Abstract
One striking feature of viruses with RNA genomes is the modification of the host membrane structure during early infection. This process requires both virus- and host-encoded proteins; however, the host factors involved and their role in this process remain largely unknown. On infection with Tobacco mosaic virus (TMV), a positive-strand RNA virus, the filamentous and tubular endoplasmic reticulum (ER) converts to aggregations at the early stage and returns to filamentous at the late infectious stage, termed the ER transition. Also, membrane- or vesicle-packaged viral replication complexes (VRCs) are induced early during infection. We used microarray assays to screen the Arabidopsis thaliana gene(s) responding to infection with TMV in the initial infection stage and identified an Arabidopsis gene, PAP85 (annotated as a vicilin-like seed storage protein), with upregulated expression during 0.5 to 6 h of TMV infection. TMV accumulation was reduced in pap85-RNA interference (RNAi) Arabidopsis and restored to wild-type levels when PAP85 was overexpressed in pap85-RNAi Arabidopsis. We did not observe the ER transition in TMV-infected PAP85-knockdown Arabidopsis protoplasts. In addition, TMV accumulation was reduced in PAP85-knockdown protoplasts. VRC accumulation was reduced, but not significantly (P = 0.06), in PAP85-knockdown protoplasts. Coexpression of PAP85 and the TMV main replicase (P126), but not their expression alone in Arabidopsis protoplasts, could induce ER aggregations.
INTRODUCTION
Most viruses causing important diseases in both animals and plants have a single-stranded RNA genome of positive polarity. One striking feature of positive-strand RNA viruses is their modification of the host membrane structure for replication. Once positive-strand RNA viruses enter host cells, they induce a change in membrane structure and form membrane or vesicle packages of 50 to 400 nm in diameter (1); these structures are free within the cytoplasm or are associated with surrounding membrane (1, 2). The virus-induced organelles contain viral proteins, viral RNAs, and host factors usually termed viroplasm or viral replication complexes (VRCs) (3–6). More viral genomic RNAs are then produced within the VRCs. The origins of membranes forming VRCs are diverse in different types of invading viruses (1).
Some viral replicases, when expressed alone, can induce membrane modifications. An example is the 1a protein of Brome mosaic virus (BMV). The 1a protein is responsible for recruiting the 2a and viral RNA to the sites of replication in yeast (7, 8). The protein 1a is localized on the endoplasmic reticulum (ER), and expression of 1a alone can induce the membrane to form spherule invaginations (9, 10). Modulating the relative levels and interactions of la and 2a can change the membrane rearrangements from small spherule invaginations to large multilayered double membranes (11). The main replicases of viruses within the genus Tombusvirus target different membrane systems and induce various changes in membranes. For example, the main replicase of Tomato bushy stunt virus P33 targets peroxisomes and causes their progressive aggregation (12); the main replicase P27, encoded by Red clover necrotic virus, induces perinuclear aggregation and many smaller aggregations derived from the ER (13). Also, some viruses encoding proteins with no predicted enzyme activity related to replication may also participate in modification of the membrane structure for replication. The 6-kDa protein encoded by Tobacco etch virus (TEV) forms a membranous vesicle at ER exit sites similar to ER alterations in TEV-infected cells (14, 15).
Besides virus-encoded proteins, some host factors, such as the chaperone proteins heat shock protein 70 (Hsp70) and Hsp90 (for BMV and tombusviruses), peroxisomal transport Pex19p (for Tomato bushy stunt virus), coat protein complex I/II for retrograde and anterograde transport (for picornavirus and TEV), and lipid synthesis-associated proteins (for BMV, tombusviruses, and Semliki Forest virus) are also involved in this process (14, 16–25). More host factors may be involved in this process, and their identification and understanding how they function may provide opportunities for developing antiviral strategies.
The first known and well-studied virus, Tobacco mosaic virus (TMV), belongs to the positive-strand RNA viruses. The TMV replicases P126 and P183 (the read-through version of P126) are the virus-encoded proteins required for virus replication. The main viral replicase, P126, can localize to the ER and modulate the formation of VRCs (26–28). P126 does not contain transmembrane domains, and TMV may hijack Arabidopsis tobamovirus multiplication 1 (TOM1) genes for membrane anchoring. TOM1 protein contains transmembrane domains that interact with TMV P126, which may serve as a tethering factor for anchoring viral replicases to the ER membrane. Genetic screening identified another gene, TOM2A, involved in accumulation of different strains of TMV. TOM2, encoding a four-pass transmembrane protein, also interacts with TOM1 and was suggested to be associated with tobamovirus VRCs (29–31).
Besides the formation of VRCs, in early TMV infection, the filamentous and tubular ER is converted into aggregations and returns to a filamentous and tubular ER in the later infection stage, called the ER transition (32, 33). In TMV, the ER transition was suggested to be the event for the conversion of smooth to rough ER by recruiting the binding of ribosomes to smooth ER during TMV infection and is important for TMV to build up the infrastructure for protein synthesis and virus replication (32). TMV P126 alone can induce VRC formation (26–28); however, whether P126 can induce the ER transition remains to be resolved.
In this study, we focused on the early stage of virus replication to screen host factors involved in TMV infection. TMV U1 strain was used to infect Arabidopsis ecotype Col-0. Arabidopsis is a symptomless host of TMV U1 (34). This feature suggests that this host has less complicated physiological responses than other symptom-developing hosts, making it simpler for our screening. We used microarray analysis to identify 2 host genes, At3g28770 and At3g22640 (PAP85), involved in TMV replication. At3g28770 encodes a protein of unknown function, and At3g22640 encodes a vicilin-like seed storage protein (PAP85) (35) (Table 1). Because seed storage protein is involved in membrane modification for transportation (36–38) and TMV infection is also involved in membrane structure modification (32, 39), we initially focused on PAP85. TMV-induced ER transition was not observed in PAP85-silenced cells. The expression of TMV P126 alone induced a VRC-like structure but not the ER transition; however, coexpression of PAP85 and TMV P126 could induce an ER structure change similar to that of the ER transition during TMV infection.
Table 1.
Genes induced in common by TMV*CP.MP at 0.5, 4, and 6 h postinfection
| Locus no. | Description | Fold expression ata: |
||
|---|---|---|---|---|
| 0.5 h | 4 h | 6 h | ||
| At5g18880b | RNA-directed DNA polymerase-related family protein (glucose transmembrane transporter activity) | 3.45 | 3.89 | 2.93 |
| At2g34700d | Pollen Ole e 1 allergen and extensin family protein | 2.45 | 2.13 | 2.16 |
| At3g22640d | PAP85 | 2.23 | 2.08 | 2.01 |
| At2g41550b | Rho transcription termination factor | 2.23 | 2.44 | 2.10 |
| At1g43910b | P-loop-containing nucleoside triphosphate hydrolase-like protein, AAA-type ATPase | 2.48 | 2.40 | 2.31 |
| At2g38970b | Zinc finger (C3HC4-type RING finger) family protein (ubiquitin-protein ligase activity) | 2.36 | 2.30 | 2.24 |
| At2g05250b | DNAJ heat shock N-terminal domain-containing protein | 2.12 | 2.05 | 2.33 |
| At1g19900b | Glyoxal oxidase related | 2.54 | 2.05 | 2.23 |
| At3g28770c | Unknown protein | 2.53 | 2.35 | 2.10 |
| At1g55940b | Member of CYP708A, cytochrome P450 | 2.29 | 2.00 | 2.01 |
| At5g12100b | Pentatricopeptide (PPR) repeat-containing protein | 2.11 | 2.04 | 2.09 |
| At3g08670d | Hypothetical protein, related to oxidative stress | 2.99 | 2.40 | 2.29 |
Relative transcriptome ratio of TMV*CP.MP to TMV*rep in inoculated samples.
Arabidopsis homologous mutant is available.
Arabidopsis heterologous mutant is available.
Arabidopsis mutant is not available.
MATERIALS AND METHODS
Constructs used in this study. (i) Clones for cDNA microarray.
The TMV wild-type infectious clone was previously described (40). Two mutated clones, TMV* coat protein (TMV*CP) and TMV* movement protein (TMV*MP), were first created by site-directed mutagenesis. TMV was used as the template, and primer pairs U1-CP-F1/U1-CP-R1 and U1-CP-F2/U1-CP-R2 were used in the PCR to generate 2 overlapping fragments. The amplified fragments were gel purified and mixed together in a molar ratio of 1:1 for 5 PCR cycles, and then the primer pair U1-CP-F1/U1-CP-R2 was added for another 30 cycles. The amplified products were digested with NcoI/BsiWI and separated on 1% agarose gels to purify the fragments. The digested fragment was ligated to NcoI/BsiWI-digested TMV by use of T4 DNA ligase (Promega, Madison, WI) to construct TMV*CP. The construction of TMV*MP was essentially the same as that of TMV*CP, except the primer pairs U1-MP-F1/U1-MP-R1 and U1-MP-F2/U1-MP-R2 were used to amplify the first 2 overlapping fragments and U1-MP-F1/U1-MP-R2 was used for the second amplification, and the amplified fragment was digested with MfeI. TMV*CP and TMV*MP were both digested with NcoI and BsiWI, and the 787- and 8,294-kb fragments were gel purified. The 2 purified fragments were ligated together with T4 DNA ligase (Promega) to create TMV*CP.MP. Two stop codons were introduced in the CP (at amino acid position 14) and MP (at amino acid position 24). TMV*rep was constructed by the cut-and-fill-in method. TMV was digested by MluI and then treated with Klenow enzyme (3′-5′ exonuclease; New England BioLabs, Beverly, MA) for 30 min to fill in the overhangs. The reaction mixture then was shifted to 65°C for 20 min for Klenow enzyme inactivation. The blunt-end products were religated by use of T4 DNA ligase (Promega), which caused a frameshift (at amino acid position 249) and induced a stop codon in the replicase open reading frame (ORF; at amino acid position 271).
(ii) Clone for RNA probe preparation.
A fragment corresponding to 448 nucleotides (nt) of the TMV CP 3′ end was amplified by PCR with the template TMV and the primer pair U1-CP-F2/U1 RE. The fragment was cloned into pGEM-T Easy vector (Promega) to generate pGEMT-CP.
(iii) Clones for plant transformation.
pB7GWIWG2(I)-PAP85 was constructed by use of Gateway technology (Invitrogen, Gaithersburg, MD). A 130-bp DNA fragment complementary to the PAP85 nucleotide positions 921 to 1050 was amplified by PCR with the primer pair AttB1-PAP85F(921)/AttB2-PAP85R(1050). The PCR products were inserted into the donor vector (provided by Invitrogen) by recombination to generate an entry vector and then by another recombination with the destination vector pB7GWIWG2(I) (VIB, Ghent, Belgium) (41) to generate pB7GWIWG2(I)-PAP85.
(iv) Clone for VRC observation.
Red fluorescent protein (mCherry) was fused to the C terminus of the MP of TMV to construct TMV-MP:mCherry. The construction of TMV-MP:mCherry was essentially the same as that of TMV*CP, except 2 overlapping fragments were generated by PCR with the template TMV and the primer pair U1-MP-F2/U1-MP-R, and also with the template pSAT6-mCherry-C1-B (a kind gift from Lan-Ying Lee, Department of Biological Sciences, Purdue University) and the primer pair MP:YFP F/MP:YFP R. The primer pair U1-MP-F2/MP:YFP R was used for secondary amplification, and the amplified products were digested with NcoI/PacI and then ligated to NcoI/PacI-digested TMV to construct TMV-MP:mCherry.
(v) Clone for PAP85 overexpression.
The fragment of PAP85-GFP was amplified by PCR with pCass2-PAP85-GFP as a template and the primer pair PAP85F(CACC)/GFP R3. The PAP85-GFP fragment was cloned into the binary vector pK2GW7 (VIB, Ghent, Belgium) by use of Gateway technology (Invitrogen). PCR products were cloned into the pENTR/D-TOPO Gateway entry vector (Invitrogen) by following the manufacturer's recommendations. The pENTR/D-TOPO recombinant construct was sequenced to confirm the accuracy of the cloned fragments, and then LR Clonase II enzyme (Invitrogen) was used to transfer the cloned fragments into pK2GW7 to generate pK2GW7-PAP85-GFP. pK2GW7-*PAP85-GFP was constructed by the cut-and-fill-in method. pK2GW7-PAP85-GFP was digested by use of AseI and then treated with Klenow enzyme (3′-5′ exonuclease; New England BioLabs) for 30 min to fill in the overhangs. The reaction mixture then was shifted to 65°C for 20 min for Klenow enzyme inactivation. The blunt-end products were religated by use of T4 DNA ligase (Promega), which caused a frameshift (at amino acid position 7) and induced a stop codon in the replicase open reading frame (ORF; at amino acid position 10).
(vi) Clones for subcellular localization analysis.
The coding regions of PAP85 and P126 were inserted in the vector pCass2 with a double 35S promoter (42) and fused with green fluorescent protein (GFP) and red fluorescent protein, respectively. The coding region of PAP85 was amplified by reverse transcription-PCR (RT-PCR) with template RNA extracted from TMV-infected plants. Total RNA was treated with a Turbo DNA-free kit to remove residual DNA (Ambion, Austin, TX), and total cDNA was synthesized by use of a high-capacity cDNA reverse transcription kit (Applied Biosystems, Tokyo, Japan). PAP85 then was amplified by PCR with the primer pair PAP85 F(GFP)/PAP85 R(GFP). The fragments were digested by use of EcoRI and ligated into EcoRI-digested pCass2 to generate pCass2-PAP85. GFP was amplified by PCR with the template 30B-GFP (40) and the primer pair GFP F2/GFP R2. The NruI (blunt-end) and HindIII sites were incorporated by use of primers into the amplified GFP fragment. pCass2-PAP85 was digested by BclI, treated with mung bean nuclease (NEB) to create a blunt end, and then digested with HindIII. The GFP fragments were digested by NruI and HindIIII and ligated to the Mung bean nuclease and HindIII-treated pCass2-PAP85 to construct pCass2-PAP85-GFP. The construction of pCass2-mCherry-P126 was essentially the same as that of pCass2-PAP85-GFP, except that TMV was used as a template and the primer pair P126 F(mCherry)/P126 R(mCherry) was used to amplify P126. The fragments were digested by KpnI and ligated into KpnI-digested pCass2 to generate pCass2-P126. mCherry fragments were amplified by PCR with the plasmid pSAT6-mCherry-C1-B (a kind gift from Lan-Ying Lee), used as a template, and the primer pair mCherry F1/mCherry R1. The PCR fragments were digested with SalI/PmlI and ligated to SalI/PmlI-digested pCass2-P126 to construct pCass2-mCherry-P126. All primer sequences are available upon request.
Plant materials and transgenic plants.
Wild-type A. thaliana ecotype Col-0, transfer DNA (T-DNA) insertion SALK lines, ER-yellow fluorescent protein (YFP) transgenic A. thaliana (YFP with an HDEL ER retention signal peptide and the AtWAK2 signal peptide, which targets the proteins to the ER) (43, 44), and pap85-RNA interference (RNAi) transgenic lines were all grown at 22°C under long-day conditions (16 h light/8 h dark, 100 to 150 μE). The pap85-RNAi transgenic lines were generated by infecting A. thaliana (Col-0) with Agrobacterium tumefaciens GV3101 carrying pB7GWIWG2(I)-PAP85 (fragment 921 to 1050) by the floral dip method (45). Progeny transformants were identified by germinating seeds on Murashige and Skoog medium containing 50 μg/ml glufosinate ammonium (GA). After 2 weeks, GA-resistant seedlings were transferred to soil, and seeds were collected later. Individual homozygous lines in the T2 generation were obtained. Three individual lines were chosen, and their T3 generations were used for the following experiments.
In vitro transcription.
Capped transcripts corresponding to the wild-type virus and the constructed virus were synthesized by use of the mMESSAGE mMACHINE T7 kit (Ambion) as described previously (46–48), except that TMV and its derived plasmids were linearized with KpnI.
Protoplast isolation and PEG transfection.
Protoplasts were isolated from 6- to 7-week-old A. thaliana expanded leaves as described previously (49), with some modifications. Leaves were cut into 0.5- to 1-mm strips with the use of a clean razor. The leaves were incubated in a petri dish with enzyme solution containing 1% cellulose R10 (Yakult Honsha, Tokyo, Japan), 0.2% macerozyme R10 (Yakult Honsha), 0.4 M mannitol, 20 mM KCl, 20 mM MES, 10 mM CaCl2, and 0.1% bovine serum albumin (BSA), pH 5.7, and incubated for 3 h in the dark. The protoplasts were harvested by spinning the enzyme solution at 100 × g to pellet the protoplasts. Protoplasts were washed twice with W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM morpholineethanesulfonic acid [MES], pH adjusted to 5.7) and then pelleted and resuspended in MMg solution (0.4 M mannitol, 15 mM MgCl2, 4 mM MES, pH 5.7). Protoplasts were transfected by the polyethylene glycol (PEG) method as described previously (49), with some modifications. Protoplasts (1 × 105 cells) were collected in a round-bottomed tube. Nucleic acids (10 μg RNA transcripts for virus inoculation, 20 μg of each plasmid DNA for subcellular localization analysis, and the amount of double-stranded RNA [dsRNA] used for transient RNAi induction) and 110 μl PEG-Ca solution (4 g PEG 4000, 3 ml H2O, 2.5 ml 0.8 M mannitol, 1 mM CaCl2) were added to the tube smoothly for incubation at 23°C for 20 min. The tube then was diluted with 0.44 ml W5 solution. The solution was gently mixed and centrifuged for 1 min to remove PEG. The protoplasts were resuspended in 10 ml W5 solution and incubated at 25°C in the dark.
RNA extraction.
RNA used in Northern blot analysis, quantitative RT-PCR (qRT-PCR), and cDNA microarray analysis was extracted from protoplasts by the Pine Tree method (50) and dissolved in diethyl pyrocarbonate-treated water. For cDNA microarray analysis, the quality of RNA was checked by use of a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
Northern blot hybridization.
T3 RNA polymerase and EcoRI-digested pGEMT-CP plasmids were used to generate negative-sense digoxigenin (DIG)-labeled minus-sense probes (Roche Applied Science, Indianapolis, IN). Northern blot hybridization was performed as described previously (48). Hybridization signals were detected by use of the chemiluminescent substrate CDP STAR (Roche Applied Science), and blots were exposed to Fuji medical X-ray film (Fuji, Tokyo, Japan).
cDNA microarray fabrication and hybridization.
The cDNA microarray glass slides (including 11,500 Arabidopsis cDNA clones corresponding to 10,452 unique genes) and techniques used in cDNA microarray screening were provided and supported by the DNA Microarray Core Laboratory, Institute of Plant and Microbial Biology, Academia Sinica (Taipei, Taiwan). Total RNA was extracted from TMV*CP.MP- or TMV*rep-inoculated protoplasts, and RNA quality was analyzed by use of a 2100 Bioanalyzer (Agilent Technologies). RNA derived from TMV*CP.MP- and TMV*rep-inoculated samples was labeled with Cy5 and Cy3, respectively. Methods for preparing the fluorescent probe and hybridization were as described elsewhere (http://ipmb.sinica.edu.tw/microarray/protocol.htm). The hybridization signals were acquired with the use of an Axon GenePix 4000B and analyzed by use of GenePix 4.0 (Axon Instruments, Foster City, CA).
Real-time qRT-PCR.
Extracted total RNA was treated with a Turbo DNA-free kit (Ambion) to remove residual DNA. An amount of 1 μg total RNA was used as a template for synthesis of cDNA by use of a high-capacity cDNA reverse transcription kit (Applied Biosystems). A one-fourth aliquot of the cDNA was used as a template, and 2× SYBR green PCR master mix was added (Applied Biosystems). qRT-PCR involved the use of the ABI Prism 7000 sequence detection system (Applied Biosystems). The primer sequences are available on request. Quantification of genes involved RNA from 3 repeated individual experiments. For qRT-PCR, each sample was analyzed in triplicate. The relative quantification was calculated according to the manufacturer's instructions (Applied Biosystems). The Arabidopsis UBQ10 gene was used as an internal quantification control.
Preparation of dsRNA and transient RNAi induction in Arabidopsis protoplasts.
dsRNA was designed and prepared as described previously (51) by use of the T7 RiboMax Express RNAi system (Promega). The dsRNA of PAP85 was targeted to 922 to 1031 nt, that of At2g34700 to 110 to 219 nt, and that of At3g08670 to 942 to 1051 nt. The dsRNA of GFP (689 to 818 nt) and At1g55940 (600 to 709 nt) were controls. The primer sequences are available on request.
Agrobacterium-mediated transient complementation assays.
Seven-week-old Arabidopsis plants were used for agroinfiltration. Agrobacterium tumefaciens strain GV3101, containing binary vector (pK2GW7, pK2GW7-PAP85-GFP, or pK2GW7-*PAP85-GFP), was incubated with YEP (10 g/liter yeast extract, 10 g/liter peptone, 5 g/liter NaCl, pH 7.0) solid medium with kanamycin (50 μg/ml) and rifampin (50 μg/ml) and grown at 28°C for 2 days. Before agroinfiltration, A. tumefaciens was maintained at 28°C overnight in YEP broth with kanamycin (50 μg/ml) and rifampin (50 μg/ml). The preparation of A. tumefaciens suspensions and the agroinfiltration method were essentially as described previously (52).
Western blot hybridization.
In total, 10 μg crude extracted protein was resolved on 12.5% SDS-PAGE, and then proteins were transferred to nitrocellulose membranes (Whatman Protran, Dassel, Germany), which were probed with the primary antibody monoclonal anti-GFP (1:2,000 dilution; Sigma-Aldrich, St. Louis, MO) and then anti-mouse IgG-horseradish peroxidase-conjugated secondary antibody (1:20,000 dilution). Signals were detected by use of enhanced chemiluminescence agents (Amersham GE Healthcare, Little Chalfont, United Kingdom), and blots were exposed to Fuji medical X-ray film (Fuji).
Confocal microscopy.
Protoplasts used for observation of subcellular localization were examined by confocal microscopy (Zeiss LSM 510 Meta NLO DuoScan; Carl Zeiss, Jena, Germany). Protoplasts were harvested at 0.5 to 36 h postinoculation by centrifugation at 100 × g for 5 min. For single fluorescent imaging of GFP or YFP, the excitation wavelength was 488 nm, with a detection wavelength of 500 to 587 nm. For imaging mCherry, the excitation wavelength was 561 nm, with a detection wavelength of 575 to 630 nm. For simultaneous imaging of GFP and YFP, we used the emission fingerprinting method and the software ZEN (Carl Zeiss). Cells solely expressing GFP or YFP were first captured and stored in Lambda mode (in ZEN) as a reference. The detection wavelength was 500 to 587 nm to capture both GFP and YFP fluorescence, and then META Unmixing (in ZEN) was used to distinguish GFP and YFP signals. Images were assembled by use of ZEN.
Microarray sequence accession number.
The microarray data determined in the course of this work were deposited in the Gene Expression Omnibus (GEO) under accession no. GSE45283.
RESULTS
Twelve genes upregulated during early TMV infection.
Because we focused on the early stage of virus replication and searched for a gene(s) whose expression responded to virus infection, we used the genome-wide screening tool DNA microarray to identify host factors involved in virus replication. To help us more easily find the host factor, we narrowed down our target gene(s) by experimental design before microarray screening. First, we used the construct TMV*CP.MP with stop codons in both the coat protein (CP) and movement protein (MP) (Fig. 1A and B). The CP and MP are dispensable in virus replication. A replication-incompetent TMV, TMV*rep, with truncated RNA-dependent RNA polymerase (RdRp), was constructed as a control (Fig. 1C). Genomic RNA (gRNA) and subgenomic RNA (sgRNA) accumulated in TMV- and TMV*CP.MP- but not TMV*rep-infected protoplasts at 12 and 24 h postinfection (hpi) (Fig. 1D).
Fig 1.
Schematic representation of Tobacco mosaic virus (TMV) and derived constructs and detection of virus accumulation. (A to C) Schematic representation of TMV genomic RNA and specific mutants used in microarray analysis. Rectangles represent open reading frames encoded by TMV genomic RNA. Wild-type TMV encodes the 126-kDa and the read-through 183-kDa replicase proteins, the movement protein (MP; 30 kDa), and coat protein (CP; 17.5 kDa). The mutated sequences in TMV*CP.MP and TMV*rep are indicated by red letters. (D) Detection of virus accumulation by Northern blot hybridization. Total RNA was purified from TMV- and TMV-derived clone-infected protoplasts collected at 0.5, 12, and 24 hpi. Viral genomic RNA (gRNA) and CP subgenomic RNA (sgRNA) are indicated.
We then selected plant protoplasts and maximized virus transfection efficiency to obtain a large amount of cells at the initial TMV replication stages for microarray analysis. We optimized the TMV-Arabidopsis transfection efficiency by using the TMV construct 30B-GFP to express GFP in cells (40). We achieved almost 70% TMV transfection efficiency in repeated experiments (data not shown).
We then determined the suitable timing for microarray analysis by conducting time course analyses of TMV*CP.MP accumulation. Viral gRNA and sgRNA were visible at 8 hpi (data not shown). Therefore, the important virus-host interaction must occur before 8 hpi in Arabidopsis protoplasts.
RNA was collected from TMV*CP.MP- and TMV*rep-infected protoplasts at 0.5, 4, and 6 hpi for microarray analysis. We used cDNA microarray (containing 10,452 genes) to screen for the relative transcriptome change of TMV*CP.MP- and TMV*rep-inoculated samples at all 3 times, with 2 microarray analyses conducted at each time (GEO accession no. GSE45283). The microarray data were analyzed as described previously (53), and the results for the 2 analyses at each time were highly correlated (R2 = 0.90 to 0.96).
Initially, we looked for genes with consistent upregulation at all 3 times and identified 12 genes (Table 1). The expression of the 12 genes was analyzed in at least 3 repeated experiments by qRT-PCR (data not shown).
TMV accumulation was reduced in At3g28770 mutant plants and At3g22640-silenced protoplasts.
From the SALK collection (http://signal.salk.edu/), we found individual T-DNA insertion lines corresponding to 9 of the 12 genes. The T-DNA insertion of each SALK line was confirmed by PCR (data not shown). We obtained 8 homozygous mutant lines and 1 heterozygous mutant line (Table 1). We inoculated TMV in the 9 Arabidopsis mutant lines and examined TMV accumulation by RT-PCR 20 days postinoculation. TMV accumulation was greatly reduced in SALK_022597 (At3g28770) mutant plants (Fig. 2A).
Fig 2.
Accumulation of TMV in Arabidopsis SALK mutant lines and double-stranded RNA (dsRNA)-treated protoplasts. (A) TMV was inoculated in Arabidopsis mutant lines. The Arabidopsis mutant lines from the SALK collection were characterized by PCR to confirm their T-DNA insertion (data not shown). Total RNA was extracted from systemic leaves at 20 days postinoculation. TMV CP accumulation was detected by RT-PCR. Ubiquitin 10 was used as a loading control. The lanes 1 to 9 represent SALK_149616C (1), SALK_131250 (2), SALK_136570C (3), SALK_059297C (4), SALK_074253C (5), SALK_020627C (6), SALK_022597 (7), SALK_115514C (8), and SALK_136572C (9) (corresponding to At5g18880, At2g41550, At1g43910, At2g38970, At2g05250, At1g19900, At3g28770, At1g55940, and At5g12100, respectively). Wild-type (WT) Arabidopsis was used as a control. H indicates healthy WT Arabidopsis. (B) qRT-PCR analysis of mRNA levels of At2g34700, At3g22640, and At3g08670 relative to that in mock-inoculated protoplasts (set to 1). Protoplasts were treated with buffer, GFP dsRNA, and gene-specific dsRNA (designed from At2g34700, At3g22640, and At3g08670). Total RNA was extracted at 24 hpi. Data are means ± standard deviations (SD) from 3 experiments. (C) Arabidopsis thaliana protoplasts pretransfected with buffer (buf.) and dsRNA from GFP, At2g34700 (lane 10), At3g22640 (11), and At3g08670 (12). After 24 h, pretreated protoplasts were transfected with TMV, and then cells were collected at 24 hpi. RT-PCR analysis of TMV CP levels in cells was performed with ubiquitin 10 as an internal control. H indicates nontransfected WT Arabidopsis protoplasts.
We were unable to find mutant lines corresponding to At2g34700, At3g22640, and At3g08670 (Table 1). Thus, we introduced in vitro-prepared dsRNAs (110 bp) corresponding to the 3 genes into wild-type Arabidopsis protoplasts to induce RNA interference (RNAi) before TMV infection. The selected dsRNA region (110 bp) was aligned with the TMV genome, and a search of the bl2seq database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) revealed no similar pairs. The expression of each gene was analyzed (Fig. 2B). TMV accumulation was inhibited in protoplasts pretransfected with At3g22640 dsRNA-treated protoplasts (Fig. 2C).
Detailed analysis of the role of At3g22640 in TMV accumulation.
According to the annotation, At3g28770 encodes a protein of unknown function, and At3g22640 encodes a vicilin-like seed-storage protein (PAP85) (35) (Table 1). Because previous reports indicated that seed storage protein is involved in membrane modification for transportation (36–38), initially we focused on PAP85. The RNA level of PAP85 was reduced to 38% of the buffer-pretreated protoplast level in PAP85 dsRNA-pretreated protoplasts at 24 hpi (Fig. 3A), and then TMV was inoculated into these protoplasts. qRT-PCR revealed that PAP85 dsRNA-treated cells showed knocked-down PAP85 levels (Fig. 3B). TMV accumulation was reduced over time in PAP85 dsRNA-treated protoplasts but was detected at a similar level in GFP-dsRNA-pretreated protoplasts compared to buffer-pretreated protoplasts (Fig. 3C). Cell viability was similar among treatments, as analyzed by fluorescein diacetate staining (54 and data not shown).
Fig 3.
Accumulation of TMV in PAP85 dsRNA-treated Arabidopsis protoplasts. (A) Arabidopsis thaliana protoplasts were transfected with buffer and dsRNA (from PAP85 and GFP). Time course analysis of the quantity of PAP85 in Arabidopsis protoplasts relative to that in mock-inoculated protoplasts at 0.5 hpi (set to 1) treated with 10 μg dsRNA (derived from PAP85 and GFP). The protoplasts were collected at 0.5, 12, and 24 hpi. (B) After 24 h, the pretreated protoplasts were inoculated with TMV and then collected at 0.5, 12, and 24 hpi. Shown is the qRT-PCR analysis of PAP85 levels in TMV-infected protoplasts pretreated with buffer, PAP85 dsRNA, and GFP dsRNA relative to that in mock-inoculated protoplasts at 0.5 hpi (set to 1). (C) qRT-PCR analysis of TMV levels in A. thaliana protoplasts pretransfected with buffer and dsRNA. The RNA level of TMV in protoplasts pretreated with buffer at 0.5 hpi was set to 1. Data are means ± SD from 3 individual experiments and were analyzed by Dunnett's t test. *, P < 0.05 compared to mock inoculation.
To further validate the role of PAP85 in TMV replication in A. thaliana, we used a 35S promoter to express PAP85 hairpin RNA and generate pap85-RNAi transgenic lines (PAP85 knockout lines are not available in the SALK collection). We randomly selected 24 homozygous T3 plants (the third-generation transgenic line) derived from 3 individual T1 plants (the first-generation transgenic line) (pap85-1, pap85-2, and pap85-3). The mRNA level of PAP85 in these lines was maximally reduced to 52% of the wild-type level (pap85-2), with no obvious phenotypes observed (data not shown).
Because T-DNA insertion plants are not available for PAP85 and we could not obtain strong PAP85 knockdown plants, adequate expression of PAP85 may be required for plant viability. However, we still used these plants for our inoculation test. The mRNA level of PAP85 in plants was checked by qRT-PCR before TMV inoculation, and the accumulation of TMV in inoculated and systemic leaves was checked by qRT-PCR at 7 and 20 days postinfection (dpi), respectively (Fig. 4). Reduced TMV accumulation was associated with PAP85 knockdown levels in both TMV initially inoculated and systemic leaves (Fig. 4).
Fig 4.

Accumulation of TMV in pap85-RNAi transgenic Arabidopsis. Eight T3 plants (1 to 8) for each T1 plant (pap85-1, pap85-2, and pap85-3) were randomly selected for TMV inoculation. qRT-PCR analysis of PAP85 and TMV accumulation relative to that in WT plants (set to 1) shows the expression of PAP85 before TMV inoculation (PAP85) and TMV accumulation in inoculated (Ino.) and systemic leaves (Sys.) in pap85-RNAi lines at 7 and 20 dpi, respectively. Data are means ± SD from 3 repeated experiments.
Overexpression of PAP85 in pap85-RNAi Arabidopsis restored the accumulation of TMV.
To further validate the role of PAP85 in TMV accumulation in Arabidopsis, we performed Agrobacterium-mediated transient overexpression assays. We used the 35S promoter to transiently express PAP85::GFP (pK2GW7-PAP85-GFP), or the same construct but with frameshift and stop codons in PAP85 (pK2GW7-*PAP85-GFP) (Fig. 5A), in wild-type and pap85-RNAi plants by Agrobacterium infiltration. The mRNA level of PAP85 in selected pap85-RNAi plants was reduced to 64% of the wild-type level (pap85-2). Agrobacterium carrying the pK2GW7 vector was used as a control. After 3 days, the protein expression of PAP85::GFP was observed in pK2GW7-PAP85-GFP-transfected plants (Fig. 5B). Similar mRNA levels of PAP85 were detected in pK2GW7-PAP85-GFP- and pK2GW7-*PAP85-GFP-transfected wild-type or pap85-RNAi plants (Fig. 5B); however, PAP85::GFP was detected only in pK2GW7-PAP85-GFP-transfected plants.
Fig 5.
Accumulation of TMV in PAP85-overexpressed pap85-RNAi transgenic Arabidopsis. (A) Schematic representation of PAP85-GFP fusion protein. Rectangles represent open reading frames encoding PAP85 and GFP. The mutated sequence in PAP85 is indicated by red letters, and the created stop codon is indicated by italics. (B) Western blot and RT-PCR analyses of PAP85 protein and mRNA levels in PAP85-overexpressed leaves. Wild-type and pap85-RNAi (pap85) leaves were transfected with Agrobacterium tumefaciens carrying pK2GW7, pK2GW7-PAP85-GFP, or pK2GW7-*PAP85-GFP. Total crude proteins and total RNA were extracted from infiltrated leaves at 3 dpi. Monoclonal anti-GFP antibody was used to detect GFP-tagged PAP85 protein. Loading control (Rubisco large subunit, RbcL) for Western blotting and corresponding positions of marker proteins (in kDa) are indicated. UBQ10 was an internal control. (C) Binary vector-infiltrated Arabidopsis leaves were inoculated with TMV. Shown is qRT-PCR analysis of TMV levels in inoculated leaves relative to that in wild-type leaves infiltrated with pK2GW7 (set to 1) at 7 dpi. Data are means ± SD from 3 individual experiments and were analyzed by Dunnett's t test. *, P < 0.05 compared to TMV-inoculated wild-type Arabidopsis leaves preinfiltrated with pK2GW7.
We next inoculated TMV transcripts into these leaves. The accumulation of TMV was higher (but not significantly so) in pK2GW7-PAP85-GFP- or pK2GW7-*PAP85-GFP-transfected plants than in vector control (pK2GW7)-transfected plants (Fig. 5C). TMV accumulation was lower in pK2GW7- or pK2GW7-*PAP85-GFP-transfected pap85-RNAi lines than in vector control-transfected wild-type Arabidopsis (P < 0.05); however, in PAP85::GFP-overexpressed leaves, the accumulation of TMV recovered to a level similar to that in vector control-transfected wild-type leaves (Fig. 5C). Thus, PAP85 protein but not mRNA is involved in TMV accumulation.
Expression pattern of PAP85 in Arabidopsis.
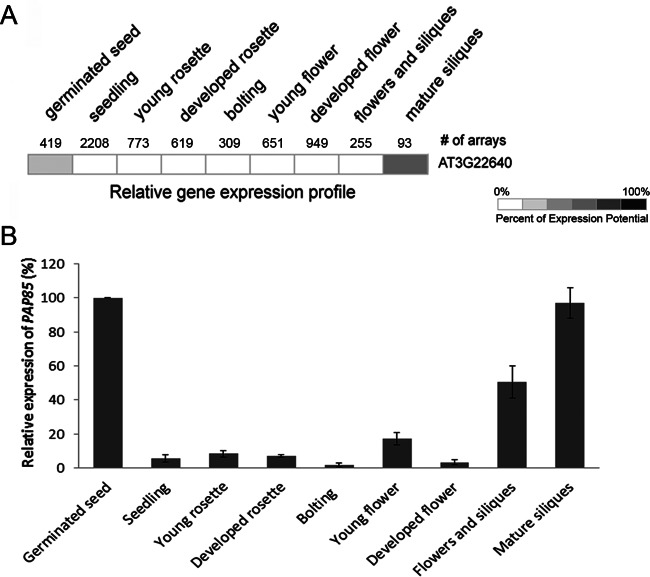
We examined the expression pattern of PAP85 in different tissues and developmental stages by use of Genevestigator (Fig. 6A) and qRT-PCR in Arabidopsis plants (Fig. 6B). PAP85 was mainly expressed in germinated seeds and mature siliques.
Fig 6.
Expression analysis by in silico and qRT-PCR analysis at different plant developmental stages. (A) In silico analysis of relative expression of PAP85 at different developmental stages. Expression levels are shown as heat maps, with dark red indicating maximal expression. Analysis involved the use of Genevestigator. (B) qRT-PCR analysis of the expression pattern of At3g22640 (PAP85) relative to that in germinated seeds (set to 100%) at different developmental stages. Data are means ± SD from 3 individual experiments.
ER morphology in TMV-infected cells.
To determine the role of PAP85 in TMV infection, we used TMV-MP:mCherry to transfect the ER marker (YFP fused with signal peptides targeting the ER derived from the Arabidopsis gene AtWAK2 and the HDEL ER retention signal peptide) transgenic Arabidopsis. A previous study revealed that TMV VRCs were easily observed in cells infected with TMV when the TMV MP is fused with green fluorescent protein (39). Thus, we could observe the ER and TMV VRCs simultaneously. During the infection process in Arabidopsis cells, the ER change in structure (Fig. 7A) was similar to that in TMV-infected N. benthamiana (32): ER aggregations were formed initially (at 8 to 24 hpi) and returned to a filamentous structure after 36 hpi, and TMV VRCs were formed and associated with ER aggregations at 8 to 24 hpi.
Fig 7.

Morphology of endoplasmic reticulum (ER) in PAP85 dsRNA-pretreated protoplasts infected with TMV-MP:mcherry. Analysis of protoplasts from ER-yellow fluorescent protein (ER-YFP) transgenic Arabidopsis leaves pretransfected with buffer (A) or control dsRNA from At1g55940 (B) and PAP85 (C) and then inoculated with TMV-MP:mcherry. Cells were collected at 0.5, 8 to 24, and 36 hpi. Scale bars represent 10 μm. (D) qRT-PCR analysis of PAP85 level relative to that in mock-inoculated protoplasts at 0.5 hpi (set to 1) and TMV-MP:mcherry-infected protoplasts pretreated with buffer, PAP85, or At1g55940 dsRNA at 0.5, 8 to 24, and 36 hpi. (E) qRT-PCR analysis of TMV-MP:mcherry level in A. thaliana protoplasts pretransfected with buffer and dsRNA. Total RNA was purified from protoplasts (as described for panel D). The mRNA level of TMV in buffer-pretreated protoplasts at 0.5 hpi was set to 1. Data are expressed as means ± SD from 3 individual experiments.
We delivered PAP85 dsRNA to ER marker transgenic Arabidopsis cells and then inoculated TMV-MP:mCherry to observe ER morphological features and VRCs over time. The ratio of cells showing VRCs was lower in PAP85 dsRNA-treated cells than in untreated or At1g55940 dsRNA-treated cells (9 versus 72 or 67%). Also, in cells showing VRCs, the ER transition was observed in all examined untreated or At1g55940 dsRNA-treated cells but not in 80% of PAP85 dsRNA-treated cells (Fig. 7A to C). Only PAP85 dsRNA-treated cells showed knocked-down PAP85 and reduced TMV levels (Fig. 7D and E).
ER structure with TMV P126 expressed alone or coexpressed with PAP85.
Previous reports indicated that TMV P126 alone can induce the VRC-like structure (26–28); however, whether TMV P126 alone can induce the ER transition has not been reported. To examine whether P126 alone can induce the ER transition, we expressed P126 only in ER marker transgenic Arabidopsis protoplasts and examined the ER structure over time. We observed the VRC-like structure but not the ER transition with P126 expression alone (Fig. 8A).
Fig 8.
ER morphology in P126-mcherry- and/or PAP85-GFP-expressed Arabidopsis protoplasts. (A) Confocal microscopy of protoplasts from ER-YFP transgenic Arabidopsis leaves inoculated with P126-mCherry (expressed by a double 35S promoter) at 0.5, 8 to 24, and 36 hpi. (B to D) Subcellular localization of PAP85 and TMV P126 in Arabidopsis protoplasts. Transient expression (by double 35S promoter) of GFP-tagged PAP85 (B) and mCherry-tagged P126 (C). (D) Transient coexpression of GFP-tagged PAP85 and mCherry-tagged P126. Cells were examined by confocal microscopy at 12 to 24 hpi. Scale bars represent 10 μm.
Our data indicated that PAP85 is involved in TMV-induced ER transition; however, neither PAP85 nor TMV P126 alone could induce the ER transition (Fig. 8A to C). TMV P126 is the main protein expressed at the early stage of TMV infection, and PAP85 is the gene upregulated in this stage. We speculated that both TMV P126 and PAP85 are required for inducing the ER transition. Thus, we coexpressed PAP85-GFP and TMV P126-mCherry (P126 fused with a red fluorescence protein) in Arabidopsis protoplasts. In all examined cells, the ER filament/tubular-like structure disappeared, and we observed ER aggregations similar to ER transition at 12 to 24 hpi (Fig. 8D).
Induction of PAP85 expression in TMV-infected or TMV P126-expressed cells.
If PAP85 and TMV P126 are both required to induce ER aggregation, similar to ER transition, TMV P126 expressed alone may not induce the ER transition, because its sole expression cannot induce the expression of PAP85. To test this hypothesis, we examined the expression of PAP85 in mock-infected, TMV-infected, and TMV P126-expressing protoplasts. We observed the induction of PAP85 only in TMV-infected protoplasts (Fig. 9).
Fig 9.

Relative expression of PAP85 in TMV P126-overexpressing or TMV-infected Arabidopsis protoplasts. qRT-PCR analysis of expression of PAP85 in pCass2- vector, pCass2-P126-, and TMV-inoculated Arabidopsis protoplasts at 0.5 (A) and 24 hpi (B) relative to that in mock-inoculated protoplasts (set to 1). Data are means ± SD from 3 individual experiments and were analyzed by Dunnett's t test. *, P < 0.01 compared to mock inoculation.
DISCUSSION
Our knowledge of viruses helps in developing antiviral strategies and has led to several landmark discoveries of cell biology (55–59). In this report, we identified a host gene, PAP85, induced by TMV and involved in TMV accumulation. Our data also suggest that PAP85, together with TMV P126, is involved in the ER transition, from a filamentous structure to aggregations and a return to a filamentous structure, a phenomenon beginning early in TMV infection. Our studies help in understanding the early event of TMV replication and provide an opportunity for developing antiviral strategies.
Our data from knockdown and overexpression of PAP85 in TMV-infected cells indicate that PAP85 is involved in TMV accumulation (Fig. 3 to 5). PAP85 encodes a vicilin-like seed storage protein (35). We searched for orthologous genes of PAP85 in the database for Solanaceae (the primary host for TMV) (http://solgenomics.net/) (60). The ortholog of PAP85 in Solanum spp. is SGN-U215172 and in Capsicum annuum is SGN-U201542. PAP85 shares 34 and 39% protein sequence identity with SGN-U215172 and SGN-U201542, respectively; however, the function of both genes is currently unknown. The BLASTP best hit for PAP85 in GenBank, besides Arabidopsis species, is another vicilin-like seed storage protein in Juglans nigra (AAM54366.1). PAP85 and AAM54366.1 share 35% protein sequence identity. However, the function of AAM54366.1 is also unknown. PAP85 contains 2 cupin conserved domains; the BLASTP best hit for each cupin domain in GenBank, besides Arabidopsis species, is the same protein in Prunus persica (EMJ26465.1). The 2 PAP85 cupin domains share 42 and 39% sequence identity with the 2 cupin domains of EMJ26465.1. The function of EMJ26465.1 has yet to be identified.
Seed storage proteins play roles as nutrient reservoirs. In Arabidopsis, the main seed proteins are the 12S globulins and the 2S albumins, and PAP85 is similar to the 12S globulins. Vicilin-like seed storage proteins share striking similarities in secondary and tertiary structure to the cupin protein superfamily of prokaryotic and eukaryotic organisms (61). The cupin protein superfamily has many functions (62). The characteristic cupin domain, the compact jelly-roll β-barrel, is thought to confer a high degree of thermal stability and resistance to protease digestion. PAP85 has not been extensively studied and is a silique development marker (35). PAP85 is expressed at a low level in most tissues and stages and abundantly in harvested seeds, embryos, and endosperm of developing seeds (63–65). Genome-wide analysis revealed the upregulation of PAP85 in alpha-amanitin- and abscisic-acid-treated seeds and MINI ZINC FINGER 1 protein-overexpressing seedlings, as well as downregulation with knockout of the abscisic acid-upregulated receptor-like kinase RPK1 (63, 66–68). However, current information about PAP85 does not help explain why PAP85 is involved in TMV accumulation.
Seed storage proteins are reported to be translated in the ER, and the translated protein is sorted to the Golgi body and then deposited in protein storage vacuoles or results from protein accretion to form a protein body (PB) (36–38). Once a PB is formed, seed proteins bypass the Golgi body and are transferred to the protein storage vacuole or sometimes remain in cytoplasm (36–38). PBs originating from the tubular ER and attaching to ribosomes were suggested to be ER bodies (36). The origin and formation of the PB show similarity to the ER changes observed in early TMV infection (Fig. 7A).
In the tobamovirus genus, several host factors are involved in virus infection. Tobacco eukaryotic elongation factor 1A; ARL8, a small GTP-binding protein; and Hsp70 are components of a replication complex (69–72). Arabidopsis Rab GDP dissociation inhibitor (GDI2) can interact with TMV P126 and may alter vesicle trafficking to enhance the establishment of virus infection (73). In addition, Arabidopsis auxin/indole acetic acid proteins interact with TMV P126/183 replicase and are involved in symptom development; the interaction may reprogram the auxin response pathway to enhance virus infection (74–76). The Arabidopsis NAC domain transcription factor ATAF2 can interact with viral replicase and is involved in plant defense responses against TMV (77, 78). Furthermore, a tobacco novel class II KNOTTED1-like protein, NTH201, was found to assist viral cell-to-cell movement and VRC formation in the early stage of TMV infection (79). TMV infection foci and VRC (size and number) are altered in ATP-synthase gamma subunit (AtpC)- and rubisco activase (RCA)-silenced leaves (80). However, not all of these host factors have been found to have a direct effect on the ER transition during tobamovirus replication. Although the detailed mechanism of the role of PAP85 in TMV accumulation remains elusive, our data show that (i) less ER transition in TMV-infected PAP85 knockdown protoplasts (Fig. 7C) and (ii) coexpression of PAP85 and TMV P126, but not either protein alone, can induce ER morphological changes similar to those of the ER transition induced by TMV infection, which supports PAP85 being involved in the ER transition during TMV infection. Our data may also explain why TMV infection, but not TMV P126 overexpression, induced the ER transition. We found increased levels of PAP85 after Arabidopsis cells were infected with TMV but not with expression of TMV P126 alone (Fig. 9).
Of note, our data suggest that the VRC-like structure formation is independent of the ER transition, because (i) in PAP85 knockdown cells, VRCs were observed in cells without the ER transition and the VRC number was reduced (but not significantly) (Fig. 7C), and (ii) the expression of TMV P126 alone induced VRCs but not the ER transition (Fig. 8A). However, small portions of ER aggregations (ER transition) may be enough for TMV P126 to induce VRCs, and we cannot rule out that the ER transition is important for the formation of a VRC-like structure.
A good plant host target for antiviral purposes should not have an essential function in plants and likely remains dormant in most developmental stages. The rationale is that if a gene is not necessary in most stages of plant growth but is induced by virus infection, then designing strategies to prevent gene induction by viruses may prevent the virus from further infection without causing undue adverse host effects. PAP85 may be needed for flower and seed development but is not expressed in most growth stages (Fig. 6A and B). Thus, strategies such as using a tissue-specific promoter to express dsRNA to prevent viruses from inducing PAP85 may be an effective antiviral strategy.
The formation of an ER body, including the PB, provides an alternative pathway for protein transport (36). However, the biological and physiological processes involved in this pathway remain largely unknown. Our data indicate that PAP85, when expressed alone, is predominately localized to the ER and does not induce ER aggregations. The expression of PAP85 modified the ER morphological features only in the presence of TMV P126. The induction of ER aggregations is protein specific, because abundant ER aggregations were not seen in all PAP85- and ER marker-coexpressed cells (Fig. 8B). Thus, PAP85 has a role in facilitating ER aggregations in a protein-selective manner. However, its role in plant growth and development remains to be further investigated.
ACKNOWLEDGMENTS
This work was supported in part by the DNA Microarray Core Laboratory, Institute of Plant and Microbial Biology, Academia Sinica, Taiwan. This work was supported in part by the Department of Medical Research, National Taiwan University Hospital. This research was funded through grants from the National Science Council, Taiwan (no. 101-2321-B-002-048).
Experiments and data analysis were performed in part with the use of the confocal microscope at the Scientific Instrument Center of Academia Sinica and with the assistance of Shu-Chen Shen. We thank Shu-Jen Chou for technical support in the microarray assay. We also thank Yi-Li Liu and I-Ching Huang for technical support in DNA sequencing.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 10 April 2013
REFERENCES
- 1. Miller S, Krijnse-Locker J. 2008. Modification of intracellular membrane structures for virus replication. Nat. Rev. 6:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laliberte JF, Sanfacon H. 2010. Cellular remodeling during plant virus infection. Annu. Rev. Phytopathol. 48:69–91 [DOI] [PubMed] [Google Scholar]
- 3. Noueiry AO, Ahlquist P. 2003. Brome mosaic virus RNA replication: revealing the role of the host in RNA virus replication. Annu. Rev. Phytopathol. 41:77–98 [DOI] [PubMed] [Google Scholar]
- 4. Ahlquist P, Noueiry AO, Lee WM, Kushner DB, Dye BT. 2003. Host factors in positive-strand RNA virus genome replication. J. Virol. 77:8181–8186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salonen A, Ahola T, Kaariainen L. 2005. Viral RNA replication in association with cellular membranes. Curr. Top. Microbiol. Immunol. 285:139–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buck KW. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47:159–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Ahlquist P. 2000. Brome mosaic virus polymerase-like protein 2a is directed to the endoplasmic reticulum by helicase-like viral protein 1a. J. Virol. 74:4310–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Noueiry A, Ahlquist P. 2001. Brome mosaic virus protein 1a recruits viral RNA2 to RNA replication through a 5′ proximal RNA2 signal. J. Virol. 75:3207–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Restrepo-Hartwig M, Ahlquist P. 1999. Brome mosaic virus RNA replication proteins 1a and 2a colocalize and 1a independently localizes on the yeast endoplasmic reticulum. J. Virol. 73:10303–10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang XF, Lee WM, Watanabe T, Schwartz M, Janda M, Ahlquist P. 2005. Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates. J. Virol. 79:13747–13758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz M, Chen J, Lee WM, Janda M, Ahlquist P. 2004. Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication. Proc. Natl. Acad. Sci. U. S. A. 101:11263–11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCartney AW, Greenwood JS, Fabian MR, White KA, Mullen RT. 2005. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell 17:3513–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner KA, Sit TL, Callaway AS, Allen NS, Lommel SA. 2004. Red clover necrotic mosaic virus replication proteins accumulate at the endoplasmic reticulum. Virology 320:276–290 [DOI] [PubMed] [Google Scholar]
- 14. Wei T, Wang A. 2008. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J. Virol. 82:12252–12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaad MC, Jensen PE, Carrington JC. 1997. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16:4049–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barajas D, Nagy PD. 2010. Ubiquitination of tombusvirus p33 replication protein plays a role in virus replication and binding to the host Vps23p ESCRT protein. Virology 397:358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomita Y, Mizuno T, Diez J, Naito S, Ahlquist P, Ishikawa M. 2003. Mutation of host DnaJ homolog inhibits brome mosaic virus negative-strand RNA synthesis. J. Virol. 77:2990–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pathak KB, Sasvari Z, Nagy PD. 2008. The host Pex19p plays a role in peroxisomal localization of tombusvirus replication proteins. Virology 379:294–305 [DOI] [PubMed] [Google Scholar]
- 19. Wang RY, Stork J, Nagy PD. 2009. A key role for heat shock protein 70 in the localization and insertion of tombusvirus replication proteins to intracellular membranes. J. Virol. 83:3276–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang RY, Stork J, Pogany J, Nagy PD. 2009. A temperature sensitive mutant of heat shock protein 70 reveals an essential role during the early steps of tombusvirus replication. Virology 394:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mine A, Hyodo K, Tajima Y, Kusumanegara K, Taniguchi T, Kaido M, Mise K, Taniguchi H, Okuno T. 2012. Differential roles of Hsp70 and Hsp90 in the assembly of the replicase complex of a positive-strand RNA plant virus. J. Virol. 86:12091–12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barajas D, Jiang Y, Nagy PD. 2009. A unique role for the host ESCRT proteins in replication of Tomato bushy stunt virus. PLoS Pathog. 5:e1000705 doi: 10.1371/journal.ppat.1000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diaz A, Wang X, Ahlquist P. 2010. Membrane-shaping host reticulon proteins play crucial roles in viral RNA replication compartment formation and function. Proc. Natl. Acad. Sci. U. S. A. 107:16291–16296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neuvonen M, Kazlauskas A, Martikainen M, Hinkkanen A, Ahola T, Saksela K. 2011. SH3 domain-mediated recruitment of host cell amphiphysins by alphavirus nsP3 promotes viral RNA replication. PLoS Pathog. 7:e1002383 doi: 10.1371/journal.ppat.1002383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verchot J. 2012. Cellular chaperones and folding enzymes are vital contributors to membrane bound replication and movement complexes during plant RNA virus infection. Front. Plant Sci. 3:275 doi: 10.3389/fpls.2012.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. dos Reis Figueira A, Golem S, Goregaoker SP, Culver JN. 2002. A nuclear localization signal and a membrane association domain contribute to the cellular localization of the Tobacco mosaic virus 126-kDa replicase protein. Virology 301:81–89 [DOI] [PubMed] [Google Scholar]
- 27. Liu JZ, Blancaflor EB, Nelson RS. 2005. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 138:1853–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Kelman Z, Culver JN. 2010. Helicase ATPase activity of the Tobacco mosaic virus 126-kDa protein modulates replicase complex assembly. Virology 402:292–302 [DOI] [PubMed] [Google Scholar]
- 29. Yamanaka T, Ohta T, Takahashi M, Meshi T, Schmidt R, Dean C, Naito S, Ishikawa M. 2000. TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc. Natl. Acad. Sci. U. S. A. 97:10107–10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsujimoto Y, Numaga T, Ohshima K, Yano MA, Ohsawa R, Goto DB, Naito S, Ishikawa M. 2003. Arabidopsis Tobamovirus multiplication (TOM) 2 locus encodes a transmembrane protein that interacts with TOM1. EMBO J. 22:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohshima K, Taniyama T, Yamanaka T, Ishikawa M, Naito S. 1998. Isolation of a mutant of Arabidopsis thaliana carrying two simultaneous mutations affecting tobacco mosaic virus multiplication within a single cell. Virology 243:472–481 [DOI] [PubMed] [Google Scholar]
- 32. Reichel C, Beachy RN. 1998. Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 95:11169–11174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moshe A, Gorovits R. 2012. Virus-induced aggregates in infected cells. Viruses 4:2218–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pereda S, Ehrenfeld N, Medina C, Delgado J, Arce-Johnson P. 2000. Comparative analysis of TMV-Cg and TMV-U1 detection methods in infected Arabidopsis thaliana. J. Virol. Methods 90:135–142 [DOI] [PubMed] [Google Scholar]
- 35. Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J. 1994. Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6:1567–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herman EM. 2008. Endoplasmic reticulum bodies: solving the insoluble. Curr. Opin. Plant Biol. 11:672–679 [DOI] [PubMed] [Google Scholar]
- 37. Vitale A, Hinz G. 2005. Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci. 10:316–323 [DOI] [PubMed] [Google Scholar]
- 38. Muntz K. 1998. Deposition of storage proteins. Plant Mol. Biol. 38:77–99 [PubMed] [Google Scholar]
- 39. Heinlein M, Padgett HS, Gens JS, Pickard BG, Casper SJ, Epel BL, Beachy RN. 1998. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 10:1107–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shivprasad S, Pogue GP, Lewandowski DJ, Hidalgo J, Donson J, Grill LK, Dawson WO. 1999. Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vectors. Virology 255:312–323 [DOI] [PubMed] [Google Scholar]
- 41. Karimi M, Inze D, Depicker A. 2002. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7:193–195 [DOI] [PubMed] [Google Scholar]
- 42. Shi BJ, Ding SW, Symons RH. 1997. Plasmid vector for cloning infectious cDNAs from plant RNA viruses: high infectivity of cDNA clones of tomato aspermy cucumovirus. J. Gen. Virol. 78(Pt 5):1181–1185 [DOI] [PubMed] [Google Scholar]
- 43. Nelson BK, Cai X, Nebenfuhr A. 2007. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51:1126–1136 [DOI] [PubMed] [Google Scholar]
- 44. He ZH, Cheeseman I, He D, Kohorn BD. 1999. A cluster of five cell wall-associated receptor kinase genes, Wak1-5, are expressed in specific organs of Arabidopsis. Plant Mol. Biol. 39:1189–1196 [DOI] [PubMed] [Google Scholar]
- 45. Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16:735–743 [DOI] [PubMed] [Google Scholar]
- 46. Yeh HH, Tian T, Rubio L, Crawford B, Falk BW. 2000. Asynchronous accumulation of lettuce infectious yellows virus RNAs 1 and 2 and identification of an RNA 1 trans enhancer of RNA 2 accumulation. J. Virol. 74:5762–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rubio L, Yeh HH, Tian T, Falk BW. 2000. A heterogeneous population of defective RNAs is associated with lettuce infectious yellows virus. Virology 271:205–212 [DOI] [PubMed] [Google Scholar]
- 48. Klaassen VA, Mayhew D, Fisher D, Falk BW. 1996. In vitro transcripts from cloned cDNAs of the lettuce infectious yellows closterovirus bipartite genomic RNAs are competent for replication in Nicotiana benthamiana protoplasts. Virology 222:169–175 [DOI] [PubMed] [Google Scholar]
- 49. Yoo SD, Cho YH, Sheen J. 2007. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2:1565–1572 [DOI] [PubMed] [Google Scholar]
- 50. Chang S, Puryear J, Cairney J. 1993. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11:113–116 [Google Scholar]
- 51. An CI, Sawada A, Kawaguchi Y, Fukusaki E, Kobayashi A. 2005. Transient RNAi induction against endogenous genes in Arabidopsis protoplasts using in vitro-prepared double-stranded RNA. Biosci. Biotechnol. Biochem. 69:415–418 [DOI] [PubMed] [Google Scholar]
- 52. Wroblewski T, Tomczak A, Michelmore R. 2005. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol. J. 3:259–273 [DOI] [PubMed] [Google Scholar]
- 53. Babu M, Griffiths JS, Huang TS, Wang A. 2008. Altered gene expression changes in Arabidopsis leaf tissues and protoplasts in response to Plum pox virus infection. BMC Genomics 9:325 doi: 10.1186/1471-2164-9-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Widholm JM. 1972. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 47:189–194 [DOI] [PubMed] [Google Scholar]
- 55. Chow LT, Gelinas RE, Broker TR, Roberts RJ. 1977. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell 12:1–8 [DOI] [PubMed] [Google Scholar]
- 56. Berget SM, Moore C, Sharp PA. 1977. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc. Natl. Acad. Sci. U. S. A. 74:3171–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hershey AD, Chase M. 1952. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J. Gen. Physiol. 36:39–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gierer A, Schramm G. 1956. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature 177:702–703 [DOI] [PubMed] [Google Scholar]
- 59. Brenner S, Jacob F, Meselson M. 1961. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature 190:576–581 [DOI] [PubMed] [Google Scholar]
- 60. Bombarely A, Menda N, Tecle IY, Buels RM, Strickler S, Fischer-York T, Pujar A, Leto J, Gosselin J, Mueller LA. 2011. The Sol Genomics Network (solgenomics.net): growing tomatoes using Perl. Nucleic Acids Res. 39:D1149–D1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dunwell JM, Khuri S, Gane PJ. 2000. Microbial relatives of the seed storage proteins of higher plants: conservation of structure and diversification of function during evolution of the cupin superfamily. Microbiol. Mol. Biol. Rev. 64:153–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dunwell JM, Purvis A, Khuri S. 2004. Cupins: the most functionally diverse protein superfamily? Phytochemistry 65:7–17 [DOI] [PubMed] [Google Scholar]
- 63. Chibani K, Ali-Rachedi S, Job C, Job D, Jullien M, Grappin P. 2006. Proteomic analysis of seed dormancy in Arabidopsis. Plant Physiol. 142:1493–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Irshad M, Canut H, Borderies G, Pont-Lezica R, Jamet E. 2008. A new picture of cell wall protein dynamics in elongating cells of Arabidopsis thaliana: confirmed actors and newcomers. BMC Plant Biol. 8:94 doi: 10.1186/1471-2229-8-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rozwadowski K, Yang W, Kagale S. 2008. Homologous recombination-mediated cloning and manipulation of genomic DNA regions using Gateway and recombineering systems. BMC Biotechnol. 8:88 doi: 10.1186/1472-6750-8-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rajjou L, Gallardo K, Debeaujon I, Vandekerckhove J, Job C, Job D. 2004. The effect of alpha-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNAs during germination. Plant Physiol. 134:1598–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Osakabe Y, Maruyama K, Seki M, Satou M, Shinozaki K, Yamaguchi-Shinozaki K. 2005. Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. Plant Cell 17:1105–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hu W, Ma H. 2006. Characterization of a novel putative zinc finger gene MIF1: involvement in multiple hormonal regulation of Arabidopsis development. Plant J. 45:399–422 [DOI] [PubMed] [Google Scholar]
- 69. Nishikiori M, Dohi K, Mori M, Meshi T, Naito S, Ishikawa M. 2006. Membrane-bound tomato mosaic virus replication proteins participate in RNA synthesis and are associated with host proteins in a pattern distinct from those that are not membrane bound. J. Virol. 80:8459–8468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nishikiori M, Mori M, Dohi K, Okamura H, Katoh E, Naito S, Meshi T, Ishikawa M. 2011. A host small GTP-binding protein ARL8 plays crucial roles in tobamovirus RNA replication. PLoS Pathog. 7:e1002409 doi: 10.1371/journal.ppat.1002409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yamaji Y, Kobayashi T, Hamada K, Sakurai K, Yoshii A, Suzuki M, Namba S, Hibi T. 2006. In vivo interaction between Tobacco mosaic virus RNA-dependent RNA polymerase and host translation elongation factor 1A. Virology 347:100–108 [DOI] [PubMed] [Google Scholar]
- 72. Patarroyo C, Laliberte JF, Zheng H. 2012. Hijack it, change it: how do plant viruses utilize the host secretory pathway for efficient viral replication and spread? Front. Plant Sci. 3:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kramer SR, Goregaoker SP, Culver JN. 2011. Association of the Tobacco mosaic virus 126 kDa replication protein with a GDI protein affects host susceptibility. Virology 414:110–118 [DOI] [PubMed] [Google Scholar]
- 74. Padmanabhan MS, Goregaoker SP, Golem S, Shiferaw H, Culver JN. 2005. Interaction of the tobacco mosaic virus replicase protein with the Aux/IAA protein PAP1/IAA26 is associated with disease development. J. Virol. 79:2549–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Padmanabhan MS, Shiferaw H, Culver JN. 2006. The Tobacco mosaic virus replicase protein disrupts the localization and function of interacting Aux/IAA proteins. Mol. Plant Microbe Interact. 19:864–873 [DOI] [PubMed] [Google Scholar]
- 76. Padmanabhan MS, Kramer SR, Wang X, Culver JN. 2008. Tobacco mosaic virus replicase-auxin/indole acetic acid protein interactions: reprogramming the auxin response pathway to enhance virus infection. J. Virol. 82:2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang X, Goregaoker SP, Culver JN. 2009. Interaction of the Tobacco mosaic virus replicase protein with a NAC domain transcription factor is associated with the suppression of systemic host defenses. J. Virol. 83:9720–9730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang X, Culver JN. 2012. DNA binding specificity of ATAF2, a NAC domain transcription factor targeted for degradation by Tobacco mosaic virus. BMC Plant Biol. 12:157 doi: 10.1186/1471-2229-12-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yoshii A, Shimizu T, Yoshida A, Hamada K, Sakurai K, Yamaji Y, Suzuki M, Namba S, Hibi T. 2008. NTH201, a novel class II KNOTTED1-like protein, facilitates the cell-to-cell movement of Tobacco mosaic virus in tobacco. Mol. Plant Microbe Interact. 21:586–596 [DOI] [PubMed] [Google Scholar]
- 80. Bhat S, Folimonova SY, Cole AB, Ballard KD, Lei Z, Watson BS, Sumner LW, Nelson RS. 2013. Influence of host chloroplast proteins on Tobacco mosaic virus accumulation and intercellular movement. Plant Physiol. 161:134–147 [DOI] [PMC free article] [PubMed] [Google Scholar]