Abstract
A vaccine against Chikungunya virus (ChikV), a reemerging pathogenic arbovirus, has been made by attenuating wild-type (WT) virus via truncation of the transmembrane domain (TMD) of E2 and selecting for host range (HR) mutants. Mice are a standard model system for ChikV disease and display the same symptoms of the disease seen in humans. Groups of mice were inoculated with one of three ChikV HR mutants to determine the ability of each mutant strain to elicit neutralizing antibody and protective immunity upon virus challenge. One mutant, ChikV TM17-2, fulfilled the criteria for a good vaccine candidate. It displayed no reactogenicity at the site of injection, no tissue disease in the foot/ankle and quadriceps, and no evidence of viral persistence in foot/ankle tissues 21 days after infection. Upon challenge with a highly pathogenic strain of ChikV, the mutant blocked viral replication in all tissues tested. This study identified a ChikV HR mutant that grows to high levels in insect cells but was restricted in the ability to assemble virus in mammalian cells in vitro. The study demonstrates that these HR strains are attenuated in the mammalian host and warrant further development as live-attenuated vaccine strains.
INTRODUCTION
Chikungunya virus (ChikV) is a member of the family Togaviridae, genus Alphavirus (1), and is known to cause severe arthralgic disease in humans (2). ChikV is an arthropod-borne virus (arbovirus) spread by the bite of an aedine mosquito (3, 4). All alphaviruses are composed of a small (∼11-kb) plus-polarity single-stranded RNA genome. The genome encodes 3 structural proteins (E1, E2, and C) and 4 nonstructural proteins (nsP1 to -4). These viruses are enveloped and derive their envelope from either the insect or vertebrate host. The genus Alphavirus contains 29 known species, which cause encephalitis, fever, and/or arthralgia (5). ChikV is endemic to Africa (6) and Southeast Asia (7; http://www.searo.who.int/index.html). However, currently, ChikV is a reemerging pathogenic virus; in 2005, it spread to the Indian Ocean and Italy, causing an epidemic with over 200 reported infections (7, 8). Of the two lineages of ChikV, the African strains remain enzootic by cycling between mosquitoes and monkeys, but the Asian strains are transmitted directly between mosquitoes and humans. This cycle of transmission may have allowed the Asian strains of the virus to become more pathogenic, as the reservoir host was eliminated (9, 10). In humans, ChikV can cause a debilitating disease characterized by arthralgia, in addition to symptoms commonly associated with dengue virus infection: fever, headache, nausea, vomiting, fatigue, rash, muscle pain, and joint pain. The incubation period can be 2 to 12 days but is generally 3 to 7 days, with “silent” infections occurring with unknown frequency (11). ChikV can be transmitted from mother to child during pregnancy (12, 13) and can produce chronic symptoms, including crippling arthralgia, encephalitis, and (rarely) myocarditis (14–17). ChikV epidemics from 2004 to 2011 resulted in 1.4 to 6.5 million reported cases, with imported cases spreading to 40 countries (8, 18). The Aedes aegypti mosquito is the primary vector of ChikV, but some recent outbreaks with fatalities have been propagated through the Aedes albopictus “Asian tiger” mosquito (8, 19). This aggressive mosquito vector has spread to 12 European countries (20, 21), Australia (22), and the Americas (23), where it is now considered endemic (24). There is currently no vaccine or antiviral treatment for ChikV approved for human use (25).
Arboviruses are a distinct virus group because they express a unique set of characteristics. Arboviruses are vectored in nature by blood-sucking insects in a complex life cycle in which an intermediate mammalian host is infected and transfers the virus during another insect's blood meal (10). These viruses have adapted over millions of years to replicate efficiently in two very different genetic and biochemical environments as natural chimeric genomes (26). The environments of the hosts differ biochemically and function optimally at different temperatures (27). The virus particles themselves are hybrid structures composed of membrane and glycosylation components derived from the host, but all protein and RNA is encoded by the virus (28). Viruses derived from insect or mammalian cells have been found to exhibit functional (7, 26, 29) and structural (30, 31) differences that emphasize their hybrid nature. It is hypothesized that these agents have developed a consensus genome capable of expression in divergent biochemical environments by interacting specifically with each of these distinct hosts through sequence elements necessary for viral replication in one host but not necessarily the other (32–35). These motifs are interpreted biochemically in the context of the specific host. “Host range” (HR) mutations of Sindbis virus (SINV) (32) and dengue virus type 2 (DV2) (36, 37) have been produced by truncating the transmembrane domain (TMD) of the E2 or E glycoprotein, respectively. These large deletion mutants are screened to select those that display the HR phenotype. The HR mutants are restricted to replicate in insect cells but are attenuated for infection in the mammalian host. These HR mutations generate viruses useful as potential vaccine strains (36–40). In this study, mutants of ChikV were produced using the same approach utilized in SINV and DV2, as mentioned above. Unlike dengue virus, which has 4 distinct serotypes and is pathogenic only in humans, only one ChikV serotype has evolved, despite known strain differences. Mice are one model system for studying ChikV disease, because they display the same symptoms of the infection as humans (41). A series of ChikV HR mutants were made, and their safety and ability to confer protection against ChikV disease were tested in mice. One HR mutant, ChikV TM17-2, produced no reactogenicity, was efficacious upon challenge, and did not induce inflammation of the feet or ankles. Importantly, this vaccine virus protected against replication of any detectable virus in serum or tissues at every time point postchallenge (p.c.). While vaccines for human use cannot be grown in mosquito cells, it was found that ChikV grows well in the Spodoptera frugiperda (Sf9) cell line in the absence of serum. This cell line is used in the production of influenza subunit vaccines awaiting FDA approval (42) and will be used for the continued development of HR mutant ChikV vaccine.
MATERIALS AND METHODS
Biosafety.
All studies involving ChikV were performed in certified biosafety level 3 (BSL3) laboratories using biosafety protocols approved by the Institutional Biosafety Committee of North Carolina State University. Animal husbandry and mouse experiments were performed by the Carolina Vaccine Institute, University of North Carolina at Chapel Hill, Chapel Hill, NC, in accordance with all University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee guidelines.
Construction of ChikV TMD deletion mutants.
A full-length cDNA clone of Chikungunya virus, West African strain 37997, in the pCHIK-37997ic vector (GenBank accession no. EU224270) was obtained from Stephen Higgs (43). Deletions in the E2 TMD of ChikV were produced by PCR, using Pfu Turbo DNA polymerase AD (Stratagene, La Jolla, CA). The ChikV TMD sequence was chosen by alignment with SINV. Sets of 9-amino-acid (aa) deletions within ChikV E2 were constructed so that the TMD was 17 aa in length (TM17-1, -2, and -3) (Table 1) (36). All ChikV deletion mutant clones were confirmed by sequence analysis (Eurofins MWG Operon, Huntsville, AL). Purified DNA producing full-length ChikV RNAs was transcribed in vitro with SP6 RNA polymerase and transfected into C7-10 cells for stock virus production.
Table 1.
Chikungunya virus host range transmembrane deletion mutations and titers compared to those of SINV
| Mutant | Sequenced | Titer (PFU/ml) |
|
|---|---|---|---|
| BHK | C7-10 | ||
| SINV | |||
| HRa | 365VYTILAVASATVAMMIGVTVAVLCAC390 | 1 × 107 | 1 × 109 |
| ChikV | |||
| 37997b | 365TMTVVIVSVASFVLLSMVGTAVGMCV390 | 3 × 108 | 5 × 108 |
| TM17-1b | 365TMTVVIVSVASFVLLSMVGTAVGMCV390 | 7 × 105 | 2 × 106 |
| TM17-2b | 365TMTVVIVSVASFVLLSMVGTAVGMCV390 | 4 × 105 | 2 × 106 |
| TM17-3c | 365TMTVVIVSVASFVLLSMVGTAVGMCV390 | 1 × 105 | 1 × 106 |
HR, heat-resistant strain. The portion of the sequence in bold represents the segment of the TMD deleted in the original SINV TM-17 mutant.
Titers from 2 days postinfection.
Titers from 3 days postinfection.
The underlined portions of the sequences represent the segments of the TMD that were deleted.
Vaccine virus TMD deletion stability.
Viral RNA from mouse ankle tissue samples 2 days postinjection (p.i.) was extracted using TRIzol LS and analyzed by reverse transcription (RT) plus two rounds of nested PCR to confirm the stability of the HR deletion in the E2 domain (42). The primary PCR program was as follows: 1 cycle of 3 min at 95°C; 45 cycles of 45 s at 95°C, 1 min at 60°C, and 1 min at 72°C; 1 cycle of 3 min at 72°C; hold at 4°C. The primer pair was as follows: Chik-9041-F, 5′-CCGCTGCCACTGCTGAGGAGATAGA-3′, and Chik-9916-R, 5′-AAGGCGGCCAGCGGGATAAGA-3′. The secondary program was as follows: 1 cycle of 3 min at 95°C; 45 cycles of 45 s at 95°C, 45 s at 55°C, and 1 min at 72°C; 1 cycle of 3 min at 72°C; hold at 4°C with nested primers Chik-9597-F, 5′-CTGGCCGCAGATGTCTACGAACG-3′, and Chik-9798-R, 5′-TGAGCAGGAAGGGAACAGTGG-3′. Sequences were confirmed by Eurofins MWG Operon. Up to 6 serial passages in mammalian cells in vitro did not reveal changes to the deleted site, and no phenotypic changes were observed.
Cell and virus culture.
Baby hamster kidney (BHK) and C7-10 mosquito cell lines were maintained as previously described (44). C7-10 cells were transfected by electroporation with wild-type (WT) ChikV and ChikV TM17 series mutant RNAs. Supernatants were harvested 2 days posttransfection and used in subsequent infections. Virus grown by infection in either type of host cells was titrated by plaque assay in C7-10 cells. Growth curves with harvest time points 6, 24, 48, and 72 h postinfection in BHK and C7-10 cells were also performed and titrated on BHK cells (Fig. 1).
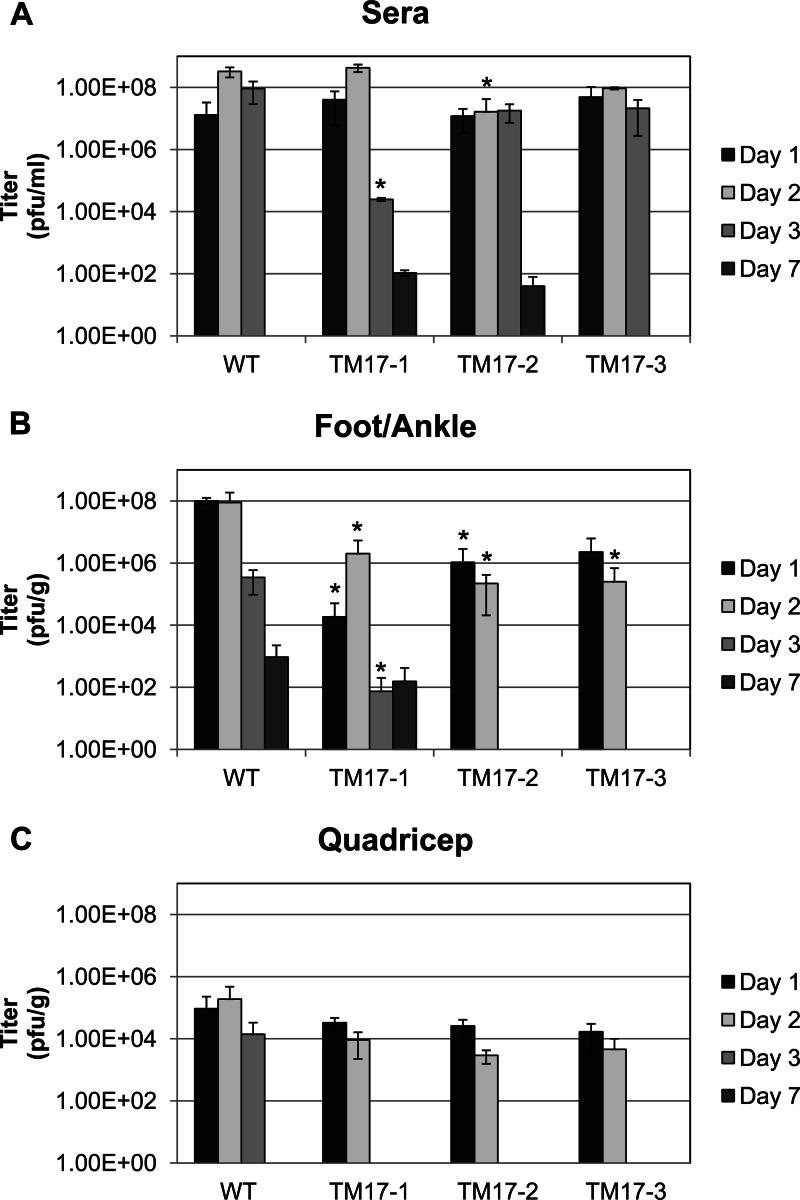
Fig 1.
Prechallenge viremia by plaque assay in mosquito cells in the designated tissues at 1, 2, 3, and 7 days after injection with 103 PFU of WT ChikV, TM17-1, TM17-2, or TM17-3. The values of the mutant virus compared to the WT viremia were analyzed by Welch-corrected Students' t test, and the asterisks indicate where significant differences were found. The error bars indicate standard deviations within sample groups. (A) Viremia detected in mouse sera; analysis of the titers showed no significant difference between the mutants and the WT until day 2 for TM17-2 (P < 0.05) and day 3 (P < 0.001) for TM17-1. (B) Foot and ankle tissue titers differed from WT as follows: day 1, P < 0.001 for TM17-1 and P < 0.01 for TM17-2; on day 2, TM17-1, -2, and -3 titers were significantly lower (P < 0.05). One day 3 virus was cleared from the TM17-2/3-infected mice. However, the WT and TM17-1 were not cleared from mouse feet/ankles at day 7. (C) Titers from quadriceps from which all the mutant viruses were cleared by day 3. No viremia was detected in mice injected with mock samples. The limit of detection of the plaque assay was 40 PFU.
Mouse studies.
Previous studies have described ChikV disease in C57BL/6J mice (45, 46). For this study, C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Twenty-eight-day-old mice were infected via subcutaneous injection into the left footpad with ∼103 PFU of WT ChikV or ChikV TM17-1, TM17-2, or TM17-3 in 10 μl of complete minimal essential medium (MEM) with 10% glycerol (45). The mice were weighed every day, and no mortality occurred from ChikV infection. Swelling and inflammation were visually inspected laterally and longitudinally along the foot below the ankle. Animals, including those from a naive group of mice injected with medium only, were sacrificed 1, 2, 3, 7, 10, and 21 days p.i. to evaluate viremia, persistence in tissues, neutralizing antibody (NAb) titer, IgG production, and disease. Observations were made 1 to 10 days postvaccination to evaluate physical stress, swelling, or disability of the mouse footpad due to virus infection. Animals from which tissues were prepared were perfused with paraformaldehyde, embedded in paraffin, and processed for hematoxylin and eosin (H&E) staining 7, 10, and 21 days p.i. Based on the results from the initial evaluation of the vaccine candidates, naive mice and mice injected with TM17-1 and TM17-2 were tested for protection by challenge with a more pathogenic strain of ChikV, SL15649. At 28 days p.i., the majority of mice from each group were challenged via subcutaneous injection in the footpad with 103 PFU WT ChikV SL15649 (45), while 3 mice from each vaccine group were injected with medium as the control. Mice were sacrificed 1, 2, 3, and 7 days p.c. to again evaluate viremia, tissue disease, and NAb.
Viremia from mice.
The ChikV mutant vaccine titers and viremias from mice were quantified by plaque assay in C7-10 cells, as described previously (44). Viremias resulting from the challenge virus ChikV SL15649 (45) were quantified by plaque assay on BHK cells due to the preference of the virus for these cells as indicator cells (this study). The limit of detection for these assays was <40 PFU/g of tissue, and the results expressed are the arithmetic means of titers obtained from 3 mice/group/day.
Persistence of infection in tissues.
To determine if mutant virus persisted in the vaccinated animals, tissues and sera from infected and naive mice 10 and 21 days postvaccination were homogenized and RNA was extracted using TRIzol LS reagent and the Purelink RNA kit (Life Technologies Inc., Grand Island, NY) and suspended in water. The extracted RNAs were then analyzed via RT-PCR (45). The plasmid ic-CHIKV SL15649 was used as a positive control, and the extracted RNA was used as a negative control. The RT-PCR had a sensitivity of detection of 12.8 pg RNA/reaction, or ∼10 PFU.
Plaque reduction neutralization test.
NAb titers were determined by plaque reduction neutralization test (PRNT) in BHK cells (37). Heat-inactivated mouse sera were serially diluted 1:2 in duplicate, starting with a 1:20 dilution. Approximately 20 PFU of WT ChikV was added to each dilution, allowed to incubate at room temperature for 15 min, and then plated on BHK cells for 2 days at 37°C. NAb titers (PRNT50) were determined to be the highest serial dilutions where ≤50% of the PFU added were observed and are expressed as the geometric mean of titers from the 3 mice/group/day.
Anti-ChikV IgG ELISA.
Poly-d-lysine-pretreated 96-well plates (Becton, Dickinson, Bedford, MA) were coated with >100 ng of purified WT ChikV/well at 37°C for 1 h and blocked with phosphate-buffered saline, 0.2% Tween 20, and 10% fetal calf serum (FCS) at 4°C overnight. Duplicate 1:100 dilutions of heat-inactivated mouse sera obtained 21 days p.i. and 7 days p.c. were added to the plate for 1.5 h at room temperature and removed. A 1:2,000 dilution of anti-mouse IgG horseradish peroxidase-conjugated (Sigma-Aldrich A8924) Ab was then added to the plate for 1.5 h at room temperature and removed. Enzyme-linked immunosorbent assays (ELISAs) were developed using TMB substrate (Promega) as instructed by the manufacturer. The plates were read using a Tecan Rainbow 96-well plate reader at an absorbance wavelength of 405 nm and a reference wavelength of 0 nm. The results are the arithmetic mean of the optical density read at 450 nm (OD450) obtained from 3 mice/group.
Histopathology.
Mice were sacrificed and perfused by intracardial injection with 4% paraformaldehyde, pH 7.3, on the days indicated. Hind limb tissues were embedded in paraffin, and 5-μm sections were prepared (45). H&E stain was used to determine the extent of inflammation of the tissue and tissue disease. Sections were evaluated for inflammation in the joint and in the muscle tissue, fasciitis, synovitis, and perivasculitis of the foot/ankle and quadriceps as described in reference 45.
RESULTS
Host range mutant design.
ChikV mutant design was based on TMD deletions made in SINV, a related alphavirus (32). In the SINV system, two HR mutants were found, which retained 16 and 17 aa of the WT 26-aa sequence in the TMD (32, 47). These mutants were designated TM16 and TM17 for the number of amino acids remaining in the membrane (32). Both mutants were tested for immunogenicity and efficacy in mice, and TM17 was found to be protective upon challenge (47). ChikV and SINV share 44% homology in the structural proteins, and the SINV deletions were used as a template for the design of the mutants made in ChikV (48). The 26-aa sequence defining the ChikV TMD was determined by comparison to the SINV TMD (28, 33, 49–51) (Table 1). The working hypothesis was that the sequence elements conferring the HR phenotype should reside in similar locations within related virus genomes. However, because of the specific geometry of the helical TMD due to the differences in the amino acid sequence, the exact deletion resulting in the desired HR phenotype may shift toward the amino or the carboxyl terminus. To address this possibility, a series of 3 TM17 mutants was made, deleting the sequences underlined in Table 1. ChikVTM17-1 replicated the location of the original SINV TM17. Two other mutants, ChikV TM17-2 and TM17-3, were designed to shift the deleted sequence toward the carboxyl terminus 2 and 1 aa, respectively. Virus titers of the ChikV mutants were determined after growth in both BHK and C7-10 cells. All 3 ChikV mutants had peak titers over a 72-h period in the range of 105 from BHK and 106 from C7-10 cells. WT ChikV, TM17-1, and TM17-2 peaked at 48 h postinfection, while TM17-3 peaked at 72 h postinfection (Table 1). These large deletions do not revert in vitro or in vivo (37) and elicit protective immunity which offers protection from challenge with WT virus (36, 37). The retention of the correct 9-aa deletion in the E2 TMD of each of the ChikV HR mutants was assessed by RT-PCR on day 2 foot/ankle tissue samples. All 3 mice in each group were confirmed to have the proper deletion (data not shown), verifying the stability of each HR mutant in vivo.
Prechallenge viremia and vaccine reactogenicity.
Chikungunya virus causes arthritis and can establish persistent infection in the joints (18). Thus, both serum and tissue surrounding the ankle were examined for viremia and tissue disease. Viremias from inoculated mice were determined from sera and tissue samples 1, 2, 3, and 7 days p.i. and are shown in Fig. 1. All mice were injected with 103 PFU of virus/10 μl in the footpad. Each of the viruses grew to a level of 107 PFU/ml within 24 h p.i. Serum virus titers were not found to be significantly different from those of WT ChikV until day 2 for TM17-2 (P < 0.05) and day 3 for TM17-1 (P < 0.001) (Fig. 1A). ChikV TM17-1 and -2 were not entirely cleared from the serum on day 7 (102 PFU/ml). While the vaccine viremia was high, no other symptoms of disease were observed (see below). The infection profile changed when the feet/ankles were examined (Fig. 1B). The values of the mutant virus compared to the WT viremias were analyzed by Welch-corrected Student's t test. Foot/ankle tissue titers differed from WT as follows: day 1 titers were significantly lower than WT for TM17-1 (P < 0.001) and for TM17-2 (P < 0.01). On day 2, TM17-1 (P < 0.05) and TM17-2 (P < 0.01) were both lower than WT. TM17-1, -2, and -3 were all significantly lower than WT on day 3 (P < 0.05). By day 7, both TM17-2 and -3 were cleared from the foot/ankle, but TM17-1 was still detected. ChikV quadriceps titers from the 3 mutants tested did not vary from WT titers on days 1 and 2 (Fig. 1C). However, for TM17-1, -2, and -3, virus was not detected on day 3, while WT-virus-inoculated animals still expressed 104 PFU/g. This study also evaluated inflammation and swelling of the foot/ankle at the site of injection of each of the vaccine viruses or WT control compared to a mock-vaccinated group. Inflammation was monitored for 10 days p.i. Swelling at the site of injection is indicative of primary reactogenicity and is a good predictor of further tissue disease (45). Severity grades were assigned as minimal, mild, moderate, or severe. WT virus produced severe swelling. TM17-3 reactogenicity was minimal, and TM17-1 and TM17-2 had no measurable swelling. WT-ChikV-infected mice displayed mild to moderate inflammation beginning 2 days p.i., which progressed to severe by days 5 and 6 and began to diminish by days 9 and 10 (data not shown). Because swelling following vaccine administration is linked to other adverse pathology, only the mutants that did not produce any inflammation at the site of injection proceeded to the challenge portion of the study. Thus, TM17-1 and TM17-2 were both challenged, while TM17-3 was eventually eliminated from the study.
Virus persistence in foot/ankle and quadriceps.
Virus persistence in mice was observed previously by the presence of virus 21 days p.i. in affected tissue (45). The persistence of the HR mutants was thus evaluated by RT-PCR at days 10 and 21 postinfection and is shown in Table 2. The presence of a PCR product was scored as plus or minus for each of 3 mice. On both days 10 and 21, 3 WT-ChikV-inoculated mice tested positive, following the same profile: 1 mouse positive in the serum, 3 in the foot/ankle, and 1 in the quadriceps. Of the mice infected with the mutant viruses, TM17-1 tested minimally positive (1 mouse) in the foot/ankle on day 21, and TM17-2 tested minimally positive (1 mouse) from serum on day 21. This may have resulted from residual RNA, which at this level would not be detectable by plaque assay. TM17-3 tested positive (2 mice) from the serum on both days 10 and 21 p.i. WT-ChikV (strain 37997) infection was found to follow the same time course as that reported for ChikV SL15649 (45). The limit of detection of this assay was 12.8 pg RNA/reaction, or 10 PFU.
Table 2.
Persistence of ChikV RNA in mouse tissue after vaccination
| Vaccine group | Mice testing positivea |
|||||
|---|---|---|---|---|---|---|
| 10 days p.i. |
21 days p.i. |
|||||
| Serum | Foot/ankle | Quadriceps | Serum | Foot/ankle | Quadriceps | |
| WT ChikV | + | +++ | + | + | +++ | + |
| TM17-1 | − | − | − | − | + | − |
| TM17-2 | − | − | − | + | − | − |
| TM17-3 | ++ | − | − | ++ | − | − |
| Naive | − | − | − | − | − | − |
The number of plus signs represents the number of mice per sample group that tested positive (n = 3). A minus sign means all mice tested negative. The limit of detection was 12.8 pg RNA/reaction, or ∼10 PFU.
Vaccine immunogenicity.
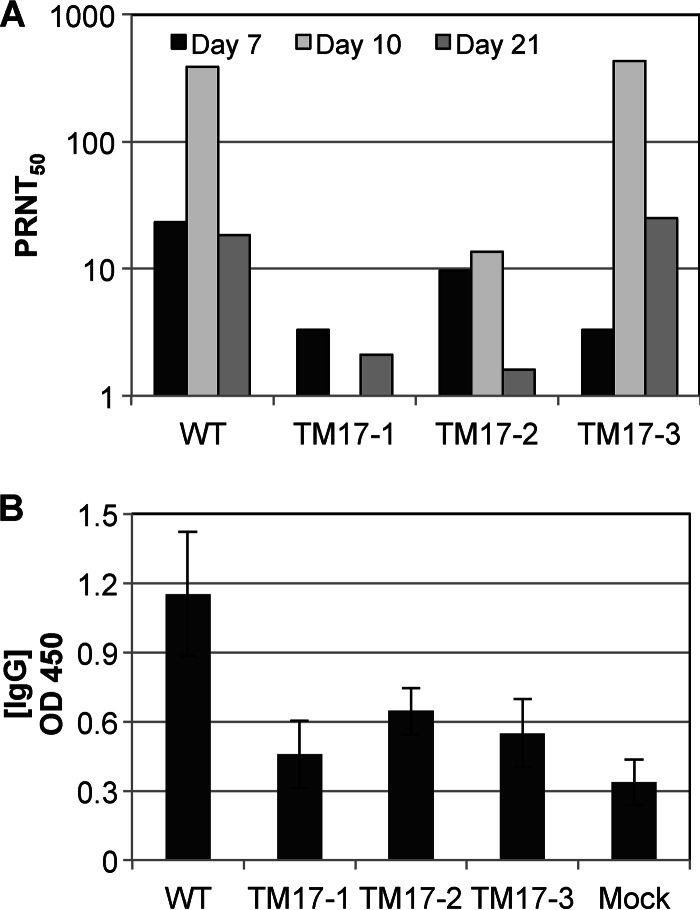
Immunogenicity was determined by evaluating the amount of serum NAb through PRNT 7, 10, and 21 days p.i. (Fig. 2A). For the majority of serum samples, little to no NAb titer was observed (PRNT50, <25). However, substantial NAb titers (PRNT50, ∼400) were present in the WT-virus- and TM17-3-injected mice 10 days p.i. Based on these results, it was also important to determine the total ChikV-specific IgG postinjection. The total concentration of ChikV-specific IgG elicited was determined by ELISA performed on the 21-day-p.i. samples. As shown in Fig. 2B, WT-ChikV-inoculated animals were found to have significantly more ChikV-specific IgG present. Animals vaccinated with TM17-1 and TM17-3 were found to elicit less total ChikV-specific Ab than WT-vaccinated animals—TM17-1, P < 0.02; TM17-3, P < 0.04; naïve, P < 0.03—while no significant difference was found between the respective mutant pairs. There was also no significant difference between WT-ChikV-inoculated mice and TM17-2-inoculated mice, and TM17-2-inoculated mice were found to produce significantly more ChikV-specific IgG than mock-inoculated mice (P ≤ 0.03). Thus, although all groups exhibited very low levels of NAb 21 days p.i., the total amount of IgG resulting from injection with WT ChikV was significantly larger than that of each with the HR mutants, except TM17-2. It is notable that TM17-2 did not induce high NAb titers by PRNT50, nor did it induce high overall titers of IgG either pre- or postchallenge. Interpretation of these data suggests that the protection conferred by ChikV TM17-2 is not solely antibody dependent.
Fig 2.
(A) NAb titers present in mouse sera 7, 10, and 21 days after vaccination with the wild type or the attenuated HR mutant ChikV 37997. (B) Total anti-ChikV IgG (OD450) present in mouse serum 21 days postvaccination. The NAb titers represent the geometric means of sera from 3 different mice per group per day. The amount of total WT IgG measured was found to be statistically larger than that of the IgG from TM17-1 and -3 (P ≤ 0.03 for naïve; P ≤ 0.02 for TM17-1; P ≤ 0.04 for TM17-3), while no significant difference was found among the respective mutant pairs. There was no significant difference between WT and TM17-2, whereas TM17-2 was statistically higher than mock (P ≤ 0.03). The error bars indicate standard deviations within sample groups.
Vaccine efficacy.
To ascertain the level of vaccine efficacy, vaccinated animals were challenged with a pathogenic strain of ChikV (ChikV SL15649) (45) and then sacrificed 1, 2, and 3 days p.c. to determine viremia and pathology. The challenge was 103 PFU of WT ChikV SL15649 injected into mice previously inoculated with TM17-1, TM17-2, WT ChikV, or no vaccine (naive). Table 3 displays the viremia levels measured for the indicated tissue on 3 consecutive days p.c. WT-ChikV-challenged mice had a titer of 8.5 × 103 PFU/g from the foot/ankle and 5.1 × 102 PFU/g from the quadriceps on day 1. WT-ChikV-infected mice displayed continued infection in the quadriceps on day 2 (1.3 × 104 PFU/g) that was cleared by day 3 p.c. It was not possible to determine if this viremia was caused by the challenge virus or continued infection with the original ChikV 37997 inoculum. As shown in Table 3, TM17-1-inoculated mice had a titer of 1.3 × 104 PFU/ml in the serum on day 1 p.c. (day 29 postvaccination). This titer is attributed to the challenge virus, because all prechallenge serum viremia was cleared for this mutant by day 21 (Table 2). ChikV TM17-1-inoculated mice also had a titer of 6.4 × 102 PFU/ml virus in the quadriceps on day 2 p.c. No further viremia was detected for this mutant from any tissue on day 3 p.c. in any mouse. ChikV TM17-2 mice had no detectable viremia in any of the tissues sampled on the 3 days p.c. The challenge virus, ChikV SL15649, gave serum titers in naive mice of 4.7 × 107 and 8.6 × 105 PFU/ml on days 1 and 2, respectively, but was cleared by day 3. These mice also had foot/ankle titers of 4.3 × 103, 7.3 × 103, and 9.8 × 102 PFU/g, respectively, on each of the 3 days p.c. and quadriceps titers of 1.7 × 105, 4.0 × 105, and 2.1 × 103 PFU/g, respectively, on each of the 3 days p.c. Collectively, these data demonstrate that ChikV infection targets the joints and surrounding musculature and that vaccination with TM17-2 protected all tissues assayed from WT-virus challenge beginning day 1 of the viremic period. Seven days p.c., naive mice challenged with ChikV SL15649 generated little to no NAb titer (Fig. 3A), while ChikV TM17-1- and TM17-2-vaccinated mice gave PRNT50 titers of >3,000 and >500, respectively. Mock-challenged animals displayed PRNT50 titers consistent with background levels. Surprisingly however, the levels of total IgG were not significantly different for TM17-1- or TM17-2-inoculated mice, whether they were challenged or remained unchallenged (Fig. 3B).
Table 3.
Viremia detected in mouse tissue after challenge with ChikV SL15649
| Vaccine | Viremiaa (PFU/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum |
Foot/ankle |
Quadriceps |
|||||||
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | |
| TM17-1 | 1.3 × 104 | ND | ND | ND | ND | ND | ND | 6.4 × 102) | ND |
| (P < 0.05) | (NS) | (NS) | (P < 0.05) | (P < 0.001) | (P < 0.05) | (P < 0.01) | (P < 0.01) | (NS) | |
| TM17-2 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| (P < 0.001) | (P < 0.001) | (NS) | (P < 0.05) | (P < 0.001) | (P < 0.05) | (P < 0.001) | (P < 0.01) | (NS) | |
| ChikV 37997 | ND | ND | ND | 8.5 × 103 | ND | ND | 5.1 × 102 | 1.3 × 104 | ND |
| (P < 0.001) | (P < 0.01) | (NS) | (P < 0.001) | (P < 0.001) | (P < 0.05) | (P < 0.001) | (P < 0.01) | (NS) | |
| Naive | 4.7 × 107 | 8.6 × 105 | ND | 4.3 × 103 | 7.3 × 103 | 9.8 × 102 | 1.7 × 105 | 4.0 × 105 | 2.1 × 103 |
ND, below the detection limit of the assay (40 PFU/ml); NS, not statistically significant. The P values demonstrate significant difference from the naive control group.
Fig 3.
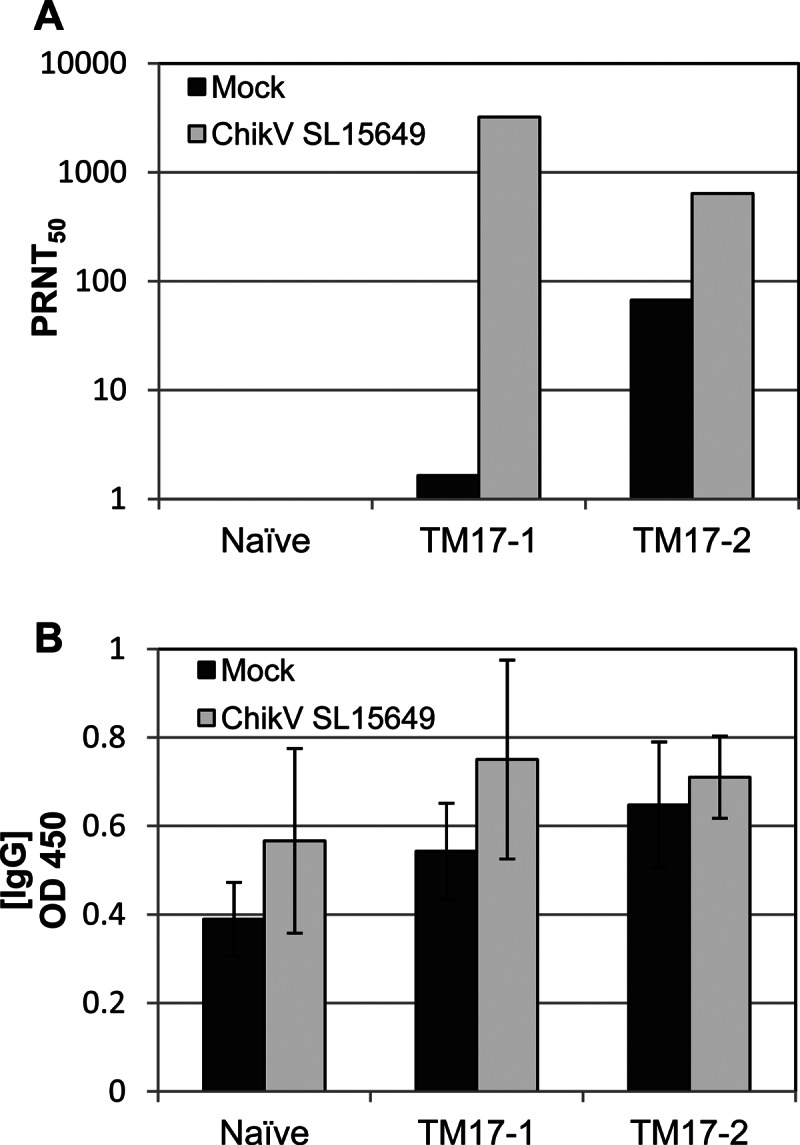
NAb titers (A) and total anti-ChikV IgG (OD450) (B) present in mouse sera 7 days after challenge with wild-type ChikV SL15649 or mock challenge (complete medium). The NAb titers represent the geometric means of sera from 3 different mice per group per day. The NAb titers in both the challenged and unchallenged naïve-mouse groups were below detection (<10), while challenge with ChikV SL15649 generated a significant boost in the NAb titers of the TM17-1- and -2-vaccinated mice compared to the mock-challenged groups. The error bars indicate standard deviations within sample groups.
Histopathology pre- and postchallenge.
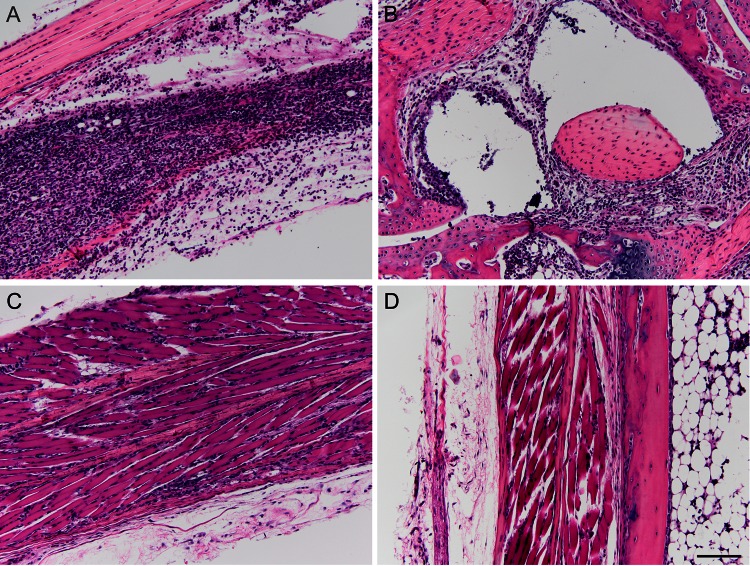
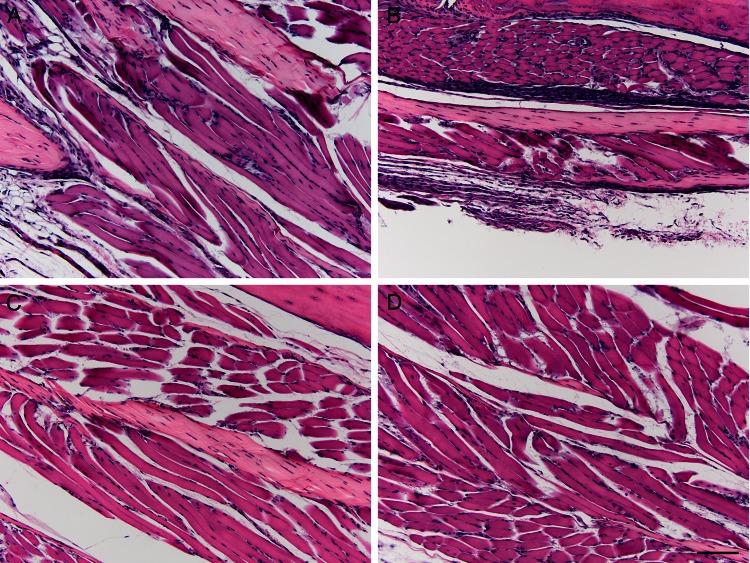
It was important to determine if any tissue pathology presented as a result of vaccination with TM17-1 or TM17-2. To determine this, sections of mouse foot/ankle joints were taken at 7 days postvaccination, H&E stained, and scored blindly. Slides from a representative animal are shown in Fig. 4 at ×20 magnification. Pathology was assessed by scoring slides from each animal based on muscle inflammation, muscle necrosis, tendonitis, synovitis, and perivasculitis. For scoring pathology, the following scale was used: 0, 0 to 2%; 1, 2 to 20%; 2, 20 to 40%; 3, 40 to 60%; 4, 60 to 80%; and 5, 80 to 100%. For scoring of synovium and perivascular inflammation, the following scale was used: 0, no change; 1, minimal; 2, mild (inflammatory infiltrate); 3, moderate; 4, severe (destruction of synovial membrane). Scores for individual animals postvaccination are shown in Table 4. As shown in Fig. 4A and B, infection of mice with WT ChikV produced severe muscle inflammation and necrosis with apparent destruction of the synovial membrane. In contrast, animals vaccinated with TM17-1 displayed mild muscle inflammation with no other signs of pathology (Fig. 4C). Importantly, animals vaccinated with TM17-2 displayed no signs of any pathology at 7 days postvaccination (Fig. 4D). These results confirm the primary reactogenicity studies, in which no swelling was seen in animals vaccinated with TM17-2.
Fig 4.
Pathology of mouse foot/ankle joint histology sections 7 days after vaccination (magnification, ×20) of a representative animal. (A) Evidence of extensive muscle inflammation and necrosis following administration of ChikV 37997. (B) Administration of ChikV 37997 also resulted in severe synovitis with destruction of the synovial membrane and moderate perivasculitis. (C) TM17-1-vaccinated animals displayed minimal muscle inflammation with no evidence of muscle necrosis, synovitis, or perivasculitis. (D) TM17-2-vaccinated animals displayed no evidence of pathology in the foot/ankle joint. Bar, 50 μm.
Table 4.
Pathology scoring assigned to slides for individual animals for foot/ankle sections taken 7 days after vaccination
| Group/mouse | Score |
||||
|---|---|---|---|---|---|
| Muscle inflammationa | Muscle necrosisa | Tendonitisa | Synovitisb | Perivasculitisb | |
| WT/1 | 5 | 5 | 1 | 3 | 3 |
| WT/2 | 5 | 5 | 1 | 4 | 2 |
| WT/3 | 5 | 5 | 1 | 4 | 2 |
| TM17-1/1 | 1 | 0 | 0 | 0 | 0 |
| TM17-1/2 | 0 | 0 | 0 | 0 | 0 |
| TM17-1/3 | 1 | 0 | 0 | 0 | 0 |
| TM17-2/1 | 0 | 0 | 0 | 0 | 0 |
| TM17-2/2 | 0 | 0 | 0 | 0 | 0 |
| TM17-2/3 | 0 | 0 | 0 | 0 | 0 |
Scale: 0, 0 to 2%; 1, 2 to 20%; 2, 20 to 40%; 3, 40 to 60%; 4, 60 to 80%; 5, 80 to 100%.
Scale: 0, no change; 1, minimal; 2, mild (inflammatory infiltrate); 3, moderate; 4, severe (destruction of synovial membrane).
To determine if vaccination with TM17-1 or TM17-2 protected animals from developing ChikV-associated pathology during challenge, foot/ankle sections of mice were taken at 7 days postchallenge, H&E stained, and scored for pathology as described above (Table 5). Slides from a representative animal taken at ×20 magnification are shown in Fig. 5, with scores from each individual animal shown in Table 5. As shown in Fig. 5A, naive mice challenged with ChikV SL15649 displayed moderate muscle inflammation and necrosis. Mice vaccinated with TM17-1 displayed minimal muscle inflammation following challenge with ChikV SL15649, with no other pathology apparent (Fig. 5B). Most importantly, samples taken from mice vaccinated with TM17-2 prior to challenge with ChikV SL15649 had no detectable pathology and appeared similar to samples taken from naive mice challenged with medium alone (Fig. 5C and D, respectively). Taken together, these data suggest that TM17-2 is not only nonreactogenic, it is also sufficient to protect mice from pathology associated with ChikV infection. This suggests that TM17-2 is a ChikV vaccine strain that warrants further investigation and development as a live-attenuated vaccine strain (LAV).
Table 5.
Pathology scoring assigned to slides for individual animals for foot/ankle sections taken 7 days postchallenge
| Group/mouse (challenge)a | Score |
||||
|---|---|---|---|---|---|
| Muscle inflammationb | Muscle necrosisb | Tendonitisb | Synovitisc | Perivasculitisc | |
| Naïve/1 (mock) | 0 | 0 | 0 | 0 | 0 |
| Naïve/2 (mock) | 0 | 0 | 0 | 0 | 0 |
| Naïve/3 (mock) | 0 | 0 | 0 | 0 | 0 |
| Naïve/1 (challenged) | 3 | 1 | 0 | 1 | 0 |
| Naïve/2 (challenged) | 4 | 3 | 0 | 2 | 0 |
| Naïve/3 (challenged) | 3 | 2 | 0 | 3 | 0 |
| TM17-1/1 (mock) | 0 | 0 | 0 | 0 | 0 |
| TM17-1/2 (mock) | 0 | 0 | 0 | 0 | 0 |
| TM17-1/3 (mock) | 0 | 0 | 0 | 0 | 0 |
| TM17-1/1 (challenged) | 0 | 0 | 0 | 0 | 0 |
| TM17-1/2 (challenged) | 2 | 0 | 0 | 0 | 0 |
| TM17-1/3 (challenged) | 0 | 0 | 0 | 0 | 0 |
| TM17-2/1 (mock) | 0 | 0 | 0 | 0 | 0 |
| TM17-2/2 (mock) | 0 | 0 | 0 | 0 | 0 |
| TM17-2/3 (mock) | 0 | 0 | 0 | 0 | 0 |
| TM17-2/1 (challenged) | 0 | 0 | 0 | 0 | 0 |
| TM17-2/2 (challenged) | 0 | 0 | 0 | 0 | 0 |
| TM17-2/3 (challenged) | 0 | 0 | 0 | 0 | 0 |
Challenge, challenged with ChikV SL15649; mock, mock challenged.
Scale: 0, 0 to 2%, 1, 2 to 20%; 2, 20 to 40%; 3, 40 to 60%; 4, 60 to 80%; 5, 80 to 100%.
Scale: 0, no change; 1, minimal; 2, mild (inflammatory infiltrate); 3, moderate; 4, severe (destruction of synovial membrane).
Fig 5.
Pathology of mouse foot/ankle joint histology sections 7 days postchallenge (magnification, ×20) of a representative animal. (A) Naive mice challenged with ChikV SL15649 displayed moderate muscle inflammation and necrosis. (B) Mice vaccinated with TM17-1 and challenged with ChikV SL15649 displayed minimal muscle inflammation. (C and D) Mice vaccinated with TM17-2 prior to challenge with ChikV SL15649 (C) displayed no notable pathology, similar to samples obtained from naive mock-challenged animals (D). Bar, 50 μm.
DISCUSSION
The search for a suitable ChikV vaccine is not new (52). In the development of inactivated LAVs, one including an internal ribosome entry site (IRES) that confers attenuation (53) and chimeric viruses (54, 55) are in various stages of study. Other platforms, such as virus-like particles (VLPs) (56) and DNA vaccines (57), have been tested (58). However, none of these potential vaccines have been approved for use in humans.
In the present study, the results shown above indicate that ChikV TM17-2 is an attenuated, nonreactogenic, efficacious vaccine strain that should be further developed for use in humans. It must be noted that ChikV TM17-2 grew to relatively high titers in mice, but this result is not sufficient to rule out the virus as a vaccine candidate. Conventionally, while high viremia is seen as an indicator of virulence, it is not a universal characteristic of all virus infections. One study of the relationship between virus fitness and virulence with vesicular stomatitis virus (VSV) concluded that, in general, viremia and viral fitness correlated with virulence, but in some cases, complex host and virus interactions could uncouple this relationship (59). For some vaccines, there are no true correlates of protection (5). There is intensive investigation for molecular markers of virus attenuation to determine the mechanisms of attenuation for current vaccines. Hypothetically, a thorough understanding of the molecular factors involved in virus attenuation and how they relate to the disease process will aid in the development of new vaccines or antiviral factors (60, 61).
While antibodies are believed to be the primary method of protection against ChikV infection (62), cell-mediated immunity has been shown to be sufficient for protection against alphavirus disease in the absence of strong antibody response (63, 64). Interestingly, the lack of IgG induced by ChikV TM17-2 could be contributing to the lack of inflammation. Severe joint pain and rheumatoid arthritis (RA)-like symptoms, which begin at the onset of infection, can persist for months or even years after virus clearance and are the most notable markers of ChikV disease (65, 66). One hypothesis for pathogenic symptoms during RA is the interaction of IgG with mast cells and cytokines (26, 67). Furthermore, increased IgG titers have been shown to correlate with disease severity and persistence in ChikV infection (68). Taken together, this suggests the low IgG titers induced by ChikV TM17-2 may be responsible for the lack of inflammation seen postvaccination but are sufficient to prevent disease upon challenge.
Further studies are currently being undertaken to determine the mechanism by which TM17-2 confers immunity as a vaccine. Although the data do not point directly to a specific mechanism for protection by this particular mutant, there is one notable point to consider. All the ChikV TM17 mutants had the same number of amino acids deleted: 9. The only distinction between these mutants is the position of the deletion with respect to the amino and carboxyl termini of the TMD. While the identical position in the SINV TM17 was found to provide protection against challenge with pathogenic Sindbis-like virus isolate S.A.AR86 (GenBank accession number U38305) (47), it is evident from the data presented here that this was not the optimal protective mutation in the ChikV sequence. While interpretation of how these small contextual changes affect virus interactions with an incredibly complex immune response is not clear, there is a possibility that slight conformational differences can and do affect interaction of the cells involved in the primary infection and effectors of the innate immune response. If this were not the case, no attenuation would result from the mutation. The different responses of HR mutants and the WT viruses could result from effects on virus receptor affinity or epitope presentation or other effects of E2 conformational changes and protein-protein interactions. The HR phenotype of these mutants has been proposed in previous studies to identify a marker of attenuation and now has additional support from studies in monkeys for DV2 (37) and ChikV (this study).
This study of ChikV HR mutants TM17-1, -2, and -3 has provided additional evidence that arbovirus HR mutants attenuated for growth in mammals provide a paradigm that can be applied to alphaviruses and flaviviruses to construct vaccine strains (32, 36, 37). There are 600 to 700 known pathogenic agents in the arbovirus class, such as dengue virus, West Nile virus, and many forms of encephalitic viruses. Any of these viruses can also be targeted using this technology. Although standard technologies have been used to produce HR mutant LAVs, there is an accumulating body of evidence that the TMD deletion method of attenuation can provide safe and efficacious vaccines. LAV vaccines provide all epitopes of the virus to the host immune system and are immunologically indistinguishable from the WT, unlike killed viruses, viral subunit proteins, chimeric viruses, or other molecular approaches. The vaccine strain ChikV TM17-2 has been found to be safe and efficacious in mice. Selection of TMD deletion mutants that are HR and restricted to growth in insect cells has been found to be a reproducible method of attenuation of arboviruses. After the target deletion was determined for each virus family, the construction of these mutants has become straightforward, as the locations and the sequences to be deleted have been found to be more predictable. By this simple and effective method of creating safe and protective LAVs, reemerging viruses, such as Chikungunya and dengue viruses, as well as other arboviruses, can be rapidly targeted and controlled.
ACKNOWLEDGMENTS
This research was supported by a grant from the Foundation for Research, Carson City, NV, and by the North Carolina Agricultural Research Service. Additional funding was supplied by the North Carolina Biotechnology Center, Research Triangle Park, NC.
Footnotes
Published ahead of print 3 April 2013
REFERENCES
- 1. Thomas S, Rai J, John L, Gunther S, Drosten C, Pützer BM, Schaefer S. 2010. Functional dissection of the alphavirus capsid protease: sequence requirements for activity. Virol. J. 7:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, Sol-Foulon N, Le Roux K, Prevost MC, Fsihi H, Frenkiel MP, Blanchet F, Afonso PV, Ceccaldi PE, Ozden S, Gessain A, Schuffenecker I, Verhasselt B, Zamborlini A, Saïb A, Rey FA, Arenzana-Seisdedos F, Desprès P, Michault A, Albert ML, Schwartz O. 2007. Characterization of reemerging Chikungunya virus. PLoS Pathog. 3:e89 doi: 10.1371/journal.ppat.0030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diallo M, Thonnon J, Traore-Lamizana M, Fontenille D. 1999. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am. J. Trop. Med. Hyg. 60:281–286 [DOI] [PubMed] [Google Scholar]
- 4. Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. 2008. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control. Parasite 15:3–13 [DOI] [PubMed] [Google Scholar]
- 5. Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peyrefitte CN, Bessaud M, Pastorino BA, Gravier P, Plumet S, Merle OL, Moltini I, Coppin E, Tock F, Daries W, Ollivier L, Pages F, Martin R, Boniface F, Tolou HJ, Grandadam M. 2008. Circulation of Chikungunya virus in Gabon, 2006–2007. J. Med. Virol. 80:430–433 [DOI] [PubMed] [Google Scholar]
- 7. Rogers KM, Heise M. 2009. Modulation of cellular tropism and innate antiviral response by viral glycans. J. Innate Immun. 1:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. 2008. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerg. Infect. Dis. 14:412–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powers AM, Brault AC, Tesh RB, Weaver SC. 2000. Re-emergence of chikungunya and o'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 81:471–479 [DOI] [PubMed] [Google Scholar]
- 10. Weaver SC, Barrett AD. 2004. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO 2007. Outbreak and spread of Chikungunya. Wkly. Epidemiol. Rec. 82:409–416 [PubMed] [Google Scholar]
- 12. Gérardin P, Barau G, Michault A, Bintner M, Randrianaivo H, Choker G, Lenglet Y, Touret Y, Bouveret A, Grivard P, Le Roux K, Blanc S, Schuffenecker I, Couderc T, Arenzana-Seisdedos F, Lecuit M, Robillard PY. 2008. Multidisciplinary prospective study of mother-to-child Chikungunya virus infections on the island of La Réunion. PLoS Med. 5:e60 doi: 10.1371/journal.pmed.0050060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fritel X, Rollot O, Gerardin P, Gauzere BA, Bideault J, Lagarde L, Dhuime B, Orvain E, Cuillier F, Ramful D, Samperiz S, Jaffar-Bandjee MC, Michault A, Cotte L, Kaminski M, Fourmaintraux A, Chikungunya-Mere-Enfant Team 2010. Chikungunya virus infection during pregnancy, Reunion, France, 2006. Emerg. Infect. Dis. 16:418–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. West J, Hernandez R, Ferreira D, Brown DT. 2006. Mutations in the endodomain of Sindbis virus glycoprotein E2 define sequences critical for virus assembly. J. Virol. 80:4458–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hafer A, Whittlesey R, Brown DT, Hernandez R. 2009. Differential incorporation of cholesterol by Sindbis virus grown in mammalian or insect cells. J. Virol. 83:9113–9121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandez R, Paredes A, Brown DT. 2008. Sindbis virus conformational changes induced by a neutralizing anti-E1 monoclonal antibody. J. Virol. 82:5750–5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernandez R, Paredes A. 2009. Sindbis virus as a model for studies of conformational changes in a metastable virus and the role of conformational changes in in vitro antibody neutralisation. Rev. Med. Virol. 19:257–272 [DOI] [PubMed] [Google Scholar]
- 18. Suhrbier A, Jaffar-Bandjee MC, Gasque P. 2012. Arthritogenic alphaviruses—an overview. Nat. Rev. Rheumatol. 8:420–429 [DOI] [PubMed] [Google Scholar]
- 19. Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux A-B. 2009. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One 4:e5895 doi: 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Napoli C, Salcuni P, Pompa MG, Declich S, Rizzo C. 2012. Estimated imported infections of Chikungunya and dengue in Italy, 2008 to 2011. J. Travel Med. 19:294–297 [DOI] [PubMed] [Google Scholar]
- 21. Queyriaux B, Armengaud A, Jeannin C, Couturier E, Peloux-Petiot F. 2008. Chikungunya in Europe. Lancet 371:723–724 [DOI] [PubMed] [Google Scholar]
- 22. van den Hurk AF, Hall-Mendelin S, Pyke AT, Smith GA, Mackenzie JS. 2010. Vector competence of Australian mosquitoes for chikungunya virus. Vector Borne Zoonotic Dis. 10:489–495 [DOI] [PubMed] [Google Scholar]
- 23. Gibney KB, Fischer M, Prince HE, Kramer LD, St. George K, Kosoy OL, Laven JJ, Staples JE. 2011. Chikungunya fever in the United States: a fifteen year review of cases. Clin. Infect. Dis. 52:e121–126 doi: 10.1093/cid/ciq214 [DOI] [PubMed] [Google Scholar]
- 24. Park JY, Lee YS, Kim BH, Park SM. 2008. Label-free detection of antibody-antigen interactions on (R)-lipo-diaza-18-crown-6 self-assembled monolayer modified gold electrodes. Anal. Chem. 80:4986–4993 [DOI] [PubMed] [Google Scholar]
- 25. Barrett AD, Stanberry LR. (ed). 2009. Vaccines for biodefence and emerging and neglected diseases. Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 26. Coffey LL, Vasilakis N, Brault AC, Powers AM, Tripet F, Weaver SC. 2008. Arbovirus evolution in vivo is constrained by host alternation. Proc. Natl. Acad. Sci. U. S. A. 105:6970–6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jozan M.1987. Of arboviruses, arthropods, and arthropod cell cultures: history and expectations, p 3–22 In Yunker C. (ed), Arboviruses in arthropod cells in vitro. CRC Press, Boca Raton, FL [Google Scholar]
- 28. Strauss JH, Strauss EG. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibbons DL, Erk I, Reilly B, Navaza J, Kielian M, Rey FA, Lepault J. 2003. Visualization of the target-membrane-inserted fusion protein of Semliki Forest virus by combined electron microscopy and crystallography. Cell 114:573–583 [DOI] [PubMed] [Google Scholar]
- 30. He L, Piper A, Meilleur F, Myles DA, Hernandez R, Brown DT, Heller WT. 2010. The structure of Sindbis virus produced from vertebrate and invertebrate hosts as determined by small-angle neutron scattering. J. Virol. 84:5270–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Casolari S, Briganti E, Zanotti M, Zauli T, Nicoletti L, Magurano F, Fortuna C, Fiorentini C, Grazia Ciufolini M, Rezza G. 2008. A fatal case of encephalitis associated with Chikungunya virus infection. Scand. J. Infect. Dis. 40:995–996 [DOI] [PubMed] [Google Scholar]
- 32. Hernandez R, Sinodis C, Horton M, Ferreira D, Yang C, Brown DT. 2003. Deletions in the transmembrane domain of a Sindbis virus glycoprotein alter virus infectivity, stability, and host range. J. Virol. 77:12710–12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernandez R, Lee H, Nelson C, Brown DT. 2000. A single deletion in the membrane-proximal region of the Sindbis virus glycoprotein E2 endodomain blocks virus assembly. J. Virol. 74:4220–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McKnight KL, Simpson DA, Lin SC, Knott TA, Polo JM, Pence DF, Johannsen DB, Heidner HW, Davis NL, Johnston RE. 1996. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J. Virol. 70:1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Presley JF, Polo JM, Johnston RE, Brown DT. 1991. Proteolytic processing of the Sindbis virus membrane protein precursor PE2 is nonessential for growth in vertebrate cells but is required for efficient growth in invertebrate cells. J. Virol. 65:1905–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith KM, Nanda K, Spears CJ, Ribeiro M, Vancini R, Piper A, Thomas GS, Thomas ME, Brown DT, Hernandez R. 2011. Structural mutants of dengue virus 2 transmembrane domains exhibit host-range phenotype. Virol. J. 8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith KM, Nanda K, Spears CJ, Piper A, Ribeiro M, Quiles M, Briggs CM, Thomas GS, Thomas ME, Brown DT, Hernandez R, McCarl V. 2012. Testing of novel dengue virus 2 vaccines in African green monkeys: safety, immunogenicity, and efficacy. Am. J. Trop. Med. Hyg. 87:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown DT, Hernandez R. U.S. patent 6,589,533 2003 Jul;
- 39. Brown DT, Hernandez R. U.S. patent 6,306,401 2001 Oct;
- 40. Brown DT, Hernandez R. U.S. patent 7,335,363 2008 Feb;
- 41. Lakshmi V, Neeraja M, Subbalaxmi MVS, Parida MM, Dash PK, Santhosh SR, Rao PV. 2008. Clinical features and molecular diagnosis of Chikungunya fever from South India. Clin. Infect. Dis. 46:1436–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mak C. 2012. Drug pipeline: 1Q12. Nat. Biotechnol. 30:383. [DOI] [PubMed] [Google Scholar]
- 43. Tsetsarkin K, Higgs S, McGee CE, De Lamballerie X, Charrel RN, Vanlandingham DL. 2006. Infectious clones of Chikungunya virus (La Reunion isolate) for vector competence studies. Vector Borne Zoonotic Dis. 6:325–337 [DOI] [PubMed] [Google Scholar]
- 44. Hernandez R, Sinodis C, Brown DT. 2010. Sindbis virus: propagation, quantification, and storage. Curr. Protoc. Microbiol. Chapter 15:Unit 15B.1 doi: 10.1002/9780471729259.mc15b01s16 [DOI] [PubMed] [Google Scholar]
- 45. Morrison TE, Oko L, Montgomery SA, Whitmore AC, Lotstein AR, Gunn BM, Elmore SA, Heise MT. 2011. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am. J. Pathol. 178:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Couderc T, Chrétien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Desprès P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. 2008. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 4:e29 doi: 10.1371/journal.ppat.0040029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown DT, Hernandez R. 2010. Host range mutants as vaccines for arthropod vectored viruses. Nova Acta Leopoldina NF 98:201–213 [Google Scholar]
- 48. Khan AH, Morita K, Parquet MC, Hasebe F, Mathenge EG, Igarashi A. 2002. Complete nucleotide sequence of Chikungunya virus and evidence for an internal polyadenylation site. J. Gen. Virol. 83:3075–3084 [DOI] [PubMed] [Google Scholar]
- 49. Rice CM, Bell JR, Hunkapiller MW, Strauss EG, Strauss JH. 1982. Isolation and characterization of the hydrophobic COOH-terminal domains of the Sindbis virion glycoproteins. J. Mol. Biol. 154:355–378 [DOI] [PubMed] [Google Scholar]
- 50. Ahlquist P, Strauss EG, Rice CM, Strauss JH, Haseloff J, Zimmern D. 1985. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J. Virol. 53:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hernandez R, Ferreira D, Sinodis C, Litton K, Brown DT. 2005. Single amino acid insertions at the junction of the Sindbis virus E2 transmembrane domain and endodomain disrupt virus envelopment and alter infectivity. J. Virol. 79:7682–7697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Jr, Lupton HW. 1986. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine 4:157–162 [DOI] [PubMed] [Google Scholar]
- 53. Plante K, Wang E, Partidos CD, Weger J, Gorchakov R, Tsetsarkin K, Borland EM, Powers AM, Seymour R, Stinchcomb DT, Osorio JE, Frolov I, Weaver SC. 2011. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 7:e1002142 doi: 10.1371/journal.ppat.1002142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang E, Kim DY, Weaver SC, Frolov I. 2011. Chimeric Chikungunya viruses are nonpathogenic in highly sensitive mouse models but efficiently induce a protective immune response. J. Virol. 85:9249–9252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim DY, Atasheva S, Foy NJ, Wang E, Frolova EI, Weaver S, Frolov I. 2011. Design of chimeric alphaviruses with a programmed, attenuated, cell type-restricted phenotype. J. Virol. 85:4363–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. 2010. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 16:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mallilankaraman K, Shedlock DJ, Bao H, Kawalekar OU, Fagone P, Ramanathan AA, Ferraro B, Stabenow J, Vijayachari P, Sundaram SG, Muruganandam N, Sarangan G, Srikanth P, Khan AS, Lewis MG, Kim JJ, Sardesai NY, Muthumani K, Weiner DB. 2011. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl. Trop. Dis. 5:e928 doi: 10.1371/journal.pntd.0000928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT. 2012. Chikungunya virus and prospects for a vaccine. Expert Rev. Vaccines 11:1087–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Furio V, Garijo R, Duran M, Moya A, Bell JC, Sanjuán R. 2012. Relationship between within-host fitness and virulence in the vesicular stomatitis virus: correlation with partial decoupling. J. Virol. 86:12228–12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Plotkin SA. 2009. Vaccines: the fourth century. Clin. Vaccine Immunol. 16:1709–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, Rénia L, Leo YS, Ng LF. 2012. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J. Infect. Dis. 205:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Couderc T, Khandoudi N, Grandadam M, Visse C, Gangneux N, Bagot S, Prost JF, Lecuit M. 2009. Prophylaxis and therapy for Chikungunya virus infection. J. Infect. Dis. 200:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Linn ML, Mateo L, Gardner J, Suhrbier A. 1998. Alphavirus-specific cytotoxic T lymphocytes recognize a cross-reactive epitope from the capsid protein and can eliminate virus from persistently infected macrophages. J. Virol. 72:5146–5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paessler S, Yun NE, Judy BM, Dziuba N, Zacks MA, Grund AH, Frolov I, Campbell GA, Weaver SC, Estes DM. 2007. Alpha-beta T cells provide protection against lethal encephalitis in the murine model of VEEV infection. Virology 367:307–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thiboutot MM, Kannan S, Kawalekar OU, Shedlock DJ, Khan AS, Sarangan G, Srikanth P, Weiner DB, Muthumani K. 2010. Chikungunya: a potentially emerging epidemic? PLoS Negl. Trop. Dis. 4:e623 doi: 10.1371/journal.pntd.0000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cavrini F, Gaibani P, Pierro AM, Rossini G, Landini MP, Sambri V. 2009. Chikungunya: an emerging and spreading arthropod-borne viral disease. J. Infect. Dev. Ctries. 3:744–752 [DOI] [PubMed] [Google Scholar]
- 67. Kamala T. 2007. Hock immunization: a humane alternative to mouse footpad injections. J. Immunol. Methods 328:204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kelvin AA, Banner D, Silvi G, Moro ML, Spataro N, Gaibani P, Cavrini F, Pierro A, Rossini G, Cameron MJ, Bermejo-Martin JF, Paquette SG, Xu L, Danesh A, Farooqui A, Borghetto I, Kelvin DJ, Sambri V, Rubino S. 2011. Inflammatory cytokine expression is associated with chikungunya virus resolution and symptom severity. PLoS Negl. Trop. Dis. 5:e1279 doi: 10.1371/journal.pntd.0001279 [DOI] [PMC free article] [PubMed] [Google Scholar]