Abstract
We previously demonstrated that vaccination of lactating rhesus monkeys with a DNA prime/vector boost strategy induces strong T-cell responses but limited envelope (Env)-specific humoral responses in breast milk. To improve vaccine-elicited antibody responses in milk, hormone-induced lactating rhesus monkeys were vaccinated with a transmitted/founder (T/F) HIV Env immunogen in a prime-boost strategy modeled after the moderately protective RV144 HIV vaccine. Lactating rhesus monkeys were intramuscularly primed with either recombinant DNA (n = 4) or modified vaccinia virus Ankara (MVA) poxvirus vector (n = 4) expressing the T/F HIV Env C.1086 and then boosted twice intramuscularly with C.1086 gp120 and the adjuvant MF59. The vaccines induced Env-binding IgG and IgA as well as neutralizing and antibody-dependent cellular cytotoxicity (ADCC) responses in plasma and milk of most vaccinated animals. Importantly, plasma neutralization titers against clade C HIV variants MW965 (P = 0.03) and CAP45 (P = 0.04) were significantly higher in MVA-primed than in DNA-primed animals. The superior systemic prime-boost regimen was then compared to a mucosal-boost regimen, in which animals were boosted twice intranasally with C.1086 gp120 and the TLR 7/8 agonist R848 following the same systemic prime. While the systemic and mucosal vaccine regimens elicited comparable levels of Env-binding IgG antibodies, mucosal immunization induced significantly stronger Env-binding IgA responses in milk (P = 0.03). However, the mucosal regimen was not as potent at inducing functional IgG responses. This study shows that systemic MVA prime followed by either intranasal or systemic protein boosts can elicit strong humoral responses in breast milk and may be a useful strategy to interrupt postnatal HIV-1 transmission.
INTRODUCTION
Breastfeeding is responsible for almost half of the 350,000 pediatric human immunodeficiency virus (HIV) infections occurring annually (1, 2). However, in areas with limited resources, breastfeeding is important for infant survival even in the context of maternal HIV infection as formula feeding is associated with increased risks of infant mortality (3, 4). Antiretroviral therapy given to the mother and/or infant throughout the breastfeeding period can significantly reduce the rate of mother-to-child transmission (MTCT) (5, 6). But, even with the optimal prophylactic regimens, in breastfeeding populations, more than 5% of infants born to HIV-infected women are still at risk of becoming infected (5). Elimination of pediatric HIV would require more than 95% of HIV-infected pregnant women to adhere to preventive programs (7). According to UNAIDS, in 2010 only 35% of pregnant women from low- and middle-income countries were tested for HIV and only 48% of women known to be infected with HIV received the optimal antiretroviral regimen to reduce the risk of MTCT (8). Thus, there is a need to develop immunologic interventions, such as a maternal or infant vaccine, to prevent postnatal HIV acquisition. It is well documented that maternal immunization can be effective in preventing neonatal infections, as maternal antibodies are transferred to infants transplacentally and during breastfeeding (9). Moreover, simian immunodeficiency virus (SIV) immunization of pregnant rhesus macaques has been shown to protect their offspring from simian-human immunodeficiency virus (SHIV) challenge (10). As the risk of HIV transmission during breastfeeding is associated with the level of HIV cell-free and cell-associated viruses in breast milk (11, 12), the induction of virus-specific immune responses in milk by maternal immunization is a potentially important strategy for preventing postnatal HIV transmission.
Interestingly, despite multiple daily mucosal exposures to the virus over several months, the majority of breastfed infants born to HIV-infected women escape infection (13). It is therefore possible that milk contains antiviral factors that protect the majority of breast milk HIV-exposed infants from acquiring HIV infection. Passive immunization of neonatal rhesus monkeys with a combination of broadly neutralizing antibodies (Abs) can protect them from oral exposure to simian-human immunodeficiency virus (SHIV) (14). Moreover, although early studies reported no association between the levels of HIV-specific binding antibodies in breast milk and infant protection (15), a recent study reported a higher magnitude of milk antibody-dependent cellular cytotoxicity (ADCC) in HIV-infected women who did not transmit HIV postnatally to their infants than in transmitters (16). Thus, inducing potent functional antibody responses in breast milk is likely to be important for an effective maternal HIV vaccine. Our previous investigations of breast milk antibody responses in HIV-infected women (17) and SIV-infected monkeys (18) indicated that functional IgG responses in breast milk mirror that of plasma but are of lower magnitude, suggesting that breast milk functional IgG antibodies mostly transudate from plasma. Thus, strong vaccine-elicited systemic antibody responses may be required to achieve potent HIV-specific antibody responses in breast milk.
Nonhuman primates are a useful model to study vaccine-elicited responses in breast milk, as lactation can be induced pharmacologically, producing milk that is immunologically similar to that of natural lactation (19). We have previously shown that systemic immunization of lactating rhesus monkeys can induce SIV-specific immune responses in milk (20). This previously studied immunization strategy with SIV DNA prime/virus vector boost with NYVAC and adenovirus type 5 (Ad5) induced strong cellular immune responses in milk of lactating rhesus monkeys. However, only weak Env-specific IgG antibodies, and no IgA responses, were detected in milk. Importantly, the RV144 poxvirus prime-protein boost vaccine regimen demonstrated a moderate 31% protection rate from HIV acquisition which was likely mediated by vaccine-elicited humoral responses (21). Thus, it would be interesting to determine if a poxvirus prime-protein boost vaccine strategy can induce humoral immune responses in breast milk resembling those elicited by the RV144 vaccine.
As an HIV vaccine would need to protect against transmitted/founder (T/F) virus variants, the use of T/F Envs as vaccine immunogens could elicit more effective immune responses. The clade C T/F virus Env C.1086, derived from an acutely infected individual, was recently reported to be highly antigenic and immunogenic and to bind to antibodies directed against important neutralizing epitopes (22). Moreover, immunization with C.1086 Env can elicit broadly neutralizing antibody responses (22). In this study, we investigated vaccine-elicited immune responses in the blood and breast milk of lactating monkeys immunized with the T/F Env C.1086 using either a DNA prime/intramuscular (i.m.) protein boost or a poxvirus prime/i.m. protein boost vaccine regimen. Furthermore, we investigated if mucosal administration of the protein boost induced more potent breast milk immune responses.
MATERIALS AND METHODS
Generation of recombinant MVA (rMVA) expressing C.1086 env gene.
The modified Ankara strain of vaccinia virus (MVA) was kindly provided by B. Moss (NIH). MVA virus (designated A660) was purified through three rounds of plaque purification in BHK21 cells maintained in minimal essential medium alpha (MEM-alpha) with 5% fetal bovine serum (FBS) (Gibco), cultured, purified, and quantified in these cells as described previously (23, 24). To facilitate subsequent genetic manipulation of MVA through use of the K1L gene as a selectable marker, the remnants of the disrupted K1L gene in MVA A660 virus were removed (25) by the transient marker stabilization procedure (26) using insertion plasmid p2573. Plasmid p2573 contains a marker cassette consisting of the functional vaccinia virus (WR strain) K1L gene next to a synthesized copy of the green fluorescent protein gene (hMGFP) originally cloned from Montastrea cavernosa and codon optimized for expression (Promega Corporation). The hMGFP gene was placed under the control of a poxvirus early/late promoter. The two genes in the marker cassette were flanked by a 250-bp direct repeat of the K2L gene to permit deletion of the marker genes after selection of the recombinant virus. The marker cassette was flanked by a 0.4-kb portion of the N2L gene and an 0.6-kb portion of the K2L gene to permit recombination to replace the K1L locus. After transfection of insertion plasmid DNA into cells infected with A660 virus, recombinant viruses capable of replication in RK13 cells (maintained in MEM supplemented with nonessential amino acids and 10% FBS; Gibco) were selected (containing the N2L gene, the K1L gene, the hMGFP gene, and the K2L gene) and then passaged in BHK21 cells to remove selective pressure for the K1L gene. A virus (A676) containing the N2L and K2L genes, designated MVA open reading frames (ORFs) 021L and 023L (27), but lacking the marker cassette was isolated. The deletion of the K1L and hMGFP genes was confirmed by PCR and Southern blot analyses.
After immunization with MVA, CD8+ T-cell memory responses can be improved by the deletion of the viral gene (MVA ORF 184R) encoding the soluble, secreted interleukin-1β receptor (IL-1βR) (28). To take advantage of this phenotype, the IL-1βR gene in MVA virus A676 was inactivated by the transient marker stabilization procedure using plasmid p2588. Plasmid p2588 contains the following: (i) a marker cassette consisting of the functional vaccinia virus (WR strain) K1L gene next to a copy of the coding region of the gene encoding β-glucuronidase (GUS) from pGUS N358S (Clontech Laboratories, Inc.) under the control of a poxvirus late promoter (the two genes in the marker cassette were flanked by a 0.3-kb direct repeat of the MVA genome [nucleotides 162700 to 163000] to permit deletion of the marker genes after selection of the recombinant virus), (ii) a copy of the luciferase gene (luc) from pGL4.10 (Promega Corporation) under the control of a poxvirus early promoter, and (iii) the luciferase gene/marker cassette flanked by sequences corresponding to MVA genome sequences 162021 to 162321 and 162700 to 163300 to promote recombinational insertion of the three genes into the MVA genome to inactivate MVA gene 184R. After transfection of insertion plasmid DNA into cells infected with A676 virus, recombinant viruses capable of replication in RK13 cells were selected (containing the luc gene, the GUS gene, and the K1L gene) and then passaged in BHK21 cells, without selective pressure for the K1L gene. A virus (A681) lacking the marker cassette but containing the inactivated MVA ORF 184R was isolated. The deletion of the K1L and GUS marker genes was confirmed by PCR and Southern blot analyses.
The HIV Env C.1086 gp140 protein corresponding to residues 1 to 669, with modifications of E489R and E497R to mutate the gp120-gp41 cleavage site, was expressed in an MVA virus. This gene, under the control of a poxvirus early/late promoter, was placed into the genome of MVA A681 virus by means of the insertion plasmid p2614. Plasmid p2614 contains a chemically synthesized 3,853-nucleotide SacI-ApaI fragment containing the following: (i) a copy of the gene encoding the C.1086 Env protein in which the nucleotide sequence was modified where possible by silent codon alterations to disrupt runs of 4 to 5 consecutive G's or C's, as well as potential early termination signals (TTTTTNT); (ii) a synthetic poxvirus early/late promoter comprising the early promoter from the cowpox virus CrmA gene (29, 30) in tandem with the late promoter of the vaccinia virus p4b gene (31) to direct transcription of the gp140 gene; (iii) an early termination signal immediately after the introduced stop codon in the gp140 gene; (iv) a marker cassette containing the K1L and GUS genes from p2588 flanked by 200-bp direct repeats of the 3′ end of the K1L gene to permit transient marker stabilization and selection of the gene insertion into the MVA genome; and (v) flanking regions to direct insertion of the gp140 gene and marker cassette into the intergenic region between the two essential viral genes I8R and G1L. The removal of runs of G's and C's was done to minimize spontaneous frameshift mutations within the gp140 sequence, and the insertion of the gp140 gene between two essential MVA genes was done to minimize the potential loss of the inserted gene through spontaneous gene deletions in the viral genome, as described previously (32). After transfection of insertion plasmid DNA into cells infected with A681 virus, recombinant viruses capable of replication in RK13 cells were selected (containing the gp140 gene, the GUS gene, and the K1L gene) and then passaged in BHK21 cells, without selective pressure for the K1L gene. A virus (A703) lacking the marker cassette but containing the gp140 gene was isolated. The deletion of the K1L and GUS genes was confirmed by PCR. The expression of the gp140 gene was confirmed by Western blot analyses of proteins in A703-infected BHK21 cells using monoclonal antibody (MAb) VRC C 16H3 to detect the C.1086 gp140 protein. A703 virus was cultured in BHK21 cells and partially purified by ultracentrifugation through a 36% sucrose cushion. The virus was quantified by plaque assay on BHK21 cells employing polyclonal rabbit antisera against vaccinia virus (Meridian Life Sciences) to visualize foci of infected cells, as described previously (23).
Generation of DNA plasmids and envelope protein production.
DNA plasmids containing the HIV C.1086 env gene were generated by cloning the virus cDNA into the pVR1012 vector, as previously described (33).
The codon-optimized HIV C.1086 gp120 env gene was commercially synthesized (Genewiz, Inc.) and cloned into the pcDNA3.1+ expression vector (Invitrogen). The expression vectors containing env gene inserts were used for polyethyleneimine-mediated transfection of 293T cells. The resulting recombinant Env gp120 glycoproteins were purified from supernatants of transfected 293T cell cultures using Galanthus nivalis lectin-agarose column chromatography (Vector Laboratories) and analyzed by SDS-polyacrylamide gel electrophoresis and by Western blotting with the anti-HIV-1 Env monoclonal antibody VRC C 16H3. Monomers of the gp120 proteins were isolated from their oligomers by fast protein liquid chromatography (FPLC) and stored at −70°C until use.
Animals.
Lactation was induced in 12 female Indian rhesus monkeys by depot medroxyprogesterone and estradiol injections followed by the oral administration of a dopamine antagonist, as previously described (19). Vaccination occurred after initiation of lactation. For the DNA prime/i.m. boost and the MVA prime/i.m. boost, all vaccine doses were administered in the quadriceps of anesthetized monkeys. For the MVA prime/intranasal (i.n.) boost, the boost was administered intranasally using a previously reported procedure with some modifications (34). Briefly, anesthetized monkeys were placed on their back with their head held steady. A total volume of 150 μl was dispensed per nostril in 30-μl aliquots at 30-s intervals with the nasal passages held closed between aliquot administrations. Milk (30 to 1,000 μl) was collected two times per week, and blood was collected weekly before and following each immunization. Milk was separated into cellular, supernatant, and fat fractions by centrifugation, as previously described (18, 20). Vaginal and rectal secretions were collected using premoistened Weck-Cel surgical sponges (Medtronic, Jacksonville, FL) as previously described (35). Animals were maintained according to the Guide for the Care and Use of Laboratory Animals.
Preparation of mucosal samples.
Mucosal secretions were eluted from the Weck-Cel sponges using a previously reported protocol (35) with slight modifications. In brief, the elution buffer for a single elution was prepared by adding 6 μl of 100× protease inhibitor cocktail set 1 (Calbiochem) to 594 μl of 1× phosphate-buffered saline containing 0.25% bovine serum albumin (Sigma). Just before elution, sponges were thawed on ice and transferred tip down to the upper chamber of a filterless Spin-X column (Corning Life Sciences). The handle was removed with scissors, and then 300 μl of ice-cold elution buffer was added directly onto the sponge and allowed to diffuse for 5 min. The columns were then centrifuged at 16,000 × g for 5 min. A second round of elution was performed using a fresh 300-μl aliquot of elution buffer. The samples were incubated for 10 min before centrifugation at 16,000 × g for 20 min. The eluted fluid was then filtered through a Spin-X column containing an 0.22-μm filter (Corning Life Sciences). The samples and buffers were incubated on ice throughout the procedure. IgG was purified from the eluted fractions using protein G columns as previously described (17, 36).
Phenotyping and intracellular cytokine staining of breast milk cells and PBMCs.
The phenotype of breast milk and blood T lymphocytes after vaccination was assessed by monoclonal antibody (MAb) staining with anti-CD3-Alexa Fluor 700 (SP34.2), anti-CD8-allophycocyanin (APC)-H7 (SK1), and anti-CD4-eFluor605NC (L200; eBiosciences, San Diego, CA). An amine dye (Aqua Vital Dye) was used to distinguish live from dead cells in all flow cytometric analyses. Intracellular cytokine staining was performed after a 6-h exposure of peripheral blood mononuclear cells (PBMCs) and breast milk cells to overlapping, protein-spanning pooled C.1086 peptides (165 peptides; 15-mers overlapping by 11) or staphylococcal enterotoxin B (SEB), as previously described (20), with the following Abs: anti-tumor necrosis factor alpha (anti-TNF-α)–eFluor450 (Mab11; eBiosciences, San Diego, CA), anti-gamma interferon (anti-IFN-γ)–phycoerythrin (PE)-Cy7 (B27; BD Biosciences), and anti-interleukin-2 (anti-IL-2)–APC (MQ1-17H12; BD Biosciences). The proportion of cytokine-producing CD3+ CD4+ and CD3+ CD8+ cells was determined by subtracting the proportion of unstimulated cytokine-producing cells from the proportion of C.1086-stimulated cytokine-producing cells. Data were collected on the LSRII flow cytometer (BD Biosciences) with FACSdiva software and analyzed with FlowJo software (TreeStar). The number of breast milk cells was considered to be too low to be analyzed in a sample if no response to the SEB positive control that was above the background was detected for at least two of the three cytokines assayed.
HIV-1 Env and V1V2-binding ELISA.
Enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with either HIV C.1086 gp120, goat anti-monkey IgG (Rockland, Gilbertsville, PA), or goat anti-monkey IgA (Rockland, Gilbertsville, PA) and then blocked with the assay diluent (phosphate-buffered saline containing 4% whey, 15% normal goat serum, and 0.5% Tween 20). Dilutions of plasma, milk, IgG-depleted vaginal/rectal secretions, and IgG purified from vaginal/rectal secretions were then added to the plates. Antibodies were detected by a horseradish peroxidase (HRP)-conjugated, polyclonal goat anti-monkey IgG (Rockland, Gilbertsville, PA) or IgA (Rockland, Gilbertsville, PA) and the addition of the ABTS-2 peroxidase substrate system (KPL, Gaithersburg, MD). Macaca mulatta purified IgG and IgA (Nonhuman Primate Reagent Resource) were used to develop standard curves, and the concentration of IgG or IgA antibody was calculated relative to the standard using a 5-parameter fit curve (WorkOut 2.5; PerkinElmer, Waltham, MA). For anti-V1V2 IgG measurement, 384-well ELISA plates (Corning Life Sciences, Lowell, MA) were coated with either gp70_B.CaseA_V1_V2 His6, gp70_B.CaseA2 V1V2/169K, gp70_B.CaseA2 V1V2 mut3, AE.A244_V1V2 Tags, C.1086C V1V2 Tags 293F, or murine leukemia virus (MuLV) gp70 His6 monomer. Serial dilutions of plasma and milk were distributed to the plates after blocking, and the Abs were detected using a peroxidase-conjugated goat anti-monkey IgG (Rockland, Gilbertsville, PA) and the SureBlue Reserve tetramethylbenzidine (TMB) peroxidase substrate (KPL, Gaithersburg, MD). End titers were calculated as the inverse of the last dilution three times above the background.
Neutralization assays.
Tier 1 and 2 neutralization was measured in TZM-bl cells by reduction in luciferase reporter gene expression after a single round of infection, as previously described (37). Autologous virus and other clade C virus neutralization was assessed in A3R5 cells using HIV infectious molecular clones expressing the Renilla luciferase gene as previously reported (38). A panel of 7 viruses was used to measure plasma neutralization: HIV MW965 (tier 1), HIV CAP45.2.00.G3.LucR.T2A.ecto, HIV Ce1086_B2.LucR.T2A.ecto (autologous), HIV Ce1176_A3.LucR.T2A.ecto, HIV Ce2010_F5.LucR.T2A.ecto, HIV Du422.LucR.T2A.ecto, and HIV DU151.2.LucR.T2A.ecto. In addition, plasma samples from the MVA prime/i.m. protein boost regimen were tested for tier 2 neutralization in TZM-bl cells using the following viruses: SHIV-1157ipd3N4, HIV TV1.21, and HIV ZM197M.PB7. Breast milk neutralization was assessed against HIV MW965 in TZM-bl cells and against HIV Ce1086_B2.LucR.T2A.ecto (autologous), HIV CAP45.2.00.G3.LucR.T2A.ecto, and HIV Ce1176_A3.LucR.T2A.ecto in A3R5 cells. The 50% inhibitory dose (ID50) titer was calculated as the plasma or milk dilution that caused a 50% reduction in relative luminescence units (RLU) compared to the virus control wells after subtraction of cell control RLU. A response was considered positive if the postimmunization ID50 was at least 3 times higher than the preimmune ID50.
Measurement of ADCC response in plasma and breast milk.
ADCC activities of plasma and delipidized breast milk samples were detected by the GranToxiLux (GTL) procedure as previously described (39). CEM.NKRCCR5 target cells (40) were coated with the recombinant HIV C.1086 gp120 used as a protein boost in the vaccine regimen. Cryopreserved human peripheral blood mononuclear cells (PBMCs) from an HIV-seronegative donor served as effector cells and were added at an effector/target ratio of 30:1. The samples were tested using 4-fold serial dilutions starting at 1:100 for plasma and 1:4 for breast milk. A minimum of 2,500 events representing viable target cells was acquired for each well using an LSRII flow cytometer (BD Biosciences). Data analysis was performed using FlowJo 9.6.1 software (Tree Star Inc.). The percent granzyme B (% GzB) activity was defined as the proportion of cells positive for proteolytically active GzB out of the total viable target cell population. The final results are expressed after subtracting the background % GzB activity observed in wells containing effector and target cells in the absence of plasma or breast milk. ADCC titers were determined by interpolating the dilutions of plasma or breast milk that intersect the positive cutoff (>average + 2 standard deviations [SD] % GzB activity of prevaccination samples) using GraphPad Prism 5 software (GraphPad).
Statistical analysis.
Immune responses were compared between vaccine regimens (either DNA prime/i.m. boost and MVA prime/i.n. boost or MVA prime/i.m. boost and MVA prime/i.n. boost) using the Mann-Whitney U test. The Wilcoxon matched-pair signed-rank test was used for comparison between vaccine-elicited blood and milk responses. Finally, the Spearman correlation coefficient was used for correlation analysis. All statistical tests were conducted using GraphPad Prism 5 software.
RESULTS
Immunization of lactating rhesus monkeys.
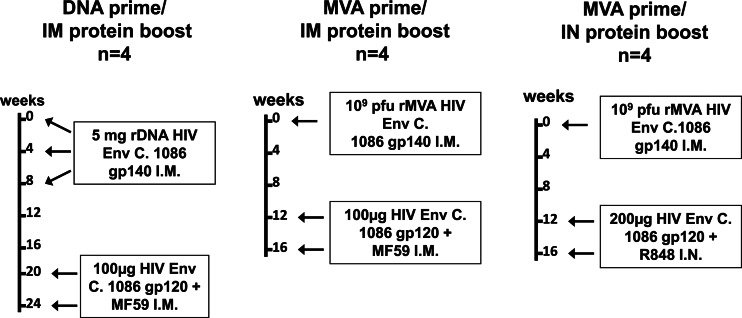
Lactation was pharmacologically induced in 12 female Indian rhesus monkeys, as previously described (19). After the initiation of lactation, the monkeys were immunized with the clade C HIV T/F Env C.1086 using one of three vaccine regimens (Fig. 1). The first group of 4 animals was primed with three doses of 5 mg of recombinant DNA (rDNA) plasmids containing the HIV C.1086 gp140 env gene insert at weeks 0, 4, and 8 and then boosted twice intramuscularly at weeks 20 and 24 with 100 μg of HIV C.1086 gp120 protein. The second group of 4 lactating female monkeys was primed with 109 PFU of rMVA expressing the HIV C.1086 gp140 env gene and boosted twice intramuscularly at weeks 12 and 16 with 100 μg of HIV C.1086 gp120 protein. Finally, four lactating rhesus monkeys were primed with 109 PFU of rMVA expressing the HIV C.1086 env gene and boosted twice intranasally at weeks 12 and 16 with 200 μg of HIV C.1086 gp120 protein (100 μg in each nostril). MF59 (41, 42) was used as the adjuvant for the intramuscular (i.m.) boosts at a 1-in-1 volume ratio with the vaccine (500 μl each). The TLR 7/8 agonist R848 (500 μg/animal) was used as the adjuvant for the intranasal (i.n.) boosts (43).
Fig 1.
Vaccine regimens. Hormone-induced, lactating female monkeys were primed with either recombinant HIV Env C.1086 gp140 DNA or recombinant MVA expressing HIV Env C.1086 gp140 and then boosted twice with HIV Env C.1086 gp120 i.m. or i.n. as indicated. Four animals were included in each vaccine group.
Systemic and mucosal immunization of lactating rhesus monkeys induces robust T-cell responses in the breast milk compartment.
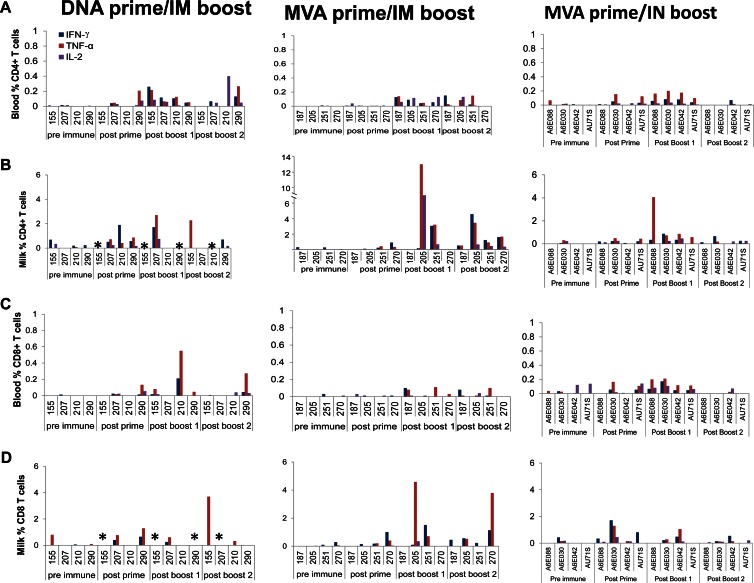
Vaccine-elicited cellular responses were assessed in the blood and breast milk compartment before immunization and then 2 to 3 weeks after the prime and each of the protein boosts. The proportion of CD4+ T cells and CD8+ T cells producing IFN-γ, TNF-α, and IL-2 after stimulation with the HIV C.1086 Env peptide pool was measured by intracellular cytokine staining. Only a small proportion of cells produced cytokines before stimulation (CD4 T cells, 0 to 0.7% and 0 to 0.06%, and CD8 T cells, 0 to 0.8% and 0 to 0.14% in milk and blood, respectively). The proportions of HIV Env-specific CD4+ T cells in blood that produced IFN-γ, TNF-α, and IL-2 after the first protein boost were comparable between the systemic regimens (P value range, 0.2 to 0.5) and between the MVA prime/i.m. boost and MVA prime/i.n. boost regimens (P value range, 0.06 to 0.7) (Fig. 2A). For animals that had adequate breast milk cell numbers for assessment, HIV Env-specific cytokine-producing CD4+ cells were also detected in the milk compartment following the first protein boost in one of two animals vaccinated with the DNA primer/i.m. protein boost regimen, two of four animals immunized with the MVA prime/i.m. boost regimen, and four of four animals that received the MVA prime/i.n. boost regimen (Fig. 2B). The proportions of milk HIV Env-specific CD4+ T cells producing IFN-γ, TNF-α, and IL-2 were not statistically different between the MVA prime/i.m. boost and MVA prime/i.n. boost regimens (P value range, 0.6 to 0.9). When detected, the proportion of HIV Env-specific CD4+ T cells producing either IFN-γ (P = 0.02) or TNF-α (P = 0.02) following the first HIV Env protein boost was consistently higher in milk than in blood across all vaccine groups.
Fig 2.
Env C.1086 prime/boost vaccination induces CD4+ and CD8+ T-cell responses in breast milk. (A and B) Percentages of HIV-specific CD4+ T cells in blood (A) and milk (B) of animals vaccinated with the DNA prime/i.m. protein boost, the MVA prime/i.m. protein boost, and the MVA prime/i.n. protein boost regimens. (C and D) Percentages of CD8+ T cells in blood (C) and milk (D) of vaccinated animals. Asterisks indicate that the numbers of breast milk cells were too low to be assessed as determined by lack of a detectable response to the SEB positive control for at least two of the three cytokines assayed.
Similarly, the proportions of HIV Env-specific cytokine-producing CD8+ T cells in blood following the first protein boost did not differ in magnitude between the systemic vaccine regimens (P value range, 0.3 to 0.9). The vaccine-elicited CD8+ T-cell responses were also similar between the MVA prime/i.m. boost and MVA prime/i.n. boost for IFN-γ (P = 0.18) and IL-2 (P = 0.10) but were higher with the i.n. boost for TNF-α (P = 0.02). HIV Env-specific CD8+ cells were detected in milk in one of two animals vaccinated with the DNA prime/i.m. boost, three of four animals vaccinated with the MVA prime/i.m. boost, and two of four animals vaccinated with the MVA prime/i.n. boost regimen. When detected, the proportions of HIV Env-specific CD8+ T cells producing IFN-γ, TNF-α, and IL-2 after the first Env protein boost were similar between breast milk and blood (P value range, 0.2 to 0.8). Overall, in accordance with previous studies (44), our results indicate that HIV immunization can induce strong cellular responses in the milk compartment of uninfected lactating rhesus monkeys.
Similar magnitudes of HIV Env-specific IgG antibody responses elicited by DNA prime/i.m. boost and MVA prime/i.m. boost in plasma and milk.
To investigate antibody responses elicited by systemic T/F Env prime/boost regimens in blood and milk, we first compared the abilities of the DNA prime/i.m. boost and MVA prime/i.m. boost regimens to induce systemic and milk IgG responses. HIV Env-specific IgG antibodies were detected in the plasma of all vaccinated animals following the prime (Fig. 3A and B), and the magnitudes of the responses were not statistically different between the two vaccine regimens (Table 1). The concentration of HIV Env-specific IgG antibodies in plasma after the first protein boost was 1 to 2 logs higher than that after the prime, and the concentrations remained comparable between the two vaccine regimens (Table 1). Only a slight increase in plasma HIV Env-specific IgG responses was observed following the second protein boost.
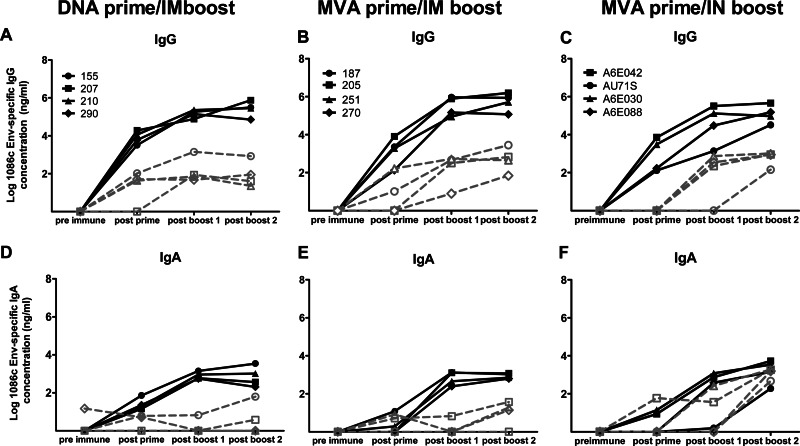
Fig 3.
MVA prime/i.n. boost immunization induces strong HIV Env-specific IgA antibodies in breast milk. (A to C) HIV Env-specific IgG antibodies in the plasma and milk of animals vaccinated with the DNA prime/i.m. protein boost (A), the MVA prime/i.m. protein boost (B), and the MVA prime/i.n. protein boost (C) regimens. (D to F) HIV Env-specific IgA responses in plasma and milk of animals vaccinated with the DNA prime/i.m. protein boost (D), the MVA prime/i.m. protein boost (E), and the MVA prime/i.n. protein boost (F) regimens. Each line represents one animal. Plasma is represented with filled symbols, and milk is represented with open symbols. The y axis represents log10.
Table 1.
Comparison of vaccine-elicited HIV Env-specific antibody responses in plasma and milk across vaccine groups
| Parameter | DNA prime/i.m. boost | MVA prime/i.m. boost | MVA prime/i.n. boost |
P valuea |
|
|---|---|---|---|---|---|
| DNA prime/i.m. boost vs MVA prime/i.m. boost | MVA prime/i.m. boost vs MVA prime/i.n. boost | ||||
| Plasma HIV Env-IgG (range, ng/ml) | |||||
| Postprime | 3,195–19,244 | 134–8,048 | 131–7,194 | 0.1 | 0.9 |
| Post-boost 1 | 76,990–229,556 | 88,532–932,526 | 1,393–320,953 | 0.5 | 0.2 |
| Post-boost 2 | 71,241–309,712 | 117,974–1,566,360 | 32,899–462,103 | 0.3 | 0.1 |
| Milk HIV Env-IgG (range, ng/ml) | |||||
| Postprime | 0–104 | 0–164 | 0–0 | 0.7 | NDb |
| Post-boost 1 | 47–1,412 | 8–525 | 1.55–1,190 | 0.9 | 0.9 |
| Post-boost 2 | 22–843 | 70–2,814 | 189–5,269 | 0.3 | 0.7 |
| Plasma HIV Env-IgA (range, ng/ml) | |||||
| Postprime | 19–72 | 0–12 | 0–13 | 0.03 | 0.9 |
| Post-boost 1 | 555–1,414 | 248–1,323 | 1.5–1,190 | 0.7 | 0.5 |
| Post-boost 2 | 202–3,466 | 625–1,187 | 189–5,269 | 0.7 | 0.3 |
| Milk HIV Env-IgA (range, ng/ml) | |||||
| Postprime | 0–6 | 0–8 | 0–59 | 0.9 | 0.3 |
| Post-boost 1 | 0–6 | 0–7 | 0–260 | 0.8 | 0.4 |
| Post-boost 2 | 0–62 | 0–37 | 462–1,841 | 0.7 | 0.03 |
| Plasma HIV Env-V1V2 IgG (range, ng/ml) | |||||
| Postprime | 810–21,870 | 30–810 | 90–2,430 | 0.05 | 0.8 |
| Post-boost 1 | 65,610–196,830 | 2,430–590,490 | 270–65,610 | 0.9 | 0.2 |
| Post-boost 2 | 65,610–196,830 | 6,5,610–198,830 | 21,870–196,830 | 0.6 | 0.2 |
| Milk HIV Env-V1V2 IgG (range, ng/ml) | |||||
| Postprime | 0 | 0–30 | 0 | ND | ND |
| Post-boost 1 | 90–810 | 0–2,430 | 0–90 | 0.3 | 0.2 |
| Post-boost 2 | 90–2,430 | 30–2,430 | 90–2,430 | 0.9 | 0.7 |
| Plasma HIV MW965, ID50 | |||||
| Postprime | 40–118 | 22–53 | <20–30 | 0.2 | 0.4 |
| Post-boost 1 | 731–2,270 | 877–12,305 | 52–2,273 | 0.2 | 0.06 |
| Post-boost 2 | 740–1,852 | 2,847–16,406 | 306–10,139 | 0.03 | 0.2 |
| Milk HIV MW965, ID50 | |||||
| Postprime | 81–154 | 29–80 | 20–49 | 0.5 | 0.2 |
| Post-boost 1 | 89–333 | 91–362 | 43–78 | 0.7 | 0.03 |
| Post-boost 2 | 65–650 (2/4) | 342–780 | 46–438 | ND | 0.06 |
| Plasma ADCC, end titer | |||||
| Postprime | 100–553 | 100 | 100–244 | ND | ND |
| Post-boost 1 | 11,691–49,438 | 1,331–93,910 | 183–5,206 | 0.34 | 0.06 |
| Post-boost 2 | 5,206–33,754 | 5,575–102,400 | 100–15,468 | 0.2 | 0.06 |
| Milk ADCC, titer | |||||
| Postprime | 4 | 4 | 4 | ND | ND |
| Post-boost 1 | 4–3,355 | 4–4,096 | 4 | 0.7 | 0.07 |
| Post-boost 2 | 4 (2/4) | 424–4,096 (3/4) | 4 | ND | ND |
Values in bold represent P values lower than 0.05.
ND, not determined.
The magnitude of the vaccine-elicited HIV Env-specific IgG response in milk was generally 2 to 4 logs lower than that in plasma. HIV Env-specific IgG antibodies were detected following the prime in the breast milk of 3 of 4 animals primed with rDNA and in 2 of 4 animals primed with rMVA (Fig. 3A and B). All animals had detectable HIV Env-specific IgG antibodies in milk following the protein boosts, and the levels were similar between the two vaccine regimens (Table 1). Thus, the two systemic vaccine regimens induced robust HIV Env-specific IgG antibody responses in plasma and lower levels in milk.
To determine if higher levels of HIV Env-specific IgG could be elicited in milk by mucosal immunization, we compared the magnitudes of the milk Env-specific IgG responses between animals immunized with the MVA prime/i.m. boost regimen (Fig. 3B) and those immunized with the MVA prime/i.n. boost regimen (Fig. 3C). Comparable levels of Env-specific IgG antibodies were elicited in milk following the systemic and the mucosal protein boosts (Table 1). As with the systemic regimen, the milk Env-specific IgG concentration in animals boosted i.n. was 2 to 4 logs lower than that in plasma. Notably, the magnitudes of the vaccine-elicited plasma Env-specific IgG responses were also similar between the i.m. and i.n. boost regimens (Table 1). Thus, the i.n. boost did not improve the magnitude of HIV Env-specific IgG responses in milk compared to the systemic boost and yet elicited similar levels of HIV Env-specific IgG in plasma.
MVA prime/i.n. boost selectively induces strong Env-specific IgA responses in milk.
HIV Env-specific IgA antibodies were detected in the plasma of all vaccinated animals after the protein boost, but the magnitude of the response was 1 to 2 logs lower than the Env-specific IgG response (Fig. 3D to F). The levels of Env-specific IgA antibodies in plasma after the two boosts were comparable between DNA prime/i.m. boost and MVA prime/i.m. boost and between MVA prime/i.m. boost and MVA prime/i.n. boost (Table 1). HIV Env-specific IgA antibodies were detected in milk of two animals vaccinated with DNA prime/i.m. boost and in three animals from the MVA prime/i.m. boost regimen (Fig. 3D and E). The magnitude of the response in milk was about 2 logs lower in milk than in plasma for the two groups of vaccinated lactating monkeys. In striking contrast, comparable levels of Env-specific IgA antibodies were detected in the plasma and milk of animals vaccinated with the MVA prime/i.n. boost (P = 0.6, Table 1). Moreover, the milk Env-specific IgA response was significantly higher among MVA prime/i.n. boost-vaccinated animals than in those vaccinated with MVA prime/i.m. boost (P = 0.03). To be sure that this strong IgA response was not due to IgG cross-reactivity in the assay, we repeated the measurement of the IgA response in milk of animals vaccinated with the MVA prime/i.n. boost after depleting IgG from the samples. The concentration of Env-specific IgA in milk remained 1 log higher in the MVA prime/i.n. boost than in the other vaccine strategies, confirming the robustness of the i.n. vaccine-elicited mucosal IgA response.
We then investigated if the high-magnitude Env-specific IgA response found in milk following MVA prime/i.n. boost was also present in other mucosal compartments. Env-specific IgA antibodies were detected in vaginal secretions but not in rectal secretions of all vaccinated animals (Table 2). However, the magnitude of the Env-specific IgA response in vaginal secretions was about 1 log lower than that in milk. This suggests that the mucosal boosting strategy (i.n. boost plus TLR agonist adjuvant) selectively induces strong HIV Env-specific IgA responses in lactating mammary glands.
Table 2.
MVA prime/i.n. boost vaccine-elicited mucosal IgA response
| Monkey | IgA response in samplea: |
||
|---|---|---|---|
| Milk | Rectal | Vaginal | |
| A6E042 | 22 | ND | 1.3 |
| AU71S | 4 | ND | 0.4 |
| A6E030 | 14 | ND | 1.1 |
| A6E088 | 23 | ND | 0.3 |
Shown in ng of HIV Env-specific IgA per μg of total IgA after the second protein boost. ND, not detected.
Systemic and mucosal HIV T/F Env vaccination elicited HIV Env-specific IgG antibodies at mucosal sites other than breast milk.
To determine if the vaccine regimens induced HIV Env-specific IgG in mucosal compartments other than milk, Env-specific IgG antibodies were measured in vaginal and rectal secretions (Table 3). While all vaccinated animals had detectable levels of Env-specific IgG in vaginal secretions after the first protein boost, only half of the animals from the DNA prime/i.m. boost and the MVA prime/i.m. boost groups and none from the MVA prime/i.n. boost group had detectable levels of Env-specific IgG in rectal secretions. The magnitudes of the vaginal Env-specific IgG responses did not statistically differ between the MVA prime/i.m. and MVA prime/i.n. boost regimens (P = 0.11). Interestingly, there was no correlation between the levels of Env-specific IgG in milk and those in vaginal secretions (r = 0.01, P = 0.9), suggesting that these mucosal responses are distinctly regulated. The function of rectal and vaginal Env-specific IgG antibodies was not assessed due to lack of sufficient material.
Table 3.
Vaccine-elicited mucosal IgG response following a single protein boost
| Regimen | Monkey no. | Response in samplea: |
||
|---|---|---|---|---|
| Milk | Rectal | Vaginal | ||
| DNA prime/i.m. boost | 155 | 1.6 | ND | 24.9 |
| 207 | 39.7 | 0.5 | 33.4 | |
| 210 | 1.2 | 1.9 | 28.9 | |
| 290 | 0.4 | ND | 16.2 | |
| MVA prime/i.m. boost | 187 | 8.2 | ND | 29.3 |
| 205 | 4.9 | ND | 37.9 | |
| 251 | 1.4 | 1.4 | 4.1 | |
| 270 | 0.08 | 0.2 | 3.1 | |
| MVA prime/i.n. boost | A6E042 | 11.5 | ND | 6.2 |
| AU71S | ND | ND | 2.3 | |
| A6E030 | 31.1 | ND | 1.0 | |
| A6E088 | 20.4 | ND | 0.3 | |
Shown as ng of HIV Env-specific IgG per μg of total IgG. ND, not detected.
Systemic and mucosal T/F Env immunization of lactating rhesus monkeys induces anti-V1V2 IgG antibodies in plasma and milk.
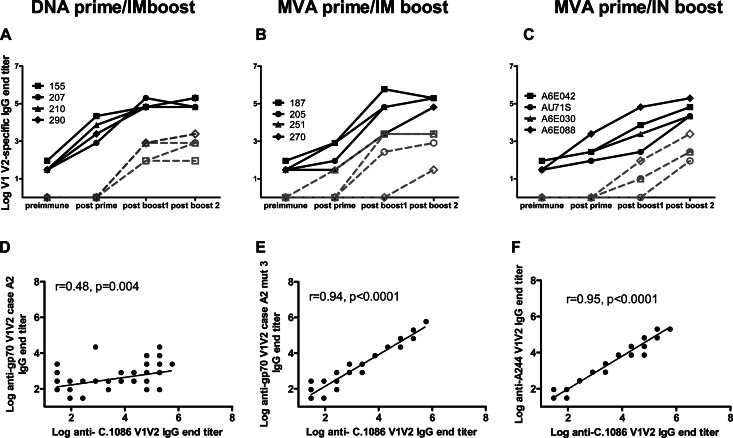
We then investigated the ability of the systemic and mucosal T/F Env vaccine regimens to elicit an anti-V1V2 IgG response, the response that was associated with a lower risk of HIV acquisition in the RV144 vaccine trial (45). Interestingly, a recent genetic analysis of breakthrough virus sequences in this RV144 trial demonstrated a vaccine efficacy of 48% against viruses matching the V2 loop of the vaccine strain with a lysine at position 169 (46), compared to the 31% overall vaccine efficacy. IgG antibodies against the autologous Env C.1086 V1V2 (containing a lysine at position 169) were also a correlate of risk of reduced transmission in the RV144 trial (G. Tomaras, H.-X. Liao, and B. F. Haynes, unpublished data), and antibodies of this specificity were elicited in plasma following immunization in all animals (Fig. 4). The magnitude of the response increased significantly between the prime and the first protein boost (P = 0.002), but in most animals, only a slight increase in antibody titers was observed after the second boost (P = 0.2). Following the prime, autologous anti-V1V2 IgG titers in plasma tended to be higher with the DNA prime/i.m. boost than with the MVA prime/i.m. boost (Table 1, P = 0.05). However, this strong anti-V1V2 IgG response following DNA prime did not lead to stronger boosting responses since the end titers did not statistically differ between the two regimens after the protein boosts (P = 0.8 and P = 0.6 for post-boost 1 and post-boost 2, respectively). The magnitudes of the plasma anti-V1V2 IgG responses were also comparable between the MVA prime/i.m. boost and MVA prime/i.n. boost regimens (P = 0.2 for both post-boost 1 and post-boost 2).
Fig 4.
Immunization of lactating monkeys induces anti-V1V2 IgG antibodies in plasma and breast milk. (A to C) End titers of IgG antibodies against C.1086 V1V2 in the plasma and milk of animals vaccinated with the DNA prime/i.m. protein boost (A), the MVA prime/i.m. protein boost (B), and the MVA prime/i.n. protein boost (C) vaccine regimens. Each line represents one animal. Plasma is represented with filled symbols, and milk is represented with open symbols. (D to F) Correlation between end titers of IgG antibodies against C.1086 V1V2 and gp70V1V2 case A2 (D), gp70V1V2 case A2 V169K/E172V/E173H (E), and A244 V1V2 (F). The y axis represents log10.
To examine the breadth of the plasma anti-V1V2 IgG response, IgG responses against the clade AE HIV A244 V1V2 (RV144 vaccine strain) and the clade B gp70V1V2 case A2 construct (used in the RV144 immune correlate analysis) (45) were also evaluated in all vaccine groups. The C.1086 Env vaccine-elicited response against the clade B gp70V1V2 case A2 (containing a valine at position 169) was less potent than that against the autologous C.1086 V1V2 (post-boost 2 end titer range, 90 to 7,290 versus 65,610 to 196,830, respectively; P = 0.002). The binding against gp70V1V2 case A2 remained low when the valine at position 169 was replaced by a lysine residue (data not shown). However, higher end titers were obtained against a gp70 V1V2 case A2 construct containing a triple mutation at positions 169, 172, and 173 (V169K/E172V/E173H) to match the C.1086 V1V2 sequence. There was a strong correlation between the magnitudes of the vaccine-elicited IgG response against A244 V1V2 (r = 0.95, P < 0.0001) and that against gp70 V1V2 case A2 V169K/E172V/E173H (r = 0.94, P < 0.0001) and C.1086 V1V2 (Fig. 4D and E).
IgG responses against the Env V1V2 region were also elicited in milk by the C.1086 Env vaccine regimens. The magnitude of the milk anti-C.1086 V1V2 IgG response was 2 to 4 logs lower than that in plasma, and the magnitudes did not differ between either DNA prime/i.m. boost and MVA prime/i.m. boost or MVA prime/i.m. boost and MVA prime/i.n. boost groups (Fig. 4A to C, post-boost 2, P = 0.9 and 0.7, respectively). Anti-V1V2 IgG antibodies tended to increase in milk following the second protein boost with the MVA prime/i.n. boost (endpoint titer range post-boost 1 versus post-boost 2, 10 to 90 versus 90 to 2,430, respectively; P = 0.09) but not with the MVA prime/i.m. boost regimen (P = 0.5). Interestingly, there was a strong correlation between the magnitudes of the milk and plasma autologous anti-V1V2 IgG responses (r = 0.8, P < 0.0001), suggesting that the vaccine-elicited anti-V1V2 IgG antibodies in milk are mostly transudate from plasma.
The MVA prime/i.m. boost vaccination regimen induced more potent neutralizing antibody responses in plasma than did the DNA prime or i.n. boost regimens.
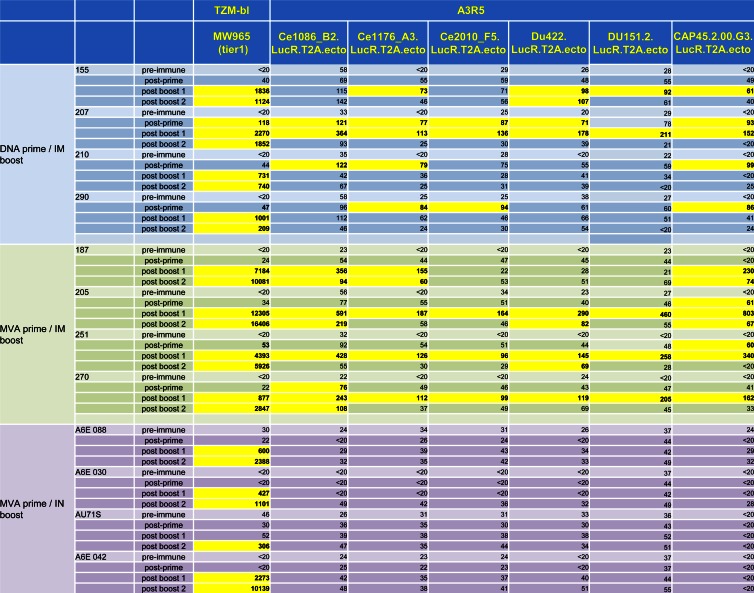
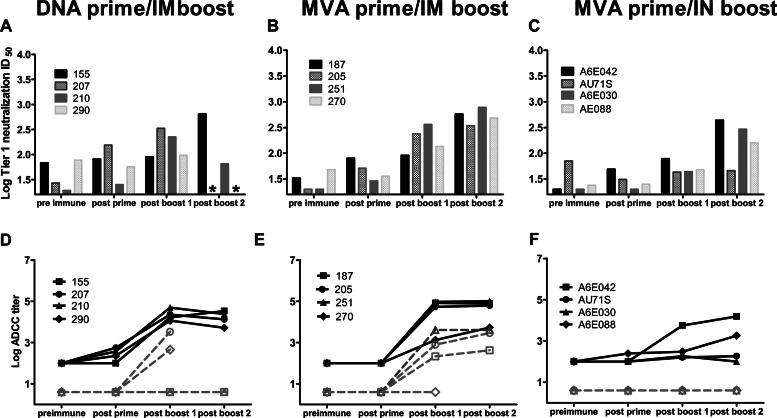
Although most HIV Env vaccines tested to date have been able to induce strong systemic binding antibody responses, only tier 1 and occasionally tier 2 neutralization responses have been elicited through vaccination (38, 47, 48). Thus, inducing potent, broadly neutralizing antibody responses remains an important goal for future HIV vaccine candidates. In this study, tier 1 plasma neutralization was assessed against HIV MW965 (clade C) in the TZM-bl cell-based assay. Neutralization against five traditional tier 2 clade C viruses (tier designation defined in the TZM-bl assay) and the autologous vaccine virus was also measured in the more sensitive A3R5 assay (38). Neutralization responses against the HIV MW965 clade C strain were detected in plasma after the first protein boost in all animals except one animal immunized with the MVA prime/i.n. boost regimen (Fig. 5). This animal (AU71S) generally showed lower potency of vaccine-elicited immune responses, potentially due to older age (age 15.4 years; immunized cohort range, 5.1 to 15.4 years). The TZM-bl tier 1 neutralization potencies in plasma after the first boost did not differ between the DNA prime/i.m. boost and the MVA prime/i.m. boost (Table 1, P = 0.2), but the neutralization titers were significantly higher in animals vaccinated with the MVA prime/i.m. boost regimen after the second boost (Table 1, P = 0.03). Plasma autologous C.1086 virus neutralization in the A3R5 assay was observed in two of four animals vaccinated with DNA prime/i.m. boost and in all animals vaccinated with the MVA prime/i.m. boost regimen (ID50 range, 94 to 594). One of the animals immunized with the DNA prime/i.m. boost had low levels of neutralization activity in plasma against the five viruses tested in the A3R5 neutralization assay, whereas 3 of 4 animals vaccinated with MVA prime/i.m. boost had low to moderate neutralizing activity against these five viruses (Fig. 5). Higher levels of neutralization were observed with the MVA prime/i.m. boost than with the DNA prime/i.m. boost against HIV CAP45.2.00.G3 in the A3R5 neutralization assay (post-boost 1 ID50 range, 162 to 803 versus <20 to 162, respectively; P = 0.04). Although classified as a tier 2 virus in the TZM-bl neutralization assay (49), HIV CAP45.2.00.G3 displays a tier 1 phenotype in the A3R5 neutralization assay (D. C. Montefiori and C. LaBranche, unpublished data). Importantly, plasma tier 2 neutralization in TZM-bl cells was assessed and found to be rare and of low magnitude in animals vaccinated with the MVA prime/i.m. boost regimen (data not shown). Thus, overall, the MVA prime/i.m. boost elicited more frequent and more potent neutralizing responses in plasma than did the DNA prime/i.m. boost.
Fig 5.
The MVA prime/i.m. protein boost regimen induces autologous and potent tier 1 neutralization in plasma. DNA prime/i.m. protein boost plasma neutralization titers (top), MVA prime/i.m. protein boost plasma neutralization titers (middle), and MVA prime/i.n. protein boost plasma neutralization titers (bottom). Positive neutralization titers defined as ID50 at least three times above the preimmune ID50 are highlighted.
We then compared the neutralization potency of antibody responses elicited by MVA priming followed by either i.m. or i.n. boost. Tier 1 neutralization titers tended to be lower in the MVA prime/i.n. boost than in the MVA prime/i.m. boost group after the first boost (Table 1, P = 0.06), but comparable neutralization was observed after the second boost (Table 1, P = 0.2). Surprisingly, although the two regimens elicited equivalent levels of Env-specific binding antibodies, no autologous or other clade C virus neutralization was detected in the plasma of i.n.-boosted animals using the A3R5 assay (Fig. 5). This finding suggests that systemic immunization was more effective than mucosal vaccination at eliciting functional systemic antibody responses.
Low to moderate levels of tier 1 neutralization are elicited in milk following T/F Env prime/boost immunization of lactating rhesus monkeys.
Our previous studies in HIV-infected lactating women and SIV-infected lactating monkeys have shown that low levels of tier 1 neutralizing antibodies can be detected in milk (17). Therefore, we investigated the ability of the adjuvanted T/F Env prime/boost regimens to induce neutralizing antibody responses in breast milk. Milk tier 1 neutralization in the TZM-bl assay was 1 to 2 logs lower than that in plasma and was detected in all animals immunized with MVA prime/i.m. boost and in 3 of 4 animals immunized with DNA prime/i.m. boost (Fig. 6A and B). The vaccine-elicited milk tier 1 neutralization responses were similar in magnitude between the two systemic vaccine regimens after the first prime (P = 0.6). Milk autologous and tier 2 neutralization responses in the A3R5 assay were observed only in one MVA-primed/i.m. boosted animal (animal 251, neutralization of 3 of 3 tier 2 viruses; ID50 range, 201 to 319; data not shown). Milk tier 1 neutralization was detected in only one animal immunized with MVA prime/i.n. boost after the first protein boost and in three animals after the second boost (Fig. 6C). After the second protein boost, tier 1 neutralization tended to be lower in animals immunized with the MVA prime/i.n. boost than in animals immunized with the MVA prime/i.m. boost (P = 0.06). No autologous or tier 2 neutralization was observed in animals who received the mucosal vaccine regimen. This weak functional antibody response in milk following i.n. immunization is in line with the weak systemic functional antibody response observed with this regimen and may indicate that neutralizing antibodies were not locally elicited.
Fig 6.
Mucosal immunization induces low levels of HIV Env-specific functional antibody responses in breast milk. (A to C) Log breast milk ID50 of the clade C tier 1 virus HIV MW965 in animals vaccinated with the DNA prime/i.m. protein boost (A), the MVA prime/i.m. protein boost (B), and the MVA prime/i.n. protein boost (C) regimens. Stars indicate samples not available for testing. (D to F) ADCC antibodies titers in the plasma and breast milk of animals which received the DNA prime/i.m. protein boost (D), the MVA prime/i.m. protein boost (E), or the MVA prime/i.n. protein boost (F) vaccine regimen. Each line represents one animal. Plasma is represented with filled symbols, and milk is represented with open symbols. The y axis represents log10.
Lack of vaccine-elicited ADCC response in milk following MVA prime/i.n. boost.
It was recently reported that HIV-infected women who did not transmit HIV to their infants during breastfeeding had a higher potency of milk ADCC-mediating antibodies than did the transmitting women (16). Thus, eliciting this nonneutralizing functional antibody response in breast milk may be important for an effective maternal HIV vaccine. The two systemic boost vaccine regimens induced potent ADCC responses in the plasma that were not statistically different after the protein boosts (Table 1; P = 0.3 and P = 0.2 for boost 1 and boost 2, respectively) (Fig. 6E and F). The ADCC response in milk was 1 to 2 logs lower than that in plasma and was detected in 2 of 4 animals from the DNA prime/i.m. boost vaccine regimen and from 3 of 4 animals vaccinated with the MVA prime/i.m. boost regimen. When detected, the magnitudes of the milk ADCC response were similar between the two groups (median DNA prime/i.m. boost ADCC titer, 1,905; range, 455 to 3,555; median MVA prime/i.m. boost ADCC titer, 784; range, 213 to 4,096). Interestingly, vaccine-elicited ADCC-mediating antibodies were present in the plasma but not in the milk of animals immunized with the MVA prime/i.n. boost. This lack of response confirms that the MVA prime/i.n. boost strategy is not potent at inducing functional antibody responses in milk.
DISCUSSION
Developing alternative prophylactic strategies that can be administered in combination with current preventive measures may be necessary to eliminate HIV MTCT in low-income areas. As ADCC activity in breast milk (16) and passive infusion of neutralizing antibodies (14) have been associated with reduction of postnatal HIV/SHIV acquisition in infants and neonatal rhesus monkeys, a maternal vaccine that provides passive functional HIV-specific antibody responses to HIV-exposed infants could contribute to achieving an HIV-free generation. In this study, we compared vaccine-elicited responses in breast milk and plasma of lactating rhesus monkeys primed with either rDNA or rMVA expressing the gp140 env gene of a highly immunogenic clade C T/F HIV strain (HIV C.1086) and then boosted twice i.m. with HIV C.1086 gp120 protein. We also compared the responses elicited when MVA priming was followed by i.m. or i.n. administration of the protein boosts. All three vaccine regimens elicited cellular immune responses in milk and plasma (Fig. 2). We previously established that robust SIV immunogen-specific CD4+ and CD8+ T-cell responses were induced in breast milk following immunization of lactating monkeys with a DNA prime virus vector NYVAC/Ad5 boost vaccine regimen (20). This DNA prime/live virus vector boost immunization strategy also induced low IgG responses but no IgA responses in breast milk. In contrast, in the present study, milk Env-specific IgG and IgA antibodies were detected in the majority of the vaccinated animals. Thus, the HIV T/F Env prime/protein boost vaccine strategies used in this study appear to be superior to the DNA prime NYVAC/Ad5 boost for inducing humoral immune responses in breast milk.
Inducing broadly neutralizing antibodies by vaccination has proven to be difficult. Most vaccines tested to date, including the moderately protective RV144 vaccine regimen, elicited tier 1 but not tier 2 neutralizing responses (38, 47, 48). As passive immunization studies have shown that neutralizing antibodies are able to protect from SHIV infection (50, 51), inducing this immune response is an important goal for an HIV vaccine. Interestingly, it was recently observed that while chronic Env immunogens induced strong tier 1 but not tier 2 neutralizing antibodies following immunization of guinea pigs, T/F Env immunogens elicited low levels of broadly neutralizing antibodies capable of neutralizing tier 1 and (weakly) tier 2 viruses (22). Consistent with the broadly neutralizing antibody responses elicited by HIV C.1086 Env in guinea pigs, lactating monkeys immunized with the MVA prime/i.m. boost regimen also developed low to moderate levels of plasma neutralizing antibodies that neutralized most of the tier 2 viruses tested in the A3R5 assay (Fig. 5). It should be noted that for this activity to be seen, it required measurement in the sensitive A3R5 neutralization assay, and as yet, the protective nature of these types of neutralizing antibodies is unknown. It is therefore important to determine if levels of these types of neutralizing antibody responses are sufficient to protect from HIV acquisition.
Only one study has investigated the association between milk neutralizing antibodies and risk of breast milk transmission (16), and although no association was observed, milk neutralization was rarely detected. Thus, it is possible that the induction of more potent neutralization in milk by a maternal vaccine could be beneficial. Surprisingly, although the systemic and mucosal MVA prime/protein boost regimens induced equivalent levels of HIV Env-binding IgG antibodies in plasma and milk, including against the Env V1V2 region, animals that received the i.n. boost had lower levels of functional antibodies in milk (Fig. 6). This low milk response likely reflects the lower magnitude of systemic functional responses observed in this vaccine group, as the breast milk functional IgG antibody response appears to be mainly derived from plasma (16, 17). Thus, systemic immunization may be required to achieve potent functional IgG antibody responses in breast milk.
High levels of plasma HIV Env-specific IgA antibodies were associated with an increased risk of HIV acquisition in RV144 vaccinees (45). However, the transmission rate among vaccine recipients with high Env-specific IgA levels was similar to that in placebo recipients, indicating that IgA antibodies did not enhance infectivity. Interestingly, although neutralization and ADCC antibody responses did not correlate with risk of HIV acquisition in the overall population of RV144 vaccine recipients, these functional responses were associated with a decreased risk of infection among vaccinees with low levels of plasma Env-specific IgA. It was therefore hypothesized that plasma (predominantly monomeric) IgA antibodies may interfere with potentially protective IgG responses (40, 69). As in most previously reported vaccine studies, the vaccine strategies used in this study induced plasma HIV Env-specific IgA responses. Importantly, although the MVA prime/i.n. boost induced high levels of Env-specific IgA antibodies in milk, there was no difference in the magnitude of the plasma response between this regimen and the systemic regimens (Fig. 3). This observation raises the important issue of balancing vaccine elicitation of immune responses that are potentially protective and those that may modulate these protective responses.
In contrast to the systemic compartment where monomeric IgA is predominant (52), mucosal IgA is mainly in the form of secretory IgA (sIgA), a dimeric antibody containing the J chain peptide and the secretory component (reviewed in reference 53). The mechanisms of sIgA induction and regulation appear to be distinct from those of systemic immune responses (54, 55), and the absence of correlation between HIV-specific IgA antibodies in plasma and those in mucosal compartments suggests that mucosal HIV Env-specific IgA antibodies are at least partially locally produced (17, 56). Importantly, mucosal IgA antibody responses were not investigated in the RV144 vaccine trial; thus, it is unclear if these responses are associated with protection or risk of HIV acquisition. Nevertheless, vaccine-elicited IgA responses in mucosal compartments are likely to exert functions distinct from those of responses in plasma. It was recently reported that HIV neutralization in rectal and vaginal secretions is mainly mediated by IgG antibodies (57). Similarly, breast milk IgA antibodies rarely neutralize HIV virions (17). However, mucosal IgA antibodies have been associated with a delayed acquisition of SIV infection following challenge of SIV-vaccinated monkeys (58). sIgA can mediate inhibitory functions other than neutralization, including aggregation of virions and inhibition of virion movement through mucus and across epithelial layers that may play protective roles for mucosal HIV transmission (59). Thus, induction of these responses by vaccination could be important to block HIV transmission at the mucosal level.
Although secretory IgA (sIgA) is the most abundant antibody isotype in mucosal compartments, only low levels of HIV-specific IgA antibodies are present in mucosal secretions of HIV-infected patients (56, 60). Similarly, the HIV Env-specific antibody response in milk of HIV-infected lactating women is predominantly IgG (17, 61). In contrast, the milk antibody response against other viral pathogens such as rotavirus and respiratory syncytial virus has been reported to be predominantly IgA (62, 63). Previous HIV/SIV immunization studies have reported higher IgG than IgA antibody responses in mucosal compartments (64–66). In this study, while the systemic vaccine strategies induced only low levels of mucosal virus-specific IgA responses, immunization with the MVA prime/i.n. boost regimen induced strong breast milk HIV Env-specific IgA responses, comparable in magnitude with the milk IgG responses (Fig. 3). Limited studies have investigated the antibody isotype distribution in milk following immunization. One of these studies reported a predominance of IgA in breast milk after immunization with pneumococcal capsular polysaccharides (67); yet, reports have also indicated that IgA is the major systemic isotype following immunization with capsular polysaccharides (68). Thus, the strong HIV Env-specific IgA response elicited in milk by the MVA prime/i.n. boost regimen is remarkable. A recent evaluation of systemic and vaginal antibody responses elicited in mice following administration of a clade C HIV Env alone or with different TLR agonists showed differences based on the route of administration and adjuvant used (43). Thus, both the use of a TLR 7/8 agonist (R848) as the adjuvant and the i.n. mucosal administration route might have contributed to the strong milk IgA response that we observed. However, this high-magnitude IgA response was specific to the breast milk compartment, suggesting that the mucosal route of HIV transmission should be taken into account when designing vaccine strategies. Vaccine-elicited Env-specific IgA responses should be evaluated in future nonhuman primate studies and human trials in order to determine if strong mucosal IgA responses are an important goal of an HIV vaccine.
As the HIV T/F Env MVA prime/i.m. boost induced robust functional antibody responses typically mediated by IgG antibodies, whereas the MVA prime/i.n. protein boost strategy induced strong HIV-specific IgA antibodies in breast milk, these two potentially protective humoral immune responses may be elicited by different pathways. If either response can be ultimately shown to be protective, then understanding the mechanism of induction of these responses will be critical for the development of an effective maternal HIV vaccine. If both functional IgG and sIgA antibody responses prove to be important for protection, our findings indicate that a vaccine strategy combining mucosal and systemic Env protein boosts may be required to induce robust protective humoral responses in breast milk.
ACKNOWLEDGMENTS
We thank Robin Shattock for helpful discussions on mucosal immunization and Krista Beck for veterinary assistance.
This work utilized services made possible with support from the Duke University Center for AIDS Research (CFAR), an NIH-funded program (5P30 AI064518), for MVA vaccine construction and for core services. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, by the Center for HIV/AIDS Vaccine Immunology (grant U19-AI067854-07), and by an SP K08 grant (K08AI087992).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published ahead of print 17 April 2013
REFERENCES
- 1. Dunn DT, Newell ML, Ades AE, Peckham CS. 1992. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet 340:585–588 [DOI] [PubMed] [Google Scholar]
- 2. Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, Kreiss J. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167–1174 [DOI] [PubMed] [Google Scholar]
- 3. Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, Gilbert PB, Stevens L, Peter T, Kim S, van Widenfelt E, Moffat C, Ndase P, Arimi P, Kebaabetswe P, Mazonde P, Makhema J, McIntosh K, Novitsky V, Lee TH, Marlink R, Lagakos S, Essex M. 2006. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi study. JAMA 296:794–805 [DOI] [PubMed] [Google Scholar]
- 4. Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, Newell ML. 2007. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 369:1107–1116 [DOI] [PubMed] [Google Scholar]
- 5. de Vincenzi I. 2011. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect. Dis. 11:171–180 [DOI] [PubMed] [Google Scholar]
- 6. Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, Makhema J, Moyo S, Thior I, McIntosh K, van Widenfelt E, Leidner J, Powis K, Asmelash A, Tumbare E, Zwerski S, Sharma U, Handelsman E, Mburu K, Jayeoba O, Moko E, Souda S, Lubega E, Akhtar M, Wester C, Tuomola R, Snowden W, Martinez-Tristani M, Mazhani L, Essex M. 2010. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N. Engl. J. Med. 362:2282–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barker PM, Mphatswe W, Rollins N. 2011. Antiretroviral drugs in the cupboard are not enough: the impact of health systems' performance on mother-to-child transmission of HIV. J. Acquir. Immune Defic. Syndr. 56:e45–8 doi: 10.1097/QAI.0b013e3181fdbf20 [DOI] [PubMed] [Google Scholar]
- 8. WHO, UNICEF, and UNAIDS 2011. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2011. World Health Organization, Geneva, Switzerland [Google Scholar]
- 9. Munoz FM, Englund JA. 2000. A step ahead. Infant protection through maternal immunization. Pediatr. Clin. North Am. 47:449–463 [DOI] [PubMed] [Google Scholar]
- 10. Van Rompay KK, Otsyula MG, Tarara RP, Canfield DR, Berardi CJ, McChesney MB, Marthas ML. 1996. Vaccination of pregnant macaques protects newborns against mucosal simian immunodeficiency virus infection. J. Infect. Dis. 173:1327–1335 [DOI] [PubMed] [Google Scholar]
- 11. John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, Overbaugh J, Bwayo J, Ndinya-Achola JO, Kreiss JK. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J. Infect. Dis. 183:206–212 [DOI] [PubMed] [Google Scholar]
- 12. Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. 2003. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J. Infect. Dis. 187:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, Jackson JB, Leroy V, Meda N, Msellati P, Newell ML, Nsuati R, Read JS, Wiktor S. 2004. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J. Infect. Dis. 189:2154–2166 [DOI] [PubMed] [Google Scholar]
- 14. Ferrantelli F, Hofmann-Lehmann R, Rasmussen RA, Wang T, Xu W, Li PL, Montefiori DC, Cavacini LA, Katinger H, Stiegler G, Anderson DC, McClure HM, Ruprecht RM. 2003. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS 17:301–309 [DOI] [PubMed] [Google Scholar]
- 15. Kuhn L, Trabattoni D, Kankasa C, Sinkala M, Lissoni F, Ghosh M, Aldrovandi G, Thea D, Clerici M. 2006. HIV-specific secretory IgA in breast milk of HIV-positive mothers is not associated with protection against HIV transmission among breast-fed infants. J. Pediatr. 149:611–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breast milk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 8:e1002739 doi: 10.1371/journal.ppat.1002739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fouda GG, Yates NL, Pollara J, Shen X, Overman GR, Mahlokozera T, Wilks AB, Kang HH, Salazar-Gonzalez JF, Salazar MG, Kalilani L, Meshnick SR, Hahn BH, Shaw GM, Lovingood RV, Denny TN, Haynes B, Letvin NL, Ferrari G, Montefiori DC, Tomaras GD, Permar SR. 2011. HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J. Virol. 85:9555–9567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Permar SR, Wilks AB, Ehlinger EP, Kang HH, Mahlokozera T, Coffey RT, Carville A, Letvin NL, Seaman MS. 2010. Limited contribution of mucosal IgA to simian immunodeficiency virus (SIV)-specific neutralizing antibody response and virus envelope evolution in breast milk of SIV-infected, lactating rhesus monkeys. J. Virol. 84:8209–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Permar SR, Kang HH, Carville A, Mansfield KG, Gelman RS, Rao SS, Whitney JB, Letvin NL. 2008. Potent simian immunodeficiency virus-specific cellular immune responses in the breast milk of simian immunodeficiency virus-infected, lactating rhesus monkeys. J. Immunol. 181:3643–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilks AB, Christian EC, Seaman MS, Sircar P, Carville A, Gomez CE, Esteban M, Pantaleo G, Barouch DH, Letvin NL, Permar SR. 2010. Robust vaccine-elicited cellular immune responses in breast milk following systemic simian immunodeficiency virus DNA prime and live virus vector boost vaccination of lactating rhesus monkeys. J. Immunol. 185:7097–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 22. Liao HX, Tsao CY, Alam SM, Muldoon M, Vandergrift N, Ma BJ, Lu X, Sutherland LL, Scearce RM, Bowman C, Parks R, Chen H, Blinn JH, Lapedes A, Watson S, Xia SM, Foulger A, Hahn BH, Shaw GM, Swanstrom R, Montefiori DC, Gao F, Haynes BF, Korber B. 2013. Antigenicity and immunogenicity of transmitted/founder, consensus and chronic envelope glycoproteins of human immunodeficiency virus type 1. J. Virol. 87:4185–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carroll MW, Moss B. 1997. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 238:198–211 [DOI] [PubMed] [Google Scholar]
- 24. Staib C, Drexler I, Sutter G. 2004. Construction and isolation of recombinant MVA. Methods Mol. Biol. 269:77–100 [DOI] [PubMed] [Google Scholar]
- 25. Staib C, Lowel M, Erfle V, Sutter G. 2003. Improved host range selection for recombinant modified vaccinia virus Ankara. Biotechniques 34:694–700 [DOI] [PubMed] [Google Scholar]
- 26. Scheiflinger F, Dorner F, Falkner FG. 1998. Transient marker stabilisation: a general procedure to construct marker-free recombinant vaccinia virus. Arch. Virol. 143:467–474 [DOI] [PubMed] [Google Scholar]
- 27. Antoine G, Scheiflinger F, Dorner F, Falkner FG. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365–396 [DOI] [PubMed] [Google Scholar]
- 28. Staib C, Kisling S, Erfle V, Sutter G. 2005. Inactivation of the viral interleukin 1beta receptor improves CD8+ T-cell memory responses elicited upon immunization with modified vaccinia virus Ankara. J. Gen. Virol. 86:1997–2006 [DOI] [PubMed] [Google Scholar]
- 29. Ink BS, Pickup DJ. 1989. Transcription of a poxvirus early gene is regulated both by a short promoter element and by a transcriptional termination signal controlling transcriptional interference. J. Virol. 63:4632–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. 1992. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69:597–604 [DOI] [PubMed] [Google Scholar]
- 31. Rosel J, Moss B. 1985. Transcriptional and translational mapping and nucleotide sequence analysis of a vaccinia virus gene encoding the precursor of the major core polypeptide 4b. J. Virol. 56:830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wyatt LS, Earl PL, Xiao W, Americo JL, Cotter CA, Vogt J, Moss B. 2009. Elucidating and minimizing the loss by recombinant vaccinia virus of human immunodeficiency virus gene expression resulting from spontaneous mutations and positive selection. J. Virol. 83:7176–7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, King SR, Montefiori DC, Nabel GJ. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 76:5357–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, Neutra MR. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169:566–574 [DOI] [PubMed] [Google Scholar]
- 35. Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. 2000. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J. Acquir. Immune Defic. Syndr. 24:297–309 [DOI] [PubMed] [Google Scholar]
- 36. Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449–12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 485:395–405 [DOI] [PubMed] [Google Scholar]
- 38. Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 206:431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. 1999. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J. Virol. 73:8966–8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Podda A. 2001. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine 19:2673–2680 [DOI] [PubMed] [Google Scholar]
- 42. Ott G, Barchfeld GL, Van Nest G. 1995. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine 13:1557–1562 [DOI] [PubMed] [Google Scholar]
- 43. Buffa V, Klein K, Fischetti L, Shattock RJ. 2012. Evaluation of TLR agonists as potential mucosal adjuvants for HIV gp140 and tetanus toxoid in mice. PLoS One 7:e50529 doi: 10.1371/journal.pone.0050529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Permar SR, Kang HH, Carville A, Wilks AB, Mansfield KG, Rao SS, Letvin NL. 2010. Preservation of memory CD4(+) T lymphocytes in breast milk of lactating rhesus monkeys during acute simian immunodeficiency virus infection. J. Infect. Dis. 201:302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490:417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vaine M, Wang S, Liu Q, Arthos J, Montefiori D, Goepfert P, McElrath MJ, Lu S. 2010. Profiles of human serum antibody responses elicited by three leading HIV vaccines focusing on the induction of Env-specific antibodies. PLoS One 5:e13916 doi: 10.1371/journal.pone.0013916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gilbert P, Wang M, Wrin T, Petropoulos C, Gurwith M, Sinangil F, D'Souza P, Rodriguez-Chavez IR, DeCamp A, Giganti M, Berman PW, Self SG, Montefiori DC. 2010. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J. Infect. Dis. 202:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 [DOI] [PubMed] [Google Scholar]
- 51. Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Delacroix DL, Dive C, Rambaud JC, Vaerman JP. 1982. IgA subclasses in various secretions and in serum. Immunology 47:383–385 [PMC free article] [PubMed] [Google Scholar]
- 53. Brandtzaeg P. 2009. Mucosal immunity: induction, dissemination, and effector functions. Scand. J. Immunol. 70:505–515 [DOI] [PubMed] [Google Scholar]
- 54. McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. 1992. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 10:75–88 [DOI] [PubMed] [Google Scholar]
- 55. Pabst O. 2012. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 12:821–832 [DOI] [PubMed] [Google Scholar]
- 56. Yates NL, Stacey AR, Nolen TL, Vandergrift NA, Moody MA, Montefiori DC, Weinhold KJ, Blattner WA, Borrow P, Shattock R, Cohen MS, Haynes BF, Tomaras GD. 9 January 2013. HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunol. [Epub ahead of print.] doi: 10.1038/mi.2012.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wei Q, Moldoveanu Z, Huang WQ, Alexander RC, Goepfert PA, Mestecky J. 2012. Comparative evaluation of HIV-1 neutralization in external secretions and sera of HIV-1-infected women. Open AIDS J. 6:293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. 2012. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J. Virol. 86:4644–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hladik F, Hope TJ. 2009. HIV infection of the genital mucosa in women. Curr. HIV/AIDS Rep. 6:20–28 [DOI] [PubMed] [Google Scholar]
- 60. Mestecky J. 2007. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J. Reprod. Immunol. 73:86–97 [DOI] [PubMed] [Google Scholar]
- 61. Van de Perre P, Hitimana DG, Lepage P. 1988. Human immunodeficiency virus antibodies of IgG, IgA, and IgM subclasses in milk of seropositive mothers. J. Pediatr. 113:1039–1041 [DOI] [PubMed] [Google Scholar]
- 62. Rahman MM, Yamauchi M, Hanada N, Nishikawa K, Morishima T. 1987. Local production of rotavirus specific IgA in breast tissue and transfer to neonates. Arch. Dis. Child. 62:401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fishaut M, Murphy D, Neifert M, McIntosh K, Ogra PL. 1981. Bronchomammary axis in the immune response to respiratory syncytial virus. J. Pediatr. 99:186–191 [DOI] [PubMed] [Google Scholar]
- 64. Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, Venzon D, Cafaro A, Ensoli B, Robert-Guroff M. 2009. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J. Immunol. 182:3718–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Leroux-Roels G, Maes C, Clement F, van Engelenburg F, van den Dobbelsteen M, Adler M, Amacker M, Lopalco L, Bomsel M, Chalifour A, Fleury S. 2013. Randomized phase I: safety, immunogenicity and mucosal antiviral activity in young healthy women vaccinated with HIV-1 Gp41 P1 peptide on virosomes. PLoS One 8:e55438 doi: 10.1371/journal.pone.0055438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Finn A, Zhang Q, Seymour L, Fasching C, Pettitt E, Janoff EN. 2002. Induction of functional secretory IgA responses in breast milk, by pneumococcal capsular polysaccharides. J. Infect. Dis. 186:1422–1429 [DOI] [PubMed] [Google Scholar]
- 68. Tarkowski A, Lue C, Moldoveanu Z, Kiyono H, McGhee JR, Mestecky J. 1990. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J. Immunol. 144:3770–3778 [PubMed] [Google Scholar]
- 69. Tomaras GD, Ferrari G, Shen X, Alam SM, Liao H, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. Vaccine induced plasma IgA specific for the C1-region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. U. S. A., in press [DOI] [PMC free article] [PubMed] [Google Scholar]