Abstract
Toscana virus (TOSV) is a phlebovirus, of the Bunyaviridae family, that is responsible for central nervous system (CNS) injury in humans. Previous data have shown that the TOSV NSs protein is a gamma interferon (IFN-β) antagonist when transiently overexpressed in mammalian cells, inhibiting IRF-3 induction (G. Gori Savellini, F. Weber, C. Terrosi, M. Habjan, B. Martorelli, and M. G. Cusi, J. Gen. Virol. 92:71–79, 2011). In this study, we investigated whether an upstream sensor, which has a role in the signaling cascade leading to the production of type I IFN, was involved. We found a significant decrease in RIG-I protein levels in cells overexpressing TOSV NSs, suggesting that the nonstructural protein interacts with RIG-I and targets it for proteasomal degradation. In fact, the MG-132 proteasome inhibitor was able to restore IFN-β promoter activation in cells expressing NSs, demonstrating the existence of an evasion mechanism based on inhibition of the RIG-I sensor. Furthermore, a C-terminal truncated NSs protein (ΔNSs), although able to interact with RIG-I, did not affect the RIG-I-mediated IFN-β promoter activation, suggesting that the NSs domains responsible for RIG-I-mediated signaling and interaction with RIG-I are mapped on different regions. These results contribute to identify a novel mechanism for bunyaviruses by which TOSV NSs counteracts the early IFN response.
INTRODUCTION
The type I interferon (IFN)-mediated immune response represents the first line of host defense against virus infection (1). When viruses infect cells, intrinsic defensive actions immediately initiate, leading to the production of type I interferons (2). IFNs are induced very rapidly by receptors that monitor the cytosol for the presence of nucleic acids, which is indicative of virus presence. Such receptors include RIG-I-like receptors that recognize RNAs. RIG-I contains a DExD/H-box RNA helicase domain and two N-terminal caspase activation and recruitment domains that allow for interaction with the MAVS mitochondrial adaptor protein (3). This, in turn, triggers the activation of transcription factors which induce transcription of IFNs. Viruses have evolved many different mechanisms to repress the effects of the type I IFN system, producing viral products able to suppress the IFN-mediated signaling pathways (4–7). Examples of viral antagonists of IFN induction include proteases that mediate recruitment of the ubiquitin proteasome system for degradation of cellular targets and proteins which lead to sequestration and inactivation of host proteins involved in the type I IFN response (8). Among the viruses which have the ability to antagonize the IFN system, Toscana virus (TOSV) has recently been recognized to have a nonstructural protein (NSs) with this activity (9). TOSV belongs to the Bunyaviridae family, and it is an important pathogen, causing aseptic meningitis, meningoencephalitis, and encephalitis with a favorable outcome, but severe and lethal infections have been reported recently (10, 11). For this reason, in Europe TOSV is considered an emerging virus, but recently a growing number of TOSV infections in travelers from the Mediterranean area during summer have indicated that this infection should be considered in the differential diagnosis of patients with central nervous system (CNS) infections (12). In a previous study, we demonstrated that TOSV NSs protein inhibits activation of IRF-3 and, hence, IFN induction (9). Like other members of the same family, the Bunyamwera virus (BUNV) and La Crosse virus (LACV) orthobunyaviruses, the Sin Nombre, Tula, and Puumala hantaviruses, and the Rift Valley fever virus (RVFV) and sandfly fever Sicilian virus (SFSV) phleboviruses all express an NSs acting as an IFN antagonist (13–20) and affecting host cell gene expression, IFN synthesis, and IFN action. So far, TOSV NSs is the only bunyaviral NSs shown to target IRF-3 or some upstream sensor involved in the signaling cascade leading to production of type I IFN (9). In this study, we demonstrated that IFN inhibition is based on the degradation of the RIG-I sensor through the action of NSs, and its functional activity is related to the carboxyl terminus of the protein itself.
MATERIALS AND METHODS
Cells, viruses, and chemicals.
Vero (ATCC CCL-81) cells were grown as a monolayer in Dulbecco's modified Eagle's medium (DMEM) (Lonza, Milan, Italy) supplemented with 5% heat-inactivated fetal calf serum (FCS) (Lonza) and 100 U/ml penicillin-streptomycin (HyClone Europe, Milan, Italy) at 37°C. Human embryonic kidney (HEK)-293FT cells (Invitrogen, Milan, Italy) were grown as a monolayer in DMEM (Lonza, Milan, Italy) supplemented with 10% heat-inactivated FCS (Lonza), 100 U/ml penicillin-streptomycin (HyClone Europe), and 300 μg/ml of G-418 (Invitrogen, Milan, Italy) at 37°C. Toscana virus, strain 1812 (TOSV) (isolated from a clinical specimen in the virology laboratory of S. Maria delle Scotte Hospital, Siena, Italy), was plaque purified and propagated on Vero cells. Transfections were performed using the SuperFect transfection reagent (Qiagen, Milan, Italy) by following the manufacturer's instructions. The MG-132 proteasome inhibitor was purchased from Enzo Life Sciences, Lausen, Switzerland.
Plasmids.
Viral RNA was extracted from TOSV-infected Vero cells using a QIAamp viral RNA minikit (Qiagen). The NSs coding gene (GenBank accession no. EU327772; nucleotide [nt] 57 to 1007) was amplified by reverse transcriptase PCR (RT-PCR) from purified viral RNA with NSs BamHI sense (nt 1 to 15), 5′-GGATCCACACAAAGACCTCCC-3′, and NSs XhoI antisense (nt 993 to 1017), 5′-CTCGAGTCATAAGGGTGGGTA-3′ primers (Sigma-Aldrich, Milan, Italy). The reaction was carried out using the SuperScript III one-step RT-PCR with Platinum Taq (Invitrogen) by one cycle of reverse transcription at 50°C for 30 min and 94°C for 2 min, followed by 40 cycles of PCR (1 min at 94°C, 30 s at 60°C, and 1 min at 72°C). The gene (nt 57 to 1007) was cloned in pcDNA4HisMax-A plasmid (Invitrogen) at the BamHI-XhoI unique sites of the polylinker in frame with the 6×His tag, generating the GC-310 recombinant plasmid. A plasmid, called GC-430, expressing a C-terminal-deleted NSs protein (ΔNSs), was generated by cloning the open reading frame (ORF) (nt 1 to 831) in the pcDNA4HisMax-A plasmid. The correct sequence of the constructs was confirmed by sequencing (Applied Biosystems, Monza, Italy). The reporter plasmid encoding firefly luciferase downstream of the complete beta interferon promoter (p125-Luc), the plasmids encoding the RIG-I CARD domains (RIG-IN), and the helicase-regulatory domain (RIG-IC) fused to the FLAG tag were kindly provided by Takashi Fujita (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) (21). The FLAG-tagged human RIG-I expression plasmid was kindly provided by A. García-Sastre (Mount Sinai School of Medicine, New York, NY). The Renilla luciferase reporter plasmid was purchased from Promega (Promega, Milan, Italy). Real-time PCR for IFN-β and RIG-I was performed as described previously (22).
Relative quantification of mRNA by real-time PCR.
Total cellular RNA was extracted from HEK293FT cells infected with TOSV or Sendai virus (SeV), or transfected with different combinations of plasmids using the RNeasy Mini kit (Qiagen), and treated with DNase I. A real-time PCR assay was performed for quantitation of IFN-β and RIG-I mRNA using 100 ng of RNA of each sample and the AgPath-ID one-step RT-PCR kit (Applied Biosystems, Monza, Italy) in an ABI Prism 7000 sequence detection system (Applied Biosystems). The reaction consisted of reverse transcription at 50°C for 30 min, an initial denaturation step at 95°C for 10 min, and then 45 PCR cycles (95°C for 20 s, 60°C for 30 s). Primers and labeled probe for human beta interferon (GenBank accession no. NM002176) and RIG-I (GenBank accession no. NM014314) were hIFN-β forward (5′-CCTCCAAATTGCTCTCCTGTT-3′) (nt 93 to 113), hIFN-β reverse (5′-CAAGCCTCCCATTCAATTGC-3′) (nt 223 to 204), hIFN-β probe (5′-6-carboxyfluorescein [FAM]-AAAGAAGCAGCAATTTTCAGTGTCAGAAGCTCC-6-carboxytetramethylrhodamine [TAMRA]-3′) (nt 167 to 199), RIG-I forward (5′-GACCCTCCCGGCACAGA-3′) (nt 2177 to 2193), RIG-I reverse (5′-TCAGCAACTGAGGTGGCAATC-3′) (nt 2260 to 2240), and RIG-I probe (5′-FAM-TGCATTCAAAGCCAGTGGAGATCACAAT-TAMRA-3′) (nt 2207 to 2234). A human β-actin probe dye VIC-MGB kit (Applied Biosystems) was used for housekeeping gene quantification. To quantify the products, a robust global normalization algorithm was used. Each sampling point was run in triplicate, and the relative quantification of IFN-β and RIG-I was determined. The threshold cycle (CT) values of positive amplicons were analyzed using the ΔΔCT method (23) to get relative fold increase of the IFN-β and RIG-I genes calibrated to the housekeeping gene and normalized with the mock-treated cell control.
Transient expression of NSs and RIG-I.
Transient expression of the recombinant proteins was carried out using SuperFect transfection reagent (Qiagen) by following the manufacturer's instructions. HEK293FT cells were seeded in a 24-well culture plate at a density of 2 × 105 cells per well and incubated at 37°C in a 5% CO2 atmosphere. Cell monolayers were transfected with 0.5 μg of GC-310 or GC-430 NSs-expressing plasmids and 0.05 μg of FLAG-RIG-I plasmid. Forty-eight hours later, cells were collected and assayed for indirect immunofluorescence (IFA). Briefly, cells were spotted on slides and fixed for 10 min at room temperature with cold methanol-acetone. Cells were then incubated for 1 h at 37°C with mouse anti-6×His antibody (GE Healthcare, Milan, Italy) or with mouse anti-FLAG M2 polyclonal serum (Sigma-Aldrich) (1:500 dilution). After cells were washed with phosphate-buffered saline (PBS), fluorescein-labeled anti-mouse IgG (Sigma-Aldrich) was added for 30 min at 37°C. Immunofluorescence was visualized by a Diaplan microscope (Leica Microsystems, Milan, Italy). Where indicated, 36 h posttransfection cells were treated with MG-132 at a final concentration of 10 μM or an equal amount of dimethylsulfoxide (DMSO) and incubated for an additional 12 h prior to further experiments.
Luciferase reporter assay.
A time course effect of TOSV infection on RIG-I and IFN-β expression was analyzed before performing this assay. Viral infection at a multiplicity of infection (MOI) of 0.05 for 48 h provided the best results without causing excessive cell damage. For this reason, we performed all assays using these parameters. For the luciferase reporter assays, 2 × 105 HEK293FT cells were seeded in a 24-well plate and left to grow at 37°C in a 5% CO2 atmosphere. The following day, cell monolayers were infected with TOSV 1812, UV-inactivated TOSV, or SeV Cantell strain (ATCC VR-907) (MOI, 0.05). Five hours postinfection, cells were transfected with the plasmids indicated above. Briefly, 0.2 μg of p125-Luc plasmid and 0.05 μg of FLAG-RIG-I-expressing plasmid were cotransfected along with 20 ng of Renilla luciferase reporter plasmid (pSV40-RL) (Promega) to normalize results. After 48 h, cells were collected and lysed, and luciferase activities were measured with dual luciferase reporter assay reagent (Promega) according to the manufacturer's instructions. In parallel, HEK293FT cells were cotransfected with the reporter plasmids, FLAG-RIG-I plasmid, and, where indicated, 0.5 μg of GC-310 or GC-430 NSs expression plasmid. The total DNA was kept constant by supplementation with an empty vector. Thirty-six hours posttransfection, cells were stimulated with 0.1 μg of poly(I·C). After an additional 12 h, cells were collected and luciferase activities were measured.
Immunoprecipitation (IP) assay.
Transfected cells (1 × 106 cells) were resuspended in a lysis buffer consisting of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100, supplemented with a complete protease inhibitor cocktail (Roche, Milan, Italy). After being clarified by centrifugation, cellular extracts (300 μg) were incubated for 3 h at room temperature with FLAG-M2 magnetic beads (Sigma-Aldrich) or polyclonal anti-6×His antibody (GE Healthcare)/G protein coupled with Sepharose beads (GE Healthcare). Beads were then washed three times with 1 ml of lysis buffer, resuspended in 30 μl of denaturing loading buffer, and assayed by immunoblotting in order to identify precipitated proteins.
Immunoblot analysis.
Cells were analyzed by immunoblotting for RIG-I, NSs, and TOSV N protein expression. Protein concentration of the whole-cell lysates was determined by bicinchoninic acid assay (Pierce, Milan, Italy). A total of 50 μg of total proteins was boiled in SDS sample buffer for 5 min, separated by SDS-PAGE, and transferred to a NitroBind nitrocellulose membrane (Santa Cruz Biotechnology, Heidelberg, Germany). After blocking with 5% nonfat dry milk, the filters were incubated overnight at room temperature with anti-Flag M2 (1:2,000), anti-NSs (1:100), or anti-TOSV N serum (1:100). After being washed three times with PBS containing 0.05% Tween 20 (PBS-T), membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology). After several washes, the target bands were visualized using an enhanced chemiluminescence kit (Pierce) and exposed to BioMax X-ray film (Kodak).
Statistics.
The mean differences were statistically analyzed using Stat View statistical software (Abacus Concepts, Berkeley, CA). P values were calculated by t test. A P value of less than 0.05 was considered statistically significant.
RESULTS
TOSV induces IFN-β through a RIG-I-dependent signaling pathway.
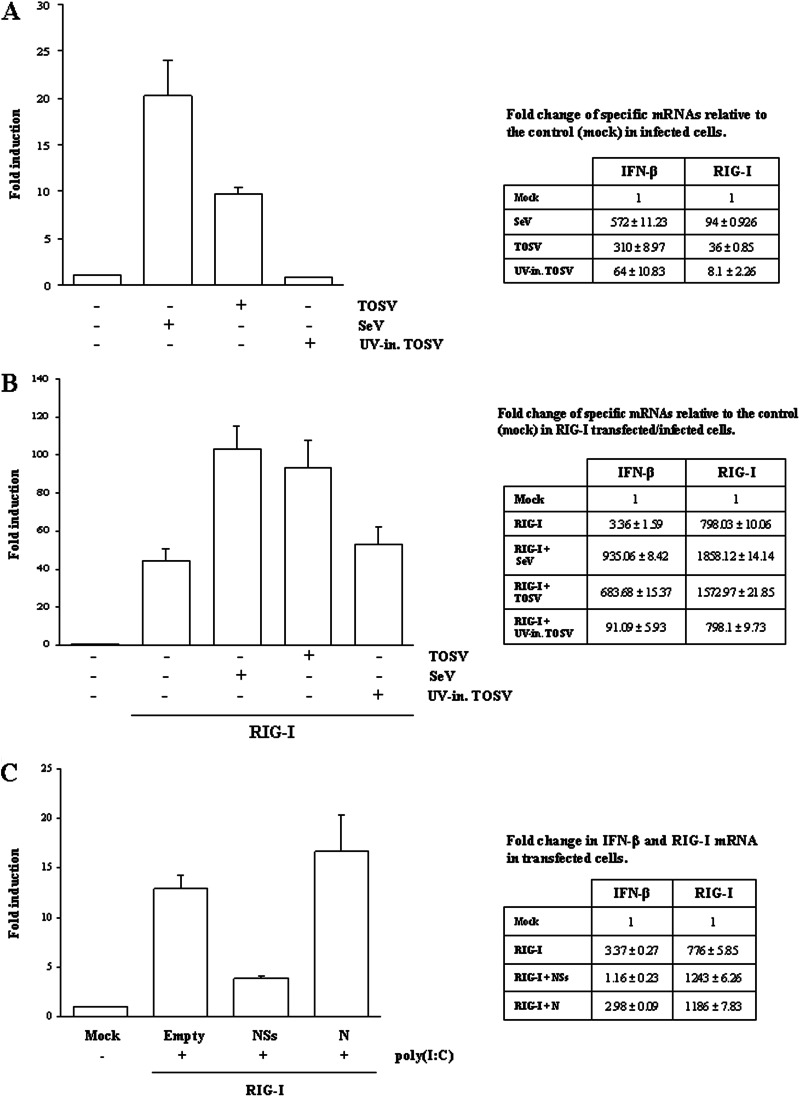
RIG-I and MDA-5 are cellular sensors involved in IFN-β production upon RNA virus infection and double-stranded RNA (dsRNA) recognition. Previous results have shown that TOSV activates transcription of IFN-β during infection, in contrast to that observed for other members of the Bunyaviridae family (9). In fact, many of these viruses possess the nonstructural protein NSs, which counteracts IFN-β production, resulting in viral escape from the host immune response (15, 17, 20, 24–29). To better address the mechanisms which regulate IFN-β production in TOSV infection, the role of RIG-I was investigated. A luciferase reporter assay performed on HEK293FT cells demonstrated that the IFN-β promoter was clearly activated, as expected, by the overexpression of RIG-I upon TOSV or SeV infection (Fig. 1A). On the contrary, this was not observed when UV-inactivated TOSV was used as an inducer. These data were further confirmed by a relative quantification of the IFN-β- and RIG-I-specific mRNA, as shown in Fig. 1A. These results suggest that TOSV was able to induce host innate immunity through RIG-I, which could bind and respond to dsRNA or single-stranded RNA (ssRNA) containing 5′-triphosphate molecules generated during viral replication (3, 30).
Fig 1.
Luciferase reporter assay. (A) HEK293FT cells were mock infected or infected with TOSV, UV-inactivated TOSV, or SeV (MOI. 0.05). Forty-eight hours later, the cleared lysates were used to measure the luciferase activities according to the manufacturer's instructions. Relative Firefly luciferase values were normalized with respect to the Renilla luciferase activity (fold induction). Relative quantitation of specific IFN β or RIG-I mRNA is reported on the right side. (B) Luciferase reporter assay on HEK293FT cells, transfected with 0.5 μg of empty plasmid or RIG-I-expressing plasmid, and mock infected or infected with TOSV, UV-inactivated TOSV, or SeV. (C) Luciferase reporter assay on HEK293FT cells transfected with reporter plasmids alone (mock) or in combination with 0.05 μg of FLAG-RIG-I and 0.5 μg of TOSV NSs, N, or empty plasmids. Thirty-six hours later, cells were stimulated with 0.1 μg of poly(I·C) and luciferase activities were measured. Results are given as mean values of several experiments ± standard deviations.
TOSV NSs affects RIG-I-mediated activation of IFN-β promoter.
Previous data showed that the TOSV NSs protein, when transiently overexpressed, counteracted IFN-β production by inhibiting IRF-3 phosphorylation and activation without affecting its expression and nuclear accumulation (9), suggesting that NSs could block a very early step of IFN-β production, most probably at RNA sensor recognition. The RIG-I like receptors (RIG-I, MDA-5, and LGP2) play a crucial role in innate immunity as sensors, and they enhance IFN-β production following specific stimuli, such as viral infections. To further investigate the role of TOSV NSs in the IFN-β pathway, we explored its effect on RIG-I-mediated activation of the IFN-β promoter by luciferase assay. As expected, IFN-β promoter activation was highly enhanced by RIG-I overexpression upon stimulation with TOSV (Fig. 1B). On the contrary, the stimulatory effect of RIG-I was strongly impaired by NSs expression, resulting in a transcriptional reduction of luciferase, while no effect was recorded when the TOSV N (nucleoprotein) was overexpressed in the same cells (Fig. 1C), indicating that NSs has a specific activity.
The NSs protein of Toscana virus interacts with RIG-I.
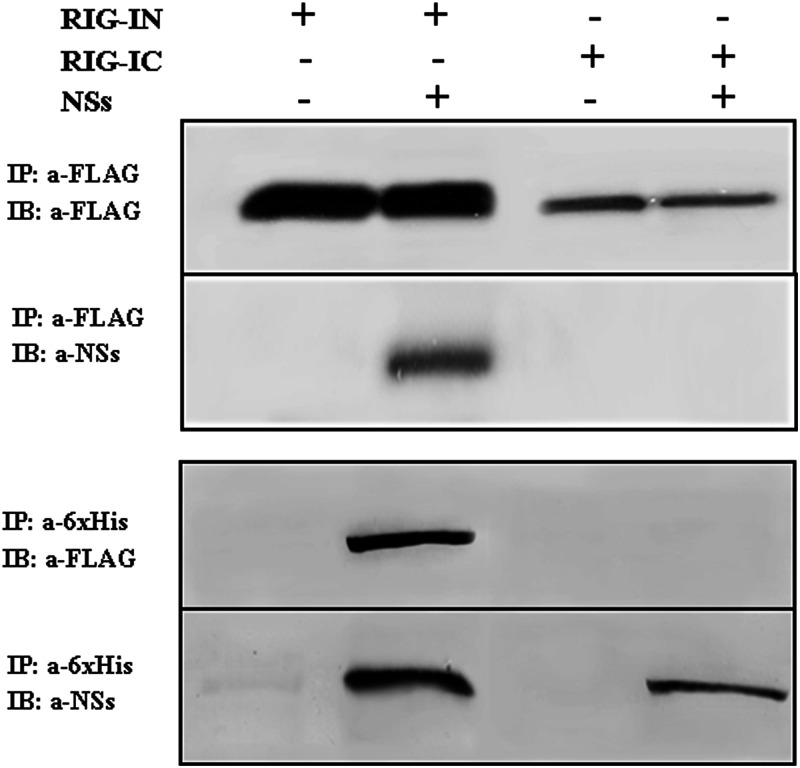
In order to verify whether the inhibitory effect of NSs was due to a direct interaction with RIG-I and to determine the potential region of RIG-I interacting with NSs, we performed cotransfection experiments using FLAG-RIG-I and 6×His-NSs expression vectors. Co-IP/Western blot analysis using anti-FLAG or anti-6×His antibody showed that NSs interacted with the RIG-I protein, although it was possible to reveal this interaction only when RIG-IN was used, implying a possible partial degradation of the whole sensor in the presence of NSs. The assay revealed that the NSs protein directly interacted with the CARD domains of RIG-I (RIG-IN) but not with RIG-IC (Fig. 2). Unfortunately, it was not possible to confirm this interaction upon TOSV infection, since the NSs protein production was too low.
Fig 2.
Coimmunoprecipitation assay. HEK293FT cells were cotransfected with FLAG-tagged RIG-I N terminus (RIG-IN) or C terminus (RIG-IC) along with 6×His-tagged TOSV NSs. Clarified lysates (300 μg) were subjected to co-IP by using either anti-FLAG M2 magnetic beads (upper panel) or polyclonal anti-6×His antibody (lower panel). The presence of RIG-I and NSs proteins in the immune complex was revealed by immunoblotting (IB) with anti-FLAG M2 or anti-NSs antibodies.
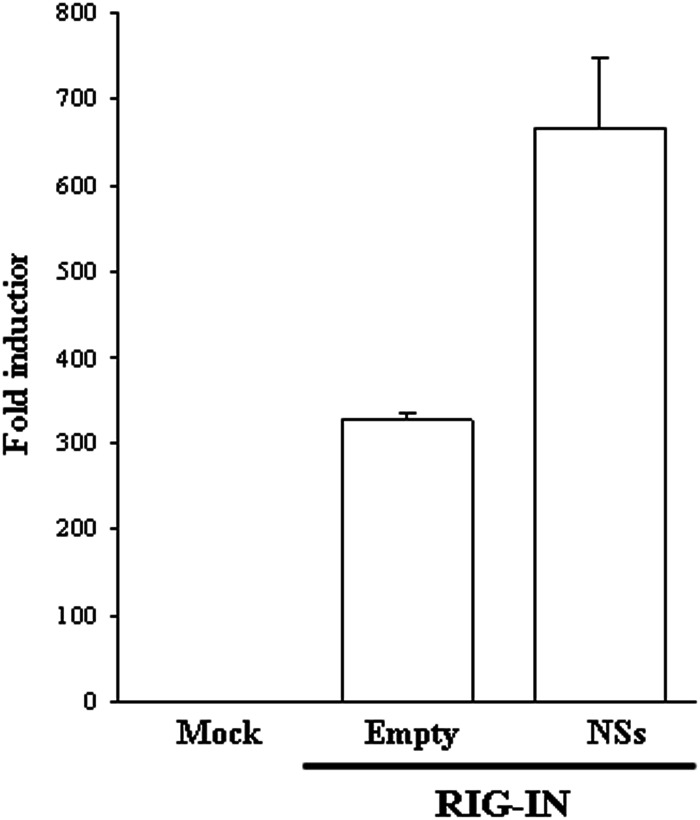
Furthermore, we examined whether the interaction between RIG-IN and NSs could have a functional consequence. Despite the interaction between RIG-IN and NSs, neither RIG-I degradation, tested by immunofluorescence (data not shown), nor the inhibition of IFN-β promoter activation by luciferase reporter assay (Fig. 3) was reported for cells coexpressing RIG-IN and NSs. A possible explanation of this phenomenon is that the domain(s) involved in the degradation process of RIG-I is located in its carboxyl terminus, and that upon the binding with NSs, a conformational change of RIG-I occurs, affecting the amino acid sequence missing in RIG-IN. NSs did not show any effect on RIG-IC, as expected.
Fig 3.
IFN-β promoter activation was evaluated by luciferase reporter assay in HEK293FT cells transfected with reporter plasmids along with FLAG-tagged RIG-IN, in combination with NSs expressing plasmid or the empty vector. Results are expressed as the average IFN-β promoter fold induction. The error bars represent the standard deviations from the mean values obtained from independent experiments.
RIG-I degradation after interaction with NSs protein.
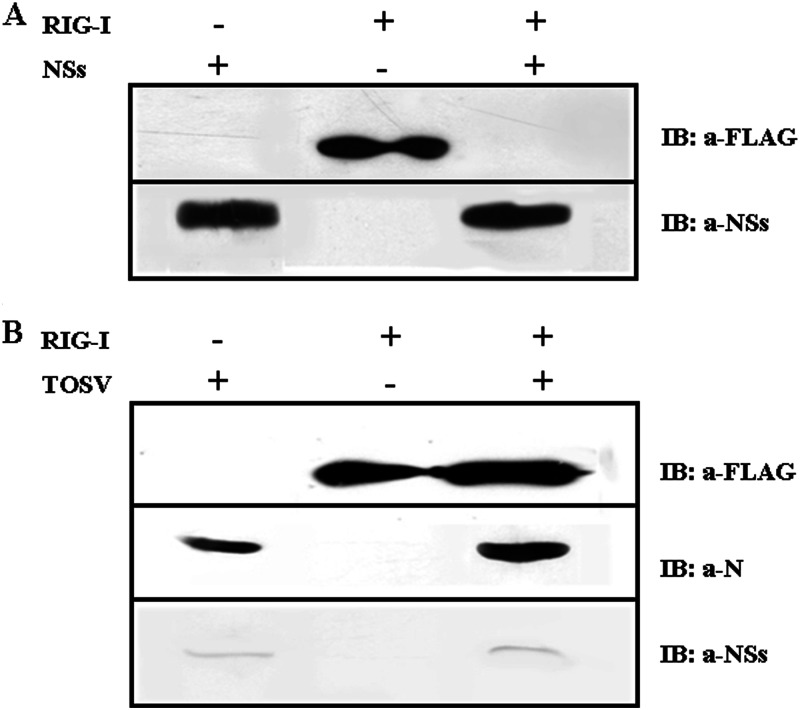
Several viruses escape from host innate immune response by inhibiting RIG-I activation and signaling or by mediating their downregulation in terms of protein degradation (5, 6, 31–37). These observations led us to investigate whether NSs have any regulatory effect on RIG-I. For this purpose, cells expressing FLAG-RIG-I, alone or in combination with NSs, after transfection were assayed for the presence of RIG-I-positive cells by immunofluorescence. The overexpression of NSs caused a marked decrease in cells expressing RIG-I compared to cells transfected with RIG-I plasmid only, as demonstrated by counting fluorescent cells (774 ± 32 positive cells versus 345 ± 46 in the presence of NSs). This result was supported by immunoblot analysis; in fact, RIG-I was not detected in cells overexpressing the NSs protein, indicating that RIG-I could be degraded (Fig. 4A). On the contrary, this sensor was revealed when cells transfected with RIG-I plasmid were infected with TOSV, suggesting that this could be due to the small amount of NSs which, produced late during virus replication, was not sufficient to interfere with RIG-I (Fig. 4B) (9). In order to check whether the regulatory effect of the NSs protein occurred at the transcriptional level, we analyzed the relative amount of RIG-I-specific mRNA in cells with or without NSs. We did not observe any decrease in the specific transcript by quantitative RT-PCR, neither in the presence nor the absence of NSs (Fig. 1C), indicating that the disappearance of RIG-I was due to a possible degradation after interaction with NSs. On the basis of these results, we believe that the antagonistic effect of the TOSV NSs protein was related to a downregulation of the RIG-I viral sensor protein rather than to a direct effect on IRF-3.
Fig 4.
Immunoblot detection of RIG-I protein in cells coexpressing TOSV NSs protein (A) or infected by TOSV (B) using anti-FLAG M2 and anti-NSs antibodies, respectively. Anti-N serum was used to evaluate the expression of viral antigen in selected samples.
Proteasome-mediated RIG-I degradation.
To better understand whether RIG-I degradation occurs through a direct activity of the NSs protein or by an indirect activation of cellular proteases, the effects of the MG-132 proteasome inhibitor were tested. The treatment of cotransfected cells with MG-132 restored RIG-I protein, abolishing the antagonistic activity of NSs (Fig. 5A). The effect of MG-132 treatment was better confirmed by an IFN-β promoter activation assay in which the inhibition exerted by the NSs on RIG-I-mediated IFN-β activation was abrogated by MG-132 in a specific manner, as the TOSV nucleoprotein expression did not affect RIG-I activity in the presence or absence of the proteasome inhibitor (Fig. 5B). Surprisingly, MG-132 strongly enhanced NSs protein expression. In fact, a robust increase in the NSs protein was noticed both when it was expressed alone and in combination with RIG-I (data not shown). This suggests the stability of NSs could be augmented by a direct effect of MG-132 on the protein itself. In this context, since MG-132 increased the amount of NSs protein, a stronger inhibitor effect of RIG-I would be expected. On the contrary, RIG-I was not degraded in the presence of MG-132, and its activity was fully restored. Further studies are in progress to better understand the effect of this drug on NSs turnover.
Fig 5.
Effects of the MG-132 proteasome inhibitor were investigated in HEK293FT cells transfected with RIG-I and NSs plasmids. (A) Cells were collected and assessed for indirect immunofluorescence with anti-FLAG M2, anti-6×His, or anti-TOSV N antibodies. (B) The ability of MG-132 to revert the antagonistic effects of the NSs protein was further investigated by luciferase reporter assay evaluating the activation of the IFN-β promoter under different conditions. Luciferase activities were measured in each sample, and results are reported as mean values ± standard deviations from at least three independent experiments.
NSs functional domains.
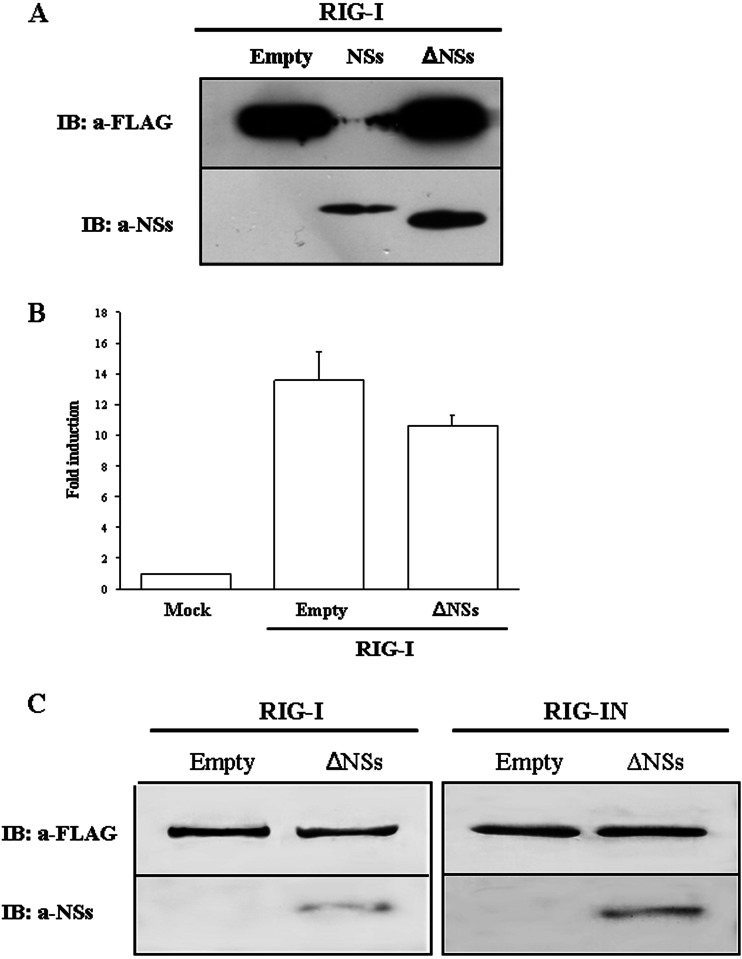
A plasmid (GC-430) expressing a carboxyl terminus-deleted NSs protein (ΔNSs) was tested to evaluate its activity on RIG-I. Immunofluorescence staining and immunoblotting performed on HEK293FT cells expressing RIG-I and ΔNSs revealed that the NSs C terminus is critical for its antagonistic activity toward IFN-β. In fact, RIG-I was expressed at the same amount in the presence or absence of ΔNSs protein without being degraded (Fig. 6A). In addition, the luciferase reporter assay confirmed that ΔNSs had no significant inhibitory activity on IFN-β promoter induction (P < 0.05) (Fig. 6B). Thus, it appeared that the antagonistic activity of NSs was strictly related to a domain located in the carboxyl-terminal sequence. Nevertheless, ΔNSs was still able to interact with both RIG-I and RIG-IN, since they coprecipitated in IP assays (Fig. 6C). This suggests that the NSs domain interacting with RIG-I was different from that involved in the inhibition/degradation of RIG-I.
Fig 6.
HEK293FT cells were transfected with RIG-I alone or in combination with full-length NSs or ΔNSs plasmids. Equal amounts of whole-cell lysates were subjected to immunoblotting (A) or to luciferase reporter assay (B). Graph bars represent mean results from different experiments ± standard deviations. (C) HEK293FT transfected with FLAG-tagged RIG-I or RIG-IN, alone or in combination with ΔNSs plasmids, were lysed and precipitated with anti-FLAG M2 magnetic beads. RIG-I and NSs proteins were detected by immunoblotting with anti-FLAG M2 and anti-NSs antibodies.
DISCUSSION
The innate immune response represents a fundamental component of the host defense against viruses, coordinating the activation of transcription factors that regulate the expression of inducible gene products with antiviral and/or inflammatory activity. As a consequence, viruses have developed strategies to evade the host immune responses, mainly by targeting the type I interferon system. Among the several viruses which have adopted different strategies to counteract the innate immune system, those belonging to the Bunyaviridae family appear to act in different ways. In fact, the Bunyamwera virus produces a nonstructural protein involved in silencing host protein expression by interfering with mRNA synthesis (27); Rift Valley fever virus promotes the degradation of double-stranded RNA-dependent protein kinase (PKR) (18, 24), and it induces a general transcriptional shutoff the infected cell (16, 18). La Crosse virus counteracts subsequent IFN gene transcription by specifically and rapidly shutting down RNAPII (38, 39). Toscana virus, which is now considered an emerging pathogen for humans (12), is a member of this large family and shows yet another behavior, different from those described above. Our previous studies showed that TOSV NSs is the only bunyaviral nonstructural protein that has been shown to target IRF-3, preventing its dimerization and, hence, the activation of IFN transcription (9). In this study, we revealed the inhibitory effect of NSs on RIG-I involved in the signaling cascade for type I IFN production, leading to degradation of RIG-I upon binding. In particular, we demonstrated that NSs inhibits IFN-β activation via a specific interaction requiring the 29-amino-acid N terminus of RIG-I. So far, viruses such as rhinoviruses, echovirus, and encephalomyocarditis virus 3C proteases have been shown to cleave RIG-I (33, 40), while respiratory syncytial virus NS2 and influenza A virus NS1 antagonize IFN-β transcription via interaction with RIG-I (5, 36). Among bunyaviruses, TOSV is the first virus to be shown to interact with RIG-I. In fact, NSs appears to directly bind the CARD domain of RIG-I and to mediate its degradation, thus abolishing the downstream signaling of IFN-β activation. We were able to identify a domain correlated with the functional activity of NSs; indeed, the deleted form of the carboxyl terminus, ΔNSs, despite its ability to bind RIG-I, appeared unable to mediate RIG-I degradation and to inhibit the downstream activation of the signaling pathway. Consequently, we deduced that the domain (or possible domains) of NSs able to associate with RIG-I is not located in the carboxyl terminus of the antigen, but it appears critical for the NSs to carry out its inhibitory role. This activity gives rise to the question of whether this viral protein has a proteolytic activity or if it activates a proteasome-mediated degradation pathway. Interestingly, NSs was scarcely expressed in HEK293FT cells upon plasmid transfection; however, treatment with MG-132 increased the level of protein, suggesting a protein-stabilizing effect of this drug, as already observed in orthobunyavirus NSs (7, 41). The addition of MG-132 to the cells widely restored IFN-β promoter activation, suggesting that RIG-I degradation, mediated by NSs, was proteasome dependent and specific, since the nucleoprotein of TOSV did not show any of these effects. It is interesting that TOSV NSs shares a protease-related activity with the nonstructural protein of other members of the Bunyaviridae family, although the target is different (24, 38, 42). Thus, it is tempting to speculate that this protein is derived from a common ancestor and has successively undergone many changes while maintaining the same activity. To support this hypothesis, a common so-called reaper-like sequence, involved in a proapoptotic activity, has been recognized in the nonstructural protein of some members of the Bunyaviridae family (43). Thus, further studies are needed to understand why this protein, which antagonizes the IFN-β system, has developed different mechanisms of action even within the same viral family. In conclusion, this study provides us with new insights into how TOSV NSs interferes in the RIG-I signaling pathway, but the domains and/or other factors involved in this function still remain to be defined.
Footnotes
Published ahead of print 3 April 2013
REFERENCES
- 1. García-Sastre A, Biron CA. 2006. Type 1 interferons and the virus-host relationship: a lesson in détente. Science 312:879–882 [DOI] [PubMed] [Google Scholar]
- 2. Samuel CE. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoneyama M, Fujita T. 2008. RNA recognition and signal transduction by RIG-like receptors. Immunity 227:54–65 [DOI] [PubMed] [Google Scholar]
- 4. Haller O, Kochs G, Weber F. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr, Garcia-Sastre A. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81:514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. 2007. IFN-beta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol. 9:930–938 [DOI] [PubMed] [Google Scholar]
- 7. Van Knippenberg I, Calton-Smith C, Eliott RM. 2010. The N-terminus of Bunyamwera orthobunyavirus NSs protein is essential for interferon antagonism. J. Gen. Virol. 91:2002–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mclnerney GM, Karlsson Hedestam GB. 2009. Direct cleavage, proteasomal degradation and sequestration: three mechanisms of viral subversion of type I interferon responses. J. Innate Immun. 1:599–606 [DOI] [PubMed] [Google Scholar]
- 9. Gori Savellini G, Weber F, Terrosi C, Habjan M, Martorelli B, Cusi MG. 2011. Toscana virus induces interferon although its NSs protein reveals antagonistic activity. J. Gen. Virol. 92:71–79 [DOI] [PubMed] [Google Scholar]
- 10. Kuhn J, Bewermeyer H, Hartmann-Klosterkoetter U, Emmerich P, Schilling S, Valassina M. 2005. Toscana virus causing severe meningoencephalitis in an elderly traveler. J. Neurol. Neurosurg. Psychiatr. 76:1605–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartels S, de Boni L, Kretzschmar HA, Heckmann JG. 2012. Lethal encephalitis caused by Toscana virus in an elderly patient. J. Neurol. 259:175–177 [DOI] [PubMed] [Google Scholar]
- 12. Jaijakul S, Arias CA, Hossain M, Arduino RC, Wootton SH, Hasbun R. 2012. Toscana meningoencephalitis: a comparison to other viral central nervous system infections. J. Clin. Virol. 55:204–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227–264 [DOI] [PubMed] [Google Scholar]
- 14. Weber F, Bridgen A, Fazakerley JK, Streitenfeld H, Kessler N, Randall RE, Elliott RM. 2002. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76:7949–7955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Billecocq A, Spiegel M, Vialat P, Kohl A, Weber F, Bouloy M, Haller O. 2004. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 78:9798–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le May N, Dubaele S, Proietti De Santis L, Billecocq A, Bouloy M, Egly JM. 2004. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell 20:541–550 [DOI] [PubMed] [Google Scholar]
- 17. Blakqori G, Delhaye S, Habjan M, Blair CD, Sánchez-Vargas I, Olson KE, Attarzadeh-Yazdi G, Fragkoudis R, Kohl A, Kalinke U, Weiss S, Michiels T, Staeheli P, Weber F. 2007. La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts. J. Virol. 81:4991–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ikegami T, Narayanan K, Won S, Kamitani W, Peters CJ, Makino S. 2009. Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation. PLoS Pathog. 5:e1000287 doi: 10.1371/journal.ppat.1000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spiropoulou CF, Albariño CG, Ksiazek TG, Rollin PE. 2007. Andes and Prospect Hill hantaviruses differ in early induction of interferon although both can downregulate interferon signaling. J. Virol. 81:2769–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jääskeläinen KM, Kaukinen P, Minskaya ES, Plyusnina A, Vapalahti O, Elliott RM, Weber F, Vaheri A, Plyusnin A. 2007. Tula and Puumala hantavirus NSs ORFs are functional and the products inhibit activation of the interferon-beta promoter. J. Med. Virol. 79:1527–1536 [DOI] [PubMed] [Google Scholar]
- 21. Yoneyama M, Suhara W, Fukuhara Y, Sato M, Ozato K, Fujita T. 1996. Autocrine amplification of type I interferon gene expression mediated by interferon stimulated gene factor 3 (ISGF3). J. Biochem. 120:160–169 [DOI] [PubMed] [Google Scholar]
- 22. Kim MJ, Hwang SJ, Imaizumi T, Yoo JY. 2008. Negative feedback regulation of RIG-I-mediated antiviral signaling by interferon-induced ISG15 conjugation. J. Virol. 82:474–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔ CT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 24. Habjan M, Pichlmair A, Elliott RM, Overby AK, Glatter T, Gstaiger M, Superti-Furga G, Unger H, Weber F. 2009. NSs protein of Rift Valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol. 83:4365–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendenhall M, Wong MH, Skirpstunas R, Morrey JD, Gowen BB. 2009. Punta Toro virus (Bunyaviridae, Phlebovirus) infection in mice: strain differences in pathogenesis and host interferon response. Virology 395:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le May N, Mansuroglu Z, Léger P, Josse T, Blot G, Billecocq A, Flick R, Jacob Y, Bonnefoy E, Bouloy M. 2008. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus infected cells. PLoS Pathog. 4:e13 doi: 10.1371/journal.ppat.0040013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bridgen AM, Weber F, Fazakerley JK, Elliott RM. 2001. Bunyamwera bunyavirus non-structural protein NSs is nonessential gene product that contributes to the viral pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 98:664–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Léonard VH, Kohl A, Hart TJ, Elliott RM. 2006. Interaction of Bunyamwera orthobunyavirus NSs protein with mediator protein MED8: a mechanism for inhibiting the interferon response. J. Virol. 80:9667–9675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weber F, Haller O. 2007. Viral suppression of the interferon system. Biochimie 89:836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Randall RE, Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1–47 [DOI] [PubMed] [Google Scholar]
- 31. Dudek SE, Wixler L, Nordhoff C, Nordmann A, Anhlan D, Wixler V, Ludwig S. 2011. The influenza virus PB1-F2 protein has interferon-antagonistic activity. Biol. Chem. 392:1135–1144 [DOI] [PubMed] [Google Scholar]
- 32. Pachler K, Vlasak R. 2011. Influenza C virus NS1 protein counteracts RIG-I-mediated IFN signalling. Virol. J. 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barral PM, Sarkar D, Fisher PB, Racaniello VR. 2009. RIG-I is cleaved during picornavirus infection. Virology 391:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo Z, Chen LM, Zeng H, Gomez JA, Plowden J, Fujita T, Katz JM, Donis RO, Sambhara S. 2007. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am. J. Respir. Cell Mol. Biol. 363:263–269 [DOI] [PubMed] [Google Scholar]
- 35. Tasaka M, Sakamoto N, Itakura Y, Nakagawa M, Itsui Y, Sekine-Osajima Y, Nishimura-Sakurai Y, Chen CH, Yoneyama M, Fujita T, Wakita T, Maekawa S, Enomoto N, Watanabe M. 2007. Hepatitis C virus non-structural proteins responsible for suppression of the RIG-I/Cardif-induced interferon response. J. Gen. Virol. 88:3323–3333 [DOI] [PubMed] [Google Scholar]
- 36. Ling Z, Tran KC, Teng MN. 2009. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J. Virol. 83:3734–3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cárdenas WB, Loo YM, Gale M, Jr, Hartman AL, Kimberlin CR, Martínez-Sobrido L, Saphire EO, Basler CF. 2006. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 80:5168–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verbruggen P, Ruf M, Blakqori G, Överby AK, Heidemann M, Eick D, Weber F. 2011. Interferon antagonist NSs of La Crosse virus triggers a DNA damage response-like degradation of transcribing RNA polymerase II. J. Biol. Chem. 286:3681–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomas D, Blakqori G, Wagner V, Banholzer M, Kessler N, Elliott RM, Haller O, Weber F. 2004. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J. Biol. Chem. 279:31471–33147 [DOI] [PubMed] [Google Scholar]
- 40. Papon L, Oteiza A, Imaizumi T, Kato H, Brocchi E, Lawson TG, Akira S, Mechti N. 2009. The viral RNA recognition sensor RIG-I is degraded during encephalomyocarditis virus (EMCV) infection. Virology 393:311–318 [DOI] [PubMed] [Google Scholar]
- 41. Hart TJ, Kohl A, Elliott RM. 2009. Role of the NSs protein in the zoonotic capacity of orthobunyaviruses. Zoonoses Public Health 56:285–296 [DOI] [PubMed] [Google Scholar]
- 42. Kalveram B, Lihoradova O, Ikegami T. 2011. NSs protein of rift valley fever virus promotes posttranslational downregulation of the TFIIH subunit p62. J. Virol. 88:6234–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colón-Ramos DA, Irusta PM, Gan EC, Olson MR, Song J, Morimoto RI, Elliott RM, Lombard M, Hollingsworth R, Hardwick JM, Smith GK, Kornbluth S. 2003. Inhibition of translation and induction of apoptosis by Bunyaviral nonstructural proteins bearing sequence similarity to reaper. Mol. Biol. Cell 14:4162–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]