Abstract
Interleukin-22 (IL-22) has redundant, protective, or pathogenic functions during autoimmune, inflammatory, and infectious diseases. Here, we addressed the potential role of IL-22 in host defense and pathogenesis during lethal and sublethal respiratory H3N2 influenza A virus (IAV) infection. We show that IL-22, as well as factors associated with its production, are expressed in the lung tissue during the early phases of IAV infection. Our data indicate that retinoic acid receptor-related orphan receptor-γt (RORγt)-positive αβ and γδ T cells, as well as innate lymphoid cells, expressed enhanced Il22 transcripts as early as 2 days postinfection. During lethal or sublethal IAV infections, endogenous IL-22 played no role in the control of IAV replication and in the development of the IAV-specific CD8+ T cell response. During lethal infection, where wild-type (WT) mice succumbed to severe pneumonia, the lack of IL-22 did not accelerate or delay IAV-associated pathogenesis and animal death. In stark contrast, during sublethal IAV infection, IL-22-deficient animals had enhanced lung injuries and showed a lower airway epithelial integrity relative to WT littermates. Of importance, the protective effect of endogenous IL-22 in pulmonary damages was associated with a more controlled secondary bacterial infection. Indeed, after challenge with Streptococcus pneumoniae, IAV-experienced Il22−/− animals were more susceptible than WT controls in terms of survival rate and bacterial burden in the lungs. Together, IL-22 plays no major role during lethal influenza but is beneficial during sublethal H3N2 IAV infection, where it limits lung inflammation and subsequent bacterial superinfections.
INTRODUCTION
Interleukin-22 (IL-22) plays a dual role in autoimmune and inflammatory diseases (for reviews, see references 1 and 2). It is produced by conventional lymphocytes and by lymphoid cells displaying “innate-like” functions (3–11). Among the later group are certain subsets of retinoic acid receptor-related orphan receptor-γt (RORγt)-positive αβ and γδ T lymphocytes and innate lymphoid cells (termed as ILC3) (4, 7–12). IL-22 solely acts on nonhematopoietic cells, including hepatocytes and epithelial cells, to exert both proinflammatory and tissue-protective properties depending on the context and the tissue in which it is expressed (2, 13–16). In experimental noninfectious systems, IL-22 exerts a potent protective effect on hepatocytes and epithelial cells at barrier surfaces, particularly in the intestine and in the thymus (17–21). IL-22 is also a key factor controlling mucosal immunity and the dissemination of commensal bacteria from the intestinal tract (22). In the lungs, IL-22 protects against experimental lung fibrosis (23) and ventilator-induced lung injury (24). IL-22 also limits Th2-mediated airway inflammation and tissue damage during asthma (25–27). On the other hand, uncontrolled IL-22 production favors dermal inflammation and acanthosis, bleomycin-induced airway inflammation, collagen-induced arthritis, and lipopolysaccharide shock, in part by enhancing tissue inflammation in concert with inflammatory factors such as tumor necrosis factor alpha (TNF-α) and IL-17 (4, 28–31).
During infection, IL-22 production by innate cells or effector conventional T cells plays a dual role depending on the pathogen and the tissue. The early production of IL-22 by innate immune cells is crucial for host protective immunity to extracellular bacteria, including Klebsiella pneumoniae in the lung and Citrobacter rodentium in the intestine (32, 33). On the other hand, IL-22 has no substantial role in host defense against Staphylococcus aureus, Mycobacterium tuberculosis, M. avium, Listeria monocytogenes, Candida albicans, or Schistosoma mansoni (34–37). Of importance, IL-22 can provide protective innate immunity when the adaptive immune system is impaired. This redundant function has been described during infection with C. albicans and Eimeria falciformis (38–40). Finally, a deleterious role for IL-22 on intestinal inflammation was reported after oral infection with Toxoplasma gondii (37, 41).
The potential role of IL-22 during viral infection has recently been addressed. IL-22 might participate in resistance to human immunodeficiency virus infection in subjects who do not seroconvert despite multiple exposures to the virus (42, 43). Recent data also indicated that reduced IL-22 production in the gut mucosa is important in human immunodeficiency virus mucosal immunopathogenesis (44). On the other hand, although hepatic IL-22 expression is upregulated in viral hepatitis, IL-22 lacks direct antiviral activity against hepatitis B and C viruses (45, 46). Of interest, IL-22 aggravates tissue inflammation during experimental hepatitis B virus infection (47) and West Nile virus encephalitis (48). The potential role of IL-22 in antiviral host defense and virus-associated inflammation has recently been studied during experimental influenza A virus (IAV) infection. Using neutralizing antibodies (Abs), Guo et al. (49) initially showed that IL-22 has little role during acute H1N1 IAV infection, as assessed by IAV-associated morbidity and mortality. In parallel, Monticelli et al. recently reported that during mild, resolving H1N1 IAV infection, neutralization of IL-22 had no impact on the morbidity, on the decreased lung function and on respiratory tissue remodeling (50). In contrast, based on in vitro assays and using IL-22-deficient mice, we suggested that during the early phase of H3N2 IAV infection (day 4), IL-22 protects bronchial epithelial cells against damages caused by IAV (51). In this report, invariant natural killer T (iNKT) cells were characterized as cellular sources of IL-22. In line with our observation, IL-22 was next reported to participate in airway epithelial regeneration during the resolution phase of H1N1 IAV infection (days 7 to 18) (52). Surprisingly, enough, in this later report, RORγt-negative conventional NK cells were shown to be the main producers of IL-22 (52). The aims of the present study were to characterize the cellular sources of IL-22 during the early phase of H3N2 IAV infection and to study its potential role on the development of viral pathogenesis and host responses during infection. To this end, an acute lethal model of IAV infection, where animals succumbed to pneumonia, and a model of infection where animals ultimately resolved inflammation, were used. Since bacterial superinfections can occur after primary IAV infection, a phenomenon accounting for a high rate of morbidity and mortality worldwide (53–60), we also examined the role of endogenous IL-22 in secondary bacterial infection following influenza virus infection. We show that IL-22 is produced by several subsets of lung RORγt-positive, but not RORγt-negative (including NK cells), cells early after infection. In both lethal and sublethal conditions, IL-22 plays no role in the control of virus replication and in the promotion of the virus-specific CD8+ T cell response. Of interest, during sublethal influenza, but not lethal influenza, IL-22 plays a protective function against respiratory tissue damages caused by IAV, a phenomenon associated with a higher resistance to secondary bacterial infections.
MATERIALS AND METHODS
Virus, bacteria, and mice.
The highly pathogenic human-origin H3N2 IAV strain Scotland/20/74 and S. pneumoniae serotype 1 clinical isolate E1586 sequence type ST304 have been described (61–64). Interleukin-22−/− mice, backcrossed at least 10 times in C57BL/6 (65), as well as WT littermate controls, were bred in the Ludwig Institute (Brussels, Belgium). RORγt- green fluorescent protein (GFP) mice were described earlier (66, 67). Mice (8- to 10-week-old male) were maintained in a biosafety level 2 facility in the Animal Resource Center at the Pasteur Institute, Lille, France. All animal work conformed to Pasteur Institute, Lille, Animal Care and Use Committee guidelines (agreement AF 16/20090 from the Comité d'Ethique en Expérimentation Animale Nord Pas-De-Calais).
Abs and reagents.
Monoclonal Abs against mouse TCRβ (allophycocyanin [APC] and V450 conjugated), TCRγδ (PerCP-Cy5.5 conjugated), CD45 (eFluor605NC, Pacific Blue, or APC-H7 conjugated), NKp46 (phycoerythrin [PE] conjugated), CD127 (PE-Cy7 conjugated), CD90.2 (Alexa Fluor 700 conjugated), streptavidin (Alexa Fluor 700 or PE conjugated), and CD4 (APC-H7-conjugated) were purchased from BD Biosciences (Le Pont de Claix, France). The biotin mouse lineage panel was from BD Biosciences. APC-conjugated and PE-conjugated phosphate-buffered saline (PBS)-57-loaded CD1d tetramers were, respectively, obtained from ProImmune (Oxford, United Kingdom) and the NIAID Tetramer Facility (Emory University, Atlanta, GA). The monoclonal Ab against mouse IL-22 (clone 3F11) was kindly provided by W. Ouyang (Genentech, San Francisco, CA). Recombinant IL-22 was produced as described previously (68).
IAV infection and assessment of gene expression by quantitative RT-PCR.
Mice were anesthetized and administered intranasally (i.n.) with 50 μl of PBS containing different dose (50 or 600 PFU) of virus (Scotland/20/74, H3N2). Total RNA from whole lungs or from cells recovered from the bronchoalveolar lavages (BAL) of mock-treated or IAV-infected mice was extracted and cDNA was synthesized by classical procedures. Quantitative reverse transcription-PCR (RT-PCR) was carried out as described previously (51). Primers specific for the gapdh, Ifng, Il17a, mx1, Ifnb, Il22, and IAV M2 genes have been described (51). In addition, the following primers were used: Il22bp, 5′-ACTCTGCCTGGACCAGGACA-3′ and 5′-GAGAAGCACCCGCAAAGATG; Il23p19, 5′-AATCTCTGCATGCTAGCCTGG-3′ and 5′-GATTCATATGTCCCGCTGGTG-3′; Il1b, 5′-TCCCCAACTGGTACATCAGCA-3′ and 5′-ACACGGATTCCATGGTGAAGTC-3′; Rorgt, 5′-TGAAAGCAGGAGCAATGGAAGT-3′ and 5′-ACAGCTCCACACCACCGTATTT-3′; Ahr, 5′-ATCGACATAACGGACGAAATCC-3′ and 5′-TTAGGTGCTGAGTCACAGGCTG-3′; Il6, 5′-AGCCTCCGACTTGTGAAGTG-3′ and 5′-CTGATGCTGGTGACAACCAC-3′; Il17f, 5′-TGTCCTCCCCTGGAGGATAAC-3′ and 5′-GAACTGGAGCGGTTCTGGAA-3′; Il21, 5′-AAACTCAAGCCATCAAACCCTG-3′ and 5′-TGTTTCTTTCCTCCCCTCCTG-3′; and Tnfa, 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ and 5′-TGGGAGTAGACAAGGTACAACCC-3′. ΔCT values were obtained by deducting the raw cycle threshold (CT values) obtained for gapdh mRNA, the internal standard, from the CT values obtained for investigated genes.
Analysis of IL-22 transcript levels in RORγt-positive cells during IAV infection.
RORγt-GFP mice were infected, or not, with IAV, and lung MNCs were prepared 60 h postinfection (p.i.). RORγt-positive αβ T lymphocytes (CD45+ TCRβ+), γδ T lymphocytes (CD45+ TCRγδ+), and TCRαβ− TCRγδ− (CD45+ TCRβ− TCRγδ−) cells were sorted from naive and IAV-infected mice. The expression of IL-22 transcript was performed by quantitative RT-PCR.
Analysis of IL-22-producing cells.
Analysis of IL-22-producing cells was assessed 2 days post-IAV infection. As a possible control, lung MNCs were cultured at 107 cells/ml in complete medium containing 10 ng of recombinant mouse IL-1β/ml and IL-23 plus 10 μg of brefeldin A (Sigma-Aldrich, Steinheim, Germany)/ml at 37°C for 4 h. After activation, cells were washed and stained with Live/Dead fixable dead cell stain kit (Life Technologies, Carlsbad, CA) in PBS for 30 min. The cells were washed and incubated with appropriate dilutions of eFluor605NC-conjugated CD45, PE-conjugated PBS-57-loaded CD1d tetramer, V450-conjugated anti-TCRβ Ab, and PerCP-Cy5.5-conjugated anti-TCRγδ Ab for 30 min in PBS containing 2% fetal calf serum (FCS). Cells were washed and fixed using IC fixation buffer (eBioscience, CliniSciences, Montrouge, France). Fixed cells were then permeabilized in permeabilization buffer (eBioscience), according to the manufacturer's instructions. Cells were stained with APC-conjugated MAb against IL-22 or control mouse IgG2a MAb and analyzed on a LSR Fortessa (BD Biosciences). To analyze the proportions of innate lymphoid cells (ILCs) in RORγt-positive TCRαβ− TCRγδ− cells, lung MNCs from RORγt-GFP mice were labeled with appropriate dilutions of APC-H7-conjugated anti-CD45, Percp-Cy5.5-conjugated anti-TCRγδ, V450-conjugated anti-TCRβ, a biotin lineage-specific Ab cocktail (TER119, CD11b, Gr1, B220, CD3, CD11c, and NK1.1) plus a PE-conjugated streptavidin, PE-Cy7-conjugated anti-CD127, and Alexa Fluor 700-conjugated CD90.2. Cells were analyzed on a LSR Fortessa.
Assessment of the mortality rate and of the pathology.
After IAV infection (600 or 50 PFU), mice were monitored daily for illness and mortality for a period of 17 days. Disease was assessed by measuring lung inflammation, viral load in the lungs, and lethality. Mice found to be moribund were euthanized and considered to have died on that day. Mice were also sacrificed at day 7 p.i. to recover the whole lung and the BALs. For histopathologic examination, lungs were fixed by inflation and immersion in PBS–3.2% paraformaldehyde and then embedded in paraffin. To evaluate airway inflammation, we subjected fixed lung slices (5-μm sections) to hematoxylin and eosin (H&E) staining. Evaluators who were blinded to genotype scored lung sections (0 [none] to 3 [extreme]) according to criteria described by Paget et al. (69).
Analysis of the viral load and of the IAV-specific CD8+ T cell response.
Lungs were homogenized and virus titers determined using a standard plaque assay on Mardin-Darby canine kidney cells. Ifnb and mx1 mRNA expression levels were determined by quantitative RT-PCR as described previously (69). The number of IAV-specific CD8+ T cells was determined as reported (69). For this, cells specific for an immunodominant Db-restricted CD8+ T epitope derived from the viral polymerase 2 protein (PA224-233) (70) were analyzed. Briefly, lung MNCs were incubated with appropriate dilutions of APC-conjugated anti-CD19, fluorescein isothiocyanate-labeled anti-CD8 and PE-conjugated Pro5 major histocompatibility complex pentamer H-2Db SSLENFRAYV. To assess the functionality of virus-specific CD8+ T cells, lung cells and cells isolated from the lung draining (mediastinal) lymph nodes (dLNs) were stimulated with the peptide SSLENFRAYV (10 μg/ml), and cytokine production was assessed by enzyme-linked immunosorbent assay (ELISA).
Secondary infection with S. pneumoniae.
Mice, infected or not with IAV (50 PFU) 7 days earlier, were i.n. inoculated with 104 S. pneumoniae serotype 1. In naive animals, this dose is self-limiting since mice cleared the bacteria within 24 h. In some circumstances, Il22−/− mice received by the i.n. route recombinant IL-22 (5 μg/mouse) or vehicle (PBS) just before IAV infection and 2 days p.i. Mice were monitored daily for illness and mortality for a period of 13 days. The number of viable bacteria in the lungs was determined 24 h after S. pneumoniae challenge. This was measured by plating lung homogenates onto blood agar plates (71). CFU were enumerated 24 h later. A morphology-based differential cell count was conducted on cytospin preparations from the BAL fluid and stained with Diff-Quik solution (Sigma).
Statistical analysis.
Results are expressed as the means ± the standard deviations (SD) or ± the standard errors of the mean (SEM). The statistical significance of differences between experimental groups was calculated by a one-way analysis of variance, followed by a Bonferroni posttest (Prism 4 Software; GraphPad, San Diego, CA). The possibility to use these parametric tests was assessed by checking whether the population is Gaussian and the variance is equal (Bartlett's test). Results with a P value of <0.05 were considered significant (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
RESULTS
IL-22 is produced in the lungs during the early stages of IAV infection.
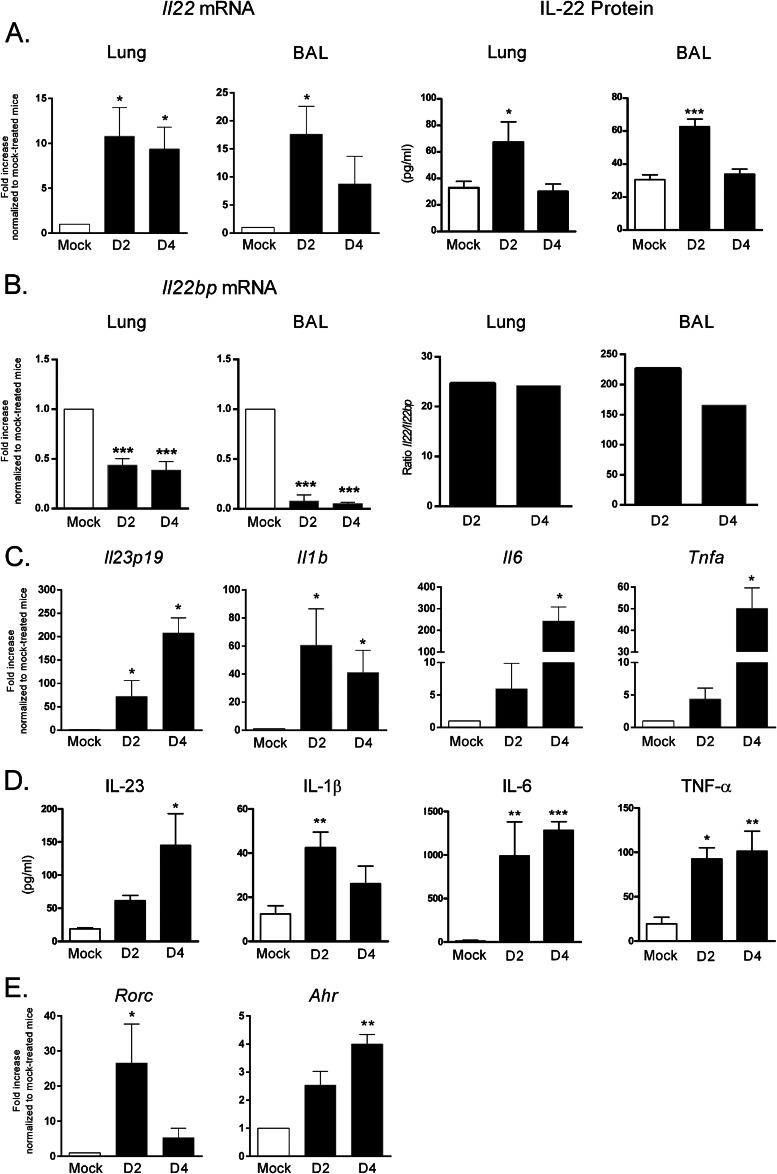
The kinetics of IL-22 expression during the early phases of IAV infection are ill-defined, although we and others have shown that natural killer (NK) cells and invariant natural killer (iNKT) cells are primary sources of IL-22 2 days after H1N1 or H3N2 IAV infection, respectively (49, 51). As shown in Fig. 1A (left panels), and relative to mock-treated animals, a higher level of Il22 gene transcript was detected in the lung tissue and alveolar spaces of IAV-infected mice 2 and 4 days p.i. (∼10-fold enhancement). An enhanced concentration of IL-22 protein was also detected 2 days, but not 4 days, p.i. (Fig. 1A, right panels), a phenomenon probably due to its rapid consumption. IL-22 activity can be neutralized in vivo by its specific opponent IL-22 binding protein (IL-22BP) (72). As seen in Fig. 1B, Il22bp messengers were slightly downregulated (∼2-fold) at day 2 and 4 p.i. in the lung tissue and the decrease was more important (∼20-fold) in the BALs. The ratio of Il22 to Il22bp was ∼10-fold higher in the BALs compared to the lung tissue and did not significantly differ at days 2 and 4 p.i. Il17a and IL17f transcripts were also found to be upregulated 2 and 4 days p.i. in the BAL cells, but not in pulmonary cells, whereas Il21, another member of the IL-17 family, was not modulated (not shown). Augmented IL-17A protein was also detected at day 2 p.i., but only in the BAL fluids (not shown).
Fig 1.
IL-22 production in the lungs during the early steps of IAV infection. C57BL/6 WT animals were infected with 600 PFU of IAV Scotland/20/74/H3N2 strain. The lungs and the BAL fluids were collected 2 and 4 days p.i. (A) Il22 mRNA copy numbers were determined by quantitative RT-PCR. The data are normalized to the expression of gapdh and are expressed as the fold increased over the average gene expression in mock-treated mice (left panels). IL-22 protein production was measured by ELISA (right panels). (B) Il22bp mRNA levels in the lungs and BAL fluids were measured (left panels). The ratio of Il22 to Il22bp are depicted (right panels). (C) Il23p19, Il1b, Il6, and Tnfa mRNA levels in the BAL fluids were measured by quantitative RT-PCR. (D) IL-23, IL-1β, IL-6, and TNF-α levels were measured in the BAL fluids by ELISA. (E) RORγt and aryl hydrocarbon receptor (AhR) mRNA levels in the BAL fluids were measured by quantitative RT-PCR. The values in panels A to E represent the means ± the SEM of four independent experiments. For quantitative RT-PCR of BAL samples, BAL fluids were pooled (5 mice/group). For quantitative RT-PCR of lung samples and for ELISA (BALs and lungs), n = 12 to 15. Significant differences were determined using a one-way analysis of variance, followed by a Bonferroni post test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
IL-23, IL-1β, IL-6, and TNF-α have been described to participate in early IL-22 production in some settings (for reviews, see references 2, 8, and 9). As seen in Fig. 1C, Il23, Il1b, Il6, and Tnfa gene transcripts were strongly upregulated in the BAL cells and in the lungs (data not shown) by 2 and 4 days p.i. Enhanced IL-23, IL-1β, IL-6, and TNF-α protein levels were also evident at these time points (Fig. 1D). Of note, RORγt, and to a lesser extent, aryl hydrocarbon receptor, transcription factors known to be crucial in IL-22 synthesis (2, 8, 9), were also upregulated at the transcript level (Fig. 1E). Collectively, IL-22, as well as factors known to regulate its expression, are produced in the lungs during the early stages of H3N2 IAV infection.
RORγt-expressing cells produce enhanced IL-22 transcripts early after IAV infection.
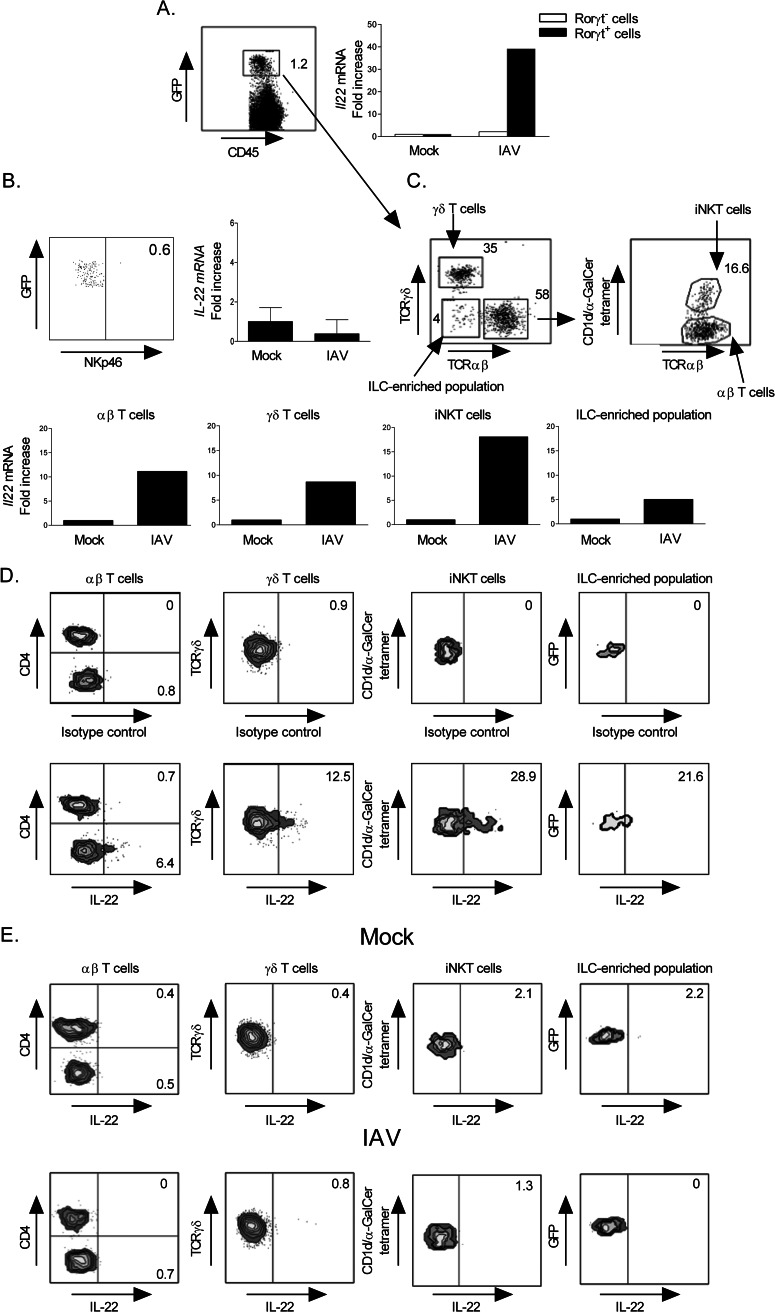
We took advantage of RORγt-GFP mice to analyze the early source(s) of IL-22 during IAV infection. As seen in Fig. 2A, RORγt-positive cells purified from IAV-infected mice express an enhanced level of Il22 gene transcripts compared to RORγt-positive cells isolated from noninfected animals. In contrast, influenza virus infection did not trigger Il22 messenger expression in RORγt negative cells. Of note, NKp46+ cells did not express RORγt in the lung tissue and failed to produce Il22 messenger in response to IAV (Fig. 2B).
Fig 2.
Expression of IL-22 transcript by pulmonary RORγt-positive cells during IAV infection. (A) RORγt-positive and RORγt-negative cells were sorted from mock-treated or IAV-infected RORγt-GFP mice (60 h p.i., 600 PFU). RNAs were prepared, and IL-22 mRNA copy numbers were measured by quantitative RT-PCR as described in Fig. 1A. Of note, the frequency of RORγt-positive cells remained stable 2 days p.i. (B) Il22 mRNA copy numbers were determined from NK cells sorted from mock-treated or IAV-infected RORγt-GFP mice. (C) RORγt-positive αβ T lymphocytes (TCRβ+) without iNKT cells, iNKT (PBS57-loaded CD1d tetramer+ TCRβ+) cells, which represent ∼15% of total RORγt-positive TCRβ+ cells, γδ T lymphocytes (TCRγδ+), and the ILC-enriched population (TCRβ−, TCRγδ−) were sorted from mock-treated or IAV-infected RORγt-GFP mice (60 h p.i.) and analyzed for IL-22 mRNA expression. For panels A to C, the data are normalized to the expression of gapdh and are expressed as the fold increased over the average gene expression in mock-treated mice. Each group is a pool of cells from six to eight mice and is representative of two independent experiments. (D) Intracellular staining of IL-22 in lung RORγt-positive lung cells. Lung MNCs from RORγt-GFP mice were treated with IL-1β and IL-23 (10 ng/ml) for 4 h in the presence of brefeldin A, and gated RORγt-positive αβ T lymphocytes, γδ T lymphocytes, an ILC-enriched population, and iNKT cells were analyzed for intracellular IL-22 production. (E) RORγt-GFP mice were infected with IAV (600 PFU), and lung MNCs were prepared 60 h p.i. Cells were cultured for 4 h in the presence of brefeldin A without restimulation, and gated RORγt-positive cell subpopulations were analyzed for intracellular IL-22 production. (D and E) Gates were set based on the isotype control. The percentage of cells expressing IL-22 is shown. The results of representative experiments out of two (D) or five (E) are depicted. (E) Similar data were obtained at 50 PFU (data not shown).
The different pulmonary RORγt-expressing cell populations were next sorted from mock-treated and IAV-infected animals and analyzed for Il22 gene transcript expression. In agreement with earlier studies (66, 73), αβ T lymphocytes and γδ T lymphocytes represented the two major populations expressing RORγt (Fig. 2C, upper panel). Invariant NKT cells were discarded from the αβ T lymphocyte pool and were analyzed separately. A fourth population of RORγt+ cells was also identified. This population, which represents a minor fraction of RORγt+ cells (∼4% of the total pool), contains ∼70% of Lin− CD127+ CD90+ CD4− cells (data not shown) and was thus termed the ILC3-enriched population (12). As shown in Fig. 2C (lower panel), αβ T lymphocytes, γδ T lymphocytes, and iNKT cells, and to a lesser extent the ILC3-enriched population, produced a higher level of Il22 mRNAs in the context of IAV infection.
Analysis of IL-22 protein expression by RORγt-expressing cells was next assessed in response to a cocktail of IL-1β and IL-23, used here as a positive control. As represented in Fig. 2D, αβ T lymphocytes (mainly CD4neg), γδ T lymphocytes, and iNKT cells and, to a lesser extent cells within the ILC-enriched population, produced IL-22. Of note, lung NKp46+ cells failed to express IL-22 in response to IL-1β and IL-23 (not shown). In the context of IAV infection, and without ex vivo restimulation, IL-22 protein expression was not evidenced by intracellular staining using fluorescence-activated cell sorting, regardless of the cell population analyzed, the kinetics (60 h and 4 days), and the dose of IAV used (Fig. 2E and data not shown). The low level of expression and/or the rapid secretion of IL-22 might explain these data.
IL-22 deficiency has no impact on mouse survival, viral clearance, and IAV-specific CD8+ T cell response.
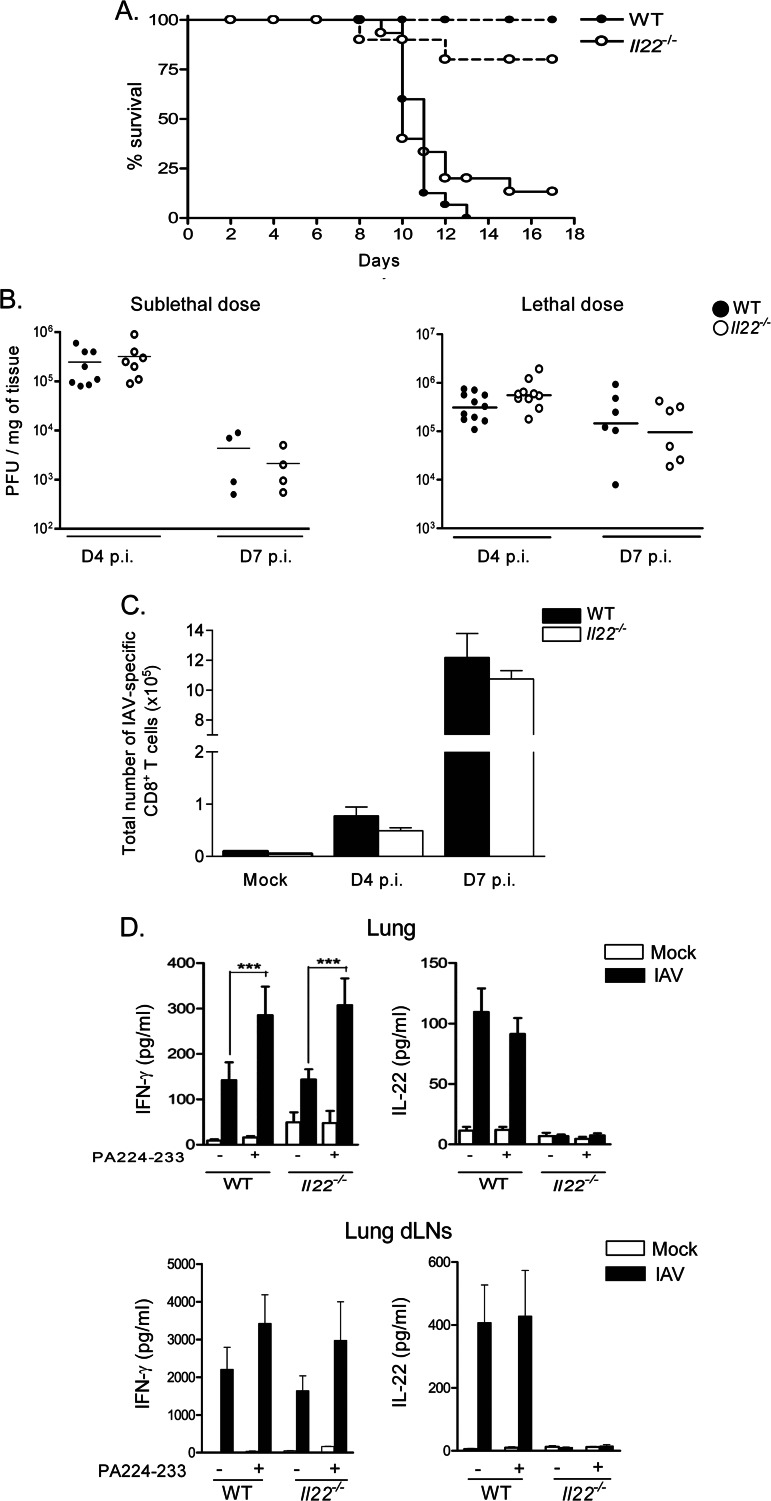
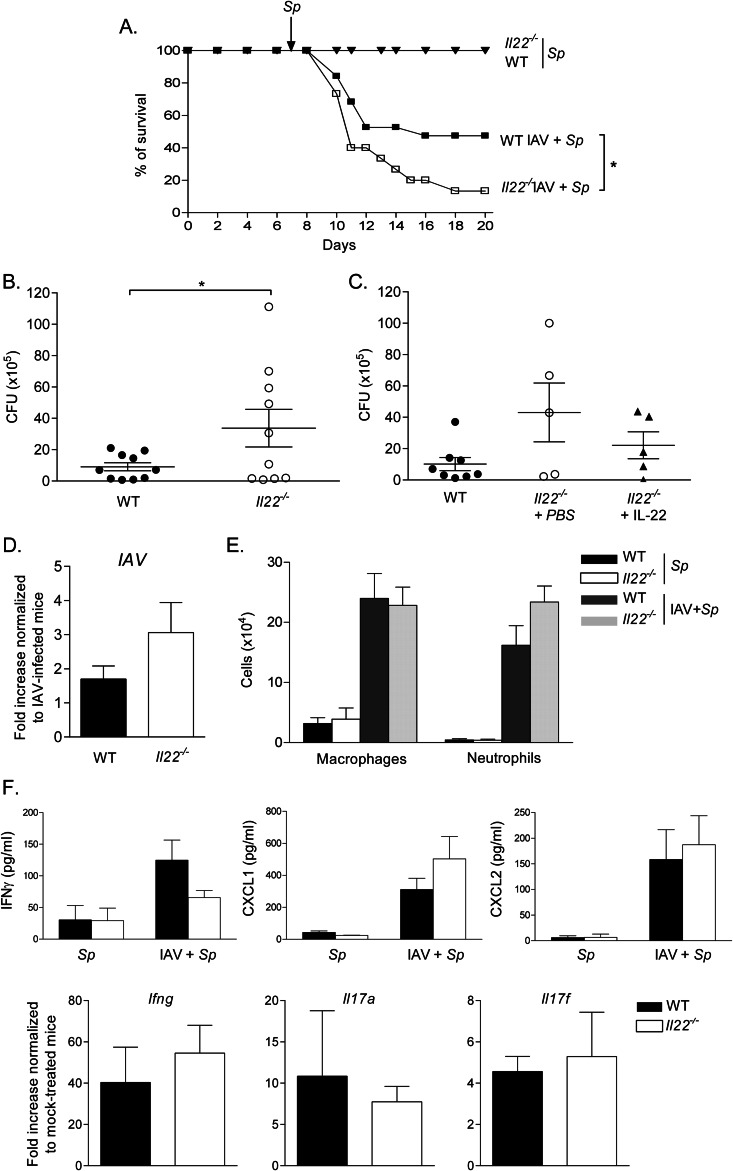
Severe lung immunopathology strongly contributes to influenza virus-related morbidity and mortality (74). It is known that IL-22 is a versatile controller of immunopathology (2, 8, 9). To address its role during influenza, WT and Il22−/− mice were used in survival studies. To rule out potential bias due to genetic background, WT and Il22−/− littermates were used. Upon a lethal dose (600 PFU) of IAV, WT animals demonstrated severe sickness ending in death at day 13 (Fig. 3A). Although not significant, 10 to 15% of Il22−/− mice survived the infection out to day 18 p.i. In contrast, using a sublethal dose (50 PFU), Il22−/− mice displayed slightly, although not significantly, decreased resistance compared to WT animals.
Fig 3.
Impact of IL-22 deficiency on mouse survival, on the control of viral clearance, and on the development of IAV-specific CD8+ T cells in the lungs. (A) Age-matched WT or Il22−/− mice were infected with a lethal (600 PFU, solid line) or a sublethal (50 PFU, dotted line) dose of IAV and then observed until death. The percentages of survival are represented (n = 10 to 15/group, at least two independent experiments). A log-rank test for comparisons of Kaplan-Meier survival curves indicated no significant difference in the mortality of Il22−/− mice compared to WT animals. (B) Viral loads in the lungs of WT and Il22−/− mice infected with 50 PFU (sublethal) or 600 PFU (lethal) of IAV were assessed at 4 and 7 days p.i. The data are given as PFU/mg of tissue (n = 4 to 11). (C) At 4 and 7 days p.i. (50 PFU), lung cells from infected WT and Il22−/− mice were recovered and analyzed for CD8+ T cell responses. The number of PA224-233-specific CD8+ T cells in the lung tissue was determined by flow cytometry. The results of one representative experiment out of three are shown. Values represent the means ± the SD (n = 4 to 6). (D) At day 7 p.i. (50 PFU), lung and lung dLN cells were restimulated with PA224-233 peptide (10 μg/ml) for 72 h. IFN-γ and IL-22 production was measured by ELISA. The results of one representative experiment out of three (lung) or two (lung dLNs) are shown. Values represent the means ± the SD (n = 4 to 6). Significant differences were determined using a one-way analysis of variance, followed by a Bonferroni post test (***, P < 0.001).
To determine whether endogenous IL-22 plays a part in viral clearance, the viral load was monitored at day 4 and at day 7 p.i. by plaque assay. Whatever the dose of IAV used, Il22−/− mice controlled virus replication in their lungs with kinetics similar to those for WT animals (Fig. 3B).
IL-22 has been shown to attenuate antigen (Ag)-induced pulmonary immune responses in some settings (23, 25–27). We thus compared the virus-specific CD8+ T cell response in IAV-infected WT and Il22−/− mice in terms of cell number and cytokine production. Whatever the dose of IAV (shown is 50 PFU), the number of lung CD8+ DbPA224-233+ cells were not different between WT and Il22−/− mice at 4 and 7 days p.i (Fig. 3C and data not shown). Similarly, upon restimulation with PA224-233, the amount of IFN-γ released by lung cells and by cells from the lung dLNs was identical (Fig. 3D). Of note, whereas pulmonary and lung dLN cells from IAV-infected mice spontaneously released IL-22 at day 7, IL-22 production was not amplified after IAV peptide restimulation. This indicates the lack of IAV-specific IL-22-producing CD8+ T cells in the context of early IAV, at least in response to the immunodominant peptide used.
Endogenous IL-22 plays a positive role in the control of sublethal, but not lethal, IAV-associated pneumonia.
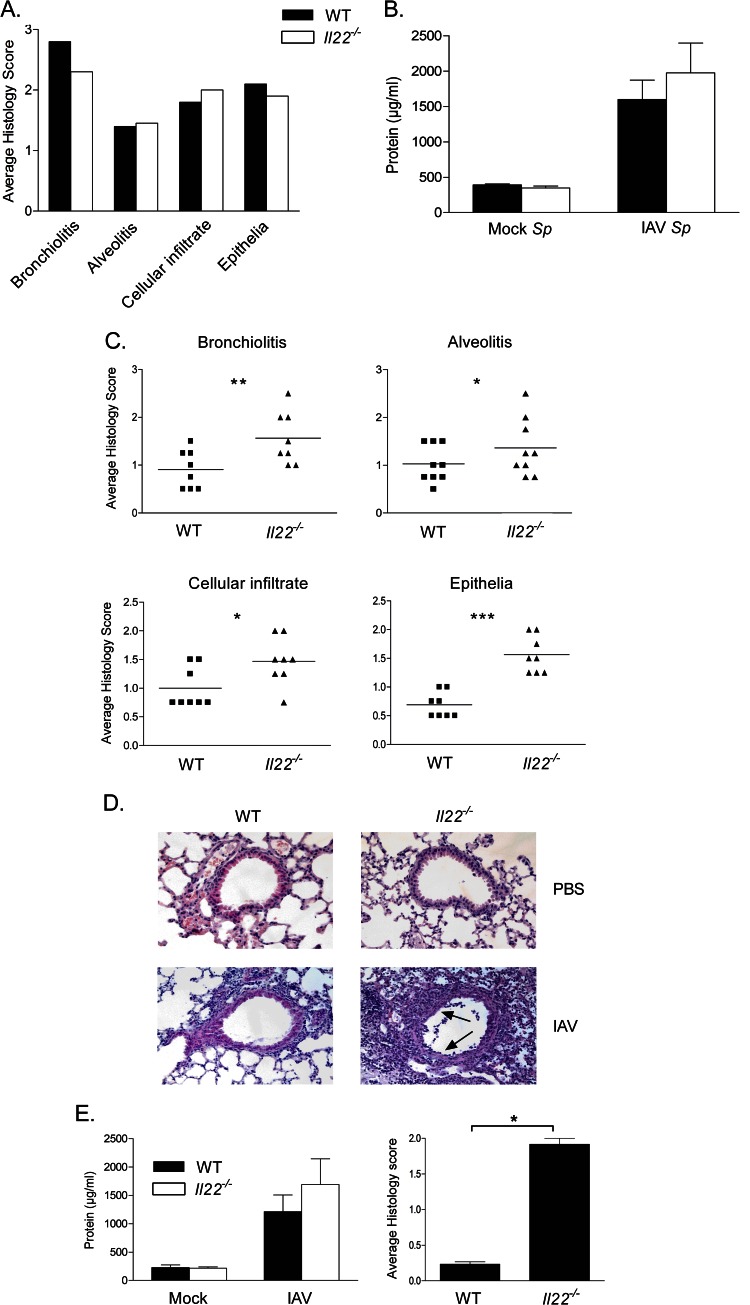
We next addressed the potential role of endogenous IL-22 in the development and/or the control of IAV-associated pulmonary pathogenesis. To do so, lungs from WT and Il22−/− mice infected with a lethal or a sublethal dose of IAV were harvested for histology during the acute-phase response. In conditions where IAV causes acute pneumonia ending by the death of the animals (600 PFU), the lack of IL-22 did not significantly modulate the intensity of the pulmonary inflammation. As shown in Fig. 4A, the cellular infiltration in the lungs, as the well as the alveolitis and bronchiolitis scores, were not significantly different between infected WT and Il22−/− mice. In both infected animal groups, bronchial epithelia were strongly damaged, and this was associated with an impaired epithelial integrity, as measured by the high total protein concentration in the BAL fluid (Fig. 4B).
Fig 4.
Analysis of the pathology of WT or Il22−/− mice infected with a lethal or sublethal dose of IAV. Age-matched WT or Il22−/− mice were infected with 600 PFU (A and B) or 50 PFU (C to E) IAV and sacrificed 7 days postchallenge. The lungs were harvested, and histopathological analysis and scoring (A and C) were performed exactly as described previously (51, 69). (B) Lung permeability of infected mice as assessed by the quantification of protein concentrations in BAL fluids from each mouse. (D) Representative H&E-stained tissue sections (magnification, ×200) are shown. Arrows indicate denuded epithelia. (E) The left panel shows the protein concentrations in BAL fluids. The right panel shows the scoring for the alveolar leakage, and lesional edema was based on the number of red blood cells in the alveoli. The data represent the means ± the SEM (B and E, left panel) or SD (E, right panel). All results are representative of two independent experiments (n = 8; *, P < 0.05; **, P < 0.01; ***, P < 0.001 [two-tailed Student t test]).
In stark contrast, upon a sublethal challenge (50 PFU), a significant enhancement of airway inflammation was noticed in Il22−/− mice relative to WT mice as reflected by the higher histopathology scores in the former group (Fig. 4C). In Il22−/− mice, alveolitis and bronchiolitis were more pronounced relative to WT littermates (Fig. 4C and D). In addition, epithelia surrounding the bronchi from Il22−/− mice were more damaged (Fig. 4D). Signs of severe injury, characterized by augmented loss of intercellular cohesion and denuded epithelium were observed in Il22−/− animals (scored in Fig. 4C). This apparent loss of epithelial integrity was associated with a slight enhancement of protein concentration in the BALs (Fig. 4E, left panel). Of note, the number of red blood cells in the luminal side of the alveoli was dramatically enhanced in Il22−/− mice indicative of alveolar leakage and lesional edema (Fig. 4E, right panel).
IL-22 deficiency leads to an enhanced susceptibility to secondary bacterial infection following influenza virus infection.
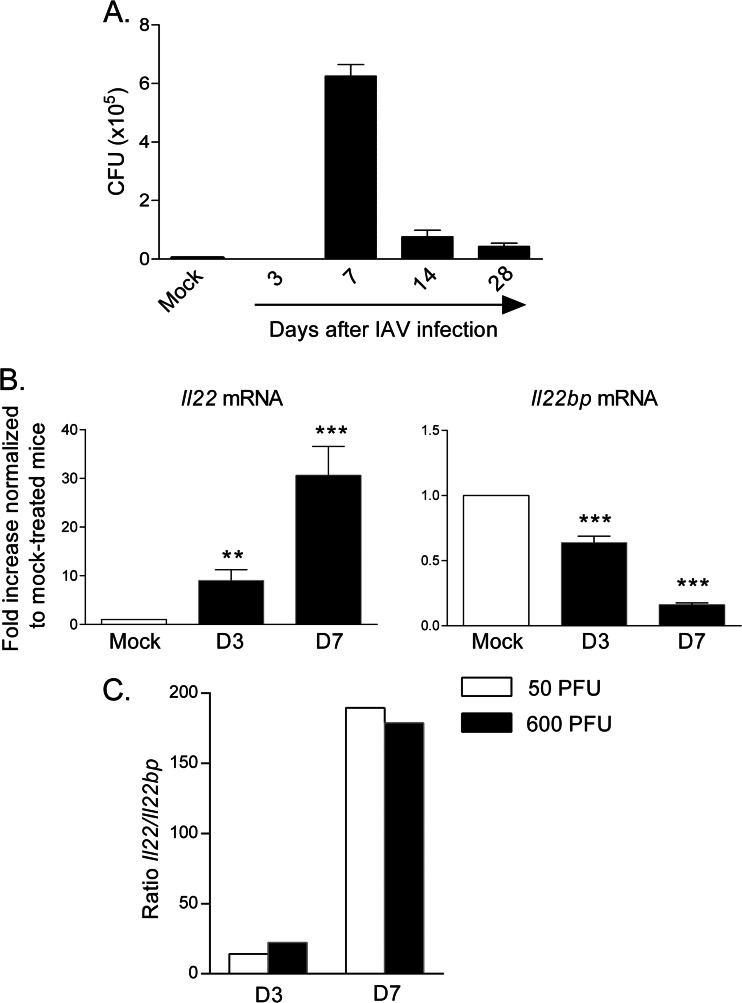
Epithelial damages post-influenza virus infection has been proposed to play a part in secondary bacterial infections such as S. pneumoniae (53, 55–57, 59, 60, 75). We thus investigated the role of endogenous IL-22 in bacterial superinfection following influenza virus infection. Before this, we determined the optimal timing between exposure to the virus and exposure to the bacteria to achieve bacterial superinfection. For this, animals were infected with a sublethal dose of IAV (50 PFU) and were then superinfected with a self-limiting dose of S. pneumoniae (104 CFU) at different time points after virus infection. As seen in Fig. 5A, mice challenged with S. pneumoniae 7 days after influenza virus infection had a high number of live bacteria in the lungs, while animals infected after 3 days completely clear the bacteria 24 h after the challenge. Of note, mice infected 2 and 4 weeks after IAV had a lower capacity to control bacterial replication, although the numbers of bacteria in the lungs were much lower relative to those in mice receiving IAV 7 days earlier. Thus, S. pneumoniae was administered 7 days post-IAV infection, the peak of bacterial susceptibility.
Fig 5.
Establishment of the superinfection model and Il22/Il22bp expression analysis. (A) Wild-type mice were infected with IAV (50 PFU) and, 3, 7, 14, or 28 days later, mice were challenged with S. pneumoniae (104 CFU). The numbers of live bacteria in the lungs were determined 24 h after S. pneumoniae infection. The results of one representative experiment out of two are shown (means ± the SEM, n = 5). (B and C) WT animals were infected with 50 PFU (B) or with 600 PFU (B and C) of IAV. The lungs were collected at 3 and 7 days p.i. (B) Il22 and Il22bp mRNA copy numbers were determined by quantitative RT-PCR. The data are expressed as the fold increased over the average gene expression in mock-treated mice (n = 6). (C) The ratios of Il22 to Il22bp (50 PFU versus 600 PFU) are depicted at days 3 and 7 p.i.
At the dose of IAV used (50 PFU), the levels of Il22 mRNA were increased, whereas those of Il22bp were decreased at day 3 and particularly at day 7 p.i. (Fig. 5B). As a consequence, the ratio of Il22 to Il22bp was higher at day 7 relative to day 3. Of note, this ratio was comparable at 50 and 600 PFU (Fig. 5C). As expected, preceding IAV infection induced animal death after S. pneumoniae challenge (Fig. 6A, IAV+Sp). Remarkably, while 50% of IAV-experienced WT mice succumbed following S. pneumoniae infection, almost all coinfected Il22−/− mice died from bacterial infection. This effect was associated with an increased number of viable bacteria in the lung tissue (Fig. 6B). Of importance, the observed effect was probably not due to a direct antibacterial effect of endogenous IL-22 per se. Indeed, both non-IAV experienced WT and Il22−/− mice survived (Fig. 6A) and equally cleared bacteria in the lungs after sublethal S. pneumoniae challenge (data not shown). To confirm our findings, Il22−/− mice were administered with recombinant IL-22. Compared to IAV-infected Il22−/− animals treated with vehicle alone, the administration of recombinant IL-22 reduced the number of CFU in the lungs (Fig. 6C). Thus, the lack of IL-22 worsens the outcome of secondary S. pneumoniae pneumonia. This effect was not associated with a significantly higher IAV burden in the lungs (Fig. 6D) or a differential recruitment of macrophages and neutrophils in the BALs of doubly infected mice, cells known to be crucial in the clearance of S. pneumoniae (76, 77) (Fig. 6E). Furthermore, the production of factors known to exert antipneumococcal effects, including IFN-γ, IL-17, and the neutrophil-attracting factors CXCL1 and CXCL2, were not different between WT and Il22−/− doubly infected mice (Fig. 6F and not shown). ICAM-1 plays a role in the control of S. pneumoniae early after infection (78). However, the transcript level of Icam1 remained unchanged between WT and Il22−/− doubly infected mice (data not shown). In the same line, REGIIIγ, a known target of IL-22 able to exert an antibacterial effect in the intestine (33) and the lung (34), was not modulated at the transcript level in doubly infected Il22−/− mice, relative to doubly infected WT animals (data not shown). Collectively, endogenous IL-22 limits bacterial superinfection post-influenza virus infection, a phenomenon probably independent from direct enhanced host defense mechanisms.
Fig 6.
Role of endogenous IL-22 on secondary bacterial infection following influenza infection. (A) WT or Il22−/− mice were infected, or not, with IAV (50 PFU). Seven days later, IAV-infected or naive animals were challenged with S. pneumoniae (104 CFU) and then observed until death. The percentages of survival are represented (n = 15, three independent experiments). A log-rank test for comparisons of Kaplan-Meier survival curves indicated a significant difference in the mortality of Il22−/− mice compared to WT animals. (B) Doubly infected WT or Il22−/− mice were sacrificed 24 h after S. pneumoniae challenge, and the number of CFU was determined in the lungs. Of note, WT and Il22−/− mice only infected with S. pneumoniae (104 CFU) had no CFU in the lungs at 1 day p.i. (data not shown). The data represent pooled results from two independent experiments (*, P < 0.05). (C) Il22−/− mice were administered with recombinant IL-22 or vehicle (PBS) at the time of IAV infection and at day 2 p.i. (D to F), The lungs of doubly infected WT or Il22−/− mice were collected 1 day after the S. pneumoniae challenge. (D) IAV M2 mRNA copy numbers were determined by quantitative RT-PCR. The data are normalized to the expression of gapdh and are expressed as the fold increased over the average gene expression in singly infected WT mice (day 7 post-IAV). (E) The numbers of macrophages and neutrophils in the BALs were determined. (F) For the upper panel, the concentrations of IFN-γ, CXCL1, and CXCL2 were determined in BAL fluids 24 h after bacterial challenge. For the lower panel, Ifng, Il17a, and Il17f mRNA copy numbers were determined by quantitative RT-PCR. The data are normalized to the expression of Gapdh and are expressed as the fold increased over the average gene expression in naive mice. Shown are the results of a representative experiment out of two performed (means ± the SEM, n = 5). For panels E and F, the data represent the means ± the SEM (n = 3 [Sp] or n = 10 [IAV+Sp]).
DISCUSSION
IL-22 participates in host defense against bacteria at mucosal surfaces and plays a part in the maintenance of the barrier integrity, with predominant effects on epithelial cells. However, this cytokine can also serve pathogenic functions in many experimental and clinical settings (1, 2, 13, 29). In the present study, we show that during nonlethal IAV-induced pathogenesis, IL-22 reduces lung inflammation and maintains pulmonary epithelial integrity, an effect associated with a more controlled secondary bacterial infection following influenza.
During H3N2 infection, and at the transcript level, IL-22 was induced as early as day 2 in the lung tissue and BALs, whereas IL-22BP, the endogenous IL-22 antagonist, was downmodulated. Of note, whatever the dose of IAV, the Il22/Il22bp ratio was enhanced in a similar extent and peaked at day 7 p.i. Along with RORγt-positive iNKT cells, other subsets of RORγt-positive cells, including αβ T lymphocytes, γδ T lymphocytes, and ILC3, express enhanced Il22 transcript during the early phases of IAV infection. In contrast, RORγt-negative cells failed to do so. The absence of IL-22 production by lung NKp46+ cells, either after IL-1β plus IL-23 stimulation or in the context of IAV infection, can be explained by the lack of RORγt expression by these cells and indicates that among ILC3, lymphoid tissue inducer cells, rather than “NK-like” cells, are a source of IL-22 during early IAV infection. Our data are in contrast to other studies (49, 52), which reported IL-22 protein production by lung conventional NK cells during the early (day 2) and the later (days 4 and 7) stages of IAV infection. The reasons for this discrepancy may be explained by differences in the virus strains used.
Whatever the dose of IAV, the overall pulmonary viral burdens of Il22−/− mice showed no difference from those of control mice. These data are in line with other studies reporting no direct or indirect antiviral activity of IL-22 (45–49). Furthermore, in agreement with a previous finding showing that IL-22 has no impact on Ag-driven lymphocyte priming and expansion (65), Il22−/− mice had a normal IAV-specific CD8+ T cell response. Thus, IL-22 does not favor or interfere with mechanisms leading to T cell priming in the dLNs. Likewise, IL-22 plays no major role on the synthesis of epithelial-derived chemokines necessary for the recruitment of expanded T cells in the lung compartment.
In response to a highly immunopathogenic IAV dose (600 PFU), Il22−/− mice did not exhibit reduced or enhanced mortality rates. In parallel, compared to WT mice, Il22−/− mice showed no improvement, or conversely dysregulation, of pneumonia. This indicated that during excessive, nonresolving, IAV-associated pneumonia, IL-22 plays no major function. In stark contrast, during sublethal IAV infection where inflammation is less intense and more controlled, endogenous IL-22 reduced lung injury. This did not significantly influence the survival outcome, although a trend toward enhanced mortality was observed in Il22−/− mice. The reason why IL-22 is redundant at a high dose of IAV and beneficial at a sublethal dose is not due to a difference in the levels of IL-22 and IL-22BP expression (at least at the transcript level). A difference in the production of IL-17 and TNF-α, known to control IL-22 activities (5, 29, 79), as well as of other inflammatory factors, might explain the different role of IL-22 during lethal and sublethal influenza. The positive role of IL-22 during sublethal IAV infection is in agreement with observations reported by Kumar et al. (H1N1) (52). During the early phase of IAV infection, the loss of epithelial integrity plays a major role in the pathology (50, 75). It leads to the loss of homeostasis and the development of pulmonary inflammation, features found to be enhanced in Il22−/− mice. Epithelial damages are caused by IAV-induced cytopathology and/or the lysis of virus-infected epithelial cells by recruited NK cells, cytotoxic T cells, apoptosis-inducing cytokines, and the activation of death receptors. Our recent data showed that IL-22 protects epithelial cells from cell death after infection with IAV in vitro (51). In line with this finding, we observed an enhanced level of epithelial damages in infected Il22−/− mice, relative to WT littermates. Although the mechanisms sustaining the protective effect of IL-22 are still unknown, we speculate that its in vivo beneficial effect is due to its role on the maintenance of epithelial integrity. In this setting, IL-22 might play a positive role in the prevention of injury (51) and, as recently demonstrated (52), in the acceleration of the epithelial repair. Recently, a population of lung RORγt-negative ILCs that resembles natural helper cells has been described to play a protective role, through amphiregulin release, on airway epithelia during IAV infection (50). It is possible that the positive role played by IL-22-producing cells during the early stages of IAV infection complements natural helper cell functions to ensure airway epithelial integrity and lung tissue homeostasis during IAV infection.
It is well documented that IAV infection enhances susceptibility to secondary bacterial infections, leading to increased morbidity and mortality (53–60). The mechanisms include alterations of mechanical and immunological defenses. For instance, epithelial cell damages caused by influenza virus improve bacterial adhesion to the mucosa and its subsequent crossing through the lung epithelium. In parallel, impairment of the host innate response is an important feature of bacterial superinfections (53, 55, 59). In the present study, we report that IAV-experienced Il22−/− mice challenged with S. pneumoniae have decreased survival compared to similarly infected WT animals. We favor the hypothesis that the protective role of IL-22 in bacterial superinfection post-IAV infection is rather due to its positive early effect on lung, including epithelial, injury due to IAV infection rather than to an antibacterial effector mechanisms per se. Indeed, molecular and cellular effectors associated with early innate defense mechanisms against bacteria were not significantly decreased in the absence of IL-22. Thus, by limiting the alteration of the epithelial barrier instigated by IAV, IL-22 might decrease the level of bacterial invasion into the lungs. This hypothesis needs to be confirmed using different IAV subtypes, including H1N1. Since in humans, dysregulation of IL-22 or IL-22 receptor production has been reported in certain pathologies, including psoriasis, Crohn's disease, and allergic diseases (80), it might be interesting to determine the potential correlation, if any, between susceptibility to bacterial superinfections and IL-22 or IL-22 receptor production in patients. Recent findings suggest that, in combination with antibiotic and antiviral therapies, treatments that protect lung epithelium and/or stimulate lung repair responses could be beneficial in improving survival in patients during influenza virus and bacterial coinfection (50, 75). Whether IL-22 supplementation in order to improve lung epithelial integrity and to prevent microbial translocation leads to less severe outcome resulting from bacterial complications during influenza virus infection is under intense investigation.
ACKNOWLEDGMENTS
We acknowledge the NIAID Tetramer Facility (Emory University, Atlanta, GA) for supplying CD1d tetramers and W. Ouyang (Genentech, San Francisco, CA) for the gift of the monoclonal Ab against mouse IL-22 (clone 3F11). We thank T. Camou (National Reference Laboratory, Ministry of Health, Montevideo, Uruguay) for the gift of the S. pneumoniae serotype 1 clinical isolate E1586.
This study was supported in part by the Institut National de la Santé et de la Recherche Médicale, the CNRS, the University of Lille Nord de France, the Pasteur Institute of Lille, and l'Agence Nationale de la Recherche (ANR) under reference ANR-08-MIEN-021-01. SI, AB, CP, EMF and LVM were recipients of a doctoral fellowship from the Ministère de l'Education Nationale de la Recherche et Technique (S.I., A.B., and E.M.F.) and the Conseil Régional Nord Pas de Calais/Inserm (C.P.). J.R. was supported by a postdoctoral fellowship from the ANR (ANR-08-MIEN-021-01). P.G. is supported by the Hopital Saint Vincent (Lille); J.C.R. and L.D. are supported by the FNRS; C.F., M.S.T., and J.C.S. are supported by Inserm; and G.E., A.B., and F.T. are supported by the CNRS.
Footnotes
Published ahead of print 17 April 2013
REFERENCES
- 1. Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 29:71–109 [DOI] [PubMed] [Google Scholar]
- 2. Sonnenberg GF, Fouser LA, Artis D. 2011. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12:383–390 [DOI] [PubMed] [Google Scholar]
- 3. Colonna M. 2009. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 31:15–23 [DOI] [PubMed] [Google Scholar]
- 4. Dumoutier L, de Heusch M, Orabona C, Satoh-Takayama N, Eberl G, Sirard JC, Di Santo JP, Renauld JC. 2011. IL-22 is produced by γC-independent CD25+ CCR6+ innate murine spleen cells upon inflammatory stimuli and contributes to LPS-induced lethality. Eur. J. Immunol. 41:1075–1085 [DOI] [PubMed] [Google Scholar]
- 5. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 119:3573–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. 2009. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31:321–330 [DOI] [PubMed] [Google Scholar]
- 8. Spits H, Cupedo T. 2012. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30:647–675 [DOI] [PubMed] [Google Scholar]
- 9. Spits H, Di Santo JP. 2011. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12:21–27 [DOI] [PubMed] [Google Scholar]
- 10. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331–341 [DOI] [PubMed] [Google Scholar]
- 11. Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, Janot L, Erard F, Bertout J, Leger H, Sebbane F, Benecke A, Renauld JC, Hardt WD, Ryffel B, Sirard JC. 2010. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3negCD127+ immune cells in spleen and mucosa. J. Immunol. 185:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. 2013. Innate lymphoid cells: a proposal for uniform nomenclature. Nat. Rev. Immunol. 13:145–149 [DOI] [PubMed] [Google Scholar]
- 13. Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. 2010. IL-17 and IL-22: siblings, not twins. Trends Immunol. 31:354–361 [DOI] [PubMed] [Google Scholar]
- 14. Renauld JC. 2003. Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat. Rev. Immunol. 3:667–676 [DOI] [PubMed] [Google Scholar]
- 15. Witte E, Witte K, Warszawska K, Sabat R, Wolk K. 2010. Interleukin-22: a cytokine produced by T, NK, and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 21:365–379 [DOI] [PubMed] [Google Scholar]
- 16. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. 2004. IL-22 increases the innate immunity of tissues. Immunity 21:241–254 [DOI] [PubMed] [Google Scholar]
- 17. Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MR. 2012. Interleukin-22 drives endogenous thymic regeneration in mice. Science 336:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. 2009. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206:1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Radaeva S, Sun R, Pan HN, Hong F, Gao B. 2004. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39:1332–1342 [DOI] [PubMed] [Google Scholar]
- 20. Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. 2008. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118:534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. 2007. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27:647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang KM, Artis D. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336:1321–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. 2010. γδ T cells protect against lung fibrosis via IL-22. J. Exp. Med. 207:2239–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, Zwissler B, Muhl H. 2011. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am. J. Respir. Cell Mol. Biol. 44:369–376 [DOI] [PubMed] [Google Scholar]
- 25. Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard F, Warszawska K, Wolk K, Quesniaux V, Ryffel B, Togbe D. 2011. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am. J. Respir. Crit. Care Med. 183:1153–1163 [DOI] [PubMed] [Google Scholar]
- 26. Nakagome K, Imamura M, Kawahata K, Harada H, Okunishi K, Matsumoto T, Sasaki O, Tanaka R, Kano MR, Chang H, Hanawa H, Miyazaki J, Yamamoto K, Dohi M. 2011. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J. Immunol. 187:5077–5089 [DOI] [PubMed] [Google Scholar]
- 27. Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, Tokoyoda K, Renauld JC, Iwamoto I, Nakayama T, Nakajima H. 2011. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J. Allergy Clin. Immunol. 128:1067–1076 [DOI] [PubMed] [Google Scholar]
- 28. Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, Matthys P. 2009. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 60:390–395 [DOI] [PubMed] [Google Scholar]
- 29. Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. 2010. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 207:1293–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, Fouser LA, Renauld JC, Dumoutier L. 2012. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 188:462–469 [DOI] [PubMed] [Google Scholar]
- 31. Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445:648–651 [DOI] [PubMed] [Google Scholar]
- 32. Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289 [DOI] [PubMed] [Google Scholar]
- 34. Choi SM, McAleer JP, Zheng M, Pociask DA, Kaplan MH, Qin S, Reinhart TA, Kolls JK. Innate Stat3-mediated induction of the antimicrobial protein Reg3γ is required for host defense against MRSA pneumonia. J. Exp. Med., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graham AC, Carr KD, Sieve AN, Indramohan M, Break TJ, Berg RE. 2011. IL-22 production is regulated by IL-23 during Listeria monocytogenes infection but is not required for bacterial clearance or tissue protection. PLoS One 6:e17171 doi: 10.1371/journal.pone.0017171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. 2010. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J. Immunol. 185:5453–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, Wynn TA. 2010. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J. Immunol. 184:4378–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, Puccetti P, Romani L. 2010. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 3:361–373 [DOI] [PubMed] [Google Scholar]
- 39. Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. 2011. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34:122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stange J, Hepworth MR, Rausch S, Zajic L, Kuhl AA, Uyttenhove C, Renauld JC, Hartmann S, Lucius R. 2012. IL-22 mediates host defense against an intestinal intracellular parasite in the absence of IFN-gamma at the cost of Th17-driven immunopathology. J. Immunol. 188:2410–2418 [DOI] [PubMed] [Google Scholar]
- 41. Munoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Holscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. 2009. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 206:3047–3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arias JF, Nishihara R, Bala M, Ikuta K. 2010. High systemic levels of interleukin-10, interleukin-22, and C-reactive protein in Indian patients are associated with low in vitro replication of HIV-1 subtype C viruses. Retrovirology 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Misse D, Yssel H, Trabattoni D, Oblet C, Lo Caputo S, Mazzotta F, Pene J, Gonzalez JP, Clerici M, Veas F. 2007. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J. Immunol. 178:407–415 [DOI] [PubMed] [Google Scholar]
- 44. Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LY, Grin A, Kandel G, Loutfy M, Ostrowski M, Gommerman JL, Kaushic C, Kaul R. 2012. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 5:670–680 [DOI] [PubMed] [Google Scholar]
- 45. Dambacher J, Beigel F, Zitzmann K, Heeg MH, Goke B, Diepolder HM, Auernhammer CJ, Brand S. 2008. The role of interleukin-22 in hepatitis C virus infection. Cytokine 41:209–216 [DOI] [PubMed] [Google Scholar]
- 46. Pagliaccetti NE, Chu EN, Bolen CR, Kleinstein SH, Robek MD. 2010. Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology 401:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD. 2011. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 141:1897–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang P, Bai F, Zenewicz LA, Dai J, Gate D, Cheng G, Yang L, Qian F, Yuan X, Montgomery RR, Flavell RA, Town T, Fikrig E. 2012. IL-22 signaling contributes to West Nile encephalitis pathogenesis. PLoS One 7:e44153 doi: 10.1371/journal.pone.0044153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo H, Topham DJ. 2010. Interleukin-22 (IL-22) production by pulmonary natural killer cells and the potential role of IL-22 during primary influenza virus infection. J. Virol. 84:7750–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12:1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P, Gosset P, Si-Tahar M, Faveeuw C, Trottein F. 2012. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J. Biol. Chem. 287:8816–8829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kumar P, Thakar MS, Ouyang W, Malarkannan S. 2013. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 6:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ballinger MN, Standiford TJ. 2010. Postinfluenza bacterial pneumonia: host defenses gone awry. J. Interferon Cytokine Res. 30:643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goulding J, Godlee A, Vekaria S, Hilty M, Snelgrove R, Hussell T. 2011. Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection. J. Infect. Dis. 204:1086–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hartshorn KL. 2010. New look at an old problem: bacterial superinfection after influenza. Am. J. Pathol. 176:536–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McCullers JA. 2006. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19:571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. 2010. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis. 202:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morens DM, Taubenberger JK, Fauci AS. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198:962–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Snelgrove RJ, Godlee A, Hussell T. 2011. Airway immune homeostasis and implications for influenza-induced inflammation. Trends Immunol. 32:328–334 [DOI] [PubMed] [Google Scholar]
- 60. van der Sluijs KF, van der Poll T, Lutter R, Juffermans NP, Schultz MJ. 2010. Bench-to-bedside review: bacterial pneumonia with influenza—pathogenesis and clinical implications. Crit. Care 14:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. 2005. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571–5580 [DOI] [PubMed] [Google Scholar]
- 62. Marques JM, Rial A, Munoz N, Pellay FX, Van Maele L, Leger H, Camou T, Sirard JC, Benecke A, Chabalgoity JA. 2012. Protection against Streptococcus pneumoniae serotype 1 acute infection shows a signature of Th17- and IFN-gamma-mediated immunity. Immunobiology 217:420–429 [DOI] [PubMed] [Google Scholar]
- 63. Munoz N, Van Maele L, Marques JM, Rial A, Sirard JC, Chabalgoity JA. 2010. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect. Immun. 78:4226–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zemlickova H, Crisostomo MI, Brandileone MC, Camou T, Castaneda E, Corso A, Echaniz-Aviles G, Pasztor M, Tomasz A. 2005. Serotypes and clonal types of penicillin-susceptible streptococcus pneumoniae causing invasive disease in children in five Latin American countries. Microb. Drug Resist. 11:195–204 [DOI] [PubMed] [Google Scholar]
- 65. Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B. 2007. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179:8098–8104 [DOI] [PubMed] [Google Scholar]
- 66. Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. 2008. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J. Exp. Med. 205:1381–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sparwasser T, Gong S, Li JY, Eberl G. 2004. General method for the modification of different BAC types and the rapid generation of BAC transgenic mice. Genesis 38:39–50 [DOI] [PubMed] [Google Scholar]
- 68. Dumoutier L, Van Roost E, Colau D, Renauld JC. 2000. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl. Acad. Sci. U. S. A. 97:10144–10149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Paget C, Ivanov S, Fontaine J, Blanc F, Pichavant M, Renneson J, Bialecki E, Pothlichet J, Vendeville C, Barba-Spaeth G, Huerre MR, Faveeuw C, Si-Tahar M, Trottein F. 2011. Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. J. Immunol. 186:5590–5602 [DOI] [PubMed] [Google Scholar]
- 70. Belz GT, Xie W, Altman JD, Doherty PC. 2000. A previously unrecognized H-2D(b)-restricted peptide prominent in the primary influenza A virus-specific CD8+ T-cell response is much less apparent following secondary challenge. J. Virol. 74:3486–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ivanov S, Fontaine J, Paget C, Machofernandez E, Van Maele L, Renneson J, Maillet I, Wolf NM, Rial A, Leger H, Ryffel B, Frisch B, Chabalgoity JA, Sirard JC, Benecke A, Faveeuw C, Trottein F. 2012. Key role for respiratory CD103+ dendritic cells, IFN-γ, and IL-17 in protection against Streptococcus pneumoniae infection in response to alpha-galactosylceramide. J. Infect. Dis. 206:723–734 [DOI] [PubMed] [Google Scholar]
- 72. Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W, Jr, Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA. 2012. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491:259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, Nikitas G, Escaliere B, Renauld JC, Dussurget O, Cossart P, Lecuit M, Vivier E, Tomasello E. 2011. Identity, regulation, and in vivo function of gut NKp46+ RORγt+ and NKp46+ RORγt− lymphoid cells. EMBO J. 30:2934–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Taubenberger JK, Morens DM. 2008. The pathology of influenza virus infections. Annu. Rev. Pathol. 3:499–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kash JC, Walters KA, Davis AS, Sandouk A, Schwartzman LM, Jagger BW, Chertow DS, Li Q, Kuestner RE, Ozinsky A, Taubenberger JK. 2011. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. mBio 2(5):e00172-11 doi: 10.1128/mBio.00172-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kadioglu A, Andrew PW. 2004. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25:143–149 [DOI] [PubMed] [Google Scholar]
- 77. Koppe U, Suttorp N, Opitz B. 2012. Recognition of Streptococcus pneumoniae by the innate immune system. Cell Microbiol. 14:460–466 [DOI] [PubMed] [Google Scholar]
- 78. Kadioglu A, De Filippo K, Bangert M, Fernandes VE, Richards L, Jones K, Andrew PW, Hogg N. 2011. The integrins Mac-1 and alpha4beta1 perform crucial roles in neutrophil and T cell recruitment to lungs during Streptococcus pneumoniae infection. J. Immunol. 186:5907–5915 [DOI] [PubMed] [Google Scholar]
- 79. Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C, Schaller M, Behrendt H, Ring J, Schmidt-Weber CB, Cavani A, Mempel M, Traidl-Hoffmann C, Eyerich K. 2011. IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur. J. Immunol. 41:1894–1901 [DOI] [PubMed] [Google Scholar]
- 80. Pan HF, Li XP, Zheng SG, Ye DQ. 2013. Emerging role of interleukin-22 in autoimmune diseases. Cytokine Growth Factor Rev. 24:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]