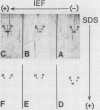
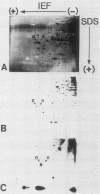
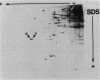
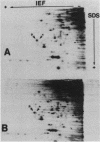
Abstract
The phosphorylation state of the alpha subunit of initiation factor 2 (eIF-2 alpha) in Saccharomyces cerevisiae has been determined by two-dimensional gel electrophoresis and autoradiography of lysates from cultures grown under a variety of conditions. The alpha subunit was maintained in a phosphorylated state during logarithmic growth on fermentable and nonfermentable carbon sources, during starvation for an essential amino acid, during heat shock, during stationary phase, and during sporulation. Only when cells were starved for a carbon source for 2 h in 1 M sorbitol was eIF-2 alpha isolated in the nonphosphorylated state. This is in contrast with the studies in rabbit reticulocyte lysates, in which arrested protein synthesis was correlated with a relative increase in the extent of phosphorylation of eIF-2 alpha.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., van Agthoven A., Pluijms W., Möller W. A comparison of the amino-terminal sequence of the L7/L12-type proteins of Artemia salina and Saccharomyces cerevisiae. FEBS Lett. 1977 Sep 15;81(2):308–310. doi: 10.1016/0014-5793(77)80541-3. [DOI] [PubMed] [Google Scholar]
- Baan R. A., Keller P. B., Dahlberg A. E. Isolation of eukaryotic initiation factor 2 from yeast Saccharomyces cerevisiae. J Biol Chem. 1981 Feb 10;256(3):1063–1066. [PubMed] [Google Scholar]
- Benne R., Edman J., Traut R. R., Hershey J. W. Phosphorylation of eukaryotic protein synthesis initiation factors. Proc Natl Acad Sci U S A. 1978 Jan;75(1):108–112. doi: 10.1073/pnas.75.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Hershey J. W. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978 May 10;253(9):3078–3087. [PubMed] [Google Scholar]
- Boucherie H. Protein synthesis during transition and stationary phases under glucose limitation in Saccharomyces cerevisiae. J Bacteriol. 1985 Jan;161(1):385–392. doi: 10.1128/jb.161.1.385-392.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Pain V. M., Wong S. T., Henshaw E. C. Phosphorylation inhibits guanine nucleotide exchange on eukaryotic initiation factor 2. Nature. 1982 Mar 4;296(5852):93–95. doi: 10.1038/296093a0. [DOI] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Heat shock-induced translational alterations in HeLa cells. Initiation factor modifications and the inhibition of translation. J Biol Chem. 1984 Oct 10;259(19):11882–11889. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Covalent modification. J Biol Chem. 1985 May 10;260(9):5493–5497. [PubMed] [Google Scholar]
- Eschenbruch M., Bürk R. R. Experimentally improved reliability of ultrasensitive silver staining of protein in polyacrylamide gels. Anal Biochem. 1982 Sep 1;125(1):96–99. doi: 10.1016/0003-2697(82)90387-6. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Issinger O. G., Benne R., Hershey J. W., Traut R. R. Phosphorylation in vitro of eukaryotic initiation factors IF-E2 and IF-E3 by protein kinases. J Biol Chem. 1976 Oct 25;251(20):6471–6474. [PubMed] [Google Scholar]
- Jagus R., Anderson W. F., Safer B. The regulation of initiation of mammalian protein synthesis. Prog Nucleic Acid Res Mol Biol. 1981;25:127–185. doi: 10.1016/s0079-6603(08)60484-5. [DOI] [PubMed] [Google Scholar]
- Jagus R., Crouch D., Konieczny A., Safer B. The role of phosphorylation in the regulation of eukaryotic initiation factor 2 activity. Curr Top Cell Regul. 1982;21:35–63. doi: 10.1016/b978-0-12-152821-8.50006-2. [DOI] [PubMed] [Google Scholar]
- Kraig E., Haber J. E. Messenger ribonucleic acid and protein metabolism during sporulation of Saccharomyces cerevisiae. J Bacteriol. 1980 Dec;144(3):1098–1112. doi: 10.1128/jb.144.3.1098-1112.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G., Hardesty B. Phosphorylation reactions that influence the activity of elF-2. Curr Top Cell Regul. 1981;20:185–203. doi: 10.1016/b978-0-12-152820-1.50009-2. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Lindquist S. Changing patterns of gene expression during sporulation in yeast. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7323–7327. doi: 10.1073/pnas.81.23.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein initiation in eukaryotes: formation and function of a ternary complex composed of a partially purified ribosomal factor, methionyl transfer RNA, and guanosine triphosphate. Proc Natl Acad Sci U S A. 1973 Jan;70(1):41–45. doi: 10.1073/pnas.70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ochoa S. Regulation of protein synthesis initiation in eucaryotes. Arch Biochem Biophys. 1983 Jun;223(2):325–349. doi: 10.1016/0003-9861(83)90598-2. [DOI] [PubMed] [Google Scholar]
- Safer B. 2B or not 2B: regulation of the catalytic utilization of eIF-2. Cell. 1983 May;33(1):7–8. doi: 10.1016/0092-8674(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Duncan R., Knutson G. S., Hershey J. W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984 Nov 10;259(21):13451–13457. [PubMed] [Google Scholar]
- Shulman R. W., Sripati C. E., Warner J. R. Noncoordinated transcription in the absence of protein synthesis in yeast. J Biol Chem. 1977 Feb 25;252(4):1344–1349. [PubMed] [Google Scholar]
- Sánchez-Madrid F., Reyes R., Conde P., Ballesta J. P. Acidic ribosomal proteins from eukaryotic cells. Effect on ribosomal functions. Eur J Biochem. 1979 Aug 1;98(2):409–416. doi: 10.1111/j.1432-1033.1979.tb13200.x. [DOI] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977 Nov;116(4):755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]