Abstract
The enzymes LinBUT and LinBMI (LinB from Sphingobium japonicum UT26 and Sphingobium sp. MI1205, respectively) catalyze the hydrolytic dechlorination of β-hexachlorocyclohexane (β-HCH) and yield different products, 2,3,4,5,6-pentachlorocyclohexanol (PCHL) and 2,3,5,6-tetrachlorocyclohexane-1,4-diol (TCDL), respectively, despite their 98% identity in amino acid sequence. To reveal the structural basis of their different enzymatic properties, we performed site-directed mutagenesis and X-ray crystallographic studies of LinBMI and its seven point mutants. The mutation analysis revealed that the seven amino acid residues uniquely found in LinBMI were categorized into three groups based on the efficiency of the first-step (from β-HCH to PCHL) and second-step (from PCHL to TCDL) conversions. Crystal structure analyses of wild-type LinBMI and its seven point mutants indicated how each mutated residue contributed to the first- and second-step conversions by LinBMI. The dynamics simulation analyses of wild-type LinBMI and LinBUT revealed that the entrance of the substrate access tunnel of LinBUT was more flexible than that of LinBMI, which could lead to the different efficiencies of dehalogenation activity between these dehalogenases.
INTRODUCTION
Hexachlorocyclohexane (HCH) is a six-chlorine-substituted cyclohexane. One of its isomers, the γ isomer, has insecticidal properties and has been widely used as an insecticide around the world (1). Although the use of γ-HCH has been prohibited in most countries due to its toxicity and long persistence, the large-scale production, widespread use, and dumping of the other noninsecticidal isomers (α-, β-, and δ-HCHs) in past decades still continue to create problems with HCH contamination in soil and groundwater (2). β-HCH in particular is a persistent and problematic isomer of HCH.
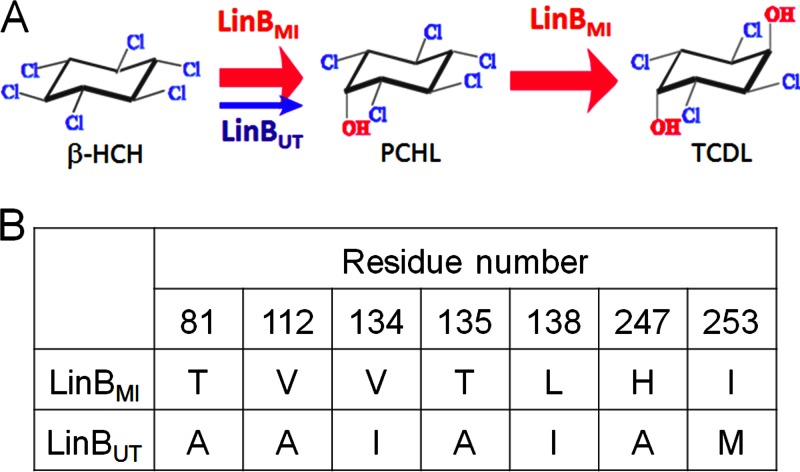
Several β-HCH-degrading bacteria whose β-HCH-degrading enzymes can be utilized for bioremediation have been identified (3–5). LinBMI and LinBUT are haloalkane dehalogenases isolated from Sphingobium sp. MI1205 and Sphingobium japonicum UT26, respectively, that can cleave the carbon-halogen bond in β-HCH. Haloalkane dehalogenases belong to the α/β-hydrolase family, and their catalytic mechanism consists of the following steps: (i) substrate binding, (ii) cleavage of the carbon-halogen bond in the substrate and formation of an intermediate covalently bound to the nucleophile, (iii) hydrolysis of the alkyl-enzyme intermediate, and (iv) release of halide ion and alcohol (6). LinBMI and LinBUT share 98% sequence identity, with only 7 different amino acid residues (at positions 81, 112, 134, 135, 138, 247, and 253) out of 296 residues, but these enzymes exhibit different enzymatic properties (Fig. 1). LinBMI catalyzes the two-step dehalogenation and converts β-HCH to 2,3,4,5,6-pentachlorocyclohexanol (PCHL) and further to 2,3,5,6-tetrachlorocyclohexane-1,4-diol (TCDL) (7) in the manner of LinB2 from Sphingomonas sp. BHC-A (8) and LinB from Sphingobium indicum B90A (9), whereas LinBUT catalyzes only the first-step dehalogenation of β-HCH to PCHL (10) and cannot degrade PCHL further. Moreover, LinBMI can catalyze the first-step conversion eight times as efficiently as LinBUT (7).
Fig 1.
Different enzymatic properties between LinBMI and LinBUT. (A) β-HCH degradation reactions catalyzed by LinBMI and LinBUT. LinBMI converts β-HCH to PCHL and further to TCDL, while LinBUT catalyzes only the first-step conversion of β-HCH to PCHL. The activity of LinBMI is approximately eight times as high as that of LinBUT in the first-step dehalogenation of β-HCH to PCHL (7). (B) The seven amino acid residues that are different between LinBMI and LinBUT.
In a previous site-directed mutagenesis study, the V134I, H247A, and V134I H247A mutants of LinBMI, in which one or two LinBMI-specific residues were mutated to a LinBUT-type residue(s), showed reduced activities in both the first- and second-step dehalogenations, with the exception that there was no reduction in the first-step dehalogenation activity of the H247A mutant (7). However, the activities of these mutants were still higher than that of LinBUT in both the first- and second-step dehalogenations, which suggested that one or more of the other five residues (T81, V112, T135, L138, and I253) uniquely found in LinBMI were also important for the high dehalogenation activity of LinBMI. To date, the crystal structure of LinBUT has been described (11–14), whereas the crystal structure of LinBMI has not. To investigate how the seven residues that are different between LinBMI and LinBUT contribute to their different enzymatic properties, we performed site-directed mutagenesis and X-ray crystallographic studies of LinBMI and its seven point mutants, where each LinBMI-specific residue is mutated to the LinBUT-type residue (T81A, V112A, V134I, T135A, L138I, H247A, and I253M). Activity measurements were made for all the mutants except for those carrying the V134I and H247A mutations, whose measurements were reported previously (7).
MATERIALS AND METHODS
Expression, purification, and crystallization.
The expression plasmids of wild-type LinBMI and the seven mutants (carrying T81A, V112A, V134I, T135A, L138I, H247A, and I253M) were constructed using the vector pAQNM, where the target proteins were expressed under the control of the tac promoter and lacIq (7). Wild-type LinBMI and the seven mutants were expressed and purified by the following procedures. Escherichia coli strain BL21(DE3) cells (Novagen) were cultured in Luria-Bertani (LB) medium containing 50 μg ml−1 ampicillin until an optical density at 600 nm (OD600) of 0.6 at 37°C. Protein expression was induced by adding isopropyl β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM, and the culture was continued at 25°C for 12 h. The cells were harvested by centrifugation at 4,500 × g at 4°C for 10 min. The harvested cells were suspended in Sol A (50 mM Tris-HCl [pH 7.5], 400 mM NaCl, and 5 mM imidazole) and disrupted by sonication. After centrifugation at 40,000 × g for 30 min at 4°C, the supernatant was loaded onto a 3-ml Ni Sepharose 6 Fast Flow column (GE Healthcare) at room temperature. After a wash step with Sol B (50 mM Tris-HCl [pH 7.5], 400 mM NaCl, and 50 mM imidazole), the protein was eluted with Sol C (50 mM Tris-HCl [pH 7.5], 400 mM NaCl, and 200 mM imidazole). The purified protein was dialyzed against 20 mM Tris-HCl (pH 8.0) and then concentrated to 25 mg ml−1 using a Vivaspin 20 concentrator (Sartorius) at 4°C.
Initial crystallization trials of LinBMI were performed by the sitting-drop vapor diffusion method in 96-well Intelli-Plate plates (Art Robbins Instruments) using Crystal Screen HT, Index HT (Hampton Research), and Wizard I and II (Emerald Biosystems) sparse-matrix screening kits. Each drop was prepared by mixing equal volumes (0.7 μl) of the protein solution and a reservoir solution and equilibrated against 70 μl of the reservoir solution at 4°C or 20°C. Further crystallization trials were carried out based on the crystallization conditions of the untagged (100 mM Tris-HCl [pH 8.8 to 9.0], 200 mM CaCl2, and 17 to 19% [wt/vol] polyethylene glycol [PEG] 6000) and His-tagged (100 mM Tris-HCl [pH 8.5], 200 mM MgCl2. and 20% [wt/vol] PEG 4000) LinBUT by the sitting-drop vapor diffusion method in 24-well plates (Hampton Research) (14, 15). The crystallization drops were prepared by mixing 1.0 μl protein solution and 1.0 μl reservoir solution and were equilibrated against 0.3 ml reservoir solution.
Data collection and processing.
The crystals of wild-type LinBMI and the seven mutants were transferred to the reservoir solution containing 25% (vol/vol) glycerol as the cryoprotectant. The X-ray diffraction data were collected at a wavelength of 1.0000 Å in a cryogenic nitrogen gas stream at beamlines BL-5A and AR-NW12A of the Photon Factory (Ibaraki, Japan). The data sets were obtained by collecting 360 frames, with an oscillation step of 0.5°. The diffraction data were indexed, integrated, and scaled using the HKL-2000 software package (16).
Structure modeling and refinement.
The crystal structure of wild-type LinBMI was determined by the molecular replacement method using the software program MOLREP (17) and the crystal structure of LinBUT (PDB code 1CV2) (11) as the initial model. Refinements were performed using the Coot (18) and Refmac5 (19) programs. Water molecules were added using ARP/wARP software (20). Then, the crystal structures of the seven mutants were solved by molecular replacement using the wild-type structure of LinBMI as the initial model. The stereochemical quality of each final model was assessed using the Ramachandran plots obtained by the RAMPAGE software program (21).
Molecular dynamics simulation.
The atomic coordinates of the crystal structures of wild-type LinBMI (PDB code 4H77), solved in this study, and LinBUT (PDB code 1CV2) (11) were used as the initial models. The following dynamics simulations were performed using the software program MOE2011.10 with the default parameter settings unless otherwise stated. The missing hydrogen atoms of wild-type LinBMI and LinBUT were generated and energy minimized using the MMFF94x (Merck molecular force field 94x) force field with distance-dependent dielectric electrostatics. Then, a few potassium ions for neutralization and explicit water molecules were added within a sphere of 10 Å from the protein surfaces. The resulting protein and solvent molecules in the spherical droplet were energy minimized using the MMFF94x force field with R-field electrostatics. Tether weight was applied to all nonhydrogen atoms during the energy minimization steps. The molecular-dynamics simulations were performed using the NVT ensemble and the Nosé-Poincaré-Anderson (NPA) algorithm at 303 K with a time step of 1 fs and without any bond constraint. As for the first 100-ps dynamics, the tether weight was applied to all nonhydrogen atoms and gradually reduced. After the first 100-ps dynamics, the dynamics simulations were performed for 14 ns without any positional restraint. The atomic coordinates were recorded every 1 ps after the first 100-ps dynamics and used for trajectory analysis.
Ligand-docking simulation.
The ligand-docking simulations were performed using the ASEDock software program, a docking program based on a shape similarity assessment between a concave portion on a protein and a ligand, in the Molecular Operating Environment (MOE) software package (Chemical Computing Group, Montreal, Canada). The three-dimensional structures of β-HCH and PCHL were modeled using the Molecule Builder in MOE. The initial models were energy minimized, employing the MMFF94x force field. The active site of the LinB structure was detected using the Alpha Site Finder in MOE. For each ligand, 250 conformations were generated using the default LowModeMD search parameters. The scoring function used by ASEDock was based on the protein-ligand interaction energies. The interaction energy (Udock) of a given conformation was calculated as the sum of Uele (electric energy), Uvdw (van der Waals energy), and Ustrain (difference of the minimal energies between the docked ligand and the ligand which was located nearest the docked ligand).
Enzymatic assays.
For enzymatic assays, E. coli BL21 Star(DE3) cells (Invitrogen) expressing LinB and its mutants were disrupted by bacteriolysis using a CelLytic B reagent (Sigma), and His-tagged enzymes were purified by using BD Talon metal affinity resins (BD Biosciences). The purified enzymes were incubated with 17 μM β-HCH in 50 mM potassium phosphate buffer (pH 7.5) containing 10% (vol/vol) glycerol at 30°C. The enzyme concentration in the reaction mixture was 150 μg/ml. The mixture (100 μl) was extracted with an equal volume of ethyl acetate and then analyzed using a Shimadzu GC-17A gas chromatograph with an 63Ni electron capture detector (ECD) and Rtx-1 capillary column (30 m by 0.25 μm by 0.25 μm; Restek). The column temperature was increased from 160°C to 200°C at a rate of 4°C/min for the separation of the peak of PCHL from that of TCDL and then from 200°C to 260°C at a rate of 20°C/min. The gas flow rate was 30 ml/min. As the internal standard, 10 μM 2,4,5-trichlorophenol was used. Kinetic data were fitted to the irreversible two-step reaction structure of HCH conversion to TCDL via PCHL (Scheme 1) by using the GEPASI 3.2 software program (22). The specificity constants and their standard errors for both reaction steps (k1 and k2) were obtained from the calculation. Evolutionary programming (23) was used to optimize the kinetic constants during the fitting of the kinetic data to Scheme 1. Values given are the means of triplicates. Due to the low solubility (17 μM) of β-HCH in water, the kcat and Km values of these mutants could not be calculated.
Scheme 1.

Protein structure accession numbers.
The atomic coordinates and structure factors (PDB codes 4H77, 4H7D, 4H7E, 4H7F, 4H7H, 4H7I, 4H7J and 4H7K) have been deposited in the Protein Data Bank.
RESULTS AND DISCUSSION
Site-directed mutagenesis.
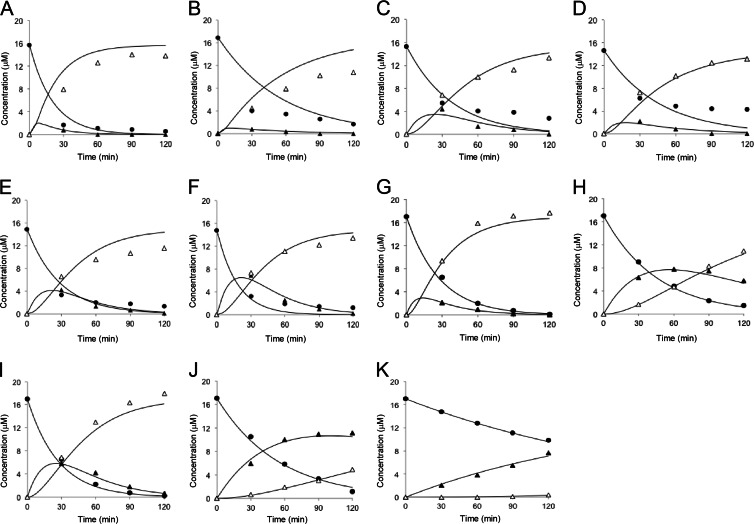
The wild-type LinBMI enzyme used in reference 7 and this study gave comparable data with the same research group, as shown in Fig. 2A and G. We examined the dehalogenation activities of the point mutants of LinBMI, in which each of the five residues (T81, V112, T135, L138, and I253) was mutated to the corresponding residue in LinBUT (Table 1 and Fig. 2B to F). The V112A (Fig. 2C), T135A (Fig. 2D), and L138I (Fig. 2E) mutants showed reduced activities in both the first- and second-step dehalogenations. The I253M (Fig. 2F) mutant retained full activity in the first-step dehalogenation but showed reduced activity in the second-step dehalogenation as in the case of the H247A (Fig. 2I) mutant (7). On the other hand, the T81A (Fig. 2B) mutant showed reduced activity in the first-step dehalogenation but retained full activity in the second-step dehalogenation. Our mutational data combined with the previous data reported by Ito et al. (7) revealed that one (T81), two (H247 and I253), and four (V112, V134, T135, and L138) of the seven different residues between LinBMI and LinBUT contributed to their different efficiencies in the first step, the second step, and both steps of dehalogenation, respectively.
Fig 2.
Degradation of β-HCH (black circles) and appearance of its metabolites, PCHL (black triangle) and TCDL (white triangle), in reaction mixtures containing LinBMI wild type (A), LinBMI T81A (B), LinBMI V112A (C), LinBMI T135A (D), LinBMI L138I (E), LinBMI I253M (F), LinBMI wild type (G), LinBMI V134I (H), LinBMI H247A (I), LinBMI V134I/H247A (J), or LinBUT wild type (K). The same data (G to K) used in reference 7 are also shown in this study. The activity data (A to F) of this study were obtained by the same research group as for reference 7 under the same reaction conditions except for the concentration of purified enzyme (100 and 150 μg/ml in the work described in reference 7 and this study, respectively).
Table 1.
Specificity constants of wild-type LinBMI and its mutants
| Enzyme | Specificity constant, kcat/Km (mM−1 s−1) |
|
|---|---|---|
| HCH → PCHL | PCHL → TCDL | |
| LinBMI wild type | 0.19 ± 0.008 | 1.0 ± 0.3 |
| LinBMI T81A | 0.070 ± 0.003 | 0.95 ± 0.5 |
| LinBMI V112A | 0.10 ± 0.009 | 0.23 ± 0.04 |
| LinBMI T135A | 0.080 ± 0.005 | 0.42 ± 0.1 |
| LinBMI L138I | 0.13 ± 0.01 | 0.22 ± 0.04 |
| LinBMI I253 M | 0.21 ± 0.03 | 0.14 ± 0.02 |
| LinBMI wild typea | 0.205 ± 0.005 | 0.716 ± 0.052 |
| LinBMI V134Ia | 0.124 ± 0.005 | 0.080 ± 0.003 |
| LinBMI H247Aa | 0.210 ± 0.015 | 0.240 ± 0.021 |
| LinBMI V134I H247Aa | 0.104 ± 0.003 | 0.027 ± 0.001 |
| LinBUTb | 0.0271 ± 0.0002 | 0.0036 ± 0.0006 |
The same data used in reference 7 are shown.
LinBUT is identical to LinBMI T81A V112A V134I T135A L138I H247A I253M.
Crystallization and data collection.
We obtained LinBMI crystals by combining the reported crystallization conditions for untagged and His-tagged LinBUT (14, 15). The best crystals, with typical dimensions of 0.2 by 0.4 by 0.01 mm, were obtained by mixing 1.0 μl of the protein solution (25 mg ml−1) and 1.0 μl of the reservoir solution (100 mM Tris-HCl (pH 8.0), 20% (wt/vol) PEG 4000, and 200 mM CaCl2) at 5°C. Similarly, the crystals of the seven mutants of LinBMI were obtained by mixing 1.0 μl of the protein solution (25 mg ml−1) and 1.0 μl of the reservoir solution (100 mM Tris-HCl (pH 7.8 to 8.1), 17 to 20% (wt/vol) PEG 4000, and 200 mM CaCl2) at 5°C.
The crystal of wild-type LinBMI belonged to the space group P21212 with the following unit cell dimensions: a = 50.4 Å, b = 72.1 Å, and c = 73.5 Å. It contained one LinBMI molecule per asymmetric unit. The Matthews coefficient (24) and the solvent content were 1.96 Å3 Da−1 and 37%, respectively. The crystals of the seven mutants had the same space group, P21212, with unit cell dimensions similar to those of the crystal of wild-type LinBMI. The diffraction data statistics for these crystals are given in Table 2.
Table 2.
Data collection and refinement statistics for wild-type LinBMI and the seven mutants
| Statistic | Value for LinBMI with mutation (PDB code) |
|||||||
|---|---|---|---|---|---|---|---|---|
| None (4H77) | T81A (4H7D) | V112A (4H7E) | V134I (4H7F) | T135A (4H7H) | L138I (4H7I) | H247A (4H7J) | I253M (4H7K) | |
| Diffraction data collection | ||||||||
| Beamline | Photon Factory AR-NW12A | Photon Factory BL-5A | Photon Factory BL-5A | Photon Factory BL-5A | Photon Factory AR-NW12A | Photon Factory BL-5A | Photon Factory BL-5A | Photon Factory AR-NW12A |
| Detector | ADSC Quantum 210 | ADSC Quantum 210 | ADSC Quantum 210 | ADSC Quantum 315r | ADSC Quantum 210 | ADSC Quantum 315r | ADSC Quantum 210 | ADSC Quantum 210 |
| Wavelength (Å) | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Space group | P21212 | P21212 | P21212 | P21212 | P21212 | P21212 | P21212 | P21212 |
| Unit-cell parameters (Å) | a = 50.4 | a = 50.4 | a = 50.4 | a = 50.5 | a = 50.4 | a = 50.4 | a = 50.4 | a = 50.5 |
| b = 72.1 | b = 72.1 | b = 72.2 | b = 72.3 | b = 71.7 | b = 72.3 | b = 72.2 | b = 72.2 | |
| c = 73.5 | c = 73.5 | c = 73.9 | c = 73.6 | c = 73.1 | c = 73.9 | c = 73.2 | c = 73.6 | |
| Resolution (Å)a | 20–1.60 (1.66–1.60) | 20–1.95 (1.98–1.95) | 20–1.80 (1.86–1.80) | 20–1.80 (1.86–1.80) | 20–2.10 (2.14–2.10) | 20–1.80 (1.86–1.80) | 20–1.80 (1.83–1.80) | 20–1.75 (1.78–1.75) |
| No. of measurements | 258,109 | 125,465 | 174,986 | 165,876 | 92,137 | 176,684 | 167,404 | 190,853 |
| No. of unique reflections | 36,060 | 19,954 | 25,428 | 25,649 | 16,096 | 25,640 | 24,924 | 27,832 |
| Completeness (%)a | 99.9 (99.9) | 99.2 (91.7) | 100.0 (100.0) | 99.8 (98.7) | 99.9 (99.6) | 99.8 (98.2) | 97.3 (97.7) | 99.8 (98.2) |
| Rsyma,b | 0.068 (0.314) | 0.087 (0.279) | 0.101 (0.339) | 0.084 (0.283) | 0.110 (0.397) | 0.075 (0.270) | 0.071 (0.194) | 0.093 (0.367) |
| <I>/<σ (I)>a | 32.0 (5.3) | 37.7 (9.3) | 31.1 (6.1) | 39.0 (7.9) | 23.7 (4.6) | 30.5 (5.6) | 41.7 (9.4) | 37.3 (5.3) |
| Refinement | ||||||||
| Resolution range (Å) | 20–1.60 | 20–1.95 | 20–1.80 | 20–1.80 | 20–2.10 | 20–1.80 | 20–1.80 | 20–1.75 |
| Rworkc (%) | 15.9 | 17.7 | 17.4 | 17.7 | 18.6 | 17.2 | 17.5 | 17.2 |
| Rfree d (%) | 19.1 | 21.0 | 19.8 | 20.0 | 25.0 | 19.3 | 19.6 | 20.5 |
| RMSD | ||||||||
| Bonds (Å) | 0.010 | 0.008 | 0.007 | 0.007 | 0.009 | 0.007 | 0.006 | 0.007 |
| Angles (°) | 1.371 | 1.310 | 1.160 | 1.253 | 1.371 | 1.230 | 1.220 | 1.298 |
| Ramachandran plot | ||||||||
| Favored region (%) | 96.6 | 96.9 | 96.9 | 96.9 | 96.2 | 96.6 | 96.9 | 96.2 |
| Allowed region (%) | 3.4 | 3.1 | 3.1 | 3.1 | 3.8 | 3.4 | 3.1 | 3.8 |
| Outlier region (%) | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Values in parentheses are for the highest-resolution shell.
Rsym = Σhkl Σi |Ii(hkl) − <I(hkl)>|/Σhkl Σi Ii(hkl), where <I(hkl)> is the average intensity of symmetry relation reflections.
Rwork = Σhkl||Fobs| − |Fcal||/Σhkl|Fobs|.
Rfree was calculated by using the 5% of reflections excluded in the refinement.
Overall structures of the wild type and seven mutants of LinBMI.
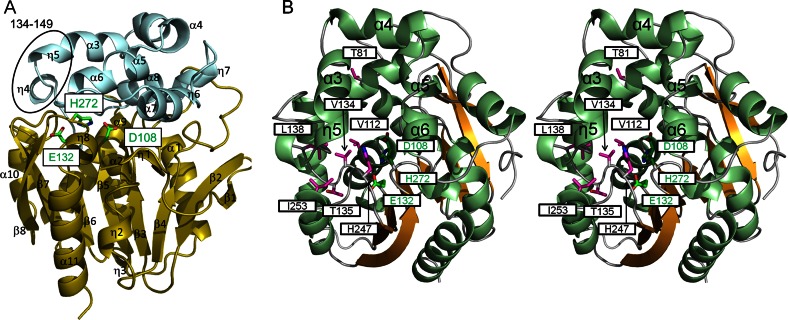
We have solved the crystal structures of wild-type LinBMI at a 1.60-Å resolution and of the seven mutants at 1.75- to 2.10-Å resolutions by molecular replacement. The LinBMI molecule existed as a monomer in the crystal and consisted of two domains, the core domain and the cap domain (Fig. 3A). The core domain (residues 2 to 132 and 214 to 295) had a typical α/β-hydrolase fold, as seen in other haloalkane dehalogenases (25–29). Unlike the core domain, the cap domain varied in the number and orientations of helices among haloalkane dehalogenases, and the cap domain (residues 133 to 213) of LinBMI was composed of four 310 and six α-helices. The crystal structures of the wild type and the seven mutants of LinBMI were very similar to one another, with root mean square deviations (RMSDs) for Cα atoms (residues 2 to 295) of 0.095 to 0.31 Å.
Fig 3.
Structure of wild-type LinBMI. (A) Ribbon diagram of wild-type LinBMI. The core domain (residues 2 to 132 and 214 to 295) and the cap domain (residues 133 to 213) are colored olive and light blue, respectively. The catalytic triad residues are shown as stick models. The most flexible region (residues 134 to 149) in LinBMI is circled in black. (B) Stereo view of wild-type LinBMI. The helices and strands are colored light green and orange, respectively. The catalytic triad residues and the residues which are different from the corresponding residues in LinBUT are shown as stick models and colored green and magenta, respectively.
In LinBMI, D108, H272, and E132 formed the catalytic triad as in LinBUT (Fig. 3A). D108, located on the β5 strand, acted as the nucleophile. The Oδ2 atom of D108 formed a hydrogen bond with the Nε atom of H272, which was located on the loop between the β8 strand and the η8 helix. The Nδ atom of H272 formed a hydrogen bond with the Oε1 atom of E132, which was located on the β6 strand.
The reservoir solution used contained 200 mM CaCl2, and the electron density of one calcium ion was clearly observed between two adjacent LinBMI molecules aligned in the crystal. The calcium ion was coordinated with the Oδ1 and Oδ2 atoms in the side chain of D166 in a LinBMI molecule, the main chain O atoms of P175 and I178 of an adjacent LinBMI molecule, and three water molecules. Thus, the calcium ion plays an important role for the growth of this crystal by mediating the above intermolecular interaction.
The electron density of one chloride ion was observed in the active site, and the chloride ion formed hydrogen bonds with two halide-stabilizing residues, N38 and W109. These hydrogen bonds would reflect the manner of recognition of a chloride ion released from the substrate.
Effects of different residues located near the active site on the specificity constants.
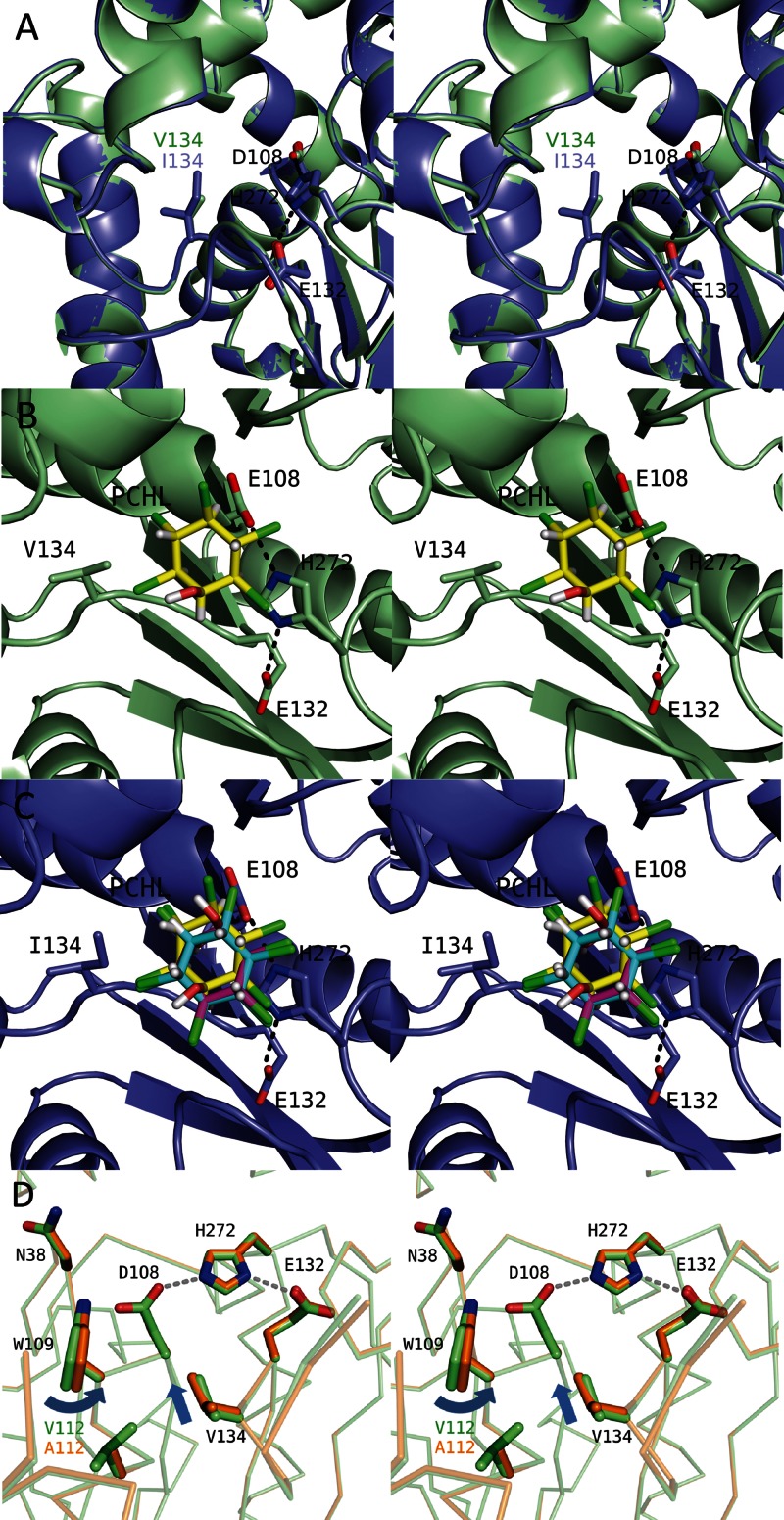
The residue at position 134 was the nearest residue to the nucleophile residue D108 among the seven residues that are different between LinBMI and LinBUT (Fig. 3B) and is likely to bind the substrate directly. The V134I mutant of LinBMI retained 60% of the first-step dehalogenation activity but showed only 11% of the second-step dehalogenation activity compared with those of wild-type LinBMI (7). The superimposition of the crystal structures of wild-type LinBMI and the V134I mutant revealed that the presence or absence of the Cδ atom at position 134 was the only difference around the active site between these two structures (Fig. 4A). To understand the effect of the Cδ atom at position 134, we performed cocrystallization and soaking experiments using β-HCH but could not obtain the crystal structure of LinBMI complexed with β-HCH. Then, we predicted the locations and orientations of β-HCH and PCHL when bound to wild-type LinBMI and the V134I mutant using the ASEDock program of MOE. The docking simulation provided reasonable binding models of β-HCH for both wild-type LinBMI and the V134I mutant. The β-HCH molecules docked in wild-type LinBMI and the V134I mutant were located at the same position with almost the same orientations (data not shown). On the other hand, the docking simulation with PCHL gave different results for wild-type LinBMI and the V134I mutant. In the top three solutions, the interaction energies of the PCHL molecule with wild-type LinBMI were −1.6, 1.8, and 3.4 kcal/mol, and those with the V134I mutant were −14.7, −1.1, and 2.3 kcal/mol. In wild-type LinBMI, the manner of binding of PCHL in the top solution could explain the occurrence of the second-step conversion from PCHL to TCDL, with the distance between the Oδ2 atom of D108 and the C-4 atom of PCHL being 3.1 Å (Fig. 4B). However, in the case of the V134I mutant, the positions and orientations of the bound PCHL models in the top two solutions (Fig. 4C, cyan and magenta) were different from those in the top solution for wild-type LinBMI. The C-4 atoms in the two PCHL models were 4.7 Å away from the Oδ2 atom of D108, and thus the second-step conversion from PCHL to TCDL was unlikely to occur. The binding manner of the third solution (Fig. 4C, yellow) for the V134I mutant was almost the same as that in the top solution for wild-type LinBMI. These docking simulation results suggested that the V134I mutant of LinBMI was not likely to bind PCHL properly for the second-step conversion to occur because of the presence of the Cδ atom at position 134.
Fig 4.
Different amino acid residues located around the active site between LinBMI and LinBUT. (A) Superimposition of the active sites of the wild type (light green) and the V134I mutant (slate) of LinBMI. The catalytic triad residues (D108, E132, and H272) and V134/I134 are labeled. (B and C) Docking simulations of the wild type (B) or the V134I mutant (C) with PCHL. The chlorine, oxygen, and hydrogen atoms of the PCHL molecules are colored green, red, and white, respectively. In wild-type LinBMI, the PCHL model with the lowest binding energy is shown, and its carbon atoms are colored yellow. In the V134I mutant, the carbon atoms are colored cyan, magenta, and yellow in the PCHL models with the lowest binding, the second-lowest binding and the highest interation energies, respectively. (D) Superimposition of the active sites between the wild type (light green) and the V112A mutant (orange).
The residue at position 112 was located at the bottom of the substrate binding pocket (Fig. 3B). The V112A mutant of LinBMI retained 53% of the first-step dehalogenation activity but showed only 23% of the second-step dehalogenation activity of wild-type LinBMI (7). In the V112A mutant, the main chain of V134 was shifted by 0.3 Å toward the catalytic residue (D108) compared with the corresponding region in wild-type LinBMI, and the side chain of W109, one of the two halide-stabilizing residues, was rotated 6° relative to that in wild-type LinBMI around the Cγ-Cδ1 bond (Fig. 4D). Such structural differences at the two residues should be due to the absence of the Cγ2 atom rather than the Cγ1 atom in the V112A mutant of LinBMI. These structural changes within the active-site pocket should cause a reduction in first- and second-step dehalogenation activities in the V112A mutant of LinBMI.
Effects of different residues lining the substrate access tunnel on specificity constants.
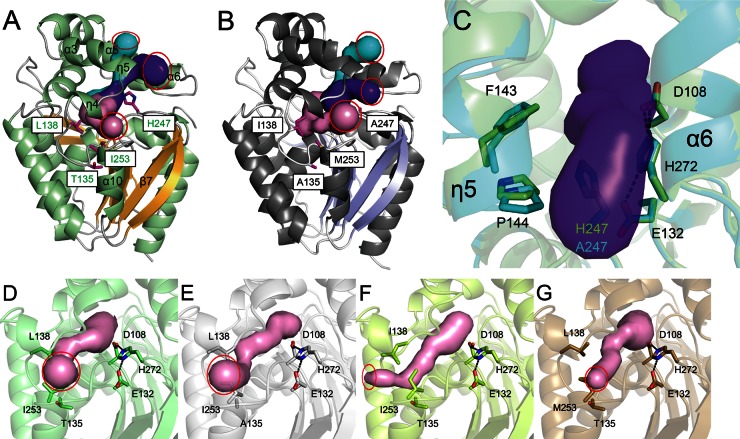
The active site of LinBMI was buried deeply inside the enzyme. Three entrances to the substrate access tunnels were found in LinBMI using the software program CAVER (Fig. 5A). Two tunnel entrances (Fig. 5A, purple and cyan) were formed by the η5, α3, α5, and α6 helices, and the other tunnel entrance (Fig. 5A, pink) was formed by the two helices (η4 and α10) and a loop between the β7 strand and the α10 helix. A tunnel entrance (Fig. 5B, purple) found in LinBUT, which was formed by the α6 helix and two loops between the η4 and η5 helices and between the β7 strand and the α10 helix, was not observed in LinBMI because the side chain of His247 covered the entrance. Ito et al. reported that H247 in LinBMI was important for the second-step conversion of PCHL to TCDL (7). In the H247A mutant structure, the η5 helix was shifted toward the α6 helix because the H247A mutation created an extra space, which resulted in conformational changes of the side chains of F143 and P144 (Fig. 5C). Thus, the side chain of H247 would contribute to the tunnel formation suitable for substrate (PCHL) entry and product (TCDL) release.
Fig 5.
Different amino acid residues lining the access tunnel between LinBMI and LinBUT. (A and B) The three access tunnels (pink, purple, and cyan) to the active site of wild-type LinBMI (A) or LinBUT (B). The catalytic triad residues (green) and amino acid residues (magenta) that are different between wild-type LinBMI and LinBUT are shown as stick models. The red circles represent the entrances of the access tunnels. (C) Superimposition between the wild type (light green) and the H247A mutant (cyan) of LinBMI. The tunnel (purple) observed in wild-type LinBMI is shown. The catalytic triad residues and the residues at positions 135, 138, and 253 are shown as sticks in the wild-type (D), T135A mutant (E), L138I mutant (F), or I253M mutant (G) structure of LinBMI. The red circles show the entrances of the access tunnels of the wild type and three mutants.
L138 and I253 of LinBMI were involved in the formation of one access tunnel (Fig. 5A, pink), while T135 was located approximately 6 Å away from the tunnel. The orientations of the side chain at position 253 were divided into two groups among the wild type and mutants of LinBMI. In wild-type LinBMI and the T81A, V112A, V134I, and H247A mutants, the Cβ-Cγ1-Cδ1 chain of I253 faced toward the side chain of T135 (Fig. 5D). In contrast, in the T135A and L138I mutants, the Cβ-Cγ1-Cδ1 chains of I253 faced toward the side chain of L138 (Fig. 5E and F). Thus, the T135A and L138I mutations caused the conformational changes of the side chain of I253, which resulted in the changes of the size and position of a tunnel entrance (Fig. 5D to F). In the I253M mutant (Fig. 5G), the side chain of M253 faced toward the side chain of L138, and the side chain of L138 was rotated approximately 90° relative to that in wild-type LinBMI along the Cβ-Cγ bond. In contrast, in LinBUT, the side chain of M253 faced toward the side chain of A135 (Fig. 6) (11). Thus, the orientation of the side chain of M253 could be influenced by the residues at position(s) 135 and/or 138. Since the residue at position 253 was located at an entrance of the access tunnel, the irregular orientation of the side chain at position 253 affected the shape of the entrance of the access tunnel in the T135A, L138I, and I253M mutants (Fig. 5E to G). The irregular forms of the tunnel entrances in these mutants should lead to the reductions in the dehalogenase activities, especially the second-step dehalogenation activity.
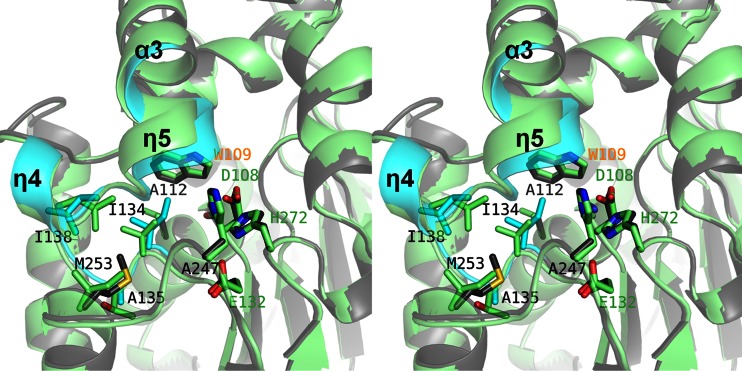
Fig 6.
Structural comparison between wild-type LinBMI and LinBUT. Superimposition between wild-type LinBMI (light green) and LinBUT (cyan and dark gray) is shown. The most noteworthy difference between wild-type LinBMI and LinBUT is colored cyan in LinBUT. The catalytic triad residues, one (W109) of two halide-stabilizing residues, and six of the residues that are different between wild-type LinBMI and LinBMI are shown as stick models and labeled.
In wild-type LinBMI, T81 was positioned outside the active site, and the side chain of T81 formed hydrogen bonds with one water molecule and the amide nitrogen of E84. The main-chain structure of the T81A mutant was very similar to that of wild-type LinBMI, with an RMSD of 0.12 Å, and no conformational change was observed either at the active site or in the access tunnel between wild-type LinBMI and the T81A mutant.
Structural comparison between LinBMI and LinBUT.
LinBMI and LinBUT share 98% sequence identity. Their overall structures were very similar to each other, with an RMSD of 0.27 Å for 292 Cα atoms. The most remarkable structural difference between LinBMI and LinBUT was observed at the N-terminal region of the cap domain (residues 134 to 149) (Fig. 3A and 6), which plays an important role in determining the shape and size of the active site and the substrate access tunnels. A structural difference similar to that between LinBMI and LinBUT was observed between wild-type LinBMI and the H247A mutant (Fig. 5C). The main chain of I134 in LinBUT was shifted by 0.8 Å toward the catalytic residue (D108) compared with that in LinBMI, and the side chain of W109 in LinBUT was rotated approximately 5° relative to that in LinBMI (Fig. 6). These structural differences would be due to the size of the amino acid residue at position 112 (Val in LinBMI versus Ala in LinBUT), considering the structural difference between wild-type LinBMI and the V112A mutant.
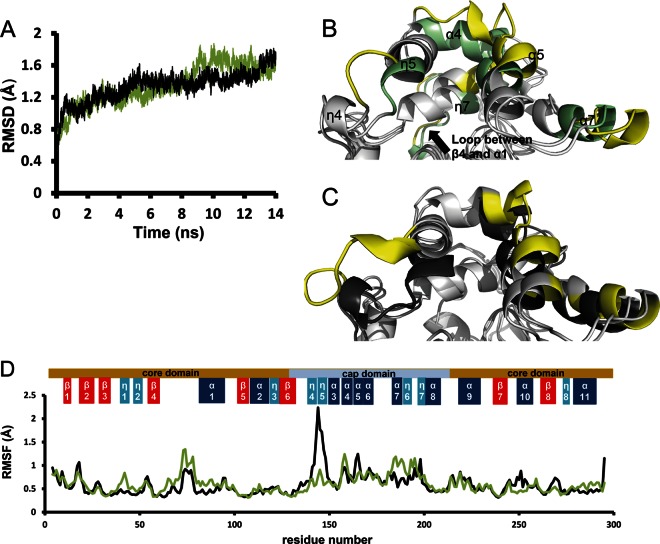
We performed molecular dynamics simulations to reveal the molecular mechanisms of the different efficiencies in dehalogenation between LinBMI and LinBUT. In both the cases of LinBMI and LinBUT, the Cα RMSDs against the initial coordinates increased sharply in the first nanosecond of the simulations, and the RMSDs were in the range of 1.4 to 1.8 Å in the last two nanoseconds (12 to 14 ns) (Fig. 7A), indicating that no global conformational change occurred. Figures 7B and C show the superpositions of the crystal structures and the structures after the simulation of LinBMI and LinBUT, respectively. In the core domain of LinBUT, the crystal and the simulated structures were almost identical. In contrast, in the core domain of LinBMI, a conformational change was observed in a loop (residues 76 to 81) between the β4 strand and the α1 helix, which would be due to T81, the only residue in this region unique to LinBMI. The conformational change in the loop could lead to a movement of the interacting η7 helix in the cap domain toward the α4 helix and a concomitant change in the shape of the substrate binding pocket, which might cause the different efficiencies in first-step dehalogenation activities between two enzymes. As for the cap domain, similar conformational changes were observed in both LinBMI and LinBUT in the following regions: η4-(loop)-η5, α4, α5, and α7. The conformational change at the entrance of the substrate access tunnel from the η4 to η5 helices in LinBUT was larger than that in LinBMI, allowing the substrates to enter the tunnel easily (Fig. 7B and C). The different residues at positions 247 and 253 should cause the different conformational changes in this region. Root mean square fluctuation (RMSF) was used as an index of structural flexibility. The RMSF analysis (Fig. 7D) clearly shows that the entrance of the substrate access tunnel from the η4 to η5 helices (residues 142 to 146) of LinBUT is much more flexible than that of LinBMI. This high flexibility in LinBUT would lead to the large conformational change at the entrance of the substrate access tunnel, as shown in Fig. 7B. In DhaA, a member of the same α/β-hydrolase family as LinB, the molecular dynamics simulation analysis revealed that the narrower substrate access tunnel in a variant than in the wild-type enzyme shielded the active site from the solvent and showed higher activity than that of the wild-type enzyme (30). Similarly, the low flexibility of the tunnel entrance in LinBMI would contribute to the increase in its dehalogenation activity by inhibiting the influx of water molecules into the active site, particularly for second-step dehalogenation activity, where the water molecules can compete with the hydroxyl group of PCHL.
Fig 7.
Molecular dynamics (MD) simulations of wild-type LinBMI and LinBUT. (A) Time course of Cα RMSDs from the initial structures of wild-type LinBMI (green) and LinBUT (black) during MD simulations. (B) Superposition of the crystal structure (gray and green) and the structure after the simulation (gray and yellow) of wild-type LinBMI. (C) Superposition of the crystal structure (gray and black) and the structure after the simulation (gray and yellow) of wild-type LinBUT. Green, black, and yellow in panels B and C indicate the most different regions observed between the crystal structures and the structures after the simulations (gray and yellow). (D) Cα RMSFs for LinBMI (green) and LinBUT (black) residues over the last 2-ns simulations.
Concluding remarks.
We have analyzed the dehalogenation activities of five of the seven amino acid residues that differ between LinBMI and LinBUT. This and previous mutagenesis analyses revealed that most of the seven residues had effects on second-step dehalogenation and none of the seven residues were critical for degradation activity. We have determined the crystal structures of the wild type and the seven mutants of LinBMI. The structural comparisons among wild-type LinBMI, LinBUT, and the seven mutants of LinBMI indicated that each mutant except the T81A mutant caused a small conformational change in the access tunnels or the active site that resulted in a reduction in the first- and second-step dehalogenation activities of LinBUT compared with those of LinBMI. The dynamics simulations of wild-type LinBMI and LinBUT suggested that the flexibility of the entrance of the substrate access tunnel led to the difference in dehalogenation activity, peculiarly the second-step activity.
ACKNOWLEDGMENTS
We thank the beamline staff at the Photon Factory for their kind help with data collection. We thank Zbynek Prokop for assistance in calculating the specificity constants of enzymatic activities. Synchrotron radiation experiments were done at the Photon Factory (Ibaraki, Japan) (proposal no. 2009G122).
This work was supported in part by the Targeted Proteins Research Program and Grants-in-Aid of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published ahead of print 5 April 2013
REFERENCES
- 1. Willett KL, Ulrich EM, Hites RA. 1998. Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environ. Sci. Technol. 32:2197–2207 [Google Scholar]
- 2. Walker K, Vallero DA, Lewis RG. 1999. Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment. Environ. Sci. Technol. 33:4373–4378 [Google Scholar]
- 3. Johri AK, Dua M, Tuteja D, Saxena R, Saxena DM, Lal R. 1998. Degradation of α, β, γ and δ-hexachlorocyclohexanes by Sphingomonas paucimobilis. Biotechnol. Lett. 20:885–887 [Google Scholar]
- 4. Gupta A, Kaushik CP, Kaushik A. 2000. Degradation of hexachlorocyclohexane (HCH; α, β, γ and δ) by Bacillus circulans and Bacillus brevis isolated from soil contaminated with HCH. Soil Biol. Biochem. 32:1803–1805 [Google Scholar]
- 5. Gupta A, Kaushik CP, Kaushik A. 2001. Degradation of hexachlorocyclohexane isomers by two strains of Alcaligenes faecalis isolated from a contaminated site. Bull. Environ. Contam. Toxicol. 66:794–800 [DOI] [PubMed] [Google Scholar]
- 6. Prokop Z, Monincová M, Chaloupková R, Klvaňa M, Nagata Y, Janssen DB, Damborský J. 2003. Catalytic mechanism of the haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26. J. Biol. Chem. 278:45094–45100 [DOI] [PubMed] [Google Scholar]
- 7. Ito M, Prokop Z, Klvaňa M, Otsubo Y, Tsuda M, Damborský J, Nagata Y. 2007. Degradation of β-hexachlorocyclohexane by haloalkane dehalogenase LinB from γ-hexachlorocyclohexane-utilizing bacterium Sphingobium sp. MI1205. Arch. Microbiol. 188:313–325 [DOI] [PubMed] [Google Scholar]
- 8. Wu J, Hong Q, Han P, He J, Li S. 2007. A gene linB2 responsible for the conversion of β-HCH and 2,3,4,5,6-pentachlorocyclohexanol in Sphingomonas sp. BHC-A. Appl. Microbiol. Biotechnol. 73:1097–1105 [DOI] [PubMed] [Google Scholar]
- 9. Sharma P, Raina V, Kumari R, Malhotra S, Dogra C, Kumari H, Kohler HP, Buser HR, Holliger C, Lal R. 2006. Haloalkane dehalogenase LinB is responsible for β- and δ-hexachlorocyclohexane transformation in Sphingobium indicum B90A. Appl. Environ. Microbiol. 72:5720–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nagata Y, Prokop Z, Sato Y, Jerabek P, Kumar A, Ohtsubo Y, Tsuda M, Damborský J. 2005. Degradation of β-hexachlorocyclohexane by haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26. Appl. Environ. Microbiol. 71:2183–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marek J, Vévodová J, Smatanová IK, Nagata Y, Svensson LA, Newman J, Takagi M, Damborský J. 2000. Crystal structure of the haloalkane dehalogenase from Sphingomonas paucimobilis UT26. Biochemistry 39:14082–14086 [DOI] [PubMed] [Google Scholar]
- 12. Oakley AJ, Prokop Z, Boháč M, Kmuniček J, Jedlička T, Monincová M, Kuta-Smatanová I, Nagata Y, Damborský J, Wilce MC. 2002. Exploring the structure and activity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26: evidence for product- and water-mediated inhibition. Biochemistry 41:4847–4855 [DOI] [PubMed] [Google Scholar]
- 13. Streltsov VA, Prokop Z, Damborský J, Nagata Y, Oakley A, Wilce MC. 2003. Haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26: X-ray crystallographic studies of dehalogenation of brominated substrates. Biochemistry 42:10104–10112 [DOI] [PubMed] [Google Scholar]
- 14. Oakley AJ, Klvaňa M, Otyepka M, Nagata Y, Wilce MC, Damborský J. 2004. Crystal structure of haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26 at 0.95 Å resolution: dynamics of catalytic residues. Biochemistry 43:870–878 [DOI] [PubMed] [Google Scholar]
- 15. Smatanová I, Nagata Y, Svensson LA, Takagi M, Marek J. 1999. Crystallization and preliminary X-ray diffraction analysis of haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26. Acta Crystallogr. D 55:1231–1233 [DOI] [PubMed] [Google Scholar]
- 16. Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307–326 [DOI] [PubMed] [Google Scholar]
- 17. Vagin A, Teplyakov A. 1997. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30:1022–1025 [Google Scholar]
- 18. Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- 19. Murshudov GN, Vagin AA, Dodson EJ. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53:240–255 [DOI] [PubMed] [Google Scholar]
- 20. Perrakis A, Morris R, Lamzin VS. 1999. Automated protein model building combined with iterative structure refinement. Nat. Struct. Biol. 6:458–463 [DOI] [PubMed] [Google Scholar]
- 21. Lovell SC, Davis IW, Arendall WB, III, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. 2003. Structure validation by Cα geometry: Φ,Ψ and Cβ deviation. Proteins 50:437–450 [DOI] [PubMed] [Google Scholar]
- 22. Mendes P. 1997. Biochemistry by numbers: simulation of biochemical pathways with Gepasi 3. Trends Biochem. Sci. 22:361–363 [DOI] [PubMed] [Google Scholar]
- 23. Baeck T, Fogel DB, Michalewicz Z. 1997. Handbook of evolutionary computation. IOP Publishing/Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 24. Matthews BW. 1968. Solvent content of protein crystals. J. Mol. Biol. 33:491–497 [DOI] [PubMed] [Google Scholar]
- 25. Verschueren KH, Franken SM, Rozeboom HJ, Kalk KH, Dijkstra BW. 1993. Refined X-ray structures of haloalkane dehalogenase at pH 6.2 and pH 8.2 and implications for the reaction mechanism. J. Mol. Biol. 232:856–872 [DOI] [PubMed] [Google Scholar]
- 26. Newman J, Peat TS, Richard R, Kan L, Swanson PE, Affholter JA, Holmes IH, Schindler JF, Unkefer CJ, Terwilliger TC. 1999. Haloalkane dehalogenases: structure of a Rhodococcus enzyme. Biochemistry 38:16105–16114 [DOI] [PubMed] [Google Scholar]
- 27. Pavlová M, Klvaňa M, Jesenská A, Prokop Z, Konečná H, Sato T, Tsuda M, Nagata Y, Damborský J. 2007. The identification of catalytic pentad in the haloalkane dehalogenase DhmA from Mycobacterium avium N85: reaction mechanism and molecular evolution. J. Struct. Biol. 157:384–392 [DOI] [PubMed] [Google Scholar]
- 28. Mazumdar PA, Hulecki JC, Cherney MM, Garen CR, James MN. 2008. X-ray crystal structure of Mycobacterium tuberculosis haloalkane dehalogenase Rv2579. Biochim. Biophys. Acta 1784:351–362 [DOI] [PubMed] [Google Scholar]
- 29. Hesseler M, Bogdanović X, Hidalgo A, Berenguer J, Palm GJ, Hinrichs W, Bornscheuer UT. 2011. Cloning, functional expression, biochemical characterization, and structural analysis of a haloalkane dehalogenase from Plesiocystis pacifica SIR-1. Appl. Microbiol. Biotechnol. 91:1049–1060 [DOI] [PubMed] [Google Scholar]
- 30. Pavlová M, Klvaňa M, Prokop Z, Chaloupková R, Banás P, Otyepka M, Wade RC, Tsuda M, Nagata Y, Damborský J. 2009. Redesigning dehalogenase access tunnels as a strategy for degrading an anthropogenic substrate. Nat. Chem. Biol. 5:727–733 [DOI] [PubMed] [Google Scholar]