of triplicates. (C) Quantitative analysis of experiments presented in panels A and B. (F) Quantitative analysis of experiments presented in panels D and E.
B. abortus-infected synoviocytes induce osteoclastogenesis.
Osteoclasts play a key role in bone resorption and originate from the fusion of precursors belonging to the monocyte/macrophage lineage in the bone marrow (46, 47). This process may involve soluble mediators from inflammatory cells or membrane-bound and soluble RANKL in conjunction with M-CSF (48). To determine if factors secreted by B. abortus-infected synoviocytes could induce osteoclast formation from monocytic THP-1 cells, these cells were stimulated with M-CSF in conjunction with supernatants from B. abortus-infected synoviocytes, and ex vivo osteoclastogenesis was determined by the generation of multinucleated vitronectin receptor- and TRAP-expressing cells. RANKL was used as a positive control. Supernatants from Brucella-infected synoviocytes induced osteoclastogenesis. The magnitude of osteoclastogenesis induced was related directly to the MOI used to infect synovial fibroblasts (Fig. 6). Osteoclastogenesis was also induced when monocytic THP-1 cells were cocultured with synoviocytes in the presence of M-CSF (not shown).
Fig 6.
Supernatants from B. abortus-infected synoviocytes induced monocyte-derived osteoclastogenesis. THP-1 cells were stimulated with supernatants from synoviocytes infected with B. abortus for 24 h at different multiplicities or uninfected in conjunction with M-CSF. RANKL was used as a positive control. After 5 days, osteoclastogenesis was determined by the generation of multinucleated TRAP-expressing (A and C) and vitronectin receptor (VR)-expressing (B and D) cells. Representative digital images were taken by light microscopy (A) or fluorescence microscopy (B), and multinucleated TRAP-positive (C) or vitronectin receptor-positive (D) cells were identified and counted. Bars express the means ± SEM of triplicates. Data shown are from a representative experiment of five performed. ***, P < 0.001 versus uninfected cells.
B. abortus-infected synoviocytes induce osteoclastogenesis mediated by RANKL.
RANKL and proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 have been shown to be important in bone resorption (48, 49). We have previously demonstrated that synoviocytes are unable to produce detectable amounts of proinflammatory cytokines in response to B. abortus infection (22). Moreover, RANKL is upregulated in other bacterial osteoarthritis (50, 51), and we have detected RANKL (400 ± 22 pg/ml) in a synovial fluid from a patient with prepatellar bursitis caused by B. abortus (52). Therefore, we investigated whether B. abortus infection would lead to membrane-bound and soluble RANKL expression from synoviocytes. To this end, cell surface RANKL protein presence was evaluated by flow cytometry and fluorometry. B. abortus infection induced the upregulation of membrane-bound RANKL expression in an MOI-dependent fashion (Fig. 7A, B, and C). Quantitative analysis of RANKL expression on synoviocytes supernatants revealed that B. abortus was also able to induce the secretion of soluble RANKL expression as determined by ELISA (Fig. 7D). These results indicated that B. abortus induced the upregulation of RANKL, which could contribute to bone destruction through activation of osteoclastogenesis.
Fig 7.
RANKL expression induced by B. abortus infection in synoviocytes. (A) RANKL surface expression on synoviocytes infected with B. abortus at MOI of 100 and 1,000 or uninfected, measured by flow cytometry. The histograms show the results of one experiment representative of three independent experiments. (B) Bars represent the arithmetic means of three experiments, and the error bars indicate the standard errors of the means. MFI, mean fluorescence intensity. (C) RANKL expression in synoviocytes infected with B. abortus at MOI of 100 to 1,000 or uninfected, measured by fluorometry. (D) RANKL measured in supernatants from B. abortus-infected synoviocytes by ELISA. Data shown are from a representative experiment of five performed. *, P < 0.1; **, P < 0.01; ***, P < 0.001 versus uninfected cells.
To assess the role of RANKL in osteoclastogenesis elicited by B. abortus, THP-1 cells were cultured with M-CSF and supernatants from B. abortus-infected synoviocytes in the presence of OPG, the RANKL decoy receptor, and osteoclastogenesis was evaluated by the generation of multinucleated TRAP-expressing cells. In contrast to what was seen in untreated cells, OPG treatment completely abrogated osteoclastogenesis induced by supernatants from B. abortus-infected synovial fibroblasts (Fig. 8A and B). These results indicate that RANKL secreted by B. abortus-infected synoviocytes could be involved in the bone loss observed in osteoarticular brucellosis.
Fig 8.
Supernatants from B. abortus-infected synoviocytes induced functional osteoclast-like cells via RANKL. Supernatants from B. abortus-infected or uninfected synoviocytes were preincubated or not with OPG. (A) Light microscopy of multinucleated TRAP-positive cells. (B) Quantitative analysis of the experiment shown in panel A. (C) The functional activity of osteoclast-like cells was determined by their ability to resorb dentine, determined by light microscopy. (D) Quantitative analysis of the experiment shown in panel C. Bars express the means ± SEM of duplicates. Data shown are from a representative experiment of five performed. ***, P < 0.001 versus cells stimulated with supernatants from uninfected synoviocytes.
B. abortus-infected synoviocytes induce functional osteoclast-like cells.
Our premise is that B. abortus infection might activate synoviocytes in a way that would promote the generation of osteoclasts, leading to bone loss. Thus, we assessed the functional activity of B. abortus-induced osteoclast-like cells by their ability to resorb dentine. THP-1 cells treated with supernatants from B. abortus-infected synoviocytes were able to induce a significant (P < 0.001) dentine resorption in an MOI-dependent manner. In contrast, supernatants from uninfected synoviocytes did not (Fig. 8C and D). Additionally, the resorption of dentine was blocked when the experiments were performed in the presence of OPG (Fig. 8C and D). Taken together, these results indicate that supernatants from B. abortus-infected synoviocytes could promote functional osteoclasts formation from human monocytes, leading to bone loss.
DISCUSSION
Osteoarticular brucellosis is the most common presentation of human active brucellosis disease (7–9, 11, 53). In this paper, we studied the role played by synoviocytes in this form of the disease, as they have been recognized as central mediators of joint damage in inflammatory arthritides of either infectious or noninfectious origins (54, 55).
Smooth Brucella species have developed several mechanisms to survive intracellularly, especially inside macrophages. We have demonstrated that B. abortus infects and replicates in human synoviocytes (22). In this study, we confirm and extend these results, demonstrating the bacterium's capacity to infect and multiply in primary human synoviocytes. The ability of B. abortus to invade, survive, and replicate within synovial fibroblast is in line with its capacity to replicate in other nonphagocytic cells, including hepatocytes, astrocytes, and osteoblasts (34, 40, 56, 57). However, unlike what happens in all these cells, the infection did not induce synoviocyte apoptosis. Moreover, it inhibits the apoptosis induced by STS and by culture supernatants from B. abortus-infected neutrophils and monocytes. Thus, B. abortus could use these cells as an alternative replicative niche in the joints. Confocal imaging confirmed this speculation, indicating that, as occurred in macrophages, B. abortus replicates within calnexin-positive vacuoles in human primary synoviocytes.
Since the first report of macrophage apoptosis following infection with Shigella flexneri (58), the ability of parasites to alter the balance between pro- and antiapoptotic signals in phagocytes has generated considerable interest (59, 60). To analyze the strategy adopted by B. abortus to develop within synovial fibroblasts, we examined the modulation of apoptotic and antiapoptotic factors during B. abortus synovial fibroblast infection. In contrast with previous results from B. abortus-infected macrophages (18), the proapoptotic BCL-2 family members were not affected in B. abortus-infected synoviocytes. We observed an increased expression of inhibitor of apoptosis (IAP) family of proteins, clusterin, and the regulator of cell cycle p27 in B. abortus-infected synoviocytes relative to their expression in uninfected cells. In addition, apoptotic factors were down-modulated or not affected after B. abortus infection. The antiapoptotic signaling mediated by live Brucella occurs downstream from phagocytosis and requires that brucellae counteract the stress induced by their internalization within phagosomes and reorient maturation of their phagosomes (16). Given that the intracellular fate of Brucella in human primary synoviocytes is similar to the one observed in its natural niche (the macrophage), the antiapoptotic phenotype observed in B. abortus-infected synoviocytes could be related to a strategy of this bacterium to survive in different host cells.
To our knowledge, there are no reports on the ability of Brucella to infect human synoviocytes in vivo. This may be explained in part by ethical restrictions, since a biopsy of the affected articulation may be justified only in very select cases. Nevertheless, in the few published studies in which a culture of synovial membrane samples was performed, Brucella was isolated from such samples (61, 62). Importantly, cultures of synovial fluid were negative in these cases, suggesting that the bacterium was located intracellularly in the synovial membrane. In support of this hypothesis, foci of intracytoplasmic granular reactions were detected by immunofluorescence with anti-Brucella antibodies in the synovial surface of joints obtained from cattle experimentally infected with B. abortus S19 (63, 64). Interestingly, synoviocytes are located in the intimal layer of the synovium, which agrees with the “synovial surface” location described in that study.
Monocytes and neutrophils are typically seen as infiltrating cells in damaged joints infected with Brucella (8, 11, 14). We have previously demonstrated that B. abortus-infected synoviocytes secrete IL-8 and MCP-1, chemokines that attract neutrophils and monocytes, respectively (22). Therefore, synoviocytes are implicated, at least in part, in leukocyte recruitment to the infected joint. The upregulation of adhesion molecules by B. abortus-infected synoviocytes would facilitate the interaction of these cells with the infiltrating leukocytes, since these intracellular adhesion molecules play a central role in the interaction between cells in the immune responses (65). In agreement with this hypothesis, we demonstrated that infection of synoviocytes with B. abortus upregulated the surface expression of CD54 and CD106 and that, as a consequence, monocytes and neutrophils adhere to B. abortus-infected synoviocytes. This can potentially increase the ability of these cells to interact with infiltrating inflammatory cells, thereby propagating immunologically mediated inflammation such as occurs in rheumatoid synovitis (54). Besides, B. abortus-infected synoviocytes inhibited the apoptosis induced by supernatants from B. abortus-infected monocytes and neutrophils. These phenomena could contribute to the establishment of a successful chronic infection by Brucella.
RANKL is a newly discovered key mediator of osteoclast differentiation and bone resorption (66). Several investigations have revealed the involvement of RANKL in the bone destruction that occurs in rheumatoid arthritis and osteoarticular infectious disease (67, 68). It has been reported that resting synovial fibroblasts do not express RANKL. However, we found increased concentrations of RANKL in a synovial fluid from a patient with bursitis caused by B. abortus infection. In addition, RANKL expression was found in surface membrane and culture supernatants from synoviocytes infected in vitro with B. abortus. The marked change in RANKL expression between uninfected and infected cells suggests that under normal conditions synovial fibroblasts may be only weakly osteoclastogenic and that after infection they become potent stimulators of osteoclast formation. This is consistent with the fact that multinucleated TRAP-positive and vitronectin receptor-positive cells were observed only when osteoclasts precursors were treated with culture supernatants from B. abortus-infected synoviocytes. These multinucleated cells also resorbed dentine, indicating that they are differentiated functional osteoclasts. Moreover, our results demonstrated that OPG (RANKL's decoy receptor) abrogates osteoclast formation and dentine resorption, further supporting the contention that RANKL determines the osteoclastogenesis induced by B. abortus and underscoring the role of this factor in the bone loss reported in osteoarticular brucellosis. In previous results, in other models of Brucella osteoclastogenesis that involved the role of macrophages and T cells in bone resorption, we reported that TNF-α plays a key role in these processes (69). Although synovial fibroblasts secrete TNF-α and other inflammatory cytokines in response to infection by other bacteria (70), B. abortus does not induce significant levels of production of TNF-α and IL-1β in these cells.
Finally, the results presented here, together with our previous observations (22, 35, 41, 56, 57, 69), are shedding light on how the interactions of B. abortus with synovial fibroblasts may have an important role in the pathogenesis of brucellar arthritis by producing RANKL and consequent osteoclastogenesis and also by attracting and adhering phagocytes and neutrophils and inducing them to produce damage in the joint.
ACKNOWLEDGMENTS
We thank Horacio Salomón and the staff at the Instituto de Investigaciones Biomédicas en Retrovirus y Sida (INBIRS) for their assistance with biosafety level 3 laboratory use. We also thank Enrique Moya and Fernando Gonzales Morán for advice with synovial samples.
This work was supported by grants PICT2010-0023 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT, Argentina), by grant UBACYT 20020090200012 from Universidad de Buenos Aires, and by grant PIP112-200801-02706 from Consejo Nacional de Investigación Científica y Tecnológica (CONICET). R.S., A.M.R., and P.C.A.B are recipients of a fellowship from CONICET. P.B., C.A.F, G.H.G., and M.V.D. are members of the Research Career of CONICET.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 18 March 2013
REFERENCES
- 1. Winter AJ, Schurig GG, Boyle SM, Sriranganathan N, Bevins JS, Enright FM, Elzer PH, Kopec JD. 1996. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am. J. Vet. Res. 57:677–683 [PubMed] [Google Scholar]
- 2. Young EJ. 1983. Human brucellosis. Rev. Infect. Dis. 5:821–842 [DOI] [PubMed] [Google Scholar]
- 3. Williams E. 1973. Brucellosis. Br. Med. J. 1:791–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Darwish M, Benkirane A. 2001. Field investigations of brucellosis in cattle and small ruminants in Syria, 1990-1996. Rev. Sci. Tech. 20:769–775 [DOI] [PubMed] [Google Scholar]
- 5. Slater PE, Costin C, Seidenbaum M, Ever-Hadani S. 1990. Epidemiology of human brucellosis in Israel. Public Health Rev. 18:159–169 [PubMed] [Google Scholar]
- 6. Picciotto D, Verso MG, Lacca G, Mangiapane N, Caracappa S, Vitale F, Vesco G. 1999. The epidemiological trend of brucellosis in the provinces of Sicily. Med. Lav. 90:786–790 (In Italian.) [PubMed] [Google Scholar]
- 7. Aydin M, Fuat Yapar A, Savas L, Reyhan M, Pourbagher A, Turunc TY, Ziya Demiroglu Y, Yologlu NA, Aktas A. 2005. Scintigraphic findings in osteoarticular brucellosis. Nucl. Med. Commun. 26:639–647 [DOI] [PubMed] [Google Scholar]
- 8. Gotuzzo E, Alarcon GS, Bocanegra TS, Carrillo C, Guerra JC, Rolando I, Espinoza LR. 1982. Articular involvement in human brucellosis: a retrospective analysis of 304 cases. Semin. Arthritis Rheum. 12:245–255 [DOI] [PubMed] [Google Scholar]
- 9. Khateeb MI, Araj GF, Majeed SA, Lulu AR. 1990. Brucella arthritis: a study of 96 cases in Kuwait. Ann. Rheum. Dis. 49:994–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colmenero JD, Ruiz-Mesa JD, Plata A, Bermudez P, Martin-Rico P, Queipo-Ortuno MI, Reguera JM. 2008. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin. Infect. Dis. 46:426–433 [DOI] [PubMed] [Google Scholar]
- 11. Madkour MM. 2001. Osteoarticular brucellosis, p 74–87 In Madkour MM. (ed), Madkour's brucellosis, 2nd ed Springer-Verlag, Berlin, Germany [Google Scholar]
- 12. Madkour MM. 2001. Bone and joint imaging, p 90–132 In Madkour MM. (ed), Madkour's brucellosis, 2nd ed Springer-Verlag, Berlin, Germany [Google Scholar]
- 13. Young EJ. 1989. Clinical manifestations of human brucellosis, p 97–126 In Young EJ, Corbel MJ. (ed), Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, FL [Google Scholar]
- 14. Bosilkovski M, Krteva L, Caparoska S, Dimzova M. 2004. Osteoarticular involvement in brucellosis: study of 196 cases in the Republic of Macedonia. Croat. Med. J. 45:727–733 [PubMed] [Google Scholar]
- 15. Starr T, Child R, Wehrly TD, Hansen B, Hwang S, Lopez-Otin C, Virgin HW, Celli J. 2012. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11:33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. 2008. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 9:678–694 [DOI] [PubMed] [Google Scholar]
- 17. Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198:545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eskra L, Mathison A, Splitter G. 2003. Microarray analysis of mRNA levels from RAW264.7 macrophages infected with Brucella abortus. Infect. Immun. 71:1125–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gross A, Terraza A, Ouahrani-Bettache S, Liautard JP, Dornand J. 2000. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68:342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barrionuevo P, Cassataro J, Delpino MV, Zwerdling A, Pasquevich KA, Garcia Samartino C, Wallach JC, Fossati CA, Giambartolomei GH. 2008. Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via Toll-like receptor 2. Infect. Immun. 76:250–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrionuevo P, Delpino MV, Pozner RG, Velasquez LN, Cassataro J, Giambartolomei GH. 29 October 2012. Brucella abortus induces intracellular retention of MHC-I molecules in human macrophages down-modulating cytotoxic CD8(+) T cell responses. Cell. Microbiol. [Epub ahead of print.] doi:10.1111/cmi.12058 [DOI] [PubMed] [Google Scholar]
- 22. Scian R, Barrionuevo P, Giambartolomei GH, De Simone EA, Vanzulli SI, Fossati CA, Baldi PC, Delpino MV. 2011. Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases. Infect. Immun. 79:3619–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krasnokutsky S, Belitskaya-Levy I, Bencardino J, Samuels J, Attur M, Regatte R, Rosenthal P, Greenberg J, Schweitzer M, Abramson SB, Rybak L. 2011. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 63:2983–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsai C, Diaz LA, Jr, Singer NG, Li LL, Kirsch AH, Mitra R, Nickoloff BJ, Crofford LJ, Fox DA. 1996. Responsiveness of human T lymphocytes to bacterial superantigens presented by cultured rheumatoid arthritis synoviocytes. Arthritis Rheum. 39:125–136 [DOI] [PubMed] [Google Scholar]
- 25. Salamone GV, Petracca Y, Fuxman Bass JI, Rumbo M, Nahmod KA, Gabelloni ML, Vermeulen ME, Matteo MJ, Geffner JR, Trevani AS. 2010. Flagellin delays spontaneous human neutrophil apoptosis. Lab. Invest. 90:1049–1059 [DOI] [PubMed] [Google Scholar]
- 26. Boyum A. 1968. Separation of leukocytes from blood and bone marrow. Introduction. Scand. J. Clin. Lab. Invest. Suppl. 97:7. [PubMed] [Google Scholar]
- 27. Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6:e1000715 doi:10.1371/journal.ppat.1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Neill S, O'Neill AJ, Conroy E, Brady HR, Fitzpatrick JM, Watson RW. 2000. Altered caspase expression results in delayed neutrophil apoptosis in acute pancreatitis. J. Leukoc. Biol. 68:15–20 [PubMed] [Google Scholar]
- 29. Godaly G, Young DB. 2005. Mycobacterium bovis bacille Calmette Guerin infection of human neutrophils induces CXCL8 secretion by MyD88-dependent TLR2 and TLR4 activation. Cell. Microbiol. 7:591–601 [DOI] [PubMed] [Google Scholar]
- 30. Agace WW, Patarroyo M, Svensson M, Carlemalm E, Svanborg C. 1995. Escherichia coli induces transuroepithelial neutrophil migration by an intercellular adhesion molecule-1-dependent mechanism. Infect. Immun. 63:4054–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li D, Dong B, Tong Z, Wang Q, Liu W, Wang Y, Liu W, Chen J, Xu L, Chen L, Duan Y. 2012. MBL-mediated opsonophagocytosis of Candida albicans by human neutrophils is coupled with intracellular Dectin-1-triggered ROS production. PLoS One 7:e50589 doi:10.1371/journal.pone.0050589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Asbeck EC, Hoepelman AI, Scharringa J, Herpers BL, Verhoef J. 2008. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol. 8:229 doi:10.1186/1471-2180-8-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giambartolomei GH, Zwerdling A, Cassataro J, Bruno L, Fossati CA, Philipp MT. 2004. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 173:4635–4642 [DOI] [PubMed] [Google Scholar]
- 34. Delpino MV, Barrionuevo P, Scian R, Fossati CA, Baldi PC. 2010. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J. Hepatol. 53:145–154 [DOI] [PubMed] [Google Scholar]
- 35. Delpino MV, Barrionuevo P, Macedo GC, Oliveira SC, Di Genaro S, Scian R, Miraglia MC, Fossati CA, Baldi PC, Giambartolomei GH. 2012. Macrophage-elicited osteoclastogenesis in response to Brucella abortus infection requires TLR2/MyD88-dependent TNF-alpha production. J. Leukoc. Biol. 91:285–298 [DOI] [PubMed] [Google Scholar]
- 36. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 37. Roop RM, II, Bellaire BH, Valderas MW, Cardelli JA. 2004. Adaptation of the Brucellae to their intracellular niche. Mol. Microbiol. 52:621–630 [DOI] [PubMed] [Google Scholar]
- 38. Gorvel JP, Moreno E. 2002. Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 90:281–297 [DOI] [PubMed] [Google Scholar]
- 39. Velasquez LN, Delpino MV, Ibanez AE, Coria LM, Miraglia MC, Scian R, Cassataro J, Giambartolomei GH, Barrionuevo P. 2012. Brucella abortus induces apoptosis of human T lymphocytes. Microbes Infect. 14:639–650 [DOI] [PubMed] [Google Scholar]
- 40. Garcia Samartino C, Delpino MV, Pott Godoy C, Di Genaro MS, Pasquevich KA, Zwerdling A, Barrionuevo P, Mathieu P, Cassataro J, Pitossi F, Giambartolomei GH. 2010. Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. Am. J. Pathol. 176:1323–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scian R, Barrionuevo P, Fossati CA, Giambartolomei GH, Delpino MV. 2012. Brucella abortus invasion of osteoblasts inhibits bone formation. Infect. Immun. 80:2333–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lambert N, Lescoulie PL, Yassine-Diab B, Enault G, Mazieres B, De Preval C, Cantagrel A. 1998. Substance P enhances cytokine-induced vascular cell adhesion molecule-1 (VCAM-1) expression on cultured rheumatoid fibroblast-like synoviocytes. Clin. Exp. Immunol. 113:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kullich W, Neff H, Pollmann G, Machreich K, Schwann H. 1999. Adhesion molecule ICAM-1 in patients with chronic polyarthritis—effects of inpatient rehabilitation. Wien. Med. Wochenschr. 149:550–553 (In German.) [PubMed] [Google Scholar]
- 44. Heard BJ, Achari Y, Chung M, Shrive NG, Frank CB. 2011. Early joint tissue changes are highly correlated with a set of inflammatory and degradative synovial biomarkers after ACL autograft and its sham surgery in an ovine model. J. Orthop. Res. 29:1185–1192 [DOI] [PubMed] [Google Scholar]
- 45. Wassilew GI, Lehnigk U, Duda GN, Taylor WR, Matziolis G, Dynybil C. 2010. The expression of proinflammatory cytokines and matrix metalloproteinases in the synovial membranes of patients with osteoarthritis compared with traumatic knee disorders. Arthroscopy 26:1096–1104 [DOI] [PubMed] [Google Scholar]
- 46. Scheven BA, Visser JW, Nijweide PJ. 1986. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature 321:79–81 [DOI] [PubMed] [Google Scholar]
- 47. Ibbotson KJ, Roodman GD, McManus LM, Mundy GR. 1984. Identification and characterization of osteoclast-like cells and their progenitors in cultures of feline marrow mononuclear cells. J. Cell Biol. 99:471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haynes DR. 2004. Bone lysis and inflammation. Inflamm. Res. 53:596–600 [DOI] [PubMed] [Google Scholar]
- 49. Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. 1996. Bacterially induced bone destruction: mechanisms and misconceptions. Infect. Immun. 64:2371–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reddi D, Brown SJ, Belibasakis GN. 2011. Porphyromonas gingivalis induces RANKL in bone marrow stromal cells: involvement of the p38 MAPK. Microb. Pathog. 51:415–420 [DOI] [PubMed] [Google Scholar]
- 51. Sakurai A, Okahashi N, Nakagawa I, Kawabata S, Amano A, Ooshima T, Hamada S. 2003. Streptococcus pyogenes infection induces septic arthritis with increased production of the receptor activator of the NF-kappaB ligand. Infect. Immun. 71:6019–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wallach JC, Delpino MV, Scian R, Deodato B, Fossati CA, Baldi PC. 2010. Prepatellar bursitis due to Brucella abortus: case report and analysis of the local immune response. J. Med. Microbiol. 59:1514–1518 [DOI] [PubMed] [Google Scholar]
- 53. Solera J, Lozano E, Martinez-Alfaro E, Espinosa A, Castillejos ML, Abad L. 1999. Brucellar spondylitis: review of 35 cases and literature survey. Clin. Infect. Dis. 29:1440–1449 [DOI] [PubMed] [Google Scholar]
- 54. Bartok B, Firestein GS. 2010. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233:233–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Inman RD, Payne U. 2003. Determinants of synoviocyte clearance of arthritogenic bacteria. J. Rheumatol. 30:1291–1297 [PubMed] [Google Scholar]
- 56. Delpino MV, Fossati CA, Baldi PC. 2009. Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with Brucella spp. Infect. Immun. 77:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scian R, Barrionuevo P, Giambartolomei GH, Fossati CA, Baldi PC, Delpino MV. 2011. Granulocyte-macrophage colony-stimulating factor- and tumor necrosis factor alpha-mediated matrix metalloproteinase production by human osteoblasts and monocytes after infection with Brucella abortus. Infect. Immun. 79:192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zychlinsky A, Prevost MC, Sansonetti PJ. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167–169 [DOI] [PubMed] [Google Scholar]
- 59. DeLeo FR. 2004. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9:399–413 [DOI] [PubMed] [Google Scholar]
- 60. Gao L, Abu Kwaik Y. 2000. Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect. 2:1705–1719 [DOI] [PubMed] [Google Scholar]
- 61. Coventry MB, Ivins JC, Nichols DR, Weed LA. 1949. Infection of the hip by Brucella suis. JAMA 141:320–325 [DOI] [PubMed] [Google Scholar]
- 62. Kelly PJ, Martin WJ, Schirger A, Weed LA. 1960. Brucellosis of the bones and joints. Experience with thirty-six patients. JAMA 174:347–353 [DOI] [PubMed] [Google Scholar]
- 63. Johnson B, Mosier DA, Morton RJ, Confer AW. 1994. Experimental Brucella abortus strain 19 arthritis in young cattle. J. Vet. Diagn. Invest. 6:56–61 [DOI] [PubMed] [Google Scholar]
- 64. Wyn-Jones G, Baker JR, Johnson PM. 1980. A clinical and immunopathological study of Brucella abortus strain 19-induced arthritis in cattle. Vet. Rec. 107:5–9 [DOI] [PubMed] [Google Scholar]
- 65. Springer TA. 1990. Adhesion receptors of the immune system. Nature 346:425–434 [DOI] [PubMed] [Google Scholar]
- 66. Takayanagi H. 2009. Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheumatol. 5:667–676 [DOI] [PubMed] [Google Scholar]
- 67. Teitelbaum SL. 2006. Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res. Ther. 8:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takayanagi H. 2007. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7:292–304 [DOI] [PubMed] [Google Scholar]
- 69. Giambartolomei GH, Scian R, Acosta-Rodriguez E, Fossati CA, Delpino MV. 2012. Brucella abortus-infected macrophages modulate T lymphocytes to promote osteoclastogenesis via IL-17. Am. J. Pathol. 181:887–896 [DOI] [PubMed] [Google Scholar]
- 70. Noss EH, Brenner MB. 2008. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol. Rev. 223:252–270 [DOI] [PubMed] [Google Scholar]