Abstract
Current influenza virus vaccine strategies stimulate immune responses toward the globular head domain of the hemagglutinin protein in order to inhibit key steps of the virus life cycle. Because this domain is highly variable across strains, new vaccine formulations are required in most years. Here we demonstrate a novel vaccine strategy that generates immunity to the highly conserved stalk domain by using chimeric hemagglutinin constructs that express unique head and stalk combinations. By repeatedly immunizing mice with constructs that expressed the same stalk but an irrelevant head, we specifically stimulated a stalk-directed response that provided broad-based heterologous and heterosubtypic immunity in mice. Notably, our vaccination scheme provides a universal vaccine approach that protects against challenge with an H5 subtype virus. Furthermore, through in vivo studies using passively transferred antibodies or depletion of CD8+ T cells, we demonstrated the critical role that humoral mechanisms of immunity play in the protection observed. The present data suggest that a vaccine strategy based on the stalk domain of the hemagglutinin protein could be used in humans to broadly protect against a variety of influenza virus subtypes.
INTRODUCTION
Each year, influenza viruses of the A and B types cause disease and death in the human population (1). In order to protect against these infections, individuals are vaccinated with preparations that drive immunity toward the viral hemagglutinin (HA), a glycoprotein that mediates viral entry into host cells. Present vaccine strategies aim primarily to elicit humoral responses toward the globular head domain of the viral HA, thereby blocking binding of the virus to host receptors on the cell membrane. Antibodies of this type display hemagglutination inhibition (HI) activity. Because these antibodies are highly strain specific, influenza virus vaccines must be reformulated annually with H1, H3, and B virus components in order to protect against the virus strains that are anticipated to circulate in the upcoming influenza season.
A second class of antibodies, directed toward the membrane-proximal portion of the HA—the highly conserved stalk domain—has been isolated from mice and humans and is cross-protective against various influenza virus subtypes (2). Impressively, many of these antibodies have broader specificities than those of antibodies directed toward the head, and they typically neutralize influenza viruses within group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, and H17) (3–6) or group 2 (H3, H4, H7, H10, H14, and H15) (7, 8). Antibodies with reactivity toward the stalk domain of influenza B virus HA have also been described (9). The increased cross-reactive nature of these antibodies is hypothesized to be the result of conservation of both the structure and sequence of the stalk domain across influenza virus subtypes (10, 11). Stalk-specific monoclonal antibodies have been shown to be prophylactically and therapeutically protective against a variety of influenza virus challenges in animal models (2, 3, 5–7), though it is thought that current vaccination strategies do not boost these antibodies to high titers (12–14).
Here we describe a vaccination regimen based on chimeric HA (cHA) structures that combine H1 stalk domains with “exotic” globular head domains derived from other influenza A virus subtypes (10). These constructs allow us to specifically induce broadly neutralizing, stalk-specific antibodies and to test their protective potential against various challenge viruses without interference from antibodies against the globular head domain. We show that these polyclonal antistalk responses are neutralizing in vitro and are able to protect mice against challenge with a panel of heterologous and heterosubtypic viruses.
MATERIALS AND METHODS
Cells and viruses.
293T and MDCK cells were obtained from the ATCC and were maintained in Dulbecco's modified Eagle's medium (DMEM) and minimal essential medium (both from Gibco). Each was supplemented with 10% fetal calf serum (HyClone) and 100 units/ml of penicillin-100 μg/ml of streptomycin (Pen/Strep; Gibco).
Recombinant and chimeric influenza A and B viruses were produced by reverse genetics as previously described (10, 15–18). Rescued viruses, A/Fort Monmouth/1/1947 (H1N1) virus (FM1; mouse adapted; a kind gift from Joshy Jacob), A/Netherlands/602/2009 virus (pH1N1; mouse adapted), a low-pathogenicity A/Vietnam/1203/04 (H5N1) (VN04):A/Puerto Rico/8/1934 (H1N1; PR8) 2:6 recombinant virus with the polybasic cleavage site removed (denominated H5N1) (19), an A/mallard/Sweden/81/2002 (H6N1; low pathogenicity):A/Puerto Rico/8/1934 (H1N1; PR8) 1:7 recombinant virus (denominated H6N1), cold-adapted A/Ann Arbor/6/1960 virus (H2N2), PR8, A/Philippines/2/1982 X-79 virus (H3N2; a kind gift from Baozhong Wang), wild-type B/Yamagata/16/1988 virus (wt fluB), and cH9/1 (H9 HA head on top of an H1 stalk)-expressing B/Yamagata/16/1988 virus (fluB-cH9/1) were propagated in 8- or 10-day-old embryonated chicken eggs for 48 h at 37°C (influenza A viruses) or for 72 h at 33°C (influenza B viruses and cold-adapted A/Ann Arbor/6/1960 virus). The cold-adapted A/Ann Arbor/6/1960 virus was handled under biosafety level 3 conditions.
Recombinant and wild-type viruses were titrated on MDCK cells (ATCC) in the presence of tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin as previously described (10). The H6N1, H5N1, H2N2, pH1N1, H3N2, FM1, and PR8 viruses were purified via gradient centrifugation and inactivated with formaldehyde to be used as positive-control vaccines or enzyme-linked immunosorbent assay (ELISA) substrates.
HA proteins.
Soluble cH6/1, cH9/1, cH5/1, and PR8 proteins containing a T4 foldon trimerization domain and a C-terminal hexahistidine tag for purification were generated using a baculovirus expression system as previously described (10, 12, 17). Soluble A/California/04/09 virus HA was expressed in a similar fashion but contained a GCN4pII trimerization domain and a C-terminal Strep-Tag II sequence in order to avoid a background signal in ELISAs for assessment of stalk-reactive antibodies induced by the vaccine constructs.
Animals.
Animal experiments were performed in accordance with the guidelines of the Mount Sinai School of Medicine Institutional Animal Care and Use Committee. Animals were allowed access to food and water ad libitum and were kept on a 12-h light-dark cycle. Female 6- to 8-week-old BALB/c mice (Jackson Laboratories) were anesthetized for all intranasal procedures by intraperitoneal (i.p.) injection of 0.1 ml of ketamine-xylazine.
Vaccination and challenge.
Naive 6- to 8-week-old female BALB/c mice were electroporated with cH9/1-encoding plasmid DNA (80 μg) (TriGrid delivery system; Ichor Medical Systems) and then boosted 3 weeks later with cH6/1 protein (replaced by full-length PR8 H1 protein for animals challenged with H6N1 virus) administered with poly(I:C) intranasally [10 μg recombinant protein plus 10 μg poly(I:C)] and intramuscularly [10 μg recombinant protein plus 10 μg poly(I:C)]. We used intranasal in combination with intramuscular vaccination to ensure that both humoral and mucosal immunity responses were induced. Mucosal immunity may play an important role in the observed protection by acting directly at the site of viral infection. The boost was repeated 3 weeks later with cH5/1 protein (replaced by full-length PR8 H1 protein for animals challenged with H5N1 virus). Control animals were electroporated with cH9/1-encoding DNA as well but were boosted twice with bovine serum albumin (BSA) (in the same manner as the treatment group, including the same amount of adjuvant). Positive-control animals received either inactivated FM1, PR8, low-pathogenicity H5N1, or H6N1 virus (1 μg) or the pH1N1 monovalent split vaccine intramuscularly (1 μg of HA per mouse; BEI). Animals were then challenged at 3.5 to 5 weeks postboost with 5 50% lethal doses (LD50) of PR8, FM1, low-pathogenicity H5N1, or H6N1 virus or 10 LD50 of pH1N1 virus. Animals used for CD8+ T-cell depletion were treated with 300 μg of anti-CD8+ T-cell antibody (20) (from hybridoma line 2.43) 48 and 24 h prior to challenge and then were challenged with 5 LD50 of PR8 virus. Weights were monitored for 14 days postchallenge. Depletion of CD8+ T cells was verified by fluorescence-activated cell sorter (FACS) analysis (data not shown). Animals used for the H3N2 challenge were vaccinated as described above, with the minor modification that the cH2/1 protein was used instead of the cH5/1 protein. Animals were then challenged with 5 LD50 of H3N2 virus.
For the experiments described for Fig. 2, mice were inoculated with fluB-cH9/1 (2 × 105 PFU) or wt fluB (2 × 105 PFU per mouse; equivalent to 0.1 LD50) virus and then vaccinated with 20 μg of BSA or cH6/1 protein as described above. A similar boost with either cH5/1 (or PR8 HA for the H5 challenge) or BSA [both adjuvanted with poly(I:C)] was administered 3 weeks later. Naive animals served as an additional control. A formaldehyde-inactivated matched challenge virus (1 μg) was administered to positive-control animals as described above. Animals were bled and challenged 4 to 5 weeks following vaccination with 250 LD50 of A/Netherlands/602/2009 virus, 10 LD50 of PR8 or FM1 virus, or 5 LD50 of the low-pathogenicity H5N1 virus (19). For determination of lung titers, animals were vaccinated and challenged with PR8 virus as described above. Animals (n = 3 per group) were euthanized on day 3 or 6 postinfection, and lungs were harvested and homogenized with a FastPrep-24 homogenizer (MP). Lung virus titers were measured by titration on MDCK cells.
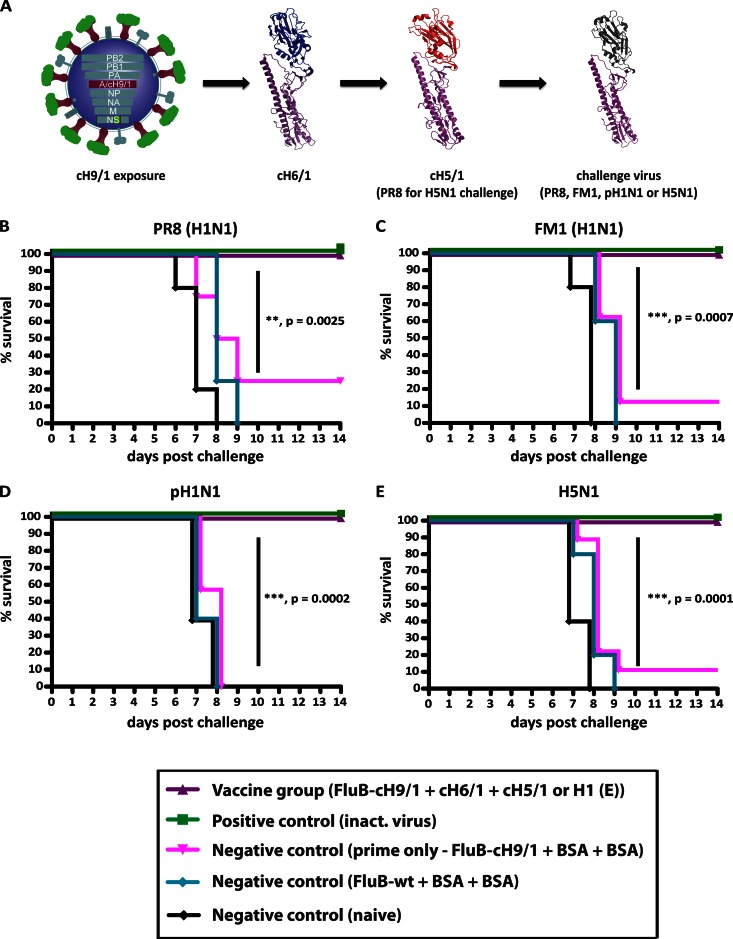
Fig 2.
Vaccination with chimeric HA constructs boosts preexisting broad-based immunity and mediates protection from heterologous and heterosubtypic virus challenges. (A to C) Animals were inoculated with fluB-H1 virus and then vaccinated with cH6/1 and boosted with cH5/1 (PR8 for the H5N1 challenge) (purple triangles; n = 10) or BSA protein (pink triangles; n = 5). Controls were inoculated with wt fluB virus and vaccinated with BSA (teal circles; n = 5), given inactivated virus (green squares; n = 5) as a positive control, or kept naive (black circles; n = 5). (A) Schematic of vaccination and challenge. Purple, H1 stalk and head; green, H9 head; blue, H6 head; red, H5 head. The conserved stalk of the challenge viruses is indicated in purple, whereas the generic head domain is shown in gray. Kaplan-Meier curves depict survival upon challenge with PR8 (H1N1) (B), FM1 (H1N1) (C), pH1N1 (D), and H5N1 (E) viruses. Differences in survival of the fluB-cH9/1+cH6/1+cH5/1 or PR8 HA (PR8 HA used for H5 challenge) group versus the fluB-cH9/1+BSA+BSA group were highly significant for all challenge experiments (P = 0.0025, 0.0007, 0.0002, and <0.0001, respectively).
For the passive transfer experiment, mice from the fluB-cH9/1 experimental series (controls and vaccine group) were bled, and serum (300 μl/mouse) was transferred into naive animals via intraperitoneal injection. At 2 h posttransfer, animals were challenged with 5 LD50 of PR8 virus, and weight was monitored for 14 days.
For the experiments shown in Fig. 1 and 2, animals were euthanized if they lost more than 30% of their initial body weight following challenge, according to institutional guidelines. A 20% cutoff was used for infection with the less pathogenic H6N1 and A/Netherlands/602/2009 viruses. For challenge experiments involving a fluB-cH9/1 prime and two protein boosts, a more stringent cutoff of 75% was used for FM1, PR8, and H5N1 virus challenges.
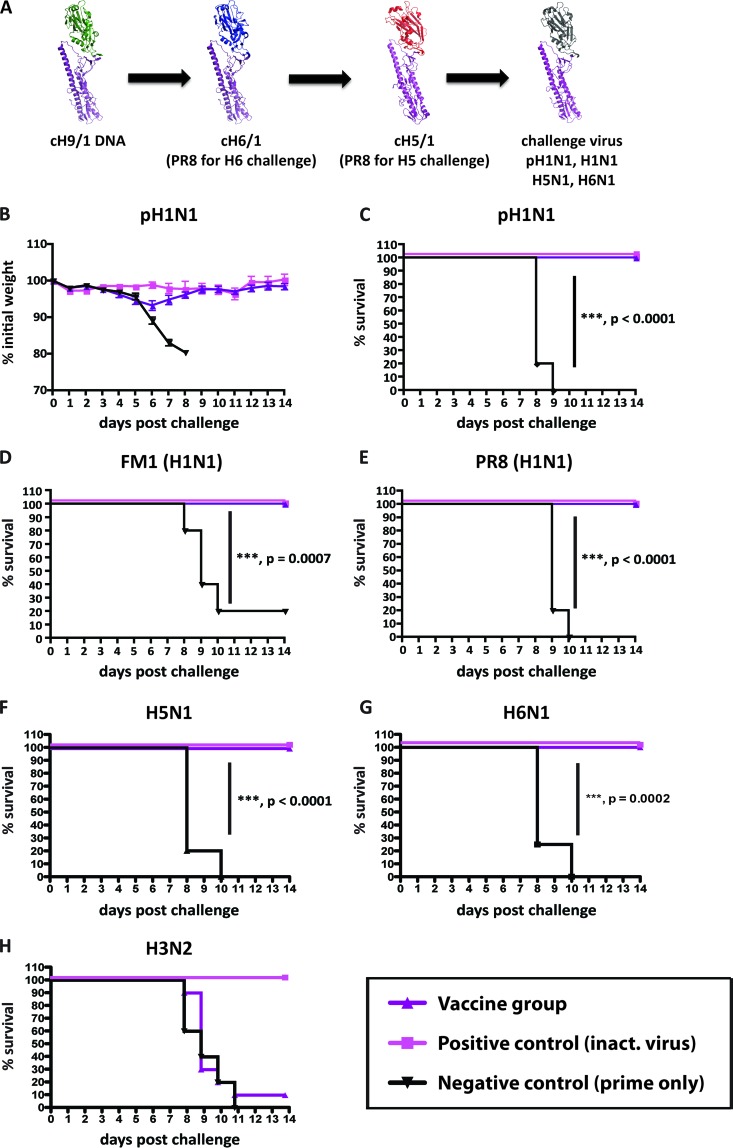
Fig 1.
Vaccination with cHA elicits broad-based immunity that mediates protection from heterologous and heterosubtypic virus challenges. Animals were electroporated with DNA encoding cH9/1 and then were vaccinated with cH6/1 (replaced by H1 for panel F) and boosted with cH5/1 (replaced by H1 for E) soluble proteins (purple triangles; n = 10) or BSA (black triangles; n = 5), while positive-control mice received inactivated virus vaccine intramuscularly (pink squares; n = 5). (A) Schematics of vaccination and challenge. Purple, H1 stalk; green, H9 head; blue; H6 head; red, H5 head. The conserved stalk of the challenge viruses is indicated in purple, whereas the generic head domain is shown in gray. (A) Animals were vaccinated and then challenged with pH1N1, H1N1, H5N1, and H6N1 viruses. (B) Mice were challenged with pH1N1 virus and weighed daily. (C) Kaplan-Meier curve depicting survival. (D to G) Kaplan-Meier curves depicting survival upon viral challenge with the FM1 (H1N1) (D), PR8 (H1N1) (E), H5N1 (F), and H6N1 (G) strains. Survival of the chimeric HA vaccine group over that of the cH9/1 DNA-plus-BSA control group was highly significant for all viral challenges (P < 0.0001 for pH1N1, PR8, and H5N1 viruses, P = 0.0007 for FM1, and P = 0.0002 for H6N1 virus). (H) Mice that were vaccinated in a similar manner to that presented in panel A were not protected against challenge with an H3N2 virus strain.
Enzyme-linked immunosorbent assay, pseudoparticle entry assay, and hemagglutination inhibition assay.
Stalk-specific antibody titers were detected by ELISAs using purified PR8, pH1N1, H5N1, and H2N2 viruses or the A/California/04/2009 virus (pH1N1) HA protein as the substrate, as previously described (17). Pseudoparticles expressing H2 HA were used in a pseudoparticle entry assay as previously described (10, 17). Antibody-pseudotyped particle mixtures were incubated for 1 h at room temperature before being transferred onto cells. HI assays with inactivated mouse serum and challenge viruses were performed as described before (17).
Flow cytometry.
In order to confirm CD8+ T-cell depletion, single-cell suspensions from whole lungs were stained with rat anti-mouse CD8a (53-6.7) and rat anti-mouse CD3e (145-2C11) (Becton Dickinson) antibodies. Following incubation with monoclonal antibodies, red blood cells were lysed and lymphocytes were fixed using BD FACS lysing solution (BD Biosciences). All flow cytometry data were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR). Levels of CD8+ CD3+ cells in T-cell-depleted mice were compared to those of control, nondepleted animals.
Statistical tests and hemagglutinin modeling.
Statistical analyses were performed using Prism4 (GraphPad). All values were plotted as averages with standard errors of the means. Differences in survival were calculated by Kaplan-Meier survival analysis with the log rank significance test. P values of ≤0.05 are considered statistically significant. In order to generate models of the wild-type and chimeric hemagglutinins, structures 3LZG, 2FK0, 1RU7, and 1JSD were downloaded from the Protein Data Bank and modeled with PyMol software (Delano Scientific).
RESULTS
Chimeric HA constructs robustly protect mice from challenge with heterologous and heterosubtypic influenza viruses.
In order to elicit stalk-specific immunity in mice, we used cHA constructs that express mismatched globular heads and a conserved H1 stalk. We reasoned that by repeatedly exposing mice to constructs that express the same stalk domain, we would stimulate a robust immune response toward this conserved region. Furthermore, preliminary experiments suggested that humoral immunity to the stalk domain would be enhanced if cHA antigens with different HA head domains were used compared to that obtained by sequential immunization with antigens that expressed the same head domain (data not shown).
To assess the protective nature of the elicited stalk-specific immune response, all experiments were designed to ensure that animals remained immunologically naive to the head domain of the challenge virus used (all cHA-vaccinated animals tested HI negative against the challenge strains used [data not shown]).
Mice were electroporated with a DNA expression vector (21) that encodes cH9/1, whose head is from an H9 isolate and whose stalk is from the PR8 (H1N1) virus (17). Mice were then boosted with the soluble cH6/1 (H6 head and H1 stalk) protein followed by the cH5/1 protein (H5 head and H1 stalk) in order to specifically boost stalk-specific immunity. Animals were then challenged with a panel of H1N1 viruses (Fig. 1A to E). Following infection with the A/Netherlands/602/2009 (pH1N1) (Fig. 1B and C), FM1 (Fig. 1D), and PR8 (Fig. 1E) viruses, all cHA-vaccinated animals were protected from challenge and displayed only minimal amounts of weight loss (Fig. 1B and data not shown). Negative-control animals also received cH9/1 DNA electroporation and were boosted in the same manner, though an irrelevant protein (BSA) was used. These animals lost considerable amounts of weight (Fig. 1B and data not shown) and, with the exception of one animal, succumbed to infection by day 9 (Fig. 1A to E). The survival of the cHA-vaccinated animals in each of the challenge experiments was significantly different from that of controls (Fig. 1A to E).
We next wanted to ascertain whether our vaccination regimen could protect mice from heterosubtypic challenges with H5N1 or H6N1 viruses as well. In order to rule out the contribution of antibodies directed toward the globular head domain of HA in the challenge viruses, we altered the vaccination regimen and replaced either the cH6/1 protein (for the H6N1 challenge) or the cH5/1 protein (for the H5N1 challenge) with the full-length PR8 H1 protein. Mice were inoculated and vaccinated as described above (Fig. 1A) and then challenged with a recombinant H5N1 (19) or H6N1 virus (Fig. 1F and G). As expected, prime-only animals were not protected from either of the challenges and succumbed to infection by day 10 in both cases. Increased protection was achieved in the cHA-vaccinated cohort, with an impressive survival rate of 100% with both challenge viruses. Differences in survival between prime-only and cHA-vaccinated cohorts were highly significant for both challenge groups (P < 0.0001 for H5N1 virus and P = 0.0002 for H6N1 virus), supporting the notion that cHAs can boost cross-protective immunity to levels that protect against heterosubtypic viruses such as H5N1 and H6N1 viruses.
Stalk-specific antibodies that have cross-reactive and neutralizing activities against group 1 and group 2 HA-expressing viruses have been described previously (22, 23). We therefore wanted to assess the ability of our group 1 stalk-based vaccination scheme to protect from group 2 virus challenge. Mice were vaccinated in a similar way to that described above and then challenged with an H3N2 virus. In this case, prime-only animals and vaccinated animals lost weight to similar extents (data not shown), with no significant difference in survival between the two groups (Fig. 1H), supporting the conclusion that the protection induced by H1 stalk-based cHAs is limited to group 1 HA-expressing viruses.
Chimeric HA constructs broadly protect against heterologous and heterosubtypic viral challenge in mice with preexisting stalk-specific immunity.
Unlike naive laboratory animals, humans are usually exposed to influenza virus, through vaccination or infection, several times over the course of their lifetime. It is noteworthy that monoclonal antibodies with specificities for the HA stalk have been isolated from individuals infected with seasonal H1N1 (sH1N1) viruses (4, 6, 7). There are also data that suggest that infection with sH1N1 and sH3N2 viruses can induce low, and most likely nonprotective, levels of serum polyclonal antistalk antibodies (12, 17, 24). We hypothesized that these low levels of stalk-reactive antibodies could effectively be boosted to protective levels by our vaccine constructs.
We therefore wished to study the efficacy of our vaccine in the context of prior exposure to influenza virus in order to better model the potential response to vaccination or infection in humans. Since immune responses against the influenza A virus neuraminidase (NA) and the internal proteins can be protective in mice, we elected not to use wild-type influenza A virus to mimic previous exposures. We engineered a recombinant influenza B virus that expresses the cH9/1 molecule described above (fluB-cH9/1) (15). Infection with this virus exposes animals to an H1 stalk domain without generating immunity toward the H1 globular head domain or to any other influenza virus proteins that could provide protection. The fluB-cH9/1 virus was therefore used to mimic preexisting immunity against the HA stalk.
Mice were inoculated with the fluB-cH9/1 virus and then boosted with the cH6/1 protein followed by the cH5/1 protein (Fig. 2A). Control mice were inoculated with fluB-cH9/1 or wt fluB virus and then vaccinated in the same manner, using an irrelevant protein (fluB-cH9/1+BSA+BSA group and wt fluB+BSA+BSA group). After vaccination, animals were challenged with a panel of H1N1 viruses, including the currently circulating pH1N1 virus. Despite being immunologically naive to H1 head structures (all animals tested HI negative against the challenge strains [data not shown]), these animals showed no clinical signs of disease and little weight loss (data not shown) and were completely protected from challenge with PR8, FM1, and pH1N1 viruses (Fig. 2B to D). In contrast, fluB-cH9/1+BSA+BSA animals showed significant amounts of weight loss and had an overall low survival rate. Control animals that were naive or received wt fluB and an irrelevant protein succumbed to infection (Fig. 2B to D) by day 9. Survival was statistically different in treatment groups compared to controls (Fig. 2B to D). The survival and weight loss data were further supported by reduced lung titers of vaccinated animals on days 3 and 6 postchallenge (data not shown).
In order to assess protection against H5N1 infection, the vaccination regimen was modified: animals were first inoculated with the fluB-cH9/1 virus followed by the cH6/1 protein, and then the full-length PR8 (H1) HA protein was used as a final boost (instead of the cH5/1 protein) (Fig. 2A). Animals were then challenged with a low-pathogenicity recombinant H5N1 virus (19). This vaccine-challenge regimen again allowed us to sequentially expose mice to the same H1 stalk yet keep the animals immunologically naive to the globular head domain of the challenge virus in an attempt to demonstrate stalk-specific protection. Following infection, 100% of vaccinated animals survived challenge, whereas minimal protection was seen in control animal groups (naive, wt fluB+BSA+BSA, and fluB-cH9/1+BSA+BSA groups) (Fig. 2E). The present data further demonstrate the efficacy of chimeric constructs as universal vaccines that provide heterosubtypic protection.
CD8+ T cells do not play a crucial role in the observed protection.
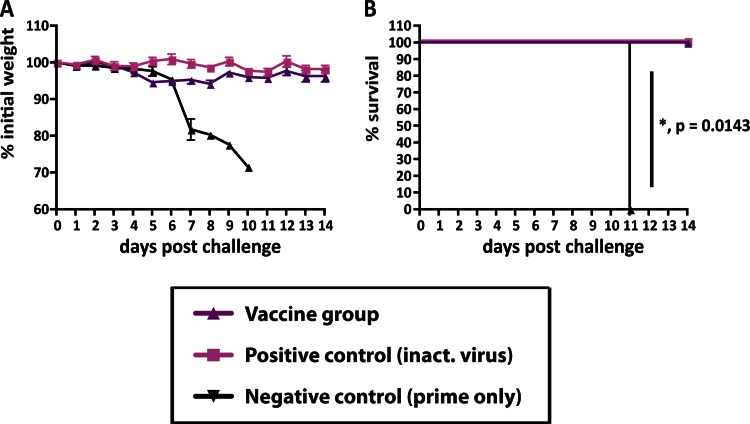
It has been shown that protection of mice from challenge with influenza virus can be mediated solely by T cells (25). Theoretically, CD8+ T cells directed toward epitopes within the HA stalk could play a role in the protection seen here (26). To test this hypothesis, we vaccinated mice again with plasmid DNA encoding the cH9/1 HA followed by the cH6/1 and cH5/1 proteins as described above. CD8+ T cells were then depleted by administration of monoclonal antibody from hybridoma 2.43 prior to the PR8 challenge (20). Depletion did not affect weight loss or survival outcomes upon challenge (Fig. 3). Although we cannot formally rule out the possibility that CD8+ T cells contributed to virus neutralization, our data suggest that an adaptive humoral immune response provided protection against the different challenge viruses.
Fig 3.
CD8 T cells do not have an essential role in mediating the broad-based protection elicited by vaccination with cHA constructs. (A) Animals were electroporated with DNA encoding cH9/1 and then were vaccinated with cH6/1 and boosted with cH5/1 soluble protein (purple triangles; n = 10) or BSA (black triangles; n = 5), while positive-control mice received inactivated virus intramuscularly (pink squares; n = 5). CD8 T cells were depleted prior to challenge with PR8 (H1N1) virus by treating the animals with an anti-CD8 T-cell antibody (hybridoma line 2.43) (17). Weight loss was monitored for 14 days. (B) Kaplan-Meier curve depicting survival (P = 0.0143).
Vaccination with cHA constructs elicits high titers of stalk-reactive antibodies.
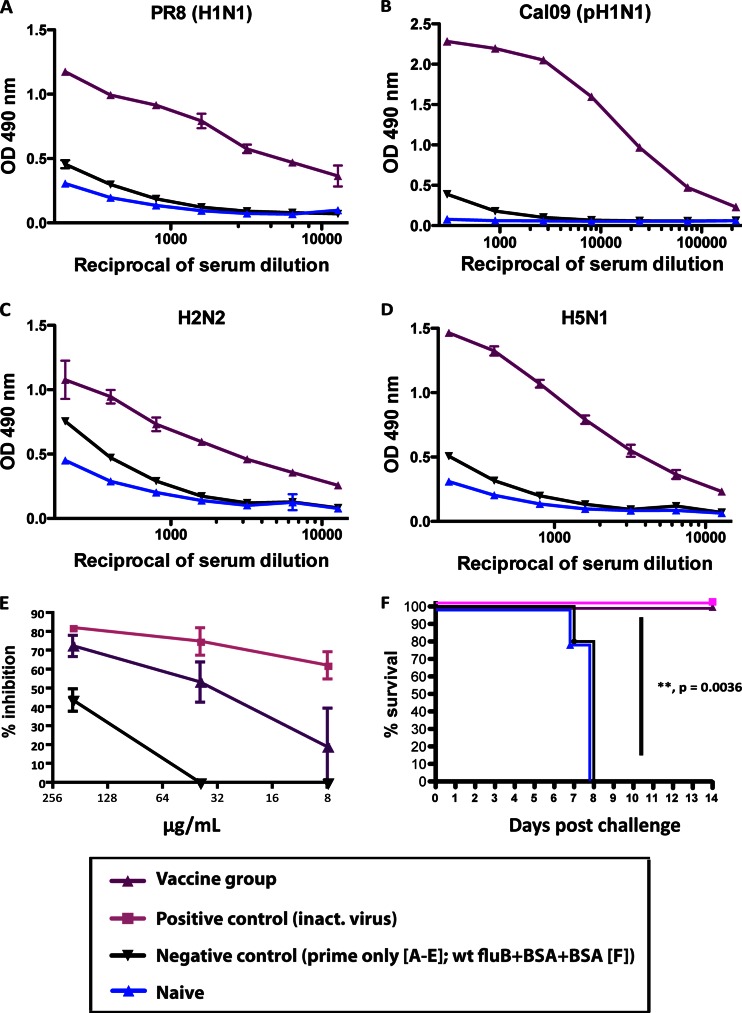
In order to further characterize the observed stalk-mediated protection, we analyzed the sera of animals vaccinated with cHA constructs by ELISA. The fact that animals were vaccinated with either DNA and protein or an influenza B virus expressing cH9/1 HA allowed us to assess seroreactivity by using purified wild-type influenza viruses as substrates. We tested the sera of animals from both vaccination groups (cH9/1 DNA+protein+protein and fluB-cH9/1+protein+protein groups) for reactivity to the PR8 (H1N1) virus, which was also used as one of the challenge strains. Naive sera as well as sera from the prime-only animals showed low, unspecific binding, as expected, whereas animals that were vaccinated with cHAs exhibited high reactivity to the substrate (Fig. 4A). Similar results were obtained for sera of animals from the fluB-cH9/1-vectored experiment (data not shown). Animals that were naive or that received wt fluB showed only background reactivity. Prime-only animals exhibited an intermediate binding phenotype, as expected, due to virus replication (12, 24). Similar results were obtained with the A/California/04/2009 (pH1N1) HA protein and an H2N2 virus substrate (Fig. 4B and C). Additionally, sera from animals from the H5N1 challenge group in the DNA-protein-protein experiment were tested for reactivity to purified H5N1 virus. Again, high antibody titers against the heterosubtypic H5 stalk domain were observed, further proving the breadth of the induced antistalk response (Fig. 4D). Despite the lack of HI activity of these sera toward the tested substrate viruses, we found strong reactivity to heterologous (H1) and heterosubtypic (H2 and H5) viruses by ELISA, confirming the production of stalk-specific antibodies by our vaccination protocol.
Fig 4.
The antibodies elicited by sequential vaccination with chimeric HAs are cross-reactive against group 1 HAs and have neutralizing activity in vitro and in vivo. (A to D) Stalk-specific ELISA reactivities of sera from animals electroporated with cH9/1 DNA and then vaccinated with cH6/1 and cH5/1 (replaced by H1 for mice used for panel C) soluble proteins (purple triangles) or BSA (black triangles) or of sera from naive animals (blue triangles) against H1 HA (purified PR8 [H1N1] virus substrate) (A), pH1 HA [purified Cal09 (pH1N1) protein substrate] (B), H2 HA (purified H2N2 virus substrate) (C), and H5 HA (purified H5N1 virus substrate) (D). (E) Animals were vaccinated as described above. Total IgG was purified for use in an H2-based pseudoparticle entry inhibition assay. Percent inhibition was assessed as the decrease in luciferase expression compared to that of controls. The Fab fragment of CR6261 (pink squares) was used as a positive control. (F) Passive transfer assay. Naive mice received sera from PR8-vaccinated animals (green squares; n = 5), fluB-cH9/1+cH6/1+cH5/1-vaccinated animals (purple triangles; n = 5), wt fluB+BSA+BSA-vaccinated animals (black triangles; n = 5), or naive mice (blue triangles; n = 5) via intraperitoneal injection and were then challenged with PR8 (H1N1) virus. The Kaplan-Meier curve depicts survival (P = 0.0036 between the groups that received sera from fluB-cH9/1+cH6/1+cH5/1-vaccinated animals and the wt fluB+BSA+BSA group).
Stalk-reactive antibodies elicited by cHA vaccine constructs are neutralizing and protect in passive transfer experiments.
To further validate the neutralizing capability of the induced stalk response against other subtypes, we tested the ability of purified IgG from vaccinated mice (cH9/1 DNA+cH6/1 protein+cH5/1 protein) to block entry of pseudoparticles harboring an H2 HA. Purified IgG was used in order to standardize the amounts used in the assay. Consistent with the protection seen following heterosubtypic challenges, IgG purified from vaccinated mice inhibited the entry of pseudoparticles in a dose-dependent manner, and with similar efficacy to that of CR6261 (3, 6). This monoclonal antibody with specificity to the HA stalk was used as the positive control (Fig. 4E). Our vaccination protocol therefore elicits stalk-specific antibodies capable of neutralizing group 1 HAs, including an H2 subtype HA.
In order to definitively show that the observed protection was the result of humoral immunity, we collected sera from mice that were immunized as described above (with fluB-cH9/1 virus, cH6/1 protein, and cH5/1 protein) for use in a passive transfer experiment. Following intraperitoneal administration of sera to naive mice, these animals were challenged with PR8 virus. Animals that received sera from cHA-vaccinated mice were completely protected from challenge, as were animals that received sera from PR8-immunized mice (Fig. 4F). However, animals that were administered sera from naive mice or from the wt fluB+BSA+BSA control group succumbed to infection by day 7 (Fig. 4F). In combination with the CD8+ T-cell depletion experiment shown in Fig. 3, our data suggest that the observed protection was mainly antibody mediated. Although we did not formally rule out the contribution of CD4+ T cells to protection, the data from the passive transfer experiment strongly indicate that humoral immunity is a key mechanism of the protection conferred by our vaccination protocol.
DISCUSSION
Our results demonstrate the efficacy of cHA constructs in eliciting broad-spectrum immunity against group 1 influenza viruses. cHA constructs not only allow for the sequential exposure of the same stalk in the context of an irrelevant globular head domain but also provide for the use of challenge viruses with globular heads to which animals have never been exposed. In this study, all animals were naive to the HA globular head domains of the challenge viruses used. This design demonstrates that protection from challenge following vaccination is based primarily on an immune response toward the HA stalk. Because cross-reactive antibodies toward the receptor binding site could possibly play a role in the protection seen here (2), we confirmed that all mice were HI negative for their respective challenge viruses. It is also possible that low levels of cross-reactive anti-globular head antibodies (not detected by HI assay) contributed to the neutralizing effect seen herein. This would, however, be considered an unexpected boon of our vaccination strategy, as this would supplement the protection already provided by antibodies with stalk specificities. Nevertheless, protection was impressively conferred following each virus challenge, even though animals were never exposed to the globular head of that particular virus subtype.
We hypothesize that the sequential exposure of the same H1 stalk stimulates adaptive immunity to this region. Note that cHA constructs contain a trimerization domain that maintains a correctly folded stalk structure in the absence of a viral membrane that normally stabilizes this interaction (27). As such, the unfettered exposure to a correctly formed HA stalk could also drive the boost in stalk titer that was seen following vaccination.
We conclusively showed that the protection induced by our vaccine was mediated by broadly neutralizing antibodies. However, the contributions of alternative mechanisms of action remain to be elucidated. We believe that processes such as antibody-dependent cell-mediated cytotoxicity and complement activation may play an important role. Other factors, such as temperature, might influence the efficacy of stalk-antibody binding. All in vitro binding assays we describe were carried out at room temperature. Even though influenza virus infection in mice induces hypothermia, prophylactic and therapeutic efficacy of stalk-reactive antibodies has been shown in ferrets as well (28). These animals develop fever upon influenza virus infection, similar to humans, suggesting that the neutralizing ability of these antibodies is not highly temperature dependent.
It is possible that a similar outcome could be achieved by using different vaccination schemes or employing other constructs that express conserved epitopes of influenza virus proteins (11, 21, 29–31), including those within the receptor binding site (32, 33) or the extracellular domain of the M2 protein (34). While we focused on a proof-of-principle experiment with group 1 influenza viruses, we believe that a similar approach could be employed for group 2 and influenza B viruses, leading to the development of a trivalent universal influenza virus vaccine.
In conclusion, we definitively demonstrated that sequential vaccination with cHA constructs can elicit polyclonal humoral responses to the HA stalk domain which are protective against heterologous and heterosubtypic challenges. The stimulation of these responses following previous influenza virus exposure supports the potential of a similar vaccine protocol in humans to provide protection against a broad range of influenza virus strains.
ACKNOWLEDGMENTS
We thank Chen Wang for technical assistance, Gene S. Tan for providing monoclonal antibodies, Christopher Seibert for purification of the H2N2 substrate virus, Ryan A. Langlois for support with FACS analysis, and Taia T. Wang for helpful discussions and critique of the manuscript. Furthermore, we thank Joshy Jacob (Emory University, Atlanta, GA) for the mouse-adapted FM1 challenge strain, Baozhong Wang (Emory University, Atlanta, GA) for the mouse-adapted H3N2 challenge strain, Damien Ekiert for the Fab of CR6261 (The Scripps Research Institute, La Jolla, CA), and Daniel Perez (University of Maryland, College Park, MD) for the H9 HA plasmid that was used to construct cH9/1.
The Mount Sinai School of Medicine has filed patent applications for the use of cHA constructs as universal influenza virus vaccines.
Partial support for this work was provided by PATH, by the National Institute for Allergy and Infectious Diseases-funded program project grant AI097092-01A1, and by Northeast Biodefense Center grant U54 AI070469. F.K. was supported by an Erwin Schrödinger fellowship (grant J 3232) from the Austrian Science Fund (FWF).
Footnotes
Published ahead of print 10 April 2013
REFERENCES
- 1. Palese P, Shaw ML. 2006. Orthomyxoviridae: the viruses and their replication, p 1648–1689 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2. Ekiert DC, Wilson IA. 2012. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr. Opin. Virol. 2:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. 2012. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J. Virol. 86:6179–6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, Cinatl J, ter Meulen J, Lasters I, Carsetti R, Peiris M, de Kruif J, Goudsmit J. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942 doi: 10.1371/journal.pone.0003942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J. 2011. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P. 2010. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 6:e1000796 doi: 10.1371/journal.ppat.1000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin O, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH. 2012. Highly conserved protective epitopes on influenza B viruses. Science 337:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol. 86:5774–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang ZY, Nabel GJ. 2010. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329:1060–1064 [DOI] [PubMed] [Google Scholar]
- 12. Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J. Virol. 87:4728–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu JS, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF. 2011. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797 doi: 10.1371/journal.pone.0025797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hai R, Garcia-Sastre A, Swayne DE, Palese P. 2011. A reassortment-incompetent live attenuated influenza virus vaccine for protection against pandemic virus strains. J. Virol. 85:6832–6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hai R, Martinez-Sobrido L, Fraser KA, Ayllon J, Garcia-Sastre A, Palese P. 2008. Influenza B virus NS1-truncated mutants: live-attenuated vaccine approach. J. Virol. 82:10580–10590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. U. S. A. 109:2573–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quinlivan M, Zamarin D, Garcia-Sastre A, Cullinane A, Chambers T, Palese P. 2005. Attenuation of equine influenza viruses through truncations of the NS1 protein. J. Virol. 79:8431–8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steel J, Lowen AC, Pena L, Angel M, Solorzano A, Albrecht R, Perez DR, Garcia-Sastre A, Palese P. 2009. Live attenuated influenza viruses containing NS1 truncations as vaccine candidates against H5N1 highly pathogenic avian influenza. J. Virol. 83:1742–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salem ML, Hossain MS. 2000. In vivo acute depletion of CD8(+) T cells before murine cytomegalovirus infection upregulated innate antiviral activity of natural killer cells. Int. J. Immunopharmacol. 22:707–718 [DOI] [PubMed] [Google Scholar]
- 21. Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P. 2010. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1:e00018–10 doi: 10.1128/mBio.00018-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856 [DOI] [PubMed] [Google Scholar]
- 23. Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R. 2012. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc. Natl. Acad. Sci. U. S. A. 109:9047–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krammer F, Pica N, Hai R, Tan GS, Palese P. 2012. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J. Virol. 86:10302–10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745–1749 [DOI] [PubMed] [Google Scholar]
- 26. Tamura M, Kuwano K, Kurane I, Ennis FA. 1998. Definition of amino acid residues on the epitope responsible for recognition by influenza A virus H1-specific, H2-specific, and H1- and H2-cross-reactive murine cytotoxic T-lymphocyte clones. J. Virol. 72:9404–9406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603 doi: 10.1371/journal.pone.0043603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friesen RH, Koudstaal W, Koldijk MH, Weverling GJ, Brakenhoff JP, Lenting PJ, Stittelaar KJ, Osterhaus AD, Kompier R, Goudsmit J. 2010. New class of monoclonal antibodies against severe influenza: prophylactic and therapeutic efficacy in ferrets. PLoS One 5:e9106 doi: 10.1371/journal.pone.0009106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bommakanti G, Citron MP, Hepler RW, Callahan C, Heidecker GJ, Najar TA, Lu X, Joyce JG, Shiver JW, Casimiro DR, ter Meulen J, Liang X, Varadarajan R. 2010. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc. Natl. Acad. Sci. U. S. A. 107:13701–13706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sagawa H, Ohshima A, Kato I, Okuno Y, Isegawa Y. 1996. The immunological activity of a deletion mutant of influenza virus haemagglutinin lacking the globular region. J. Gen. Virol. 77:1483–1487 [DOI] [PubMed] [Google Scholar]
- 31. Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, Garcia-Sastre A, Moran TM, Palese P. 2010. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl. Acad. Sci. U. S. A. 107:18979–18984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Albrecht R, Blum DL, Ramos I, Fernandez-Sesma A, Edwards KM, Garcia-Sastre A, Basler CF, Crowe JE., Jr 2012. Human monoclonal antibodies to pandemic 1957 H2N2 and pandemic 1968 H3N2 influenza viruses. J. Virol. 86:6334–6340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohshima N, Iba Y, Kubota-Koketsu R, Asano Y, Okuno Y, Kurosawa Y. 2011. Naturally occurring antibodies in humans can neutralize a variety of influenza virus strains, including H3, H1, H2, and H5. J. Virol. 85:11048–11057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. 1999. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 5:1157–1163 [DOI] [PubMed] [Google Scholar]