Abstract
DNA vaccines formulated with the cationic lipid-based adjuvant Vaxfectin induce protective immunity in macaques after intradermal (i.d.) or intramuscular (i.m.) delivery of 0.5 to 1 mg of codon-optimized DNA encoding the hemagglutinin (H) and fusion (F) proteins of measles virus (MeV). To characterize the effect of Vaxfectin at lower doses of H+F DNA, rhesus macaques were vaccinated twice with 20 μg of DNA plus Vaxfectin i.d., 100 μg of DNA plus Vaxfectin i.d., 100 μg of DNA plus Vaxfectin i.m. or 100 μg of DNA plus phosphate-buffered saline (PBS) i.m. using a needleless Biojector device. The levels of neutralizing (P = 0.036) and binding (P = 0.0001) antibodies were higher after 20 or 100 μg of DNA plus Vaxfectin than after 100 μg of DNA plus PBS. Gamma interferon (IFN-γ)-producing T cells were induced more rapidly than antibody, but were not improved with Vaxfectin. At 18 months after vaccination, monkeys were challenged with wild-type MeV. None developed rash or viremia, but all showed evidence of infection. Antibody levels increased, and IFN-γ- and interleukin-17-producing T cells, including cells specific for the nucleoprotein absent from the vaccine, were induced. At 3 months after challenge, MeV RNA was detected in the leukocytes of two monkeys. The levels of antibody peaked 2 to 4 weeks after challenge and then declined in vaccinated animals reflecting low numbers of bone marrow-resident plasma cells. Therefore, Vaxfectin was dose sparing and substantially improved the antibody response to the H+F DNA vaccine. This immune response led to protection from disease (rash/viremia) but not from infection. Antibody responses after challenge were more transient in vaccinated animals than in an unvaccinated animal.
INTRODUCTION
Measles remains an important cause of child morbidity and mortality in developing countries despite the availability of a safe and effective live attenuated virus vaccine (1–3). Recent efforts to reduce mortality through increased routine vaccination combined with supplemental immunization activities have improved measles control but have been difficult to sustain (4–6). One impediment is the inability to reliably immunize infants younger than 9 months of age due to immaturity of the immune system and the interference of maternal antibody (7, 8). In high-transmission settings, this leads to a window of susceptibility, and many infants, particularly those born to HIV-infected mothers, acquire measles during the first year of life (9–11).
A measles vaccine for infants under the age of 6 months could improve measles control by allowing delivery with other infant vaccines. DNA vaccines are attractive candidates for development because they are safe, are relatively inexpensive to produce, may not require a cold chain, induce strong cellular immune responses, and can be delivered without the use of a syringe and needle (12). However, DNA vaccines have often been disappointing when tested in humans and nonhuman primates because of the relatively poor induction of antibody (13). Approaches to improving responses have included increasing the amount of DNA given, microparticle formulation, plasmid redesign, altered delivery methods, and use of adjuvants (14–18).
One safe and easily manufactured adjuvant class consists of cationic lipids (19, 20). Vaxfectin, an equimolar mixture of the cationic lipid GAP-DMORIE [(±)-N-(3-aminopropyl)-N,N-dimethyl-2,3-bis(cis-9-tetradecenyloxy)-1-propanaminium bromide)] and a neutral colipid DPyPE (1,2-diphytanoyl-sn-glydero-3-phosphoethanolamine) (21, 22) is dose sparing and enhances antigen-specific antibody production in small animals (21–31). Limited evaluation in humans and nonhuman primates (21, 32–35) has also indicated that it is well tolerated.
Measles virus (MeV) encodes two surface glycoproteins, hemagglutinin (H) and fusion (F), involved in attachment and entry. MeV uses at least three cellular receptors for entry of different target cells: membrane cofactor protein or CD46, a complement regulatory protein present on all nucleated cells (36, 37); signaling lymphocytic activation molecule (SLAM) or CD150, present on activated immune cells (38); and poliovirus receptor-related 4 (PVRL4) or nectin 4, present on epithelial cells (39, 40). Both vaccine and wild-type (WT) strains can use SLAM as a receptor, and most H proteins can bind both CD46 and SLAM, but receptor affinity and efficiency of entry differ, and most WT viruses cannot use CD46 as an entry receptor (41–48). Antibodies that inhibit MeV infection in neutralization assays are directed primarily against the H protein, which also contains important CD8+ T cell epitopes (49). Vaccine-induced antibody that does not neutralize WT virus can lead to enhanced disease (50, 51).
Because protection from measles correlates best with the quality and quantity of neutralizing antibodies at the time of exposure (52, 53), most experimental vaccines have used H alone or H and F for induction of MeV protective immunity (52, 54–56). We have previously shown that juvenile and infant macaques are protected from rash and viremia after intradermal (i.d.; 500 μg) or intramuscular (i.m.; 1 mg) vaccination with two doses of Vaxfectin-formulated plasmid DNA encoding codon-optimized H and F (32). In the present study, we have directly compared the immune responses and protective efficacy in rhesus macaques of low doses of Vaxfectin-formulated and naked MeV H+F DNA delivered i.d. (100 and 20 μg) and i.m. (100 μg) with a needle-free Biojector device.
MATERIALS AND METHODS
Animals.
Thirteen 1-year-old juvenile rhesus macaques (Macaca mulatta) born to measles naive mothers were obtained from the Johns Hopkins Primate Breeding Facility. Monkeys were anesthetized with ketamine (10 to 15 mg/kg) during procedures. All animals were maintained within the guidelines, and studies were performed in accordance with experimental protocols approved by the Animal Care and Use Committee for Johns Hopkins University.
Vaccine, vaccination, and challenge.
As previously described (32), codon-optimized DNA encoding MeV Moraten strain H and F proteins were used to produce plasmids VR7302 (H) and VR7303 (F). Prior to vaccination DNA was formulated with Vaxfectin (22) or with phosphate-buffered saline (PBS) and delivered intramuscularly (i.m.) or intradermally (i.d.) with a Biojector 2000 needle-free injection system (Bioject, Inc., Tualatin, OR). Groups of three monkeys were vaccinated with either 100 μg of Vaxfectin-formulated H+F i.d. (14V, 30V, and 33V), 20 μg of Vaxfectin-formulated H+F i.d. (32V, 37V, and 39V), 100 μg of PBS-formulated H+F i.m. (20V, 22V, and 28V), or 100 μg of Vaxfectin-formulated H+F i.m. (24V, 26V, and 31V) in a volume of 100 μl on days 0 and 28. Monkeys were bled at 1- to 2-week intervals to monitor vaccine-induced immune responses. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized blood by density gradient centrifugation using Lympholyte Mammal (Cedarlane Laboratories). Plasma was collected and stored at −20°C. Monkey 32V was euthanized 11 months after vaccination for reasons unrelated to the present study.
All vaccinated monkeys and an unvaccinated measles-naive monkey (55V) were challenged intratracheally with 104 tissue culture 50% infectious doses (TCID50) of the WT Bilthoven strain of MeV (A. Osterhaus, Erasmus University, Rotterdam, Netherlands) 18 months after vaccination. Monkeys were shaved, examined for rash, and bled at regular intervals to monitor viremia and immune responses. Plasma from three monkeys (46U, 55U, and 67U) that were similarly challenged was used for comparison of MeV neutralization.
Virus assays.
Viremia was assessed by cocultivation in triplicate of serial dilutions of PBMCs with Vero cells expressing human CD150 (Vero/hSLAM cells) (47) in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. Wells were scored at 120 h for MeV-positive syncytia. The data are reported as numbers of infected cells/106 PBMCs. MeV RNA was measured by quantitative reverse transcription-PCR (RT-PCR) for the nucleocapsid (N) gene as previously described (57).
Antibody assays.
Neutralizing antibodies were measured by the ability of serially diluted plasma to reduce plaque formation by the Chicago-1 strain of MeV on Vero cells by 50% (i.e., plaque reduction neutralization [PRN]). Plasma from 48 weeks after immunization was tested for neutralization of Bilthoven WT MeV infection of Vero/hSLAM cells, as well as for neutralization of Chicago-1 MeV infection of Vero cells. The data are expressed as geometric means of the reciprocal titer.
For enzyme immunoassays (EIAs), MeV-infected Vero cell lysate (Advanced Biotechnologies, Columbia, MD) was used (1.16 μg of protein/well) to coat 96-well Maxisorp plates (Nunc, Rochester, NY) and then incubated overnight at 4°C with plasma diluted 1:100 to 1:300, followed by alkaline phosphatase-conjugated rabbit antibody to monkey IgG (Biomakor; Accurate Chemicals, Westbury, NJ) or horseradish peroxidase (HRP)-conjugated goat antibody to monkey IgM (Nordic, Capistrano Beach, CA). The data are presented as optical density (OD) values.
To measure antibody-secreting cells (ASCs) in blood and bone marrow, mononuclear cells were isolated by density gradient centrifugation as described above. Cells were added to Multiscreen HTS HA Opaque ELIspot plates (Millipore) coated with MeV-infected Vero cell lysate or purified goat anti-monkey IgG, IgM, and IgA (H and L; Open Biosystems) for 6 h. To measure MeV-specific ASCs in bone marrow, 5 × 105 cells were added to each of 8 replicate wells. To measure MeV-specific ASCs in PBMCs and total ASCs, 2-fold serial dilutions, starting at 5 × 105 cells, were added to each of six wells. After incubation, bound immunoglobulin was detected with HRP-conjugated goat anti-monkey IgG, developed with stable diaminobenzidine solution (DAB; Invitrogen, Carlsbad, CA). Plates were read on an ImmunoSpot plate reader and analyzed using ImmunoSpot 5.0 software, both obtained from Cellular Technology, Cleveland, OH. The data on MeV-specific ASCs are presented as spot-forming cells (SFCs)/106 PBMCs or 5 × 106 bone marrow cells.
T cell ELISPOT assays.
A total of 1 × 105 to 5 × 105 PBMCs was added to plates coated with antibody to human gamma interferon (IFN-γ; 2 μg/ml), interleukin-4 (IL-4; 5 μg/ml; BD Pharmingen), or IL-17 (2 μg/ml; eBioscience, clone eBio64cap17), along with 1 μg of pooled MeV H, F, or N peptides/ml, 5 μg of concanavalin A/ml, 5.8 μg of MeV-infected Vero cell lysate/ml, or medium alone. After 40 h at 37°C, the plates were washed and incubated with 1 μg of biotinylated antibody to IFN-γ (Mabtech)/ml, 2 μg of biotinylated antibody to IL-4 (Pharmingen)/ml, or 1 μg of biotinylated antibody to IL-17 (eBioscience, clone eBio64Dec17)/ml at 37°C for 2 h. After washing, 50 μl of HRP-conjugated avidin (Vector Laboratories) was added to each well, followed by incubation for 1 h at 37°C. The assays were developed with DAB. Wells were scanned in an ImmunoSpot reader and analyzed using ImmunoSpot 5.0 software. MeV-specific data are presented as SFCs/106 PBMCs after subtraction of the medium control.
Statistical analysis.
Student unpaired t test was used for comparisons between two groups, and one-way analysis of variance (ANOVA) was used for comparison between multiple groups of monkeys using Prism 4 software.
RESULTS
Antibody response to vaccination.
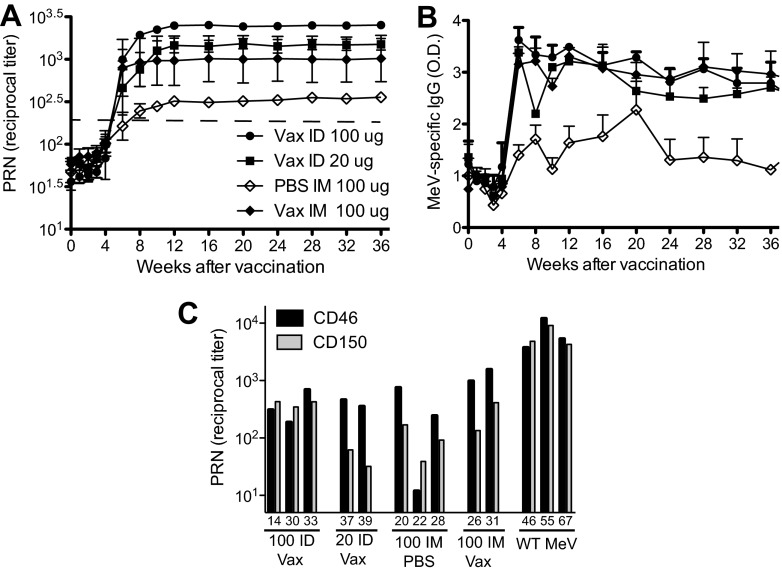
Groups of three monkeys were vaccinated with H+F DNA either Vaxfectin-formulated (100 or 20 μg i.d.), Vaxfectin-formulated (100 μg i.m.), or PBS-formulated (100 μg i.m.) on days 0 and 28. All animals developed neutralizing antibody titers predicted to be protective (>120) (52, 53) within 2 weeks after the boost with the highest titers in the group that received 100 μg of Vaxfectin-formulated DNA i.d. and the lowest titers in the group that received 100 μg of PBS-formulated DNA i.m. (Fig. 1A). Differences between i.d. and i.m. delivery were not significant (P = 0.52), while animals receiving Vaxfectin-formulated DNA i.m. had higher titers than animals receiving PBS-formulated DNA i.m. (P = 0.036). MeV-specific binding IgG measured by EIA was also induced (Fig. 1B). The lowest levels of EIA antibody were in the monkeys that received the unadjuvanted DNA while the groups of monkeys receiving Vaxfectin-adjuvanted DNA developed similar higher levels of antibody (P = 0.0001).
Fig 1.
Antibody responses to vaccination. Monkeys were vaccinated on day 0 and boosted 4 weeks later with codon-optimized DNA plasmids expressing the MeV H and F proteins. The vaccine was delivered either intradermally (i.d.) or intramuscularly (i.m.) either naked (PBS) or formulated with Vaxfectin (Vax). (A) Reciprocal titers of neutralizing antibody as measured by 50% plaque reduction of Chicago-1infection of Vero cells. The data are plotted as geometric means ± the standard errors of the mean (SEM). A dashed line indicates the generally accepted protective levels. Comparison of 100 μg of DNA i.m. with or without Vaxfectin (P = 0.0356, Student t test). (B) Enzyme immunoassay of plasma (1:100) IgG binding to MeV lysate-coated wells. The data are expressed as optical density + the SEM (P = 0.0001, one-way ANOVA). (C) Plasma taken 48 weeks after vaccination or 135 days after infection with Bilthoven compared for neutralization of Chicago-1 infection of Vero cells (interaction with CD46; black bars) and neutralization of Bilthoven infection of Vero/hSLAM cells (interaction with CD150; gray bars).
The Chicago strain of MeV used for neutralization assays can use CD46 as a receptor and infects Vero cells efficiently. To determine the ability of antibody to neutralize WT virus that uses CD150/hSLAM as a receptor, plasma samples obtained 48 weeks after vaccination or after WT Bilthoven infection were simultaneously tested in PRN assays with Vero cells and Chicago as the challenge virus and in Vero/SLAM cells with Bilthoven as the challenge virus (Fig. 1C). The responses of vaccinated animals were compared to those elicited by infection with WT virus. The antibody induced by 100 μg of Vaxfectin-formulated DNA given i.d. neutralized the Chicago and Bilthoven viruses equivalently, as was also observed after recovery from WT infection. However, plasma from most (6/7) monkeys in the other immunization groups neutralized Bilthoven 3- to 11-fold less well than Chicago.
IFN-γ and IL-4 T cell response to vaccination.
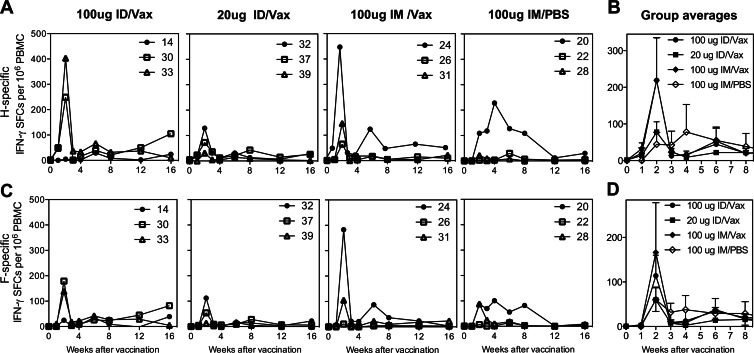
T cell responses to pooled H peptides (Fig. 2A and B) and pooled F peptides (Fig. 2C and D) were measured by IFN-γ ELISPOT assays. T cell responses to both H and F were generally highest 2 weeks after the initial vaccine dose and in monkeys that received 100 μg of Vaxfectin-formulated DNA either i.m. or i.d., but differences were not significant. Very few IL-4-producing cells were generated at 2 weeks after vaccination (Fig. 3).
Fig 2.
IFN-γ T cell responses to vaccination. PBMCs from monkeys vaccinated as described above were assessed for production of IFN-γ in response to stimulation with pooled overlapping peptides from the H protein (A and B) and the F protein (C and D). The results are presented as SFCs/106 PBMCs for individual monkeys (A and C) and as averaged values ± the SEM for each vaccine group (B and D).
Fig 3.
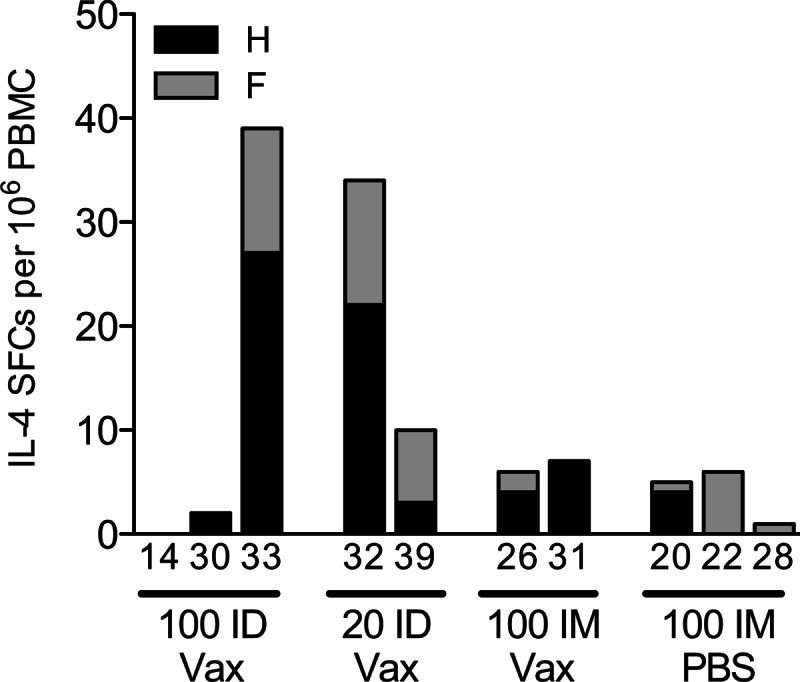
IL-4 T cell responses to vaccination. At 2 weeks after the first dose of vaccine, PBMCs were assessed for IL-4 production after stimulation with overlapping peptides from the H protein and the F protein. The results are presented as SFCs/106 PBMCs.
Protection from infection and disease after challenge.
Monkeys were challenged 18 months after vaccination with 104 TCID50 of Bilthoven WT MeV. All vaccinated animals were protected from disease defined by presence of a rash or viremia, while the unvaccinated naive monkey developed a rash and a viremia that peaked at day 10 (Fig. 4). MeV RNA was not detected in PBMCs from vaccinated monkeys during the first weeks after challenge but was detected at low levels in two monkeys (33V [100 μg i.d., Vaxfectin] and 28V [100 μg i.m., PBS]) at 88 days.
Fig 4.
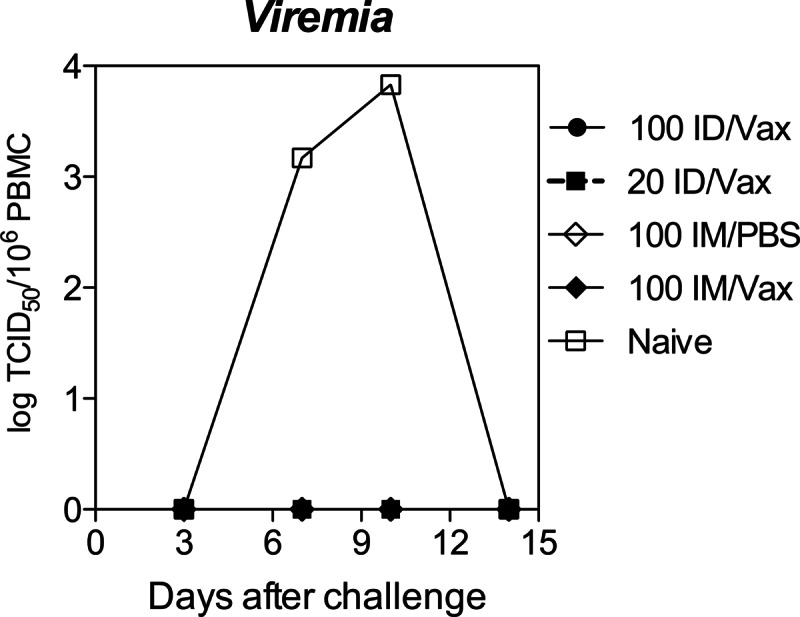
Protection from viremia after challenge. PBMCs collected at days 3, 7, 10, and 14 after intratracheal challenge with 104 TCID50 of the Bilthoven strain of WT MeV were cocultivated with Vero/hSLAM cells and read for cytopathic effect. None of the vaccinated animals developed viremia or rash after challenge, while the unvaccinated animal developed both rash and viremia. The data are presented as TCID50/106 PBMCs.
Antibody response to challenge.
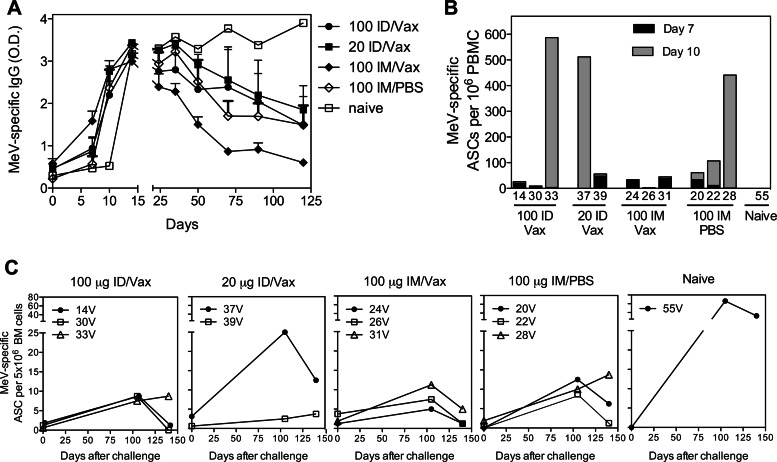
Changes in levels of MeV-specific antibody after challenge were measured by EIA (Fig. 5A). All vaccinated monkeys had an anamnestic response to challenge with an increase in IgG by 7 to 10 days compared to day 14 for the naive monkey. However, antibody titers for vaccinated monkeys declined over the next 3 to 4 months, while they continued to increase for the unvaccinated monkey. To further characterize the induction of antibody-secreting cells (ASCs) and the generation of long-lived plasma cells after challenge, PBMCs and bone marrow (BM) were examined for the presence of MeV-specific ASCs. Plasmablasts secreting antibody to MeV, indicating the stimulation of memory B cells to become ASCs, were detectable in circulation 7 and 10 days after challenge in most vaccinated animals but not in the unvaccinated animal (Fig. 5B). Bone marrow, the site of residence of most long-lived plasma cells that produce the antibody found in plasma (58), was sampled at the time of challenge (17 months after the vaccine boost) and at 105 and 140 days after challenge (Fig. 5C). Small numbers of bone marrow plasma cells (0 to 4/5 × 106 cells) were established after DNA vaccination and present at the time of challenge. After challenge, the numbers of ASCs in the bone marrow increased in all animals, but at 3 to 4 months were substantially higher in the unvaccinated animal than in the previously vaccinated animals.
Fig 5.
B cell responses after challenge. Plasma, PBMCs, and bone marrow (BM) cells were collected from vaccinated and unvaccinated monkeys after challenge with WT MeV. (A) MeV-specific IgG in plasma as determined by enzyme immunoassay. The data are presented as the mean of OD values + the SEM. (B) Numbers of plasmablasts producing MeV-specific antibody present in circulation 7 and 10 days after challenge. ASCs, antibody-secreting cells. (C) Numbers of plasma cells in bone marrow producing MeV-specific antibody before (d0, 17 months after vaccine boost) and after (105 and 140 days) challenge.
IFN-γ and IL-17 T cell responses to challenge.
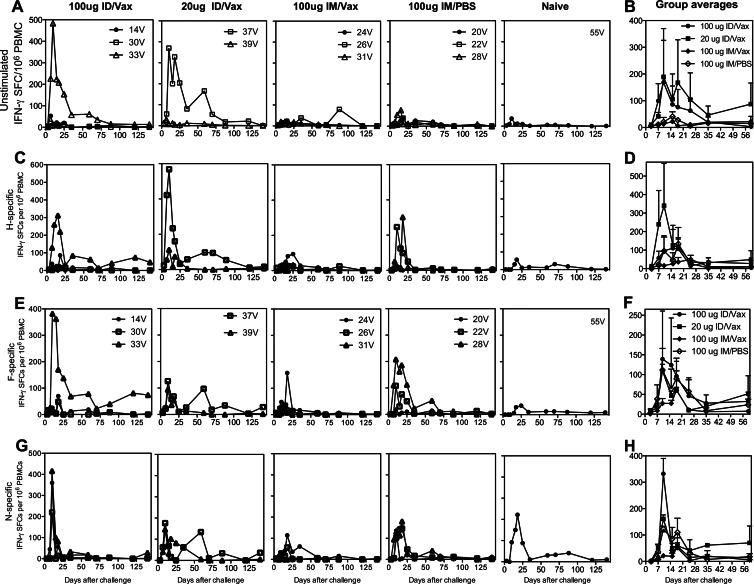
After challenge, PBMCs were cultured in the absence (Fig. 6A and B) or presence of overlapping peptides from the H (Fig. 6C and D) and F (Fig. 6E and F) proteins and assessed for the production of IFN-γ. Substantial numbers of IFN-γ-secreting cells were detected without in vitro stimulation, indicating in vivo activation. These numbers were further increased after peptide stimulation in vitro. In vaccinated animals, maximum stimulated and unstimulated ex vivo IFN-γ responses were at 10 and 18 days after challenge and at 18 days in the unvaccinated monkey reflecting vaccine-induced priming of the T cell responses to H and F. Primary T cell IFN-γ responses to overlapping peptides from N, which was not present in the vaccine, were also induced in all of the vaccinated animals, as well as the naive monkey (Fig. 6G and H).
Fig 6.
IFN-γ T cell responses after challenge. Vaccinated and unvaccinated monkeys were challenged intratracheally with the Bilthoven WT strain of MeV. PBMCs were examined ex vivo without stimulation for production of IFN-γ (A and B) and after stimulation with pools of overlapping peptides from the H (C and D), F (E and F), and N (G and H) proteins. The data are presented as SFCs per 106 PBMCs for individual animals (A, C, E, and G) and as averages plus the SEM for each vaccine group (B, D, F, and H). For stimulated cells (C to H), the numbers of SFCs present without stimulation (A and B) have been subtracted.
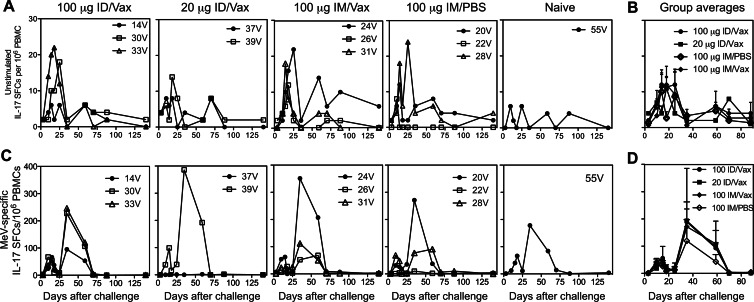
In vitro stimulation of PBMCs from both vaccinated and unvaccinated animals with MeV antigen induced the secretion of IL-17 in a biphasic pattern (Fig. 7A and B). Vaccinated animals had a small early peak at day 10, which coincided with ex vivo production of IL-17 by unstimulated cells, and a second larger peak at day 35. No differences based on the dose of DNA, the route of administration, or the adjuvant were identified between vaccine groups. For the naive monkey, very few unstimulated cells produced IL-17, and the peaks for stimulated cells were at day 18 and 35.
Fig 7.
IL-17 T cell responses after challenge. After intratracheal challenge, PBMCs were examined ex vivo without stimulation (A and B) and after stimulation with MeV-infected cell lysate (C and D) for IL-17 production. The data are presented as SFCs/106 PBMCs for individual animals (A and C) and as means + the SEM for each group (B and D). For stimulated cells (C), the numbers of SFCs present without stimulation (A) have been subtracted.
DISCUSSION
This study has shown that Vaxfectin is an effective adjuvant for increasing antibody responses to measles DNA vaccination. MeV-specific neutralizing and EIA antibody increased after the second dose of vaccine, and the levels were significantly improved with Vaxfectin as an adjuvant. The levels of antibody after two doses of either 20 or 100 μg of Vaxfectin-formulated DNA were higher than levels after 100 μg of DNA without Vaxfectin indicative of a dose-sparing effect. MeV-specific IFN-γ-producing T cell numbers were not significantly improved by Vaxfectin formulation. Animals in all vaccine groups were protected from disease, as manifested by rash and viremia, but not from infection, as evidenced by the late appearance of MeV RNA in PBMCs and the induction of an immune response to the N protein. After challenge, anamnestic MeV-specific B cell responses resulted in the appearance of ASCs in blood at 7 to 10 days and rapid increases in the levels of antibody. The amount of antibody produced after the challenge of vaccinated animals was similar to that of an unvaccinated animal at 2 to 4 weeks but was less durable, with decreasing levels of antibody in plasma and few MeV-specific long-lived plasma cells resident in the bone marrow at 2 to 4 months. Prior DNA vaccination had little effect on the induction of MeV-specific IFN-γ- and IL-17-producing T cells after challenge.
As has been observed in some prior studies of DNA vaccinated nonhuman primates, T cell responses were induced more readily than antibody (32, 59). MeV-specific T cells were abundant in circulation 10 to 14 days after the first dose of vaccine and were minimally boosted by the second dose. The T cell response was characteristic of a Th1-type response with production of IFN-γ rather than IL-4. No differences related to dose of DNA, route of injection, or Vaxfectin adjuvant were noted.
The production of detectable levels of MeV-specific antibody occurred more slowly than the T cell response and not until after the second dose. Previous studies using 500 μg of DNA elicited detectable antibody after a single dose, with a further increase after the second dose (32). DNA vaccines are often used for priming the immune response, followed by another type of vaccine as a boost to increase antibody responses (18, 60). In the present study, both the prime and the boost were with DNA. Vaxfectin significantly increased the antibody response to the boosting dose compared to the same vaccine given without the adjuvant that may serve to stimulate innate responses. The route of delivery did not appear to be an important variable, because similar responses were induced after i.m. and i.d. needleless injection.
After challenge, both antibody and T cells showed anamnestic responses. As previously observed with other immunizations (59), ASCs derived from memory B cells appeared in circulation 7 to 10 days after challenge, indicating some level of infection. However, few of these cells became resident in the bone marrow to increase the numbers of long-lived plasma cells necessary for maintaining high levels of antibody in plasma. Accordingly, levels of antibody waned quickly in previously vaccinated animals, while primary infection in the unvaccinated monkey led to a slower response, but many more ASCs in bone marrow and sustained plasma antibody levels. Plasma cell longevity is dependent on cell cycle exit, expression of appropriate regulatory factors and receptors, and migration to and occupancy of survival niches in the bone marrow or sites of inflammation (61–63). How the ASCs produced by DNA vaccine-primed animals differ from those produced by naive animals is a topic worthy of further study. It is possible that the low level of virus replication after challenge in previously vaccinated animals programmed responding B cells for short lives as plasma cells (64).
The vaccine-induced immune response to H and F prevented the appearance of infected lymphocytes in circulation early after infection but did not prevent infection. In two animals MeV RNA was detected in PBMCs 3 months after challenge and may have been present and missed in other animals due to infrequent sampling at late times after infection. Because the amount of antigen present in the challenge is insufficient to efficiently stimulate the immune system without replication, the induction of a T cell response to N, not present in the vaccine, in all of the vaccinated animals further indicated that the challenge virus caused infection. Evidence of immune stimulation by challenge has also been observed in lymphoid tissue even after vaccination with LAV, suggesting that some level of infection is common in vaccinated animals after exposure to WT MeV (65). The site of replication is unclear, but both the respiratory tract and local lymphoid tissues are possibilities, and the late appearance of MeV RNA in PBMCs has been observed in previous studies of other MeV vaccines that protect from initial viremia (66).
Challenge also induced the appearance of PBMCs producing IL-17 in all groups. In contrast to the appearance of IFN-γ-producing cells, MeV-specific IL-17-producing cells had a biphasic pattern after challenge. In mice, IL-17 can be produced by CD4+ T cells, CD8+ T cells, γδ T cells, lymphoid tissue inducer-like cells, and invariant NKT cells associated with both adaptive and innate immune responses to infection (67–71). IL-17 induces release of an array of immune signaling molecules and has a recognized role in defense against bacterial infections, maintenance of epithelial integrity, and autoimmune diseases (67, 72–76). The role of IL-17 in the pathogenesis of acute viral infections is only beginning to be explored. Circulating Th17 cells are increased during enterovirus 71 infection, but the timing and duration of the increase has not been examined (77). IL-17 has not been previously described as a part of the response to MeV infection of humans or nonhuman primates. Biphasic appearance after infection with MeV may reflect activation of different populations of IL-17-producing cells, changes in lymphocyte trafficking or different phases of memory T cell generation in response to continued presence of MeV RNA (78). Infections with human and simian immunodeficiency viruses are associated with an early decrease in Th17, but not Tc17, cells in blood and mucosal tissues that is predictive of chronic immune activation and disease progression (79–82). Further characterization of MeV-specific IL-17-producing cells will be of interest as in vitro studies show that IL-17 enhances virus-induced inflammatory cytokines (83).
ACKNOWLEDGMENTS
This study was funded by a research grant from the Bill and Melinda Gates Foundation.
The assistance of Brandyn Lau, Jane Morrow, Jenny Chaplin, and Peggy Lalor is appreciated.
Footnotes
Published ahead of print 3 April 2013
REFERENCES
- 1. Nandy R, Handzel T, Zaneidou M, Biey J, Coddy RZ, Perry R, Strebel P, Cairns L. 2006. Case-fatality rate during a measles outbreak in eastern Niger in 2003. Clin. Infect. Dis. 42:322–328 [DOI] [PubMed] [Google Scholar]
- 2. Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. 2009. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int. J. Epidemiol. 38:192–205 [DOI] [PubMed] [Google Scholar]
- 3. Moss WJ, Griffin DE. 2006. Global measles elimination. Nat. Rev. Microbiol. 4:900–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Center for Disease Control 2007. Progress in global measles control and mortality reduction, 2000–2006. MMWR Morb. Mortal. Wkly. Rep. 56:1237–1242 [PubMed] [Google Scholar]
- 5. Moss WJ, Griffin DE. 2012. Measles. Lancet 379:153–164 [DOI] [PubMed] [Google Scholar]
- 6. Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, Strebel P. 2012. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet 379:2173–2178 [DOI] [PubMed] [Google Scholar]
- 7. Albrecht P, Ennis FA, Saltzman EJ, Krugman S. 1977. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J. Pediatr. 91:715–718 [DOI] [PubMed] [Google Scholar]
- 8. Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, Maldonado Y. 1998. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 280:527–532 [DOI] [PubMed] [Google Scholar]
- 9. Scott S, Moss WJ, Cousens S, Beeler JA, Audet SA, Mugala N, Quinn TC, Griffin DE, Cutts FT. 7 A.D. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin. Infect. Dis. 45:1417–1424 [DOI] [PubMed] [Google Scholar]
- 10. Moss WJ, Monze M, Ryon JJ, Quinn TC, Griffin DE, Cutts F. 2002. Prospective study of measles in hospitalized, human immunodeficiency virus (HIV)-infected and HIV-uninfected children in Zambia. Clin. Infect. Dis. 35:189–196 [DOI] [PubMed] [Google Scholar]
- 11. Embree JE, Datta P, Stackiw W, Sekla L, Braddick M, Kreiss JK, Pamba H, Wamola I, Ndinya-Achola JO, Law BJ. 1992. Increased risk of early measles in infants of human immunodeficiency virus type 1-seropositive mothers. J. Infect. Dis. 165:262–267 [DOI] [PubMed] [Google Scholar]
- 12. Schalk JA, Mooi FR, Berbers GA, van Aerts LA, Ovelgonne H, Kimman TG. 2006. Preclinical and clinical safety studies on DNA vaccines. Hum. Vaccin. 2:45–53 [DOI] [PubMed] [Google Scholar]
- 13. Donnelly JJ, Wahren B, Liu MA. 2005. DNA vaccines: progress and challenges. J. Immunol. 175:633–639 [DOI] [PubMed] [Google Scholar]
- 14. Denis-Mize KS, Dupuis M, Singh M, Woo C, Ugozzoli M, O'Hagan DT, Donnelly JJ, Ott G, III, McDonald DM. 2003. Mechanisms of increased immunogenicity for DNA-based vaccines adsorbed onto cationic microparticles. Cell. Immunol. 225:12–20 [DOI] [PubMed] [Google Scholar]
- 15. Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, Kumar S, Wu TC. 2003. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J. Clin. Invest. 112:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leitner WW, Hwang LN, deVeer MJ, Zhou A, Silverman RH, Williams BR, Dubensky TW, Ying H, Restifo NP. 2003. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 9:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kutzler MA, Weiner DB. 2004. Developing DNA vaccines that call to dendritic cells. J. Clin. Invest. 114:1241–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kutzler MA, Weiner DB. 2008. DNA vaccines: ready for prime time? Nat. Rev. Genet. 9:776–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nabel GJ, Nabel EG, Yang ZY, Fox BA, Plautz GE, Gao X, Huang L, Shu S, Gordon D, Chang AE. 1993. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc. Natl. Acad. Sci. U. S. A. 90:11307–11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parker SE, Vahlsing HL, Serfilippi LM, Franklin CL, Doh SG, Gromkowski SH, Lew D, Manthorpe M, Norman J. 1995. Cancer gene therapy using plasmid DNA: safety evaluation in rodents and non-human primates. Hum. Gene Ther. 6:575–590 [DOI] [PubMed] [Google Scholar]
- 21. Sullivan SM, Doukas J, Hartikka J, Smith L, Rolland A. 2010. Vaxfectin: a versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert. Opin. Drug Deliv. 7:1433–1446 [DOI] [PubMed] [Google Scholar]
- 22. Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, Sawdey M, Wloch MK, Tonsky K, Norman J, Manthorpe M, Wheeler CJ. 2001. Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine 19:1911–1923 [DOI] [PubMed] [Google Scholar]
- 23. Nukuzuma C, Ajiro N, Wheeler CJ, Konishi E. 2003. Enhancing effect of vaxfectin on the ability of a Japanese encephalitis DNA vaccine to induce neutralizing antibody in mice. Viral Immunol. 16:183–189 [DOI] [PubMed] [Google Scholar]
- 24. Hermanson G, Whitlow V, Parker S, Tonsky K, Rusalov D, Ferrari M, Lalor P, Komai M, Mere R, Bell M, Brenneman K, Mateczun A, Evans T, Kaslow D, Galloway D, Hobart P. 2004. A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. Proc. Natl. Acad. Sci. U. S. A. 101:13601–13606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sedegah M, Rogers WO, Belmonte A, Belmonte M, Banania G, Patterson N, Ferrari M, Kaslow DC, Carucci DJ, Richie TL, Doolan DL. 2006. Vaxfectin enhances immunogenicity and protective efficacy of P. yoelii circumsporozoite DNA vaccines. Vaccine 24:1921–1927 [DOI] [PubMed] [Google Scholar]
- 26. Hahn UK, Aichler M, Boehm R, Beyer W. 2006. Comparison of the immunological memory after DNA vaccination and protein vaccination against anthrax in sheep. Vaccine 24:4595–4597 [DOI] [PubMed] [Google Scholar]
- 27. Margalith M, Vilalta A. 2006. Sustained protective rabies neutralizing antibody titers after administration of cationic lipid-formulated pDNA vaccine. Genet. Vaccines Ther. 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jimenez GS, Planchon R, Wei Q, Rusalov D, Geall A, Enas J, Lalor P, Leamy V, Vahle R, Luke CJ, Rolland A, Kaslow DC, Smith LR. 2007. Vaxfectin-formulated influenza DNA vaccines encoding NP and M2 viral proteins protect mice against lethal viral challenge. Hum. Vaccine 3:157–164 [DOI] [PubMed] [Google Scholar]
- 29. Shlapobersky M, Marshak JO, Dong L, Huang ML, Wei Q, Chu A, Rolland A, Sullivan S, Koelle DM. 2012. Vaxfectin-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J. Gen. Virol. 93:1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hartikka J, Bozoukova V, Morrow J, Rusalov D, Shiapobersky M, Wei Q, Boutsaboualoy S, Ye M, Wloch MK, Doukas J, Sullivan S, Rolland A, Smith LR. 2012. Preclinical evaluation of the immunogenicity and safety of plasmid DNA-based prophylactic vaccines for human cytomegalovirus. Hum. Vaccin. Immunother. 8:1595–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne N. 2012. A Vaxfectin®-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine 30:7046–7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan CH, Jimenez GS, Nair N, Wei Q, Adams RJ, Polack FP, Rolland A, Vilalta A, Griffin DE. 2008. Use of Vaxfectin adjuvant with DNA vaccine encoding the measles virus hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques against measles virus. Clin. Vaccine Immunol. 15:1214–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Porter KR, Ewing D, Chen L, Wu SJ, Hayes CG, Ferrari M, Teneza-Mora N, Raviprakash K. 2012. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine 30:336–341 [DOI] [PubMed] [Google Scholar]
- 34. Locher CP, Witt SA, Ashlock BM, Polacino P, Hu SL, Shiboski S, Schmidt AM, Agy MB, Anderson DM, Staprans SI, zur Megede J, Levy JA. 2004. Human immunodeficiency virus type 2 DNA vaccine provides partial protection from acute baboon infection. Vaccine 22:2261–2272 [DOI] [PubMed] [Google Scholar]
- 35. Smith LR, Wloch MK, Ye M, Reyes LR, Boutsaboualoy S, Dunne CE, Chaplin JA, Rusalov D, Rolland AP, Fisher CL, Al-Ibrahim MS, Kabongo ML, Steigbigel R, Belshe RB, Kitt ER, Chu AH, Moss RB. 2010. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 28:2565–2572 [DOI] [PubMed] [Google Scholar]
- 36. Dorig RE, Marcil A, Chopra A, Richardson CD. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295–305 [DOI] [PubMed] [Google Scholar]
- 37. Naniche D, Varior-Krishnan G, Cervoni F, Wild F, Rossi B, Rabourdin-Combe C, Gerlier D. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tatsuo H, Ono N, Tanaka K, Yanagi Y. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893–898 [DOI] [PubMed] [Google Scholar]
- 39. Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VHJ, Navaratnarajah CK, Frenzke M, Wong XX, Sawatsky B, Ramachandran S, McCray PBJ, Cichutek K, von Messling V, Lopez M, Cattaneo R. 2011. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480:530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noyce RS, Bondre DG, Lin Sisson L-TG, Tsao MS, Richardson CD, Ha MN. 2011. Tumor cell marker PVRL4 (Nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 7:1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Santiago C, Bjorling E, Stehle T, Casasnovas JM. 2002. Distinct kinetics for binding of the CD46 and SLAM receptors to overlapping sites in the measles virus hemagglutinin protein. J. Biol. Chem. 277:32294–32301 [DOI] [PubMed] [Google Scholar]
- 42. Masse N, Barrett T, Muller CP, Wild TF, Buckland R. 2002. Identification of a second major site for CD46 binding in the hemagglutinin protein from a laboratory strain of measles virus (MV): potential consequences for wild-type MV infection. J. Virol. 76:13034–13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manchester M, Eto DS, Valsamakis A, Liton PB, Fernandez-Munoz R, Rota PA, Bellini WJ, Forthal DN, Oldstone MB. 2000. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 74:3967–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vongpunsawad S, Oezgun N, Braun W, Cattaneo R. 2004. Selectively receptor-blind measles viruses: identification of residues necessary for SLAM or CD46-induced fusion and their localization on a new hemagglutinin structural model. J. Virol. 78:302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schneider U, von Messling V, Devaux P, Cattaneo R. 2002. Efficiency of measles virus entry and dissemination through different receptors. J. Virol. 76:7460–7467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erlenhofer C, Duprex WP, Rima BK, ter Meulen V, Schneider-Schaulies J. 2002. Analysis of receptor (CD46, CD150) usage by measles virus. J. Gen. Virol. 83:1431–1436 [DOI] [PubMed] [Google Scholar]
- 47. Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa HY. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yanagi Y, Ono N, Tatsuo H, Hashimoto K, Minagawa H. 2002. Measles virus receptor SLAM (CD150). Virology 299:155–161 [DOI] [PubMed] [Google Scholar]
- 49. Ota MO, Ndhlovu Z, Oh S, Piyasirisilp S, Berzofsky JA, Moss WJ, Griffin DE. 2007. Hemagglutinin protein is a primary target of the measles virus-specific HLA-A2-restricted CD8+ T cell response during measles and after vaccination. J. Infect. Dis. 195:1799–1807 [DOI] [PubMed] [Google Scholar]
- 50. Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. 2003. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat. Med. 9:1209–1213 [DOI] [PubMed] [Google Scholar]
- 51. Polack FP, Auwaerter PG, Lee S-H, Nousari HC, Valsamakis A, Leiferman KM, Diwan A, Adams RJ, Griffin DE. 1999. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat. Med. 5:629–634 [DOI] [PubMed] [Google Scholar]
- 52. Polack F, Lee S, Permar S, Manyara E, Nousari H, Jeng Y, Mustafa F, Valsamakis A, Adams R, Robinson H, Griffin D. 2000. Successful DNA immunization against measles: neutralizing antibody against either the hemagglutinin or fusion glycoprotein protects rhesus macaques without evidence of atypical measles. Nat. Med. 6:776–781 [DOI] [PubMed] [Google Scholar]
- 53. Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036–1042 [DOI] [PubMed] [Google Scholar]
- 54. Van Binnendijk RS, Poelen MCM, van Amerongen G, de Vries P, Osterhaus ADME. 1997. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J. Infect. Dis. 175:524–532 [DOI] [PubMed] [Google Scholar]
- 55. Pan CH, Valsamakis A, Colella T, Nair N, Adams RJ, Polack FP, Greer CE, Perri S, Polo JM, Griffin DE. 2005. Inaugural article: modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles. Proc. Natl. Acad. Sci. U. S. A. 102:11581–11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu Y, Rota P, Wyatt L, Tamin A, Rozenblatt S, Lerche N, Moss B, Bellini W, McChesney M. 2000. Evaluation of recombinant vaccinia virus–measles vaccines in infant rhesus macaques with preexisting measles antibody. Virology 276:202–213 [DOI] [PubMed] [Google Scholar]
- 57. Griffin DE, Lin WH, Pan CH. 2012. Measles virus, immune control and persistence. FEMS Microbiol. Rev. 36:649–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Benner R, Hijmans W, Haaijman JJ. 1981. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin. Exp. Immunol. 46:1–8 [PMC free article] [PubMed] [Google Scholar]
- 59. Pasetti MF, Resendiz-Albor A, Ramirez K, Stout R, Papania M, Adams RJ, Polack FP, Ward BJ, Burt D, Chabot S, Ulmer J, Barry EM, Levine MM. 2007. Heterologous prime-boost strategy to immunize very young infants against measles: pre-clinical studies in rhesus macaques. Clin. Pharmacol. Ther. 82:672–685 [DOI] [PubMed] [Google Scholar]
- 60. Ramshaw IA, Ramsay AJ. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21:163–165 [DOI] [PubMed] [Google Scholar]
- 61. Elgueta R, de Vries VC, Noelle RJ. 2010. The immortality of humoral immunity. Immunol. Rev. 236:139–150 [DOI] [PubMed] [Google Scholar]
- 62. Tourigny MR, Ursini-Siegel J, Lee H, Toellner KM, Cunningham AF, Franklin DS, Ely S, Chen M, Qin XF, Xiong Y, MacLennan IC, Chen-Kiang S. 2002. CDK inhibitor p18INK4c is required for the generation of functional plasma cells. Immunity 17:179–189 [DOI] [PubMed] [Google Scholar]
- 63. Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. 2010. Plasma cell development and survival. Immunol. Rev. 237:140–159 [DOI] [PubMed] [Google Scholar]
- 64. Amanna IJ, Slifka MK. 2010. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 236:125–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McChesney MB, Miller CJ, Rota PA, Zhu Y, Antipa L, Lerche NW, Ahmed R, Bellini WJ. 1997. Experimental measles. 1. Pathogenesis in the normal and the immunized host. Virology 233:74–84 [DOI] [PubMed] [Google Scholar]
- 66. Pan C-H, Greer CE, Hauer D, Legg HS, Lee E-Y, Bergen MJ, Lau B, Adams RJ, Polo JM, Griffin DE. 2010. A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J. Virol. 84:3798–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Blaschitz C, Raffatellu M. 2010. Th17 cytokines and the gut mucosal barrier. J. Clin. Immunol. 30:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gaffen SL. 2011. Recent advances in the IL-17 cytokine family. Curr. Opin. Immunol. 23:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim JS, Jordan MS. 2012. Diversity of IL-17-producing T lymphocytes. Cell. Mol. Life Sci. doi: 10.1007/s00018-012-1163-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chien YH, Zeng X, Prinz I. 2012. The natural and the inducible: interleukin (IL)-17-producing γδ T cells. Trends Immunol. 34:151–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. 2009. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, de VJ, Balazs M, Gonzalez L, Jr, Singh H, Ouyang W, Pappu R. 2011. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 12:1159–1166 [DOI] [PubMed] [Google Scholar]
- 73. Pappu R, Rutz S, Ouyang W. 2012. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 33:343–349 [DOI] [PubMed] [Google Scholar]
- 74. Reynolds JM, Martinez GJ, Nallaparaju KC, Chang SH, Wang YH, Dong C. 2012. Cutting edge: regulation of intestinal inflammation and barrier function by IL-17C. J. Immunol. 189:4226–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Onishi RM, Gaffen SL. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen J, Tong J, Liu H, Liu Y, Su Z, Wang S, Shi Y, Zheng D, Sandoghchian S, Geng J, Xu H. 2012. Increased frequency of Th17 cells in the peripheral blood of children infected with enterovirus 71. J. Med. Virol. 84:763–767 [DOI] [PubMed] [Google Scholar]
- 78. Lin WH, Kouyos RD, Adams RJ, Grenfell BT, Griffin DE. 2012. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. Proc. Natl. Acad. Sci. U. S. A. 109:14989–14994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hartigan-O'Connor DJ, Hirao LA, McCune JM, Dandekar S. 2011. Th17 cells and regulatory T cells in elite control over HIV and SIV. Curr. Opin. HIV AIDS 6:221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, Zaffiri L, Tryniszewska E, Tsai WP, Vaccari M, Parks RW, Venzon D, Douek DC, O'Shea JJ, Franchini G. 2008. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal. Immunol. 1:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. 2009. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 5:e1000295 doi: 10.1371/journal.ppat.1000295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nigam P, Kwa S, Velu V, Amara RR. 2011. Loss of IL-17-producing CD8 T cells during late chronic stage of pathogenic simian immunodeficiency virus infection. J. Immunol. 186:745–753 [DOI] [PubMed] [Google Scholar]
- 83. Ryzhakov G, Lai CC, Blazek K, To KW, Hussell T, Udalova I. 2011. IL-17 boosts proinflammatory outcome of antiviral response in human cells. J. Immunol. 187:5357–5362 [DOI] [PubMed] [Google Scholar]