Abstract
Sigma B (σB) is an alternative sigma factor that regulates the general stress response in Bacillus subtilis and in many other Gram-positive organisms. σB activity in B. subtilis is tightly regulated via at least three distinct pathways within a complex signal transduction cascade in response to a variety of stresses, including environmental stress, energy stress, and growth at high or low temperatures. We probed the ability of fluoro-phenyl-styrene-sulfonamide (FPSS), a small-molecule inhibitor of σB activity in Listeria monocytogenes, to inhibit σB activity in B. subtilis through perturbation of signal transduction cascades under various stress conditions. FPSS inhibited the activation of σB in response to multiple categories of stress known to induce σB activity in B. subtilis. Specifically, FPSS prevented the induction of σB activity in response to energy stress, including entry into stationary phase, phosphate limitation, and azide stress. FPSS also inhibited chill induction of σB activity in a ΔrsbV strain, suggesting that FPSS does not exclusively target the RsbU and RsbP phosphatases or the anti–anti-sigma factor RsbV, all of which contribute to posttranslational regulation of σB activity. Genetic and biochemical experiments, including artificial induction of σB, analysis of the phosphorylation state of the anti–anti-sigma factor RsbV, and in vitro transcription assays, indicate that while FPSS does not bind directly to σB to inhibit activity, it appears to prevent the release of B. subtilis σB from its anti-sigma factor RsbW.
INTRODUCTION
Sigma B (σB), an alternative sigma factor that regulates the general stress response in Bacillus subtilis, is conserved in many Gram-positive bacteria, including the pathogens Listeria monocytogenes, Staphylococcus aureus, and Bacillus anthracis (1). In both L. monocytogenes (2, 3) and B. subtilis (4, 5), activation of σB leads to the rapid, coordinated induction of more than 100 genes that collectively enhance survival under changing and often harsh physiological conditions. In addition to its regulation of the general stress response, σB modulates the expression of virulence factors important for pathogenesis in L. monocytogenes (6, 7), B. anthracis (8), and S. aureus (9, 10). The importance of σB as a regulator of both the stress response and virulence factor expression suggests that this alternative sigma factor may serve as a potential target for therapeutic intervention strategies during infection by these pathogens.
Our group previously used high-throughput screening of small-molecule libraries to identify inhibitors of σB activity in L. monocytogenes (11). The goal of these efforts was to identify novel tools that would enable study of the complex signaling pathways used by Gram-positive organisms to respond to environmental changes, with the potential for devising more-effective strategies to control the virulence of this pathogen and related organisms. We identified fluoro-phenyl-styrene-sulfonamide (FPSS) as a novel inhibitor of σB activity in L. monocytogenes and showed that FPSS also inhibits σB activity in B. subtilis in response to an environmental stress, the presence of 0.3 M NaCl (11). However, the mode of FPSS action was not identified.
In the present study, we sought to determine the mechanism by which FPSS prevents σB activity. Because FPSS inhibits σB activity in both L. monocytogenes and B. subtilis, we hypothesized that the small molecule operates through similar mechanisms in these related organisms, which share highly conserved sigB operons (1). We chose to exploit the multiple σB-activating systems in B. subtilis to conduct experiments with the goal of identifying the protein(s) with which FPSS interacts to inhibit σB.
The activity of σB is tightly regulated in B. subtilis by three distinct pathways that integrate responses to stress (reviewed in reference 12) (Fig. 1). One branch of the signal transduction cascade relays the response to environmental stresses (such as the presence of high levels of salt, acid, or ethanol) through a 1.8-MDa multiprotein stressosome complex comprising the RsbS antagonist, RsbR coantagonists, and the RsbT serine/threonine kinase (13–15). In stressed cells, RsbT phosphorylates the antagonist RsbS and the coantagonist RsbRA, allowing RsbT to be released from the stressosome, which activates the phosphatase RsbU (16–19). Active RsbU, in turn, dephosphorylates the anti–anti-sigma factor RsbV, allowing it to bind to the anti-sigma factor RsbW, thus promoting the release of σB from RsbW. In a second branch, activation of σB in response to energy stresses (such as limitation of glucose, ATP, GTP, or phosphate) requires RsbP and RsbQ (20–22). The phosphatase RsbP dephosphorylates RsbV-P (phosphorylated RsbV), again resulting in a partner switch and the release of σB from RsbW. Finally, σB activation occurs in response to growth at low temperatures independently of RsbT, RsbU, and RsbV, in a manner not fully understood (23).
Fig 1.
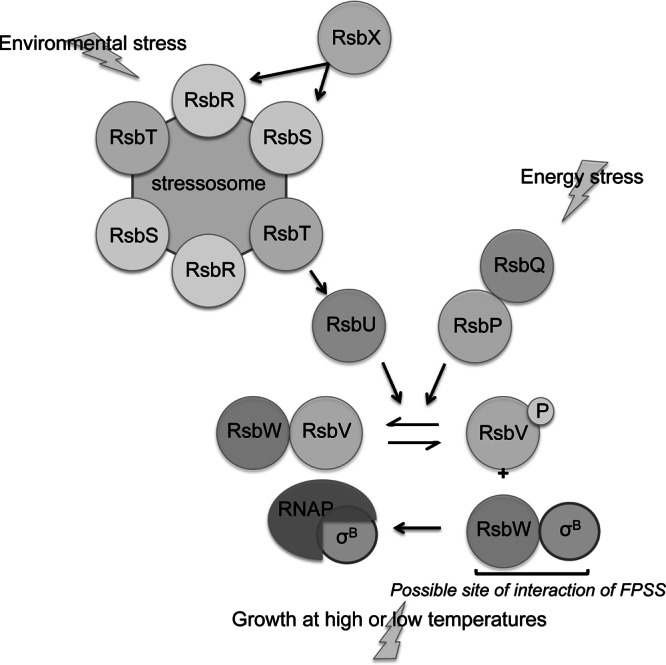
Model of σB regulation in B. subtilis. The stressosome senses environmental stress signals and activates the positive regulator RsbU, which dephosphorylates RsbV-P. Unphosphorylated RsbV binds to RsbW, freeing σB. RsbQ and RsbP are required for σB activation in response to energy stress; RsbP dephosphorylates RsbV-P. Growth at high or low temperatures leads to σB activation independently of RsbV. References are cited in the text. The possible site of FPSS interaction is noted at the RsbW-SigB interface. (Modified from the Annual Review of Microbiology [12] with permission of the publisher.)
In contrast, several lines of evidence indicate that the L. monocytogenes σB response to environmental and energy stresses occurs through a single pathway via the stressosome (24–26). For example, L. monocytogenes lacks genes encoding homologs of the B. subtilis RsbP and RsbQ energy stress response pathway proteins (25, 26). Further, replacement of the four B. subtilis rsbR paralogs with L. monocytogenes rsbR within the sigB operon in B. subtilis allows for the activation of σB by energy stresses, suggesting that this L. monocytogenes paralog may integrate responses to both energy and environmental stresses (27). Finally, RsbU is necessary for σB activation in response to environmental and energy stresses (24–26), further supporting the single-pathway model of σB activation in L. monocytogenes.
We methodically investigated all known proteins involved in the signal transduction cascade that regulates B. subtilis σB in order to determine at which point FPSS disrupts σB activity. In particular, we explored the four shared components of the branches of the signal transduction pathway: the protein phosphatase domains of RsbU and RsbP, the components downstream of RsbV, the ability of σB to initiate transcription by association with RNA polymerase (RNAP), and the recognition of σB promoters by the sigma factor. Our experiments show (i) that FPSS appears to act independently and downstream of RsbV and (ii) that FPSS does not interact with σB in vivo or in vitro. Therefore, we conclude that FPSS interferes with a shared component of the two pathways, and the most likely target is the partner-switching mechanism involving σB and RsbW.
MATERIALS AND METHODS
Bacterial strains and genetic methods.
All strains used in this study are listed in Table 1. Strains PB2, PB198, PB206, PB213, and PB345 were provided by C. W. Price (University of California, Davis). Strains (renamed FSL B2-273 and FSL B2-274 for this study) for the overexpression of His6-SigB and His6-SigA were provided by W. Goebel (Universität Würzburg, Germany).
Table 1.
Plasmids and strains used in this study
| Plasmid or strain | Relevant genotype | Source |
|---|---|---|
| Plasmids | ||
| pDLR1 | Pfri Lm SigA2 SigB in pUC19; Apr | This study |
| pDLR2 | Pspac rsbVBs; Neor | This study |
| pCK35 | Pspac Δ(rsbR rsbS) rsbT+; Neor | 17 |
| pUC19 | High-copy-number cloning vector; Apr | 28 |
| pQE-30 | N-terminal His6 expression vector; PT5/Olac; ColEI ori; Apr | Qiagen |
| Strains | ||
| B. subtilis | ||
| FSL B2-303 | Pspac rsbVBs amyE::ctc-lacZ trpC2 | pDLR2→PB198 |
| FSL B2-304 | Pspac rsbVBs amyE::ctc-lacZ trpC2 sigBΔ3::spc trpC2 | pDLR2→PB345 |
| PB2 | trpC2 | 29 |
| PB198 | amyE::ctc-lacZ trpC2 | 30 |
| PB206 | rsbVΔ1 amyE::pDH32-ctc | 30 |
| PB213 | Pspac (rsbV+ rsbWΔ1 sigB+ rsbX+) amyE::pDH32-ctc trpC2 | 30 |
| PB345 | amyE::ctc-lacZ trpC2 sigBΔ3::spc trpC2 | 31 |
| E. coli | ||
| FSL B2-273 | M15 pREP4 pQE-30 His6-SigALm | 32 |
| FSL B2-274 | M15 pREP4 pQE-30 His6-SigBLm | 32 |
| FSL B2-302 | TOP10 pUC19-Pfri Lm | pDLR1→TOP10 |
| TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| L. monocytogenes | ||
| 10403S | Wild type; serotype 1/2a | 33 |
| 10403SΔsigB | ΔsigB | 34 |
Strains expressing B. subtilis RsbV were constructed by cloning a PCR fragment containing rsbV amplified using primers DR34 and DR35 (Table 2) into the pCK35 vector (17) at HindIII and SphI sites and confirming the presence of rsbV by sequencing at the Cornell University Life Sciences Core Laboratories Center. The resulting plasmid, pDLR2, was transformed by electroporation (35) into strains PB198 and PB345. For in vitro transcription assays, a 276-bp PCR fragment amplified using primers DR1 and DR2 (Table 2), which contain SigA2- and SigB-dependent promoter elements from the L. monocytogenes fri promoter region (37), was cloned into PUC19 at HindIII and XbaI sites and was transformed into Escherichia coli TOP10, resulting in strain FSL B2-302. Chloramphenicol (10 μg/ml), streptomycin (100 μg/ml), kanamycin (25 μg/ml), ampicillin (100 μg/ml), and neomycin (5 μg/ml) were added to media as appropriate.
Table 2.
Primers and probes used in this study
| Primer or probe | Sequence (5′→3′)a | Source or reference |
|---|---|---|
| DR1 FRI Fwd | CGAAGCTTCACCTGAAAGCGGTGAGAAT | This study |
| DR2 FRI Rev | CTCTAGACCAGTGTGGAAACCATCACA | This study |
| DR34 rsbV Fwd | GATAAGCTTAAAGCAACTAGTGATTTGAAGGAAAA | This study |
| DR35 rsbV Rev | GATGCATGCCGGCACTTTCATTTCGATGT | This study |
| sigB TaqMan F | GCCGCTTACCAAGAAAATGG | S. Chaturongakul, unpublished |
| sigB TaqMan R | TTCGGGCGATGGACTCTACT | S. Chaturongakul, unpublished |
| sigB MGB probe | ATCAAGACGCCCAATAT | S. Chaturongakul, unpublished |
| rpoB TaqMan F | CCGGACGTCACGGTAACAA | 36 |
| rpoB TaqMan R | CAGGTGTTCCGTCTGGCATA | 36 |
| rpoB MGB probe | CCGGACGTCACGGTAACAA | 36 |
Restriction sites are underlined.
Overproduction and purification of σB and σA for in vitro transcription.
His6-SigB and His6-SigA proteins were overexpressed from strains FSL B2-273 and FSL B2-274. Cells were grown in 500 ml Luria broth (LB) containing ampicillin and kanamycin at 37°C with shaking (225 rpm). At an optical density at 600 nm (OD600) of 0.7 to 1.0, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added. Cells were pelleted by centrifugation (10,000 × g, 15 min, 4°C) after 3 to 4 h of growth with IPTG and were frozen at −80°C. To purify proteins, cell pellets were thawed and resuspended in 5 ml lysis buffer (20 mM Tris [pH 8.0], 0.5 M NaCl, 5 mM imidazole, 6 M guanidine hydrochloride, 1 mM β-mercaptoethanol, 1 mg/ml lysozyme). Cells were sonicated (4 times, with 20 s on and 1 min off, at 33 W) on ice, and the lysate was centrifuged (10,000 × g, 15 min, 4°C) to remove cell debris. Supernatants were applied to a Ni-nitrilotriacetic acid (NTA) column (HisTrap HP; GE Life Sciences, Pittsburgh, PA) using a 10-ml syringe. On-column refolding was performed using a stepwise gradient of a buffer (20 mM Tris [pH 8.0], 0.5 M NaCl, 5 mM imidazole, 1 mM β-mercaptoethanol) containing decreasing concentrations of urea, according to the manufacturer's instructions. Following elution, protein fractions were analyzed using SDS-PAGE and Coomassie blue staining. Fractions containing target protein were concentrated (Vivaspin 2; molecular weight cutoff [MWCO], 10,000; GE Life Sciences), exchanged into protein storage buffer (10 mM Tris [pH 8.0], 10 mM MgCl2, 0.1 mM EDTA, 0.1 mM dithiothreitol [DTT], 0.1 M NaCl, 50% glycerol), and stored at −20°C.
Growth conditions and β-galactosidase assays.
All B. subtilis strains were grown with shaking (225 rpm) in 300-ml Nephelo flasks (Bellco, Vineland, NJ). Strains PB213, FSL B2-303, and FSL B2-304 were grown in buffered LB (BLB) (31). For azide and salt stress experiments, strains grown overnight in LB at 37°C were diluted (1:25) into 30 ml of fresh LB at 37°C and were then passaged again (1:25) at mid-exponential phase (OD at 600 nm [OD600], 0.2) into fresh LB (28.8 ml) at 37°C. At an OD600 of 0.2, sodium azide (200 mM) or sodium chloride (5 M NaCl) was added to a final concentration of 2 mM or 0.3 M NaCl, respectively. (E)-N-(4-fluorophenyl)-2-phenylethenesulfonamide (FPSS; Enamine Ltd., Kiev, Ukraine) (11) stock solutions (10 mM) were diluted in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) and were filtered with 0.2-μm nylon membrane syringe filters (Acrodisc; Pall, Port Washington, NY). FPSS was added to a final concentration of 64 μM, except where noted differently. For cold growth experiments, exponential-phase cultures that had been grown in LB at 37°C were moved to 16°C with shaking (225 rpm) immediately following the addition of either FPSS or an equal volume of DMSO. Phosphate limitation experiments were conducted in a synthetic medium (20). A low-phosphate (0.15 mM) synthetic medium (28.8 ml) was inoculated with 1.2 ml of culture (1:25) that had been grown in the same medium overnight at 37°C with shaking. At an OD600 of 0.2, FPSS or an equal volume of DMSO was added, and samples were removed at regular intervals for β-galactosidase activity assays performed using cell permeabilization with chloroform as described by Kenney and Moran (38). OD600 values of cell suspensions were used to calculate Miller units, while the protein concentration, determined by the Bradford assay (Bio-Rad, Hercules, CA), was used to calculate the specific activity of β-galactosidase, defined as the change in A420 min−1 mg of protein−1. At least two biological replicates were performed for each experiment.
In vitro transcription assays.
The phenol-chloroform-purified PCR product (amplified using primers DR1 and DR2 and plasmid pDLR1) was used as a DNA template for in vitro transcription assays. In vitro transcription that is initiated from the SigA2- and SigB-dependent promoters of the L. monocytogenes fri promoter generates RNA fragments of 185 and 120 bp, respectively. Reaction mixtures (40 μl) containing an in vitro transcription buffer (10 mM Tris-HCl [pH 7.8], 10 mM MgCl2, 0.5 mM EDTA, 1 mM DTT, 7.5 mM KCl, and 10 μg/ml acetylated bovine serum albumin [BSA]), His6-SigA or His6-SigB, and FPSS or DMSO were incubated at room temperature for 5 min. Purified B. subtilis RNA polymerase was added to the mixtures for a final protein concentration of 150 nM. The PCR product (330 ng) was added, followed by incubation at 37°C for 10 min. Transcription reactions were started by adding a mixture of nucleoside triphosphates (NTPs) (approximately 0.2 mM [final concentration] each ATP, CTP, GTP, and UTP and 1.7 nmol [α-32P]UTP [6,000 Ci mmol−1]) and incubating at 37°C for 10 min. Reactions were stopped by adding 60 μl of stop solution (0.5 M sodium acetate, 17 mM EDTA). RNA was precipitated by adding 330 μl ethanol (EtOH) and 2 μl glycogen (GlycoBlue; Invitrogen, Carlsbad, CA), followed by overnight storage at −20°C. RNA was collected by centrifugation (16,000 × g, 10 min, room temperature), washed with 70% EtOH, and resuspended in formamide loading dye. Samples were heated at 95°C for 5 min and were loaded onto a 6% Tris-borate-EDTA (TBE)-urea gel for separation. Transcripts were visualized using phosphorimaging.
IEF and immunostaining.
Aliquots (15 ml) of mid-exponential-phase (OD600, 0.3) cells grown in LB at 37°C with shaking were harvested before and after the addition of FPSS (final concentration, 64 μM) or an equal volume of DMSO, at designated time points. Cells were centrifuged (6,000 × g, 3 min, 4°C), and pellets were resuspended in lysis buffer (100 mM Tris-HCl [pH 7.5], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 mM NaF). Cells were mechanically lysed using a beadbeater (Mini-Beadbeater-8; Biospec) for 3 min. Lysates were centrifuged (16,000 × g, 10 min, 4°C), and proteins were precipitated from the supernatant with ethanol. Protein pellets were resuspended in Novex isoelectric focusing (IEF) sample buffer (pH 3 to 7; Invitrogen) and were quantified by a Bradford assay (Bio-Rad). Equivalent amounts of protein (40 μg) were loaded onto an IEF minigel (Novex pH 3–7 IEF gel; Invitrogen) and were run according to the manufacturer's instructions. Proteins from gels were transferred to nitrocellulose membranes (0.2 μm) in Towbin buffer (25 mM Tris-HCl [pH 8.3], 192 mM glycine) containing 20% (vol/vol) methanol. Bound proteins were probed with monoclonal anti-RsbV antibodies (39), provided by W. G. Haldenwang (University of Texas Health Science Center at San Antonio), and an alkaline phosphatase-conjugated anti-mouse secondary antibody (Invitrogen) and were visualized with the chromogenic substrate NBT (nitroblue tetrazolium)-BCIP (5-bromo-4-chloro-3-indolylphosphate) (Invitrogen).
RNA extraction, cDNA synthesis, and quantitative reverse transcription-PCR (qRT-PCR).
L. monocytogenes strain 10403S ΔsigB was streaked from frozen stocks onto brain heart infusion (BHI) agar plates and was incubated overnight at 37°C. An isolated colony was used to inoculate 5 ml BHI broth, which was incubated at 37°C overnight with shaking (225 rpm). A 50-μl aliquot was transferred from this culture to 5 ml fresh, prewarmed BHI (1:100) and was grown to an OD600 of 0.4. A 300-μl aliquot was transferred to two Nephelo flasks containing 300 ml prewarmed BHI and was grown to an OD600 of 0.4. An aliquot (5 ml) was removed from each culture and was added to 5 ml RNAprotect (Qiagen) to stop transcription. To the remaining cultures, 64 μM FPSS or an equal volume of DMSO was added, and the flasks were returned to the incubator. After 15 min, another 5 ml was removed and treated as described above. Salt (final concentration, 0.3 M NaCl) was added to the cultures, and the cultures were returned to the incubator. After 15 min, a final 5-ml aliquot was removed and was treated as described above. Cells were pelleted (3,600 × g, 10 min, 4°C) after 5 min at room temperature in RNAprotect. Pellets were kept on ice until RNA extraction, which was performed as described previously (40). cDNA was synthesized as described elsewhere (41). TaqMan qPCR was performed on 10−1, 10−2, and 10−3 dilutions of cDNA by using sigB and rpoB (36) primers and probes. A standard curve was generated using genomic chromosomal DNA isolated from L. monocytogenes 10403S. Copy numbers of sigB transcript levels were calculated from standard curves and were normalized to rpoB transcript levels, as described by Sue et al. (42).
Statistical analysis.
Statistical analysis of sigB transcript levels from three biological replicates was performed using Student t tests at each time point with a statistical significance (P) value of <0.05 (JMP 9.0; SAS, Inc., Cary, NC).
RESULTS AND DISCUSSION
In previous work, we showed that FPSS, a small molecule initially identified through high-throughput screening as an L. monocytogenes σB inhibitor, inhibited B. subtilis σB activity induced by salt exposure (0.3 M NaCl) (11), as monitored by a well-characterized σB-dependent single-copy ctc-lacZ transcriptional fusion (30). Here we aimed to define the molecular mechanism through which FPSS inhibits σB activity. We hypothesized that FPSS could inhibit σB activity by blocking the transcription of the gene encoding the sigma factor; therefore, we investigated the question of whether FPSS prevents the transcription of sigB in L. monocytogenes. We measured sigB transcript levels in L. monocytogenes 10403S ΔsigB with sigB TaqMan qRT-PCR primers and a probe that can detect a signal in the null mutant due to the residual ∼200 bp of the 5′ end of the gene (34). The use of the ΔsigB strain allowed us to monitor sigB transcript levels in response to FPSS addition in the presence of an environmental stress but in the absence of positive upregulation by the autoregulatory feedback loop formed by a second σB-dependent promoter located upstream of rsbV.
Treatment with FPSS did not significantly (P, >0.05 by the t test) reduce sigB mRNA transcript levels in L. monocytogenes before or after exposure to 0.3 M NaCl from those in DMSO-treated control cultures (Fig. 2). These findings indicate that FPSS does not appear to operate at a transcriptional level to inhibit σB activity during mid-exponential-phase growth or in response to a sudden environmental stress.
Fig 2.
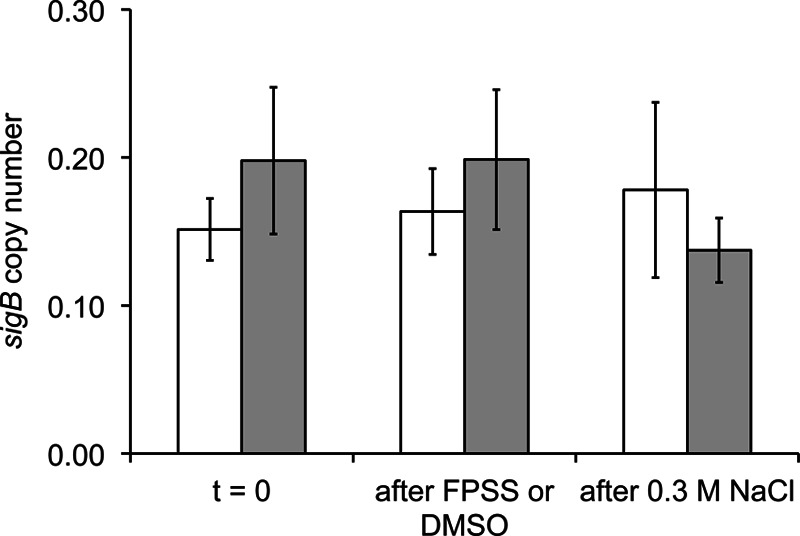
sigB transcript copy numbers after addition of FPSS or DMSO. L. monocytogenes 10403S ΔsigB was grown in BHI at 37°C with shaking (225 rpm). At an OD600 of 0.4, 5 ml of culture was removed and was added to 5 ml RNAprotect (Qiagen) to stop transcription prior to RNA isolation (t = 0). FPSS (64 μM) or an equal volume of DMSO was added to the remaining cultures. After 15 min, another 5-ml aliquot was removed for RNA isolation (“after FPSS or DMSO”). Salt (0.3 M [final concentration] NaCl) was added to both cultures, and after 15 min, another 5 ml of culture was collected (“after 0.3 M NaCl”). Mean values for sigB transcript copy numbers (in arbitrary units) from a DMSO-treated culture (open bars) and an FPSS-treated culture (shaded bars), calculated from three biological replicates, are shown with standard deviations. sigB transcript copy numbers were normalized to rpoB transcript copy numbers in order to calculate relative transcript levels.
FPSS prevents σB activity in response to environmental stress, energy stress, and growth at low temperatures in B. subtilis.
Because we saw no transcriptional effect of FPSS in L. monocytogenes, we hypothesized that the molecule operates posttranslationally to inhibit σB activity. Therefore, to determine the effects of FPSS on σB activity, we chose to perform genetic experiments in B. subtilis, in which the σB signal transduction cascade has been well characterized. Initial sporulation experiments showed that addition of FPSS to mid-exponential-phase cultures of strain PB198 and the ΔsigB mutant strain PB345 failed to alter sporulation significantly (data not shown) in either strain. We therefore concluded that FPSS specifically targets the signaling pathway that regulates σB rather than interfering more globally with other homologous partner-switching systems (e.g., SpoIIAB and SpoIIAA) (43, 44).
Energy and environmental stresses induce σB activity through two distinct pathways in B. subtilis. The response to energy stresses requires the phosphatase RsbP to dephosphorylate RsbV-P (16, 20, 22). An rsbP-null strain demonstrates normal σB activity in response to high-salt and ethanol exposure (22), indicating that RsbP is not an essential regulatory protein for the induction of σB activity in response to environmental stress. Therefore, to determine whether FPSS interacts with a member of the stressosome or the phosphatase RsbU, we investigated whether FPSS prevents the induction of σB activity in response to energy stresses. We hypothesized that if FPSS interferes solely with the environmental stress pathway, then the response to energy stress should be unaffected. We assayed three energy stress conditions in B. subtilis: entry into stationary phase, phosphate limitation, and azide stress.
The presence of FPSS at 64 μM, a concentration previously shown to inhibit σB activity (11), prevented σB activity during entry into stationary phase (Fig. 3A) in PB198 cells grown at 37°C in LB, an effect that lasted for at least 5 h after entry into stationary phase, in contrast to the results for control cells treated with DMSO. Furthermore, FPSS delayed σB activity in response to sodium azide (2 mM), an inhibitor of ATP synthesis (Fig. 3B). The FPSS-treated culture showed induction of σB activity about 4 h after induction in the DMSO-treated culture, despite concurrent cessation of growth in the two cultures. Finally, we grew strain PB198 in synthetic medium with limited phosphate (15 μM) to induce phosphate starvation, and we treated parallel cultures with 0, 32, 64, or 128 μM FPSS. We observed a delay in σB activity in cultures treated with FPSS, as well as decreased activity dependent on the concentration of FPSS added to the cultures (Fig. 3C). The addition of 64 μM or 128 μM FPSS resulted in ∼46% or ∼24% of the activity of the wild type, respectively. The perturbation of σB activity in response to both environmental and energy stresses suggests that FPSS does not interfere only with the function of the positive regulator RsbU or upstream members of the stressosome, which are not required for the response to energy stress (16), or only with RsbP or RsbQ, which are not required for σB activity in response to salt stress (22).
Fig 3.
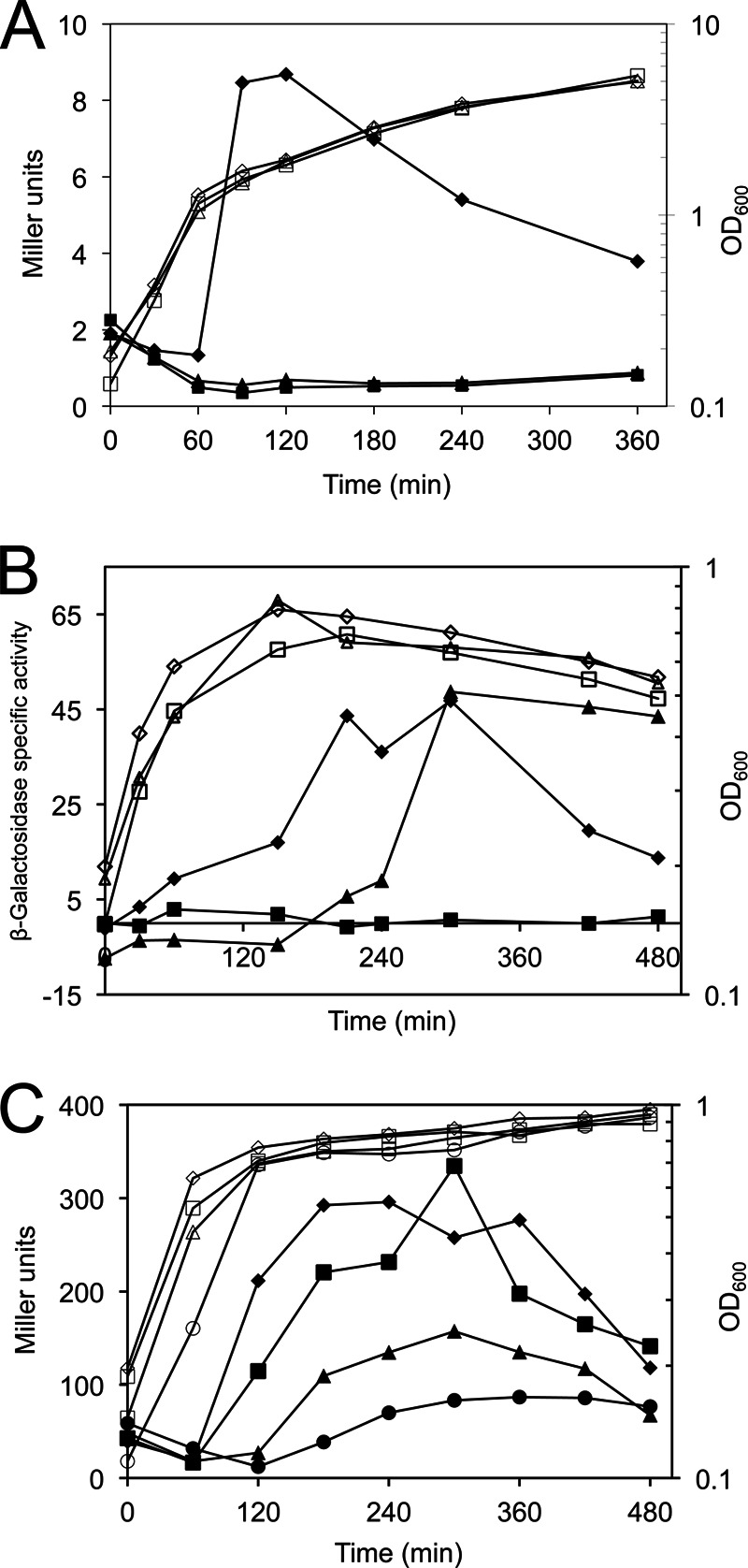
Effect of FPSS on σB activity during energy stress. β-Galactosidase activity (filled symbols) and cell growth, measured by the OD600 (open symbols), from one representative experiment are shown. (A) Entry into stationary phase. Strains PB198 (amyE::ctc-lacZ trpC2) and PB345 (amyE::ctc-lacZ trpC2 sigBΔ3::spc trpC2) were grown in LB at 37°C with shaking (225 rpm). At mid-exponential phase (OD600, 0.2), a sample of culture was removed, and 64 μM FPSS (triangles) or an equal volume of DMSO (diamonds) was added to PB198, while PB345 was treated with DMSO (squares). (B) Azide stress. Strains PB198 and PB345 were grown in LB at 37°C with shaking (225 rpm) to mid-exponential phase. At an OD600 of 0.2 (time zero), cultures were treated with sodium azide (final concentration, 2 mM) and either 64 μM FPSS (PB198) (triangles) or an equal volume of DMSO (diamonds [PB198] or squares [PB345]). (C) Phosphate limitation. Strain PB198 was grown at 37°C with shaking (225 rpm) in a low-phosphate (15 μM) defined medium with FPSS at 32 μM (squares), 64 μM (triangles), or 128 μM (circles), or with a volume of DMSO equal to the volume of FPSS added to the culture treated with 128 μM FPSS (diamonds).
FPSS inhibits σB chill induction independently of RsbV.
Another type of stress, growth at a low (16°C) temperature, induces σB activity in B. subtilis. Interestingly, the gradual induction of σB activity observed under these conditions occurs independently of the RsbT/RsbU/RsbV pathway in B. subtilis (23), indicating that induction of σB activity under these conditions does not depend on the phosphorylation state of RsbV. To determine whether FPSS prevents the induction of σB by interaction with RsbV or is dependent on the phosphorylation state of RsbV, we tested whether FPSS could prevent “chill induction” of σB activity. If FPSS interacts with RsbV to disrupt the release of σB from RsbW, we would expect to see no effect of FPSS on σB activity during growth at 16°C. Conversely, if FPSS interacts in an RsbV-independent manner, we would expect to see inhibition of σB activity in a ΔrsbV mutant during cold growth. We grew B. subtilis PB198 and the ΔrsbV strain PB206 (30) at 37°C in LB, treated the cells with FPSS or DMSO, shifted them to 16°C, and monitored σB activity (Fig. 4). DMSO-treated PB198 and PB206 showed induction of σB activity approximately 18 h after a shift to 16°C, compared to cultures treated with 64 μM FPSS prior to the temperature shift. The inhibition of σB activity by FPSS in a B. subtilis strain lacking rsbV suggests that FPSS acts via at least one mechanism that is independent of RsbV and independent of either the RsbU or the RsbP phosphatase.
Fig 4.
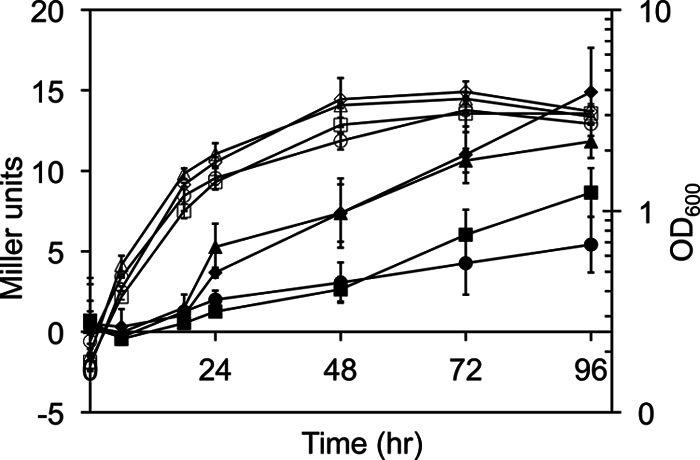
Effect of FPSS on σB activation by growth at 16°C. Mean Miller units (filled symbols) and cell growth, monitored by the OD600 (open symbols), for two biological replicates are shown. Error bars, standard deviations. Strains PB198 (amyE::ctc-lacZ trpC2) and PB206 (rsbVΔ1 amyE::pDH32-ctc) were grown at 37°C with shaking (225 rpm) in LB to mid-exponential phase (OD600, 0.2). The strains were treated with 64 μM FPSS or an equal volume of DMSO and were then transferred to 16°C with shaking (225 rpm). Results for PB198 treated with DMSO (diamonds) or 64 μM FPSS (squares) and for PB206 treated with DMSO (triangles) or 64 μM FPSS (circles) are shown.
FPSS prevents σB activation.
Having determined that FPSS prevents σB activity in response to the three known categories of stress in B. subtilis, we asked: does FPSS prevent σB activity by altering the activation of σB (meaning its switch from an inactive, bound state to its free, active state) or, rather, does it inhibit the activity of the sigma factor, i.e., its transcriptional function once σB is released from its antagonist, RsbW? To address this question, we measured the effect of adding FPSS 15 min after the induction of σB activity by salt stress (0.3 M NaCl). We hypothesized that if FPSS affects either the activation of σB or the recruitment of the RNAP holoenzyme to σB-dependent promoter sites, the addition of FPSS after the induction of σB activity should not affect the development of σB activity in response to an environmental stress. We observed a level of accumulation of the β-galactosidase enzyme in cultures treated with FPSS after exposure to salt similar to that in control cultures treated with DMSO (Fig. 5). In agreement with our previous work, we saw no σB activity in cultures treated with FPSS prior to the addition of salt. The absence of an effect on σB activity after σB activity had been induced suggests that FPSS inhibits the activation of σB rather than interfering with σB-dependent transcription once σB is active and is promoting transcription from σB-dependent binding sites.
Fig 5.
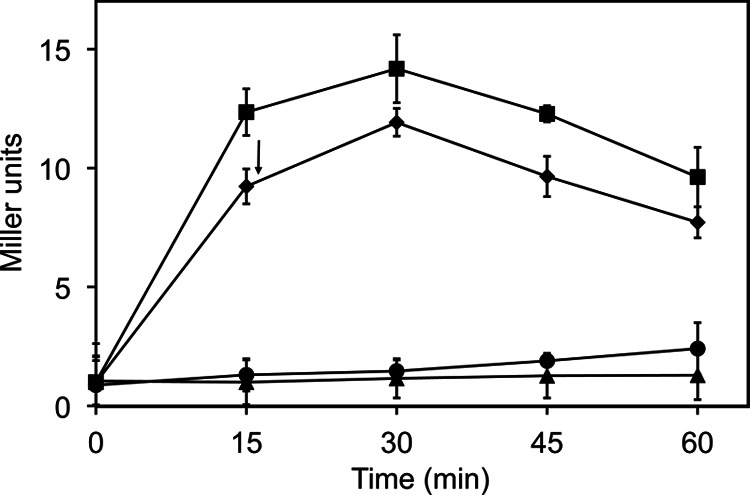
Effect of delayed addition of FPSS on salt-induced σB activity. At mid-exponential phase (OD600, 0.2), PB198 (amyE::ctc-lacZ trpC2) grown in LB at 37°C with shaking (225 rpm) was treated with either H2O and DMSO (●), 0.3 M NaCl and DMSO (■), 0.3 M NaCl and 64 μM FPSS (▲), or 0.3 M NaCl, with 64 μM FPSS added after 15 min of sampling (indicated by an arrow) (◆). Mean β-galactosidase activity for two biological replicates is shown. Error bars, standard deviations.
FPSS does not inhibit σB transcription activity in vivo and in vitro.
To further investigate the ability of FPSS to inhibit σB activity, we used genetic experiments to investigate the effect of FPSS on the transcriptional role of σB as an RNAP subunit. In B. subtilis and L. monocytogenes, the gene encoding σB lies within an eight-gene operon (PA-rsbR-rsbS-rsbT-rsbU-PB-rsbV-rsbW-sigB-rsbX) known as the sigB operon (45, 46). A σB-dependent promoter lies upstream of sigB, creating an autoregulatory feedback loop after σB becomes active. We used B. subtilis strain PB213 (30), which contains an inducible promoter upstream of rsbV, to enable IPTG-induced expression of σB. The addition of IPTG to PB213 induces σB activity, as measured by a σB-dependent reporter fusion, in this rsbW-null mutant (30), presumably by increasing the amount of active, unbound σB in the absence of the anti-sigma factor antagonist.
As shown in Fig. 6, σB activity in strain PB213 was rapidly induced upon addition of IPTG, even when 64 μM FPSS was added immediately before the addition of IPTG. This result, again, suggests that FPSS has no effect on the transcriptional function of σB once σB has become active. These results provide additional evidence to support our hypothesis that FPSS interferes with the regulation of σB rather than with its role in transcription initiation.
Fig 6.
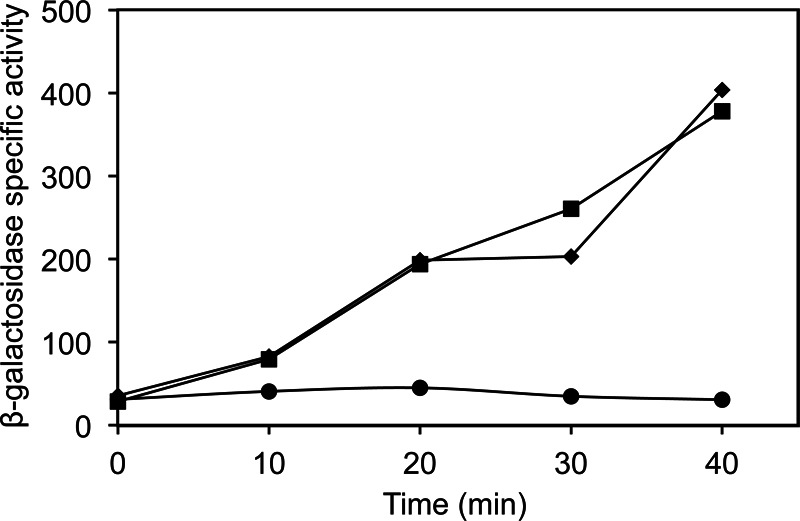
Effect of FPSS on σB activity by artificial induction. PB213 [Pspac (rsbV+ rsbWΔ1 sigB+ rsbX+) amyE::pDH32-ctc trpC2] was grown in BLB at 37°C with shaking (225 rpm). At an OD600 of 0.4 (time zero), 64 μM FPSS or DMSO and IPTG (final concentration, 1 mM) or distilled H2O were added. β-Galactosidase activities were determined for PB213 with H2O and DMSO (●), IPTG and DMSO (■), and IPTG and FPSS (◆). Results from a representative experiment are shown.
We next sought to rule out the possibility that FPSS binds directly to σB by using in vitro transcription assays. If FPSS binds directly to σB, it might interfere either with the sigma factor's ability to bind to RNAP or with its release from RsbW. We performed transcription assays in a simplified in vitro system containing B. subtilis RNAP, His6-tagged L. monocytogenes σB or σA (as a control), and a fragment of the L. monocytogenes fri promoter region containing one σB- and one σA-dependent promoter site (37).
The reconstituted holoenzymes were successfully transcribed from both L. monocytogenes promoter sites (Fig. 7). Control reactions without any added sigma factors showed that residual B. subtilis σA contained in the purified RNAP protein fraction initiated transcription from the L. monocytogenes σA-dependent promoter site (Fig. 7, lane 11), but the addition of exogenous, purified L. monocytogenes σA and σB promoted higher transcript levels from the template's σA- and σB-dependent promoter sites (lanes 13 and 12, respectively). DMSO addition did not affect the abilities of σA and σB to drive transcription from either promoter (Fig. 7, lanes 14 and 15). We performed two titration experiments to test the effect of FPSS on transcription from a σB-dependent promoter site. In the first titration experiment, increasing amounts of σB added to reaction mixtures caused higher levels of transcripts to be produced from the σB promoter (Fig. 7, lanes 1 to 5). Transcription in these reactions from the σB-dependent promoter site was uninhibited by the presence of FPSS up to ∼1,000-fold the concentration of σB and B. subtilis RNAP (Fig. 7, lanes 6 to 10). In the second experiment, titration with increasing amounts of FPSS (up to 1,000-fold higher) relative to σB (Fig. 7, lanes 17 to 21) did not inhibit transcription relative to that in a control reaction mixture containing DMSO (lane 16). Since the RNAP-σB holoenyzme was able to transcribe from a σB-dependent promoter despite the presence of a relatively high concentration of FPSS, these data provide additional support for the idea that in vitro, FPSS does not prevent the binding of σB to core RNAP and does not prevent the recognition of σB-dependent promoter sites.
Fig 7.
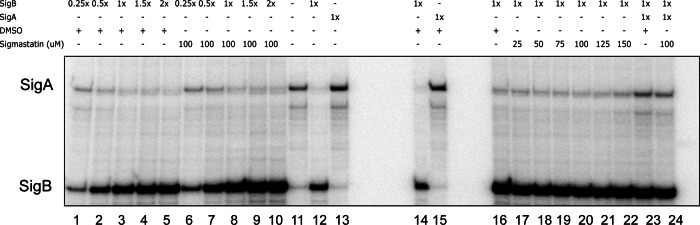
Effect of FPSS on in vitro transcription of the L. monocytogenes σB-dependent promoter. (Left lanes) B. subtilis RNAP (150 nM) was added to reaction mixtures containing various concentrations of σB (37.5, 75, 150, 225, or 300 nM) in the presence of DMSO (lanes 1 to 5) or 100 μM FPSS (lanes 6 to 10). Control reactions (lanes 12 and 13) exhibit sigma factor-initiated transcription from both promoters, even in the presence of DMSO (lanes 14 and 15). (Right lanes) B. subtilis RNAP (150 nM) was added to reaction mixtures containing σB (150 nM) in the presence of DMSO (lane 16) or various concentrations of FPSS (25, 50, 75, 100, 125, or 150 μM) (lanes 17 to 22).
FPSS inhibits σB activity specifically, likely by preventing RsbW-σB partner switching.
The discovery that FPSS inhibits the activation of σB led us to investigate potential targets to which it might bind as a mechanism for its inhibitory activity. We speculated that since FPSS does not appear to interact with either the RsbU or the RsbP phosphatase alone, it might interfere with the partner-switching module RsbV-RsbW-σB, a shared component of both pathways that plays a central role in regulating σB. To test that hypothesis, we explored the phosphorylation state of RsbV in response to environmental stress in the presence of FPSS. Isoelectric focusing and immunostaining of RsbV, used to separate the unphosphorylated and phosphorylated forms of RsbV, revealed that FPSS prevents the in vivo dephosphorylation of RsbV-P in B. subtilis in response to salt stress (Fig. 8). The dephosphorylation of RsbV-P is a crucial step necessary for freeing and activating σB upon stress signaling. These results suggest that FPSS disrupts the ability of RsbV to switch partners with σB, thus leaving σB bound to RsbW and hence inactive.
Fig 8.
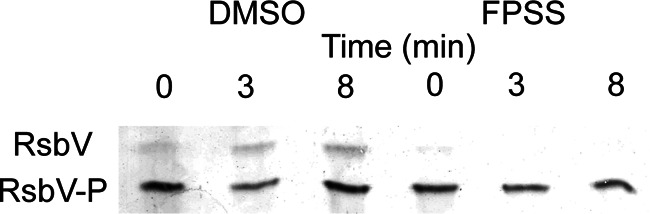
IEF analysis of phosphorylation of B. subtilis RsbV after exposure to salt stress. B. subtilis PB2 was grown in LB at 37°C with shaking (225 rpm) to the mid-exponential phase (OD600, 0.3) and was treated with 64 μM FPSS or an equivalent volume of DMSO. Cultures were then treated with 0.3 M (final concentration) NaCl and were incubated at 37°C with shaking. Samples were removed before (0 min) and after (3 and 8 min) salt treatment. Crude extracts (40 μg protein) were separated using vertical IEF, transferred to nitrocellulose membranes, and probed using a monoclonal anti-RsbV antibody.
Central to the regulation of σB in response to energy and sudden environmental stress in B. subtilis is the dephosphorylation of the anti–anti-sigma factor RsbV, a member of the partner-switching module directly responsible for controlling the free or RsbW-bound state of σB (30, 39, 47). RsbW has a higher affinity for unphosphorylated RsbV than for σB (48), but the kinase function of RsbW keeps a majority of RsbV phosphorylated at baseline expression levels of the sigB operon in unstressed cells, and thus, RsbW remains bound to σB (49). The phosphatases RsbU and RsbP dephosphorylate RsbV-P and modulate σB activity in a tightly controlled circuit, countering the antagonistic role of RsbW (49). In B. subtilis, RsbU and RsbP function independently, responding to separate stress-sensing components. Our finding that the response to stress controlled by both pathways possessing these phosphatases is inhibited by FPSS allows us to conclude that neither RsbU nor RsbP is the exclusive target of FPSS and that FPSS interacts with a component shared by both stress activation pathways.
Our analysis of the phosphorylation state of RsbV in B. subtilis exposed to salt stress indicates that FPSS prevents the dephosphorylation of RsbV that is seen in untreated control cells in response to the stress. The sum of our findings, namely, (i) that RsbV is not necessary for inhibition by FPSS and (ii) that dephosphorylation of RsbV does not appear to occur in FPSS-treated cells in response to stress, leads us to hypothesize that the likely target of FPSS is RsbW. We speculate that FPSS may act to prevent the release of RsbW from σB, by binding either to RsbW alone or to the interface of the proteins. Additional biochemical experiments are needed to determine the precise molecular mechanism of the inhibitory action of FPSS on the RsbW and σB components of the partner-switching module that plays a central role in regulating σB activity.
Novel inhibitors of σB in B. subtilis and L. monocytogenes may inhibit homologous sigma factors in other organisms, such as S. aureus, and such inhibitors could offer novel therapeutic approaches as alternatives to broad-spectrum antibiotics. Identification of the mechanism of FPSS for inhibiting σB activity will improve our understanding of how σB activity is regulated in B. subtilis and L. monocytogenes, and how similar, homologous systems are regulated in other Gram-positive organisms with similar regulatory elements.
ACKNOWLEDGMENTS
The work was supported by National Institutes of Health awards 5R01AI052151-09 (to K.J.B.) and GM-047446 (to J.D.H.). Support for student training was provided by USDA National Needs Graduate Fellowship Competitive Grant 2007-38420-17751 from the National Institute of Food and Agriculture.
The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print 22 March 2013
REFERENCES
- 1. Ferreira A, Gray M, Wiedmann M, Boor KJ. 2004. Comparative genomic analysis of the sigB operon in Listeria monocytogenes and in other Gram-positive bacteria. Curr. Microbiol. 48:39–46 [DOI] [PubMed] [Google Scholar]
- 2. Oliver H, Orsi R, Ponnala L, Keich U, Wang W, Sun Q, Cartinhour S, Filiatrault M, Wiedmann M, Boor KJ. 2009. Deep RNA sequencing of L. monocytogenes reveals overlapping and extensive stationary phase and sigma B-dependent transcriptomes, including multiple highly transcribed noncoding RNAs. BMC Genomics 10:641 doi:10.1186/1471-2164-10-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raengpradub S, Wiedmann M, Boor KJ. 2008. Comparative analysis of the σB-dependent stress responses in Listeria monocytogenes and Listeria innocua strains exposed to selected stress conditions. Appl. Environ. Microbiol. 74:158–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nannapaneni P, Hertwig F, Depke M, Hecker M, Mäder U, Völker U, Steil L, van Hijum SAFT. 2012. Defining the structure of the general stress regulon of Bacillus subtilis using targeted microarray analysis and random forest classification. Microbiology 158:696–707 [DOI] [PubMed] [Google Scholar]
- 5. Price CW, Fawcett P, Ceremonie H, Su N, Murphy CK, Youngman P. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757–774 [DOI] [PubMed] [Google Scholar]
- 6. Ollinger J, Bowen B, Wiedmann M, Boor KJ, Bergholz TM. 2009. Listeria monocytogenes σB modulates PrfA-mediated virulence factor expression. Infect. Immun. 77:2113–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nadon CA, Bowen BM, Wiedmann M, Boor KJ. 2002. Sigma B contributes to PrfA-mediated virulence in Listeria monocytogenes. Infect. Immun. 70:3948–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fouet A, Namy O, Lambert G. 2000. Characterization of the operon encoding the alternative sigma B factor from Bacillus anthracis and its role in virulence. J. Bacteriol. 182:5036–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bischoff M, Dunman P, Kormanec J, Macapagal D, Murphy E, Mounts W, Berger-Bachi B, Projan S. 2004. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 186:4085–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palmer ME, Chaturongakul S, Wiedmann M, Boor KJ. 2011. The Listeria monocytogenes σB regulon and its virulence-associated functions are inhibited by a small molecule. mBio 2(6):e00241–11 doi:10.1128/mBio.00241-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hecker M, Pané-Farré J, Völker U. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215–236 [DOI] [PubMed] [Google Scholar]
- 13. Marles-Wright J, Grant T, Delumeau O, van Duinen G, Firbank SJ, Lewis PJ, Murray JW, Newman JA, Quin MB, Race PR, Rohou A, Tichelaar W, van Heel M, Lewis RJ. 2008. Molecular architecture of the “stressosome,” a signal integration and transduction hub. Science 322:92–96 [DOI] [PubMed] [Google Scholar]
- 14. Kim T-J, Gaidenko TA, Price CW. 2004. In vivo phosphorylation of partner switching regulators correlates with stress transmission in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 186:6124–6132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delumeau O, Chen CC, Murray JW, Yudkin MD, Lewis RJ. 2006. High-molecular-weight complexes of RsbR and paralogues in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 188:7885–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voelker U, Voelker A, Maul B, Hecker M, Dufour A, Haldenwang WG. 1995. Separate mechanisms activate sigma B of Bacillus subtilis in response to environmental and metabolic stresses. J. Bacteriol. 177:3771–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang X, Kang CM, Brody MS, Price CW. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265–2275 [DOI] [PubMed] [Google Scholar]
- 18. Gaidenko TA, Bie X, Baldwin EP, Price CW. 2011. Substitutions in the presumed sensing domain of the Bacillus subtilis stressosome affect its basal output but not response to environmental signals. J. Bacteriol. 193:3588–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eymann C, Schulz S, Gronau K, Becher D, Hecker M, Price CW. 2011. In vivo phosphorylation patterns of key stressosome proteins define a second feedback loop that limits activation of Bacillus subtilis σB. Mol. Microbiol. 80:798–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang S, Haldenwang WG. 2005. Contributions of ATP, GTP, and redox state to nutritional stress activation of the Bacillus subtilis σB transcription factor. J. Bacteriol. 187:7554–7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brody MS, Vijay K, Price CW. 2001. Catalytic function of an α/β hydrolase is required for energy stress activation of the σB transcription factor in Bacillus subtilis. J. Bacteriol. 183:6422–6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vijay K, Brody MS, Fredlund E, Price CW. 2000. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the σB transcription factor of Bacillus subtilis. Mol. Microbiol. 35:180–188 [DOI] [PubMed] [Google Scholar]
- 23. Brigulla M, Hoffmann T, Krisp A, Volker A, Bremer E, Volker U. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and Its contribution to low-temperature adaptation. J. Bacteriol. 185:4305–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin J-H, Brody MS, Price CW. 2010. Physical and antibiotic stresses require activation of the RsbU phosphatase to induce the general stress response in Listeria monocytogenes. Microbiology 156:2660–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaturongakul S, Boor KJ. 2006. σB activation under environmental and energy stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 72:5197–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaturongakul S, Boor KJ. 2004. RsbT and RsbV contribute to σB-dependent survival under environmental, energy, and intracellular stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 70:5349–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinez L, Reeves A, Haldenwang W. 2010. Stressosomes formed in Bacillus subtilis from the RsbR protein of Listeria monocytogenes allow σB activation following exposure to either physical or nutritional stress. J. Bacteriol. 192:6279–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 29. Price CW, Doi RH. 1985. Genetic mapping of rpoD implicates the major sigma factor of Bacillus subtilis RNA polymerase in sporulation initiation. Mol. Gen. Genet. 201:88–95 [DOI] [PubMed] [Google Scholar]
- 30. Boylan SA, Rutherford A, Thomas SM, Price CW. 1992. Activation of Bacillus subtilis transcription factor sigma B by a regulatory pathway responsive to stationary-phase signals. J. Bacteriol. 174:3695–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boylan SA, Redfield AR, Price CW. 1993. Transcription factor sigma B of Bacillus subtilis controls a large stationary-phase regulon. J. Bacteriol. 175:3957–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rauch M, Luo Q, Muller-Altrock S, Goebel W. 2005. SigB-dependent in vitro transcription of prfA and some newly identified genes of Listeria monocytogenes whose expression is affected by PrfA in vivo. J. Bacteriol. 187:800–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bishop DK, Hinrichs DJ. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005–2009 [PubMed] [Google Scholar]
- 34. Wiedmann M, Arvik TJ, Hurley RJ, Boor KJ. 1998. General stress transcription factor sigma B and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xue G-P, Johnson JS, Dalrymple BP. 1999. High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J. Microbiol. Methods 34:183–191 [Google Scholar]
- 36. Bergholz TM, den Bakker HC, Fortes ED, Boor KJ, Wiedmann M. 2010. Salt stress phenotypes in Listeria monocytogenes vary by genetic lineage and temperature. Foodborne Pathog. Dis. 7:1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olsen KN, Larsen MH, Gahan CGM, Kallipolitis B, Wolf XA, Rea R, Hill C, Ingmer H. 2005. The Dps-like protein Fri of Listeria monocytogenes promotes stress tolerance and intracellular multiplication in macrophage-like cells. Microbiology 151:925–933 [DOI] [PubMed] [Google Scholar]
- 38. Kenney TJ, Moran CP., Jr 1987. Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J. Bacteriol. 169:3329–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benson AK, Haldenwang WG. 1993. Bacillus subtilis sigma B is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc. Natl. Acad. Sci. U. S. A. 90:2330–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bergholz TM, Bowen B, Wiedmann M, Boor KJ. 2012. Listeria monocytogenes shows temperature-dependent and -independent responses to salt stress, including responses that induce cross-protection against other stresses. Appl. Environ. Microbiol. 78:2602–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oliver HF, Orsi RH, Wiedmann M, Boor KJ. 2010. Listeria monocytogenes σB has a small core regulon and a conserved role in virulence but makes differential contributions to stress tolerance across a diverse collection of strains. Appl. Environ. Microbiol. 76:4216–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sue D, Fink D, Wiedmann M, Boor KJ. 2004. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology 150:3843–3855 [DOI] [PubMed] [Google Scholar]
- 43. Alper S, Dufour A, Garsin DA, Duncan L, Losick R. 1996. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J. Mol. Biol. 260:165–177 [DOI] [PubMed] [Google Scholar]
- 44. Alper S, Duncan L, Losick R. 1994. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell 77:195–205 [DOI] [PubMed] [Google Scholar]
- 45. Kalman S, Duncan ML, Thomas SM, Price CW. 1990. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J. Bacteriol. 172:5575–5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wise AA, Price CW. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor sigma B in response to environmental signals. J. Bacteriol. 177:123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benson AK, Haldenwang WG. 1993. Regulation of sigma B levels and activity in Bacillus subtilis. J. Bacteriol. 175:2347–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Delumeau O, Lewis RJ, Yudkin MD. 2002. Protein-protein interactions that regulate the energy stress activation of σB in Bacillus subtilis. J. Bacteriol. 184:5583–5589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Locke JCW, Young JW, Fontes M, Jiménez MJH, Elowitz MB. 2011. Stochastic pulse regulation in bacterial stress response. Science 334:366–369 [DOI] [PMC free article] [PubMed] [Google Scholar]


