Abstract
We investigated the role of interleukin-10 (IL-10) in cutaneous and pulmonary infection with Francisella tularensis. We found that after intradermal challenge of mice with the live vaccine strain (LVS) of F. tularensis, splenic IL-10 levels increased rapidly and reached a peak 5 days after infection. However, IL-10 expression after infection was detrimental, since IL-10−/− mice showed increased bacterial clearance and were resistant to an infectious dose (>106 CFU/mouse) that was uniformly lethal for IL-10+/+ mice. Furthermore, IL-10+/+ mice treated with neutralizing anti-IL-10R monoclonal antibody were able to survive lethal cutaneous LVS challenge. The presence of IL-10 appeared to restrain the expression of IL-17, since high levels of splenic IL-17 were observed after intradermal LVS infection only in IL-10−/− mice. Furthermore, treatment with neutralizing anti-IL-17R antibody ablated the enhanced survival observed in IL-10−/− mice. However, neutralization of IL-10 activity in IL-17R−/− mice failed to provide protection. Thus, IL-10 suppresses a protective IL-17 response that is necessary for resistance to cutaneous LVS infection. Surprisingly, however, IL-10−/− mice were significantly more susceptible to pulmonary infection with LVS. Finally, although IL-10 is a critical and novel regulator of immunity to F. tularensis LVS infection, its effects were masked during infection with the highly virulent SchuS4 strain. Taken together, these findings suggest that differentially regulating expression of the IL-10 pathway in various tissues could ultimately have prophylactic and therapeutic benefits for protection against tularemia.
INTRODUCTION
Tularemia is a vector-borne zoonosis caused by Francisella tularensis, a Gram-negative, intracellular bacterium. Infection can occur through various routes, including bites from infected insects, handling of infected carcasses, drinking of contaminated water, and inhalation of infectious aerosols. The ulceroglandular form of disease is the most common infection in humans and is usually acquired by a skin bite from an infected arthropod. Pneumonic tularemia is less common, but inhalation of ≤10 CFU of the fully virulent SchuS4 strain of F. tularensis can cause serious disease with a 50% mortality rate. Due to its extreme infectivity and ease of dissemination, F. tularensis is classified as a tier 1 biothreat agent (1–3).
Immune correlates of protection against tularemia are still poorly defined, especially against the highly virulent SchuS4 strain, although the results of multiple studies with the attenuated live vaccine strain (LVS) have indicated that T cells and T cell-associated cytokines such as gamma interferon (IFN-γ) (4–6), interleukin-12 (IL-12) (6, 7), and IL-17 (8–11) may play an important role in protection (reviewed in reference 12). Francisella has the ability to suppress or avoid immune responses early after infection, which likely contributes to its virulence (13, 14). It has been reported that transforming growth factor β (TGF-β) can mediate immunosuppression of initial immunity following pulmonary infection (15). In addition, it has been found that signaling through the type 1 IFN receptor suppresses IL-17 production that is important for resistance to infection with F. novicida (9) and LVS (16). Nevertheless, little is currently known about the potential importance of anti-inflammatory cytokines in resistance or susceptibility to tularemia. In particular, a role for IL-10 has not been examined in detail.
The present study was designed specifically to determine the possible importance of IL-10 during Francisella infection. We (17) and others (18–20) have previously reported that IL-10 can be detrimental for the protection against other pathogens, including influenza virus and mycobacteria. Unexpectedly, we now report that absence of IL-10 activity is detrimental for pulmonary tularemia and yet enhances resistance to cutaneous infection. The findings underscore the importance of anti-inflammatory pathways during tularemia and the possibility that they may play unique roles in different infection sites.
MATERIALS AND METHODS
Mice and bacteria.
Five- to six-week-old C57BL/6 and BALB/c mice were purchased from Taconic, Inc. (Germantown, NY). BALB/c IL-17R−/− mice were kindly provided by Sarah Gaffen (University of Pittsburgh, Division of Rheumatology and Clinical Immunology, Pittsburgh, PA). C57BL/6 IL-10−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were bred and maintained at the Albany Medical College Animal Facility, and all animal procedures were approved by the institutional animal care and use committee.
Animal LVS and SchuS4 challenge and bacterial burden analysis.
For sublethal and lethal intradermal (i.d.) challenges, 105 and >106 CFU, respectively, of LVS were injected in 50 μl of phosphate-buffered saline (PBS). For sublethal intranasal (i.n.) challenge, 1.6 × 103 CFU of LVS in 50 μl of PBS were inoculated into anesthetized mice. For SchuS4 challenge, 64 CFU were delivered i.d. or i.n. in 50 μl of PBS. Each group consisted of eight mice, and the health of all mice was monitored daily. In addition, on days 1, 3, 5, 7, 14, and 21 postchallenge, three mice/day were sacrificed by pentobarbital injection (5 mg/mouse). The lungs, livers, and spleens were harvested aseptically and homogenized in 1 ml of PBS. Dilutions of individual tissue homogenates were then plated on chocolate agar plates and incubated for 3 days at 37°C to enumerate organ CFU.
Cytokine levels.
For quantitative real-time PCR, total RNA was prepared from spleen and lung homogenates with TRIzol reagent (Invitrogen, Grand Island, NY). cDNA was synthesized with superscript reverse transcriptase and oligo(dT) primers (Invitrogen). The primers used to quantify IL-10 and IL-17 transcripts were obtained from Invitrogen and had the following sequences: IL-10 forward, CAGAGCCACATGCTCCTAGA; IL-10 reverse, TGTCCAGCTGGTCCTTTGTT; IL-17 forward, CAAGAAATCCTGGTCCTTCG; and IL-17 reverse, GAGCATCTTCTCCAACCTGAA. Gene expression was examined using a Bio-Rad iCycler optical system and an iQ SYBR green real-time PCR kit (Bio-Rad Laboratories, Hercules, CA). The ΔΔCT method was used to normalize transcripts to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and to calculate expression relative to untreated mice.
Protein levels of IL-10 and IL-17 in spleen and lung tissue homogenates were determined by the BD OptEIA (BD Biosciences) and the DuoSet ELISA (R&D Systems, Minneapolis, MN) kits, respectively, according to the manufacturer's instructions.
Anti-IL-10R and anti-IL-17 MAb treatment.
To neutralize IL-10 activity, 200 μg of rat anti-murine IL-10R1 monoclonal antibody (MAb; BioXCell, West Lebanon, NH) or control rat IgG (Jackson Immunoresearch Laboratories, West Grove, PA) were inoculated intraperitoneally (i.p.) on days 0, 1, 3, and 5 following bacterial challenge. To neutralize IL-17, rat anti-murine IL-17 MAb (kindly provided by Amgen, CA) or control rat IgG was inoculated i.p. in a similar manner but at a dose of 50 μg per mouse.
Statistics.
Survival curves were analyzed by the Kaplan-Meier log-rank test using GraphPad Prism 4 software. Analysis of variance (for comparison of multiple groups) or Student t test (for comparison of two groups) was used for analyses of bacterial burden and cytokine levels. Probability values for significance were set at 0.05. Values for all measurements are expressed as means ± the standard deviations.
RESULTS
Levels of splenic IL-10 after cutaneous F. tularensis LVS infection.
To examine the potential role of IL-10 during cutaneous F. tularensis LVS infection, C57BL/6 mice were injected i.d. with 105 CFU of LVS and IL-10 transcripts in the spleen were quantified by real-time reverse transcription-PCR (RT-PCR) on various days thereafter. Within 1 day after infection, IL-10 levels increased ∼20-fold compared to levels in uninfected mice, and reached an ∼60-fold increase on day 5, decreasing thereafter (Fig. 1, left panel). As expected, no IL-10 transcripts were detected in IL-10−/− mice injected with LVS.
Fig 1.
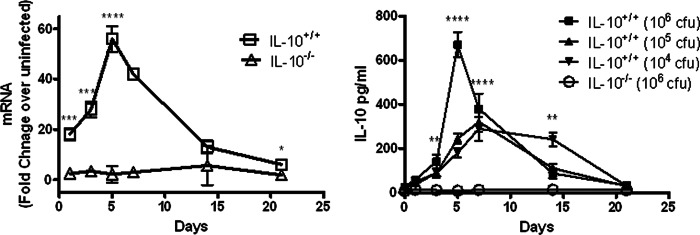
Levels of IL-10 in spleens of IL-10+/+ and IL-10−/− mice infected i.d. with F. tularensis LVS. (Left panel) RNA transcript levels; (right panel) protein levels. Mice were infected with 105 CFU of LVS/mouse (left panel) or 104 to 106 CFU of LVS/mouse (right panel) as indicated. The spleens were harvested from 3 mice/group on days 1, 3, 5, 7, 14, and 21 after infection, homogenized, and analyzed for IL-10 by RT-PCR and ELISA, respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (compared to IL-10−/− mice).
A similar experiment was conducted with various doses of i.d. LVS and measurement of protein levels of splenic IL-10 by enzyme-linked immunosorbent assay (ELISA). Again, it was seen that IL-10 protein levels increased rapidly after i.d. infection and peaked on day 5 (Fig. 1, right panel). The greatest amounts of IL-10 (>600 pg/ml) were produced after challenge with the highest dose of F. tularensis (106 CFU), although significant levels of IL-10 were detected even after a relatively low challenge dose of 104 CFU. Again, IL-10−/− mice inoculated with 106 CFU failed to produce detectable IL-10, which confirmed the specificity of the assay.
Role of IL-10 in susceptibility to cutaneous F. tularensis LVS infection.
Given the large amounts of IL-10 produced after LVS infection, it was of interest to determine whether the presence of this IL-10 influenced susceptibility to infection. To assess this, survival rates of C57BL/6 IL-10+/+ and IL-10−/− mice were monitored following a lethal i.d. infection with F. tularensis LVS. Although all IL-10+/+ mice succumbed within 9 days to an i.d. challenge dose of 2 × 106 CFU, nearly all IL-10−/− mice survived (Fig. 2, top panel). Analysis of splenic bacterial burdens showed a steady increase in splenic CFU in IL-10+/+ mice, which reached >108 CFU/spleen before the mice began to die on day 5 (Fig. 2, middle panel). IL-10−/− mice, on the other hand, demonstrated similar increases in bacterial burdens during the first few days of infection, but the levels of bacteria reached a peak of only 106 CFU/spleen by day 7, and the bacteria were then effectively cleared. By day 21 after infection, few bacteria remained in the spleens of the surviving IL-10−/− mice.
Fig 2.
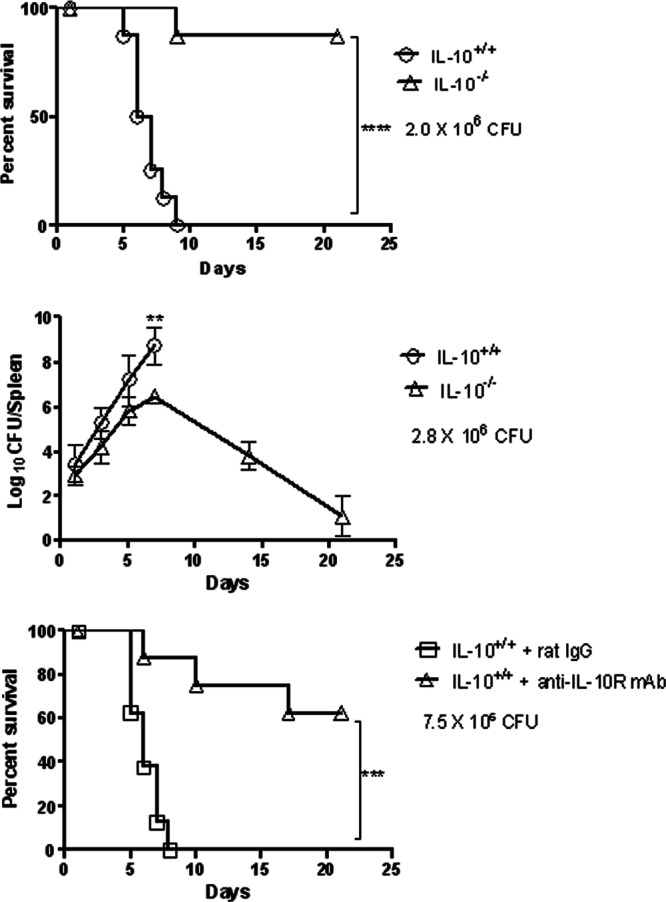
Susceptibility of IL-10+/+ and IL-10−/− mice to cutaneous LVS infection. C57BL/6 mice were inoculated i.d. with 2.0 × 106 CFU of LVS/mouse (top panel), 2.8 × 106 CFU of LVS/mouse (middle panel), or 7.5 × 106 CFU of LVS/mouse (bottom panel). In the bottom panel, the mice were also injected i.p. with 200 μg of rat IgG or neutralizing anti-IL-10R MAb on days 0, 1, 3, and 5 after bacterial challenge. In the top and bottom panels, survival was monitored daily (8 mice/group). The results are representative of two independent experiments. For animals tested for the middle panel, three mice/group were sacrificed on days 1, 3, 5, 7, 14, and 21 after infection, and their spleens were analyzed for bacterial burdens. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To confirm the deleterious effect of IL-10 on survival, C57BL/6 IL-10+/+ mice were i.d. infected with a lethal dose of LVS and then treated with anti-IL-10R MAb or an isotype control MAb. Again, neutralization of IL-10 activity led to significantly increased survival (Fig. 2, bottom panel). Although all control mice died within 8 days, >60% of mice treated with anti-IL-10R MAb survived to day 21. Taken together, these results demonstrate that IL-10 significantly inhibits systemic bacterial clearance and decreases survival after cutaneous F. tularensis LVS infection.
IL-10 suppresses IL-17A expression following cutaneous LVS infection.
We (9) and others (8, 10) have reported a critical role for IL-17 in protection of mice against F. tularensis LVS infection. In addition, it has been shown in other experimental models that IL-10 strongly inhibits IL-17 expression (18, 21, 22). Thus, we next determined whether the presence of IL-10 during cutaneous F. tularensis infection would result in decreased expression of IL-17. After i.d. infection of IL-10+/+ mice with LVS, levels of IL-17 transcripts (Fig. 3, top panel) and protein (Fig. 3, bottom panel) increased only slightly in the spleen, reaching a peak around day 7. This agrees with the work of Woolard et al. (23), who observed only small increases in splenic IL-17 in mice infected i.d. with LVS. In IL-10−/− mice, however, IL-17 levels were significantly higher after infection. Thus, absence of IL-10 allows enhanced production of normally low levels of IL-17 in LVS-infected mice.
Fig 3.
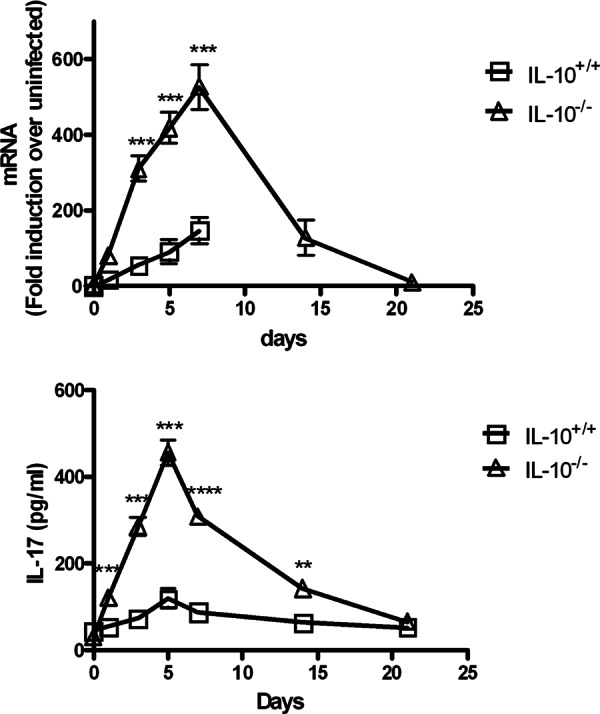
Levels of IL-17 in spleens of IL-10+/+ and IL-10−/− mice infected i.d. with F. tularensis LVS. (Top panel) RNA transcript levels; (bottom panel) protein levels. Mice were infected with 2.5 × 105 CFU of LVS/mouse. Spleens were harvested from three mice/group on days 1, 3, 5, 7, 14, and 21 after infection, homogenized, and analyzed for IL-17 by RT-PCR and ELISA, respectively. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (compared to IL-10−/− mice).
IL-17 is required for the increased resistance of IL-10−/− mice to F. tularensis LVS infection.
It was next determined whether the resistance of IL-10−/− mice to cutaneous tularemia observed above was due to their increased expression of IL-17. For this purpose, IL-10+/+ and IL-10−/− mice were infected i.d. with LVS, and IL-17 was then neutralized by anti-IL-17 MAb injection. Control IL-10+/+ and IL-10−/− mice received normal rat IgG. In the case of IL-10+/+ mice, anti-IL-17 MAb treatment tended to somewhat increase susceptibility to a sublethal LVS challenge dose (104 CFU), although the differences were not statistically significant (Fig. 4). On the other hand, and in keeping with the results presented above, IL-10−/− mice survived a 100-fold higher dose of LVS (106 CFU) compared to IL-10+/+ mice, but neutralization of IL-17 caused all of the IL-10−/− mice to succumb to this infectious dose within 10 days. Thus, IL-17 is required for the increased resistance to F. tularensis LVS that is seen in the absence of IL-10.
Fig 4.
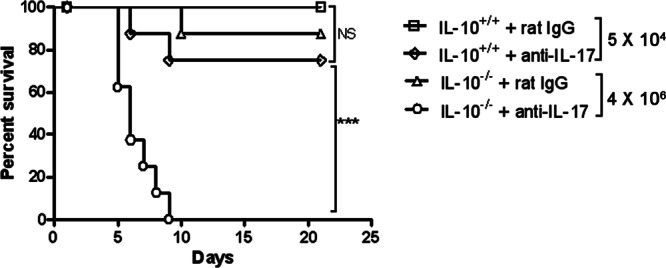
Susceptibility of IL-10+/+ and IL-10−/− mice to cutaneous LVS infection after neutralization of IL-17. C57BL/6 IL-10+/+ mice were inoculated i.d. with 5.0 × 104 CFU of LVS/mouse, while C57BL/6 IL-10−/− mice were inoculated i.d. with 4 × 106 CFU of LVS/mouse. The mice were also injected i.p. with 50 μg of rat IgG or neutralizing anti-IL-17 MAb on days 0, 1, 3, and 5 after bacterial challenge. Survival was monitored daily (eight mice/group). ***, P < 0.001; NS, not significant.
IL-10 neutralization fails to protect IL-17R−/− mice.
The results presented above showed that absence of IL-10 activity caused increased protection against cutaneous LVS infection and suggested that this effect is due to increased levels of IL-17. Thus, we tested whether BALB/c IL-17R−/− mice would be more susceptible to infection and, in addition, whether neutralization of IL-10 activity would have any effect on susceptibility. IL-17R−/− mice demonstrated a high level of susceptibility to i.d. infection, with all mice dying at a challenge dose that was 103-fold lower than that required to kill IL-17R+/+ mice (Fig. 5). Although anti-IL-10R MAb treatment protected nearly all wild-type mice, this same treatment did not improved survival of IL-17R−/− mice. Thus, neutralization of IL-10 has no protective effect in the absence of a functional Th17 pathway.
Fig 5.
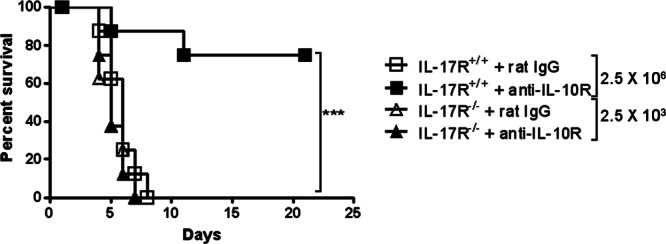
Susceptibility of IL-17R+/+ and IL-17R−/− mice to cutaneous LVS infection after neutralization of IL-10. BALB/c IL-17R+/+ mice were inoculated i.d. with 2.5 × 106 CFU of LVS/mouse, while BALB/c IL-17R−/− mice were inoculated i.d. with 2.5 × 103 CFU of LVS/mouse. The mice were also injected i.p. with 200 μg of rat IgG or neutralizing anti-IL-10R MAb on days 0, 1, 3, and 5 after bacterial challenge. Survival was monitored daily (eight mice/group). ***, P < 0.001.
IL-10−/− mice are more susceptible to pulmonary LVS infection.
The findings described above showed that IL-10 is detrimental for protection against cutaneous LVS infection. Thus, we next tested the influence of this cytokine on pulmonary F. tularensis infection, the route that is most deadly for humans and animals. Others (24) have reported increased expression of IL-10 in the lungs of mice after LVS infection. However, in contrast to their resistance to cutaneous infection, IL-10−/− mice showed significantly increased susceptibility to pulmonary F. tularensis infection, succumbing to doses of LVS that were sublethal for IL-10+/+ mice (Fig. 6). This finding confirms our previous results (16) and indicates that while IL-10 is harmful for survival against cutaneous Francisella infection, it appears to be required for protection from i.n. challenge.
Fig 6.
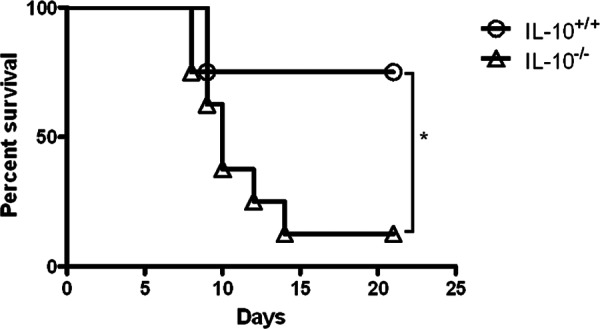
Susceptibility of IL-10+/+ and IL-10−/− mice to pulmonary LVS infection. C57BL/6 IL-10+/+ and IL-10−/− mice were inoculated i.n. with 1.6 × 103 CFU of LVS/mouse, and survival was monitored daily (eight mice/group). The results are representative of two independent experiments. *, P < 0.05.
Absence of IL-10 fails to influence SchuS4 infection.
Given the high level of virulence of F. tularensis SchuS4, it was of interest to determine whether IL-10−/− mice would be similarly resistant to this bacterial strain. However, regardless of the route of challenge, all SchuS4-infected IL-10+/+ and IL-10−/− mice succumbed to infection with nearly identical survival kinetics (Fig. 7).
Fig 7.
Susceptibility of IL-10+/+ and IL-10−/− mice to cutaneous and pulmonary F. tularensis SchuS4 infection. C57BL/6 IL-10+/+ and IL-10−/− mice were inoculated i.d. (left panel) or i.n. (right panel) with 64 CFU of SchuS4/mouse, and survival was monitored daily (eight mice/group).
DISCUSSION
In the present study, we have shown that IL-10 has a significant impact on infection with F. tularensis LVS. For cutaneous tularemia, IL-10 appears to be detrimental and inhibits a potentially protective IL-17 immune response, whereas IL-10 reduces susceptibility to lung infection. These findings indicate that neutralization of IL-10 would be useful for enhancing bacterial clearance from cutaneous sites but could exacerbate pulmonary infection.
A major influence of IL-10 appears to be in the suppression of IL-17 expression since the absence of IL-10 did not influence the survival of mice treated with neutralizing anti-IL-17 MAb. Furthermore, the protection seen after neutralization of IL-10 with anti-IL-10R MAb was not observed in IL-17R−/− mice. We have previously reported that the IL-17 pathway is protective against i.d. infection (9), and others (8, 10, 11) have observed that IL-17 plays a protective role following i.n. LVS infection of mice, findings that have been confirmed in our laboratory using IL-17R−/− mice (unpublished observations). One group (10) reported that mice lacking a particular member of the IL-17 family, IL-17A−/− mice, were more susceptible to i.n. infection with LVS compared to i.d. infection; nevertheless, enhanced bacterial burden was still seen at low i.d. challenge doses and decreased survival was found at high doses, similar to what has been observed in the present study with IL-17R−/− mice. It is possible that other IL-17 family members that bind the IL-17R, such as IL-17F, are more important for protection against cutaneous versus pulmonary tularemia. It should be noted that type 1 IFN can also restrain IL-17 expression, thus explaining the resistance of IFN-αβR−/− mice to F. novicida and LVS infection (9, 16). However, IFN-αβR−/− mice are more resistant to both subcutaneous and pulmonary Francisella infection, whereas IL-10−/− mice were found in the present study to have enhanced resistance to only cutaneous LVS infection and, in fact, showed heightened susceptibility to pulmonary LVS infection compared to IL-10+/+ mice. Thus, it is likely that IL-10 has a different regulatory activity in the lung than type I IFN. Others (25) have reported that i.n. treatment of wild-type mice with IL-17A has a limited and yet consistent protective effect against pulmonary LVS infection; it will be of interest in future studies to determine whether this protective effect might be magnified in IL-10−/− mice.
A cardinal feature of F. tularensis infection is the apparent lack of a protective immune response during the initial 48 to 72 h after infection. Some studies have suggested that this effect is due to lack of a stimulatory lipopolysaccharide (LPS) on F. tularensis (26, 27), while others suggest active suppression of immunity through preferential induction of anti-inflammatory cytokine pathways (9, 15, 24, 28). TGF-β has been reported to inhibit early immunity to F. tularensis pulmonary infection (15). Although TGF-β is produced in the lung following infection (24), in vivo neutralization of TGF-β leads to minimal effects on SchuS4 bacterial load (15) or animal survival (S. Roberts and D. W. Metzger, unpublished findings). On the other hand, the results presented here indicate that IL-10 is a critical immune regulator during F. tularensis LVS infection. The effects of IL-10 depend upon the site of infection and timing is also apparently critical. Although IL-10 transcripts were increased systemically as early as 1 day after cutaneous LVS infection, protein levels were increased only after 3 days. Similarly, in the absence of IL-10, the expression of IL-17 was increased only 3 days after bacterial challenge. Thus, suppression of the IL-17 pathway by IL-10 appears to be a later event during infection and likely regulates ultimate clearance of bacteria, rather than preventing initial immune responsiveness.
It was particularly surprising in the present study that IL-10−/− mice were more susceptible to pulmonary LVS infection, given that in other experimental models IL-10−/− mice have actually shown increased resistance to pulmonary pathogens such as influenza virus (17, 18) and mycobacteria (19, 20), as well as to systemic listeria infection (29, 30). Especially in the influenza virus infection model, IL-10 can be detrimental during the early stages of infection by suppressing development of protective adaptive immunity. Influenza virus-infected IL-10−/− mice are better able to clear virus from the lungs due to the accelerated development of humoral and T cell-mediated immunity in the lungs (17, 18). However, if IL-10 is neutralized specifically in the latter stages of viral infection, the ensuing high levels of inflammation can lead to decreased survival (31). Control of inflammation may be the predominant role for IL-10 in pulmonary tularemia as previously suggested (24), but enhanced induction of protective immunity in IL-10−/− mice seems to be beneficial for cutaneous infection. The ultimate effects of IL-10 on morbidity and mortality during tularemia are clearly unique from those reported for other pathogens.
In summary, we have demonstrated that IL-10 is a critical and novel regulator of immunity to F. tularensis LVS infection, although its effects are masked during infection with the highly virulent SchuS4 strain. Although IL-10 suppresses an IL-17 response that can be protective for cutaneous infection, it is likely that in the lung, partial control of pulmonary IL-17 by IL-10 leads to improved resistance. Taken together, it appears that the host can tolerate the lack of an IL-10 anti-inflammatory response to Francisella in the skin, but this response cannot be tolerated in the pulmonary tract. Further studies are in progress to investigate the potential prophylactic and therapeutic benefits of manipulating the IL-10 pathway during Francisella infection. In addition, it will be important to examine in detail whether manipulation of IL-10 expression has any ultimate impact on infection with the virulent F. tularensis SchuS4 strain.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grant PO1 AI056320.
The authors declare that they have no financial conflicts related to this study.
Footnotes
Published ahead of print 25 March 2013
REFERENCES
- 1. Sjostedt A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105:1–29 [DOI] [PubMed] [Google Scholar]
- 2. Federal Register 2012. Possession, use, and transfer of select agents and toxins; biennial review; final rule. Fed. Regist. 77:61083–61115 [PubMed] [Google Scholar]
- 3. Conlan JW. 2011. Francisella tularensis: a red-blooded pathogen. J. Infect. Dis. 204:6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sjostedt A, North RJ, Conlan JW. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142:1369–1374 [DOI] [PubMed] [Google Scholar]
- 5. Rhinehart-Jones TR, Fortier AH, Elkins KL. 1994. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host γ-interferon and T cells. Infect. Immun. 62:3129–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duckett NS, Olmos S, Durrant DM, Metzger DW. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 73:2306–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elkins KL, Cooper A, Colombini SM, Cowley SC, Kieffer TL. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. 2009. Interleukin-17 Is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henry T, Kirimanjeswara GS, Ruby T, Jones JW, Peng K, Perret M, Ho L, Sauer JD, Iwakura Y, Metzger DW, Monack DM. 2010. Type I IFN signaling constrains IL-17A/F secretion by gd T cells during bacterial infections. J. Immunol. 184:3755–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cowley SC, Meierovics AI, Frelinger JA, Iwakura Y, Elkins KL. 2010. Lung CD4− CD8− double-negative T cells are prominent producers of IL-17A and IFN-gamma during primary respiratory murine infection with Francisella tularensis live vaccine strain. J. Immunol. 184:5791–5801 [DOI] [PubMed] [Google Scholar]
- 11. Shen H, Harris G, Chen W, Sjostedt A, Ryden P, Conlan JW. 2010. Molecular immune responses to aerosol challenge with Francisella tularensis in mice inoculated with live vaccine candidates of varying efficacy. PLoS One 5:e13349 doi:10.1371/journal.pone.0013349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cowley SC, Elkins KL. 2011. Immunity to Francisella. Front. Microbiol. 2:26 doi:10.3389/fmicb.2011.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bosio C. 2011. The subversion of the immune system by Francisella tularensis. Front. Microbiol. 2:9 doi:10.3389/fmicb.2011.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones CL, Napier BA, Sampson TR, Llewellyn AC, Schroeder MR, Weiss DS. 2012. Subversion of host recognition and defense systems by Francisella spp. Microbiol. Mol. Biol. Rev. 76:383–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bosio CM, Bielefeldt-Ohmann H, Belisle JT. 2007. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178:4538–4547 [DOI] [PubMed] [Google Scholar]
- 16. Metzger DW, Bakshi CS, Kirimanjeswara G. 2007. Mucosal immunopathogenesis of Francisella tularensis. Ann. N. Y. Acad. Sci. 1105:266–283 [DOI] [PubMed] [Google Scholar]
- 17. Sun K, Torres L, Metzger DW. 2010. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J. Virol. 84:5007–5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. 2009. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J. Immunol. 182:7353–7363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beamer GL, Flaherty DK, Assogba BD, Stromberg P, Gonzalez-Juarrero M, De Waal Malefyt R, Vesosky B, Turner J. 2008. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J. Immunol. 181:5545–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft GJ, O'Garra A. 2010. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur. J. Immunol. 40:2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Durrant DM, Metzger DW. 2010. IL-12 can alleviate Th17-mediated allergic lung inflammation through induction of pulmonary IL-10 expression. Mucosal Immunol. 3:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu Y, Yang J, Ouyang X, Liu W, Li H, Yang J, Bromberg J, Chen SH, Mayer L, Unkeless J, Xiong H. 2008. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur. J. Immunol. 38:1807–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woolard MD, Hensley LL, Kawula TH, Frelinger JA. 2008. Respiratory Francisella tularensis live vaccine strain infection induces Th17 Cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect. Immun. 76:2651–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Periasamy S, Singh A, Sahay B, Rahman T, Feustel PJ, Pham GH, Gosselin EJ, Sellati TJ. 2011. Development of tolerogenic dendritic cells and regulatory T cells favors exponential bacterial growth and survival during early respiratory tularemia. J. Leukoc. Biol. 90:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Markel G, Bar-Haim E, Zahavy E, Cohen H, Cohen O, Shafferman A, Velan B. 2010. The involvement of IL-17A in the murine response to sublethal inhalational infection with Francisella tularensis. PLoS One 5:e11176 doi:10.1371/journal.pone.0011176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duenas AI, Aceves M, Orduna A, Diaz R, Sanchez CM, Garcia-Rodriguez C. 2006. Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than Escherichia coli LPS. Int. Immunol. 18:785–795 [DOI] [PubMed] [Google Scholar]
- 27. Phillips NJ, Schilling B, McLendon MK, Apicella MA, Gibson BW. 2004. Novel modification of lipid A of Francisella tularensis. Infect. Immun. 72:5340–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woolard MD, Wilson JE, Hensley LL, Jania LA, Kawula TH, Drake JR, Frelinger JA. 2007. Francisella tularensis-infected macrophages release prostaglandin E2 that blocks T cell proliferation and promotes a Th2-like response. J. Immunol. 178:2065–2074 [DOI] [PubMed] [Google Scholar]
- 29. Dai WJ, Köhler G, Brombacher F. 1997. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J. Immunol. 158:2259–2267 [PubMed] [Google Scholar]
- 30. Kelly JP, Bancroft GJ. 1996. Administration of interleukin-10 abolishes innate resistance to Listeria monocytogenes. Eur. J. Immunol. 26:356–364 [DOI] [PubMed] [Google Scholar]
- 31. Sun J, Madan R, Karp CL, Braciale TJ. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 15:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]


