Abstract
Animal models indicate that exposure to choline in utero improves visual memory through cholinergic transmission and/or epigenetic mechanisms. Among 895 mothers in Project Viva (eastern Massachusetts, 1999–2002 to 2008–2011), we estimated the associations between intakes of choline, vitamin B12, betaine, and folate during the first and second trimesters of pregnancy and offspring visual memory (measured by the Wide Range Assessment of Memory and Learning, Second Edition (WRAML2), Design and Picture Memory subtests) and intelligence (measured using the Kaufman Brief Intelligence Test, Second Edition (KBIT-2)) at age 7 years. Mean second-trimester intakes were 328 (standard deviation (SD), 63) mg/day for choline, 10.5 (SD, 5.1) µg/day for vitamin B12, 240 (SD, 104) mg/day for betaine, and 1,268 (SD, 381) µg/day for folate. Mean age 7 test scores were 17.2 (SD, 4.4) points on the WRAML 2 Design and Picture Memory subtests, 114.3 (SD, 13.9) points on the verbal KBIT-2, and 107.8 (SD, 16.5) points on the nonverbal KBIT-2. In a model adjusting for maternal characteristics, the other nutrients, and child's age and sex, the top quartile of second-trimester choline intake was associated with a child WRAML2 score 1.4 points higher (95% confidence interval: 0.5, 2.4) than the bottom quartile (P-trend = 0.003). Results for first-trimester intake were in the same direction but weaker. Intake of the other nutrients was not associated with the cognitive tests administered. Higher gestational choline intake was associated with modestly better child visual memory at age 7 years.
Keywords: choline, cognition, folate, memory, pregnancy
Methyl donor nutrients like choline, vitamin B12, betaine, and folate may affect brain development through involvement in methylation processes (1), and choline may be particularly important because of its role in cholinergic transmission (2). Animal studies demonstrate that higher choline exposure in utero improves memory (3). Offspring of rat dams given choline supplementation during mid- to late gestation displayed better visuospatial memory than those of unsupplemented dams (4–6). In rodents, choline supplementation in utero changed gene-specific methylation and protein expression, increased cell proliferation, and decreased apoptosis in the fetal hippocampus, the region of the brain associated with memory (7–9).
Few studies in humans have examined the association of maternal gestational choline intake with child cognition. Among US mother-child pairs, serum choline concentrations in maternal and umbilical cord blood were not associated with child intelligence quotient (IQ) at age 5 years (e.g., 0.61 IQ points per unit difference in cord serum choline level (P = 0.36)) (10). In Project Viva, gestational choline intake was not associated with score on the Peabody Picture Vocabulary Test, Third Edition (PPVT-III), a test of receptive language (per 450-mg/day increase in second-trimester choline intake, adjusted difference = 0.8, 95% confidence interval (CI): −7.4, 9.0), or the Wide Range Assessment of Visual Motor Abilities (WRAVMA) (adjusted difference = −0.4, 95% CI: −7.6, 6.8) at age 3 years (11). However, the IQ, PPVT-III, and WRAVMA tests do not specifically measure visuospatial memory, which is the cognitive domain that is consistently affected by choline intake in animal models. In the Framingham Offspring Cohort, choline intake during adulthood was associated with better performance on visual and verbal memory tests (12).
In contrast to choline, more studies have examined maternal intakes of folate and vitamin B12 in relation to child cognition in humans. In the Pune (13) and Mysore Parthenon (14) studies in India, higher maternal plasma B12 and folate levels at approximately 28–30 weeks of pregnancy were associated with improved offspring cognition, including attention and short-term memory at ages 9–10 years. In a cohort study in Mexico, maternal dietary vitamin B12 deficiency was associated with a reduced neurodevelopmental index in offspring within the first year of life (15). However, these previous studies did not carefully assess total diet to control for confounding and examined only a few methyl donor nutrients.
We sought to examine maternal first- and second-trimester dietary intake of methyl donor nutrients during pregnancy in relation to child visual memory in a generally well-nourished US population. We hypothesized that high maternal intake of choline and other methyl donor nutrients would be associated with higher memory test scores in children at age 7 years.
MATERIALS AND METHODS
Study sample
We studied participants in Project Viva, an ongoing prospective prebirth cohort study initiated in eastern Massachusetts in 1999. Women joined the study during their first prenatal visit at Harvard Vanguard Medical Associations, a large multispecialty group practice. Eligibility criteria included fluency in English, gestational age less than 22 weeks at the first prenatal visit, and singleton pregnancy. Additional details of recruitment and retention procedures have been published elsewhere (16). Of the 2,128 mother-infant pairs in the cohort, we obtained first- and/or second-trimester dietary data from 1,896 women. We administered cognitive tests to 1,038 of their children who were still in follow-up at age 7 years. We excluded mother-child pairs without complete covariate data (n = 131) and children for whom English was not the primary language (n = 12), leaving 895 mother-child pairs for this study. In the primary analysis of choline intake in relation to score on the Wide Range Assessment of Memory and Learning, Second Edition (WRAML2), Design and Picture Memory subtests, there were 861 mother-child pairs with data on first-trimester choline and 808 with data on second-trimester choline (n = 890 with WRAML2 score and at least 1 choline measurement). The institutional review boards of Harvard Pilgrim Health Care, Brigham and Women's Hospital, and Beth Israel Deaconess Medical Center approved the study protocols, and all mothers provided written informed consent.
Measurements
At each of the first- and second-trimester study visits, to assess intake of choline and the other methyl donors from food, we administered an approximately 130-item semiquantitative food frequency questionnaire (FFQ) modified from the well-validated instrument used in the Nurses' Health Study and other large cohort studies (17, 18) and further calibrated for use in pregnancy (19). Previous investigators have shown that similar FFQs measure choline, vitamin B12, betaine, and folate accurately compared with other dietary assessment methods and/or relevant biomarker concentrations (20, 21). In the Framingham Offspring Study, which used a similar approximately 130-item FFQ, Cho et al. (20) found an inverse dose-response association between dietary choline intake and plasma total homocysteine concentration, suggesting that the FFQ validly assessed choline at physiologically relevant levels. For the FFQ used at the first-trimester visit (mean gestational age at visit = 11.7 (standard deviation (SD), 3.2) weeks), the time referent was “during this pregnancy,” that is, from the date of the last menstrual period to the assessment. We also conducted a 33-item detailed interview about use (frequency, brand/type, dosage, and timing) of nutritional supplements in early pregnancy. We calculated total first-trimester maternal intake of the nutrients examined by summing food and supplement contributions. For the FFQ used at the second-trimester visit (mean gestational age at visit = 29.1 (SD, 2.4) weeks), the time referent was “during the last 3 months.” The second-trimester FFQ itself also included questions about use of nutritional supplements, which we used to calculate total second-trimester intake. We adjusted micronutrient intake for total energy intake using a residuals method (22).
We administered cognitive tests at the children's age 7 in-person visits and performed periodic quality assurance tests to ensure correct administration and scoring of the tests. The WRAML2 Design and Picture Memory subtests assess visuospatial memory (23). The Kaufman Brief Intelligence Test, Second Edition (KBIT-2), assesses verbal and nonverbal intelligence to create a composite IQ score and is reliable and valid for use in children aged 4 years or more (24). We also administered this test to mothers at the child's age 7 visit to assess maternal verbal and nonverbal intelligence. All test scores were age-standardized. We assessed cognition on a continuous scale.
Using a combination of questionnaires and interviews, we collected information about a range of sociodemographic factors, lifestyle habits, and medical and reproductive history (16). Mothers reported their educational level, smoking during pregnancy, date of birth, and parity, the father's educational level, the child's race/ethnicity, and the child's primary language. For women with missing data on smoking status, we reviewed clinical records to assess smoking during pregnancy. We used the FFQ to obtain information on maternal fish consumption and total energy intake. We previously calibrated the questionnaire against elongated n-3 fatty acids in erythrocytes (19) and found associations between maternal fish intake during pregnancy and child cognition at age 3 years (25, 26). Mothers completed the Home Observation for Measurement of the Environment (HOME) middle childhood questionnaire, which assesses maternal interactions and the home environment. The HOME score independently predicts cognitive development and has been used in previous longitudinal studies such as the National Longitudinal Study of Youth (27).
Statistical analysis
After examining baseline characteristics of the study population, we calculated Spearman correlation coefficients for correlations between first- and second-trimester intakes of choline and the other methyl donor nutrients.
We conducted linear regression of the cognitive tests on maternal intake of choline using quartiles of choline intake to minimize the influence of outliers. We calculated P values for trend based on the median value within each quartile of intake, using the Wald test. We assessed the bivariate association between choline and the cognitive test outcomes; then adjusted choline for vitamin B12, betaine, and folate; and finally added variables that we considered a priori to be confounders or that were associated with maternal dietary intake of methyl donors and/or child cognitive function. We present results from sequential multivariable models to illustrate the extent to which addition of covariates changed effect estimates. The final model included adjustment for maternal intake of other methyl donors, age, race/ethnicity, education, KBIT-2 score, parity, smoking, and fish and energy intakes during pregnancy, paternal education, HOME score, and child's age and sex.
We additionally considered household income, maternal prepregnancy body mass index (weight (kg)/height (m)2), maternal iron intake, exercise, and alcohol consumption during pregnancy as potential confounders in the multivariable models, but none of these variables materially changed (i.e., by >10%) exposure-outcome associations, so we did not include them in the final model. We also examined the extent to which associations were modified by child sex by examining associations separately in females versus males and by including an interaction term in the final model.
We examined maternal intake of vitamin B12 and folate/folic acid from food or supplements separately to see if one had a stronger association with the cognitive test results than the other. Finally, we separately examined whether vitamin B2, vitamin B6, methionine, iron, cadmium, and zinc—other nutrients involved in the methylation pathways—were individually associated with child cognition in the fully adjusted model.
We performed all calculations in SAS, version 9.2 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Characteristics of the 813 mother-child pairs are shown in Tables 1 and 2. Daily mean maternal first-trimester nutrient intake was 335 (SD, 64) mg for choline, 11.1 (SD, 17.5) µg for vitamin B12, 250 (SD, 110) mg for betaine, and 972 (SD, 392) µg for folate; second-trimester mean nutrient intake was 328 (SD, 63) mg for choline, 10.5 (SD, 5.1) µg for vitamin B12, 240 (SD, 104) mg for betaine, and 1,268 (SD, 381) µg for folate. Mean age 7 cognitive test scores were 17.2 (SD, 4.4) points on the WRAML2 Design and Picture Memory subtests, 114.3 (SD, 13.9) points on the verbal KBIT-2, and 107.8 (SD, 16.5) points on the nonverbal KBIT-2. The Spearman correlation between first- and second-trimester choline intakes was 0.53, while the correlations between concurrent intakes of choline and vitamin B12, betaine, or folate were moderate to low (rs = 0.03–0.40).
Table 1.
Selected Characteristics of 813 Mother-Child Pairs in Project Viva According to Quartile of Second-Trimester Choline Intake, Eastern Massachusetts, 1999–2002 to 2008–2011a
| Overall | Quartile of Second-Trimester Choline Intake |
||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Mother | |||||
| Age, years | 32.9 (4.6) | 32.1 (4.9) | 32.6 (4.3) | 33.3 (4.4) | 33.6 (4.7) |
| Prepregnancy BMIb | 24.4 (4.9) | 24.7 (5.2) | 24.5 (5.1) | 24.3 (4.5) | 23.9 (4.8) |
| Gestational weight gain, poundsc | 34.3 (11.4) | 34.6 (10.6) | 32.9 (11.9) | 34.5 (11.2) | 35.0 (11.8) |
| Physical activity during pregnancy, hours/week | 6.7 (6.3) | 7.0 (7.5) | 6.8 (5.9) | 6.5 (5.4) | 6.7 (6.1) |
| Second-trimester energy intake, calories/day | 2,149 (606) | 2,007 (558) | 2181 (626) | 2178 (573) | 2229 (643) |
| Energy-adjusted daily dietary intake during second trimester | |||||
| Choline, mg | 328 (63) | 253 (29) | 308 (11) | 344 (11) | 406 (49) |
| Vitamin B12, µg | 10.5 (5.1) | 8.8 (2.8) | 9.8 (3.3) | 10.7 (3.3) | 12.7 (8.1) |
| Betaine, mg | 240 (104) | 224 (101) | 244 (105) | 233 (89) | 258 (118) |
| Folate, µg | 1,268 (381) | 1,197 (418) | 1261 (382) | 1308 (338) | 1306 (376) |
| Second-trimester fish intake, servings/day | 0.2 (0.2) | 0.2 (0.2) | 0.2 (0.2) | 0.2 (0.2) | 0.3 (0.3) |
| KBIT-2 composite score | 108.8 (14.8) | 107.2 (14.4) | 110.2 (13.7) | 110.3 (14.4) | 107.4 (16.4) |
| Child | |||||
| Birth-weight-for-gestational-age z score | 0.2 (1.0) | 0.1 (0.9) | 0.3 (1.0) | 0.3 (1.0) | 0.3 (1.0) |
| Age at 7-year visit, years | 7.8 (0.8) | 7.9 (0.7) | 7.8 (0.8) | 7.9 (0.9) | 7.8 (0.8) |
| WRAML2 score | 17.2 (4.4) | 16.6 (4.6) | 17.2 (4.6) | 17.2 (4.4) | 17.8 (3.9) |
| KBIT-2 verbal score | 114.3 (13.9) | 112.9 (14.5) | 114.5 (13.3) | 115.2 (14.1) | 114.5 (13.8) |
| KBIT-2 nonverbal score | 107.8 (16.5) | 106.3 (16.3) | 108.0 (16.3) | 108.0 (17.2) | 109.1 (16.3) |
| BMI z score at age 7 years | 0.3 (1.0) | 0.4 (0.9) | 0.2 (1.0) | 0.2 (1.0) | 0.3 (1.0) |
Abbreviations: BMI, body mass index; KBIT-2, Kaufman Brief Intelligence Test, Second Edition; WRAML2, Wide Range Assessment of Memory and Learning, Second Edition (Design and Picture Memory subtests).
a All data shown are mean values with standard deviations in parentheses. Median choline intakes (and ranges) were as follows: overall, 326 mg/day (range, 141–806); quartile 1, 260 mg/day (range, 141–288); quartile 2, 309 mg/day (range, 288–326); quartile 3, 344 mg/day (range, 326–364); quartile 4, 392 mg/day (range, 364–806).
b Weight (kg)/height (m)2.
c 1 pound = 0.45 kg.
Table 2.
Selected Characteristics (Number and Percentage) of 813 Mother-Child Pairs in Project Viva According to Quartile of Second-Trimester Choline Intake, Eastern Massachusetts, 1999–2002 to 2008–2011
| Quartile of Second-Trimester Choline Intake |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Mother | ||||||||||
| Race/ethnicity | ||||||||||
| Asian | 48 | 5.9 | 6 | 3.0 | 11 | 5.4 | 8 | 3.9 | 23 | 11.3 |
| Black | 86 | 10.6 | 23 | 11.3 | 21 | 10.3 | 17 | 8.3 | 25 | 12.3 |
| Hispanic | 31 | 3.8 | 3 | 1.5 | 8 | 3.9 | 10 | 4.9 | 10 | 4.9 |
| White | 619 | 76.1 | 162 | 79.8 | 158 | 77.8 | 165 | 80.9 | 134 | 66.0 |
| >1 race/other | 29 | 3.6 | 9 | 4.4 | 5 | 2.5 | 4 | 2.0 | 11 | 5.4 |
| Education | ||||||||||
| Completed high school or less | 197 | 24.2 | 60 | 29.6 | 41 | 20.2 | 44 | 21.6 | 52 | 25.6 |
| Completed college | 294 | 36.2 | 75 | 37.0 | 77 | 37.9 | 75 | 36.8 | 67 | 33.0 |
| Completed graduate degree | 322 | 39.6 | 68 | 33.5 | 85 | 41.9 | 85 | 41.7 | 84 | 41.4 |
| Multipara | 420 | 51.7 | 95 | 46.8 | 109 | 53.7 | 105 | 51.5 | 111 | 54.7 |
| Smoking status | ||||||||||
| Never smoker | 574 | 70.6 | 143 | 70.4 | 143 | 70.4 | 139 | 68.1 | 149 | 73.4 |
| Smoked during early pregnancy | 64 | 7.9 | 19 | 9.4 | 12 | 5.9 | 17 | 8.3 | 16 | 7.9 |
| Former smoker | 175 | 21.5 | 41 | 20.2 | 48 | 23.7 | 48 | 23.5 | 38 | 18.7 |
| Child | ||||||||||
| Male sex | 402 | 49.5 | 98 | 48.3 | 96 | 47.3 | 106 | 52.0 | 102 | 50.3 |
| Household | ||||||||||
| Income >$70,000/year | 538 | 69.4 | 122 | 63.9 | 140 | 70.0 | 150 | 76.5 | 126 | 67.0 |
| Biological father | ||||||||||
| Completed high school or less | 235 | 28.9 | 70 | 34.5 | 57 | 28.1 | 52 | 25.5 | 56 | 27.6 |
| Completed college | 307 | 37.8 | 65 | 32.0 | 85 | 41.9 | 83 | 40.7 | 74 | 36.5 |
| Completed graduate degree | 271 | 33.3 | 68 | 33.5 | 61 | 30.1 | 69 | 33.8 | 73 | 36.0 |
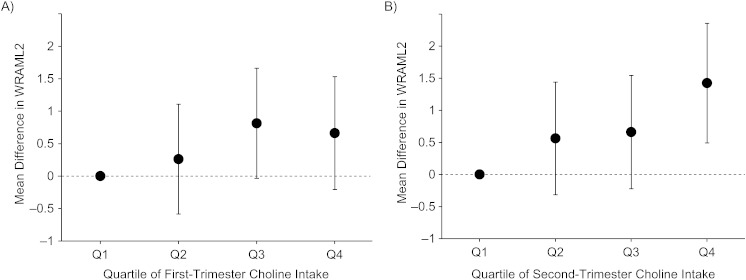
In bivariate models, a higher quartile of choline intake was associated with higher WRAML2 visual memory score, and multivariable adjustment for confounders changed the association very little (Table 3). In the fully adjusted model, children exposed to the highest quartile of first- and second-trimester choline intake had WRAML2 test scores 0.7 (95% CI: −0.2, 1.5; P-trend = 0.08) and 1.4 (95% CI: 0.5, 2.4; P-trend = 0.003) points higher, respectively, than those in the lowest quartile (Table 3, Figure 1). Males exposed to the highest quartile of second-trimester choline intake had WRAML2 scores 2.3 points higher (95% CI: 0.9, 3.7; P-trend = 0.001) than the lowest quartile, compared with 0.8 points higher (95% CI: −0.5, 2.1; P-trend = 0.22) in females. However, the P value for interaction between diet and child's sex was 0.44, indicating that we cannot be confident that there is truly a different association in males and females.
Table 3.
Association Between Maternal Choline Intake During the First and Second Trimesters of Pregnancy and Child WRAML2 Score at Age 7 Years, Project Viva (n = 890 Mother-Child Pairs), Eastern Massachusetts, 1999–2002 to 2008–2011a
| Trimester and Modelb |
Quartile of Material Choline Intake |
P for Trend |
||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 (Referent) |
Quartile 2 |
Quartile 3 |
Quartile 4 |
|||||
| β | 95% CI | β | 95% CI | β | 95% CI | |||
| First trimester (n = 861) | 266 (162–293)c | 312 (294–332) | 349 (332–375) | 404 (375–668) | ||||
| 1 | 0 | 0.4 | −0.4, 1.2 | 1.0 | 0.1, 1.8 | 0.7 | −0.1, 1.5 | 0.06 |
| 2 | 0 | 0.3 | −0.5, 1.1 | 0.8 | −0.1, 1.6 | 0.6 | −0.3, 1.4 | 0.12 |
| 3 | 0 | 0.2 | −0.6, 1.1 | 0.7 | −0.1, 1.6 | 0.6 | −0.2, 1.5 | 0.09 |
| 4 | 0 | 0.3 | −0.6, 1.1 | 0.8 | 0.0, 1.7 | 0.7 | −0.2, 1.5 | 0.08 |
| Second trimester (n = 808) | 260 (141–288) | 309 (288–326) | 344 (326–364) | 392 (364–806) | ||||
| 1 | 0 | 0.5 | −0.3, 1.4 | 0.6 | −0.3, 1.4 | 1.2 | 0.3, 2.0 | 0.01 |
| 2 | 0 | 0.5 | −0.3, 1.4 | 0.6 | −0.2, 1.5 | 1.2 | 0.3, 2.1 | 0.01 |
| 3 | 0 | 0.5 | −0.4, 1.3 | 0.5 | −0.3, 1.4 | 1.2 | 0.3, 2.1 | 0.01 |
| 4 | 0 | 0.6 | −0.3, 1.4 | 0.7 | −0.2, 1.5 | 1.4 | 0.5, 2.4 | 0.003 |
Abbreviations: CI, confidence interval; HOME, Home Observation for Measurement of the Environment; KBIT-2, Kaufman Brief Intelligence Test, Second Edition; WRAML2, Wide Range Assessment of Memory and Learning, Second Edition (Design and Picture Memory subtests).
a The β coefficient represents the mean difference in WRAML2 score from the lowest quartile (referent).
b Model 1, unadjusted; model 2, adjusted for intakes of vitamin B12, betaine, and folate; model 3, additionally adjusted for maternal KBIT-2 score; model 4, additionally adjusted for maternal age, race/ethnicity, education, parity, smoking, and fish and energy intake during pregnancy; paternal education; HOME score; and child's sex and age at the 7-year visit.
c Median maternal choline intake (range), in mg/day.
Figure 1.
Associations of first-trimester (A) and second-trimester (B) choline intakes with score on the Wide Range Assessment of Memory and Learning, Second Edition (WRAML2), Design and Picture Memory subtests at age 7 years among 890 mother-child pairs in Project Viva, eastern Massachusetts, 1999–2002 to 2008–2011. Black dots show the mean difference from the lowest quartile (referent) after adjustment for maternal age, race/ethnicity, education, parity, smoking, intakes of vitamin B12, betaine, folate, fish, and energy during pregnancy, and KBIT-2 score; paternal education; HOME score; and child's sex and age at the 7-year visit. Median values (in mg/day) within quartiles (Q) of choline intake were: first trimester—Q1, 266; Q2, 312; Q3, 349; and Q4, 404 (P-trend = 0.08); second trimester—Q1, 260; Q2, 309; Q3, 344; Q4, 392 (P-trend = 0.003). Bars, 95% confidence interval. HOME, Home Observation for Measurement of the Environment; KBIT-2, Kaufman Brief Intelligence Test, Second Edition.
Maternal intakes of vitamin B12, betaine, and folate were directly associated with the cognitive test results in some bivariate models, but mutually adjusting for intake of all 4 nutrients and other covariates attenuated these associations (Table 4). There was a suggestion of a positive association between second-trimester choline intake and child nonverbal KBIT-2 score (quartile 4 vs. quartile 1 effect estimate = 3.5, 95% CI: 0.1, 6.9; P-trend = 0.06). Associations of folate and vitamin B12 with the cognitive test results were null regardless of whether we assessed intake from food and supplements separately. Intakes of methionine, vitamins B2 and B6, iron, cadmium, and zinc also were not associated with the cognitive test results in multivariable models.
Table 4.
Association Between Maternal Methyl Donor Intake During the First and Second Trimesters of Pregnancy and Child Cognitive Outcomes at Age 7 Years (Final Modela), Project Viva (n = 895 Mother-Child Pairs), Eastern Massachusetts, 1999–2002 to 2008–2011b
| Methyl Donor and Cognitive Test | Quartile of Maternal Methyl Donor Intake |
P for Trend | ||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 (Referent) |
Quartile 2 |
Quartile 3 |
Quartile 4 |
|||||
| β | 95% CI | β | 95% CI | β | 95% CI | |||
| First Trimester | ||||||||
| Choline | ||||||||
| WRAML2 | 0.00 | 0.3 | −0.6, 1.1 | 0.8 | 0.0, 1.7 | 0.7 | −0.2, 1.5 | 0.08 |
| KBIT-2 verbal | 0.00 | 1.4 | −0.9, 3.7 | 1.4 | −1.0, 3.7 | 1.3 | −1.1, 3.7 | 0.32 |
| KBIT-2 nonverbal | 0.00 | 0.3 | −2.8, 3.4 | −0.6 | −3.7, 2.5 | 1.0 | −2.2, 4.2 | 0.63 |
| Vitamin B12 | ||||||||
| WRAML2 | 0.00 | 0.1 | −0.8, 1.0 | −0.1 | −1.1, 0.8 | 0.0 | −1.0, 1.0 | 0.94 |
| KBIT-2 verbal | 0.00 | 3.0 | 0.5, 5.5 | 0.9 | −1.7, 3.5 | 0.4 | −2.3, 3.1 | 0.59 |
| KBIT-2 nonverbal | 0.00 | 0.0 | −3.3, 3.4 | 2.3 | −1.2, 5.8 | 1.1 | −2.5, 4.7 | 0.47 |
| Betaine | ||||||||
| WRAML2 | 0.00 | 0.5 | −0.4, 1.3 | 0.2 | −0.7, 1.0 | 0.8 | −0.1, 1.7 | 0.14 |
| KBIT-2 verbal | 0.00 | −2.0 | −4.3, 0.3 | −1.4 | −3.8, 1.0 | −0.8 | −3.2, 1.6 | 0.84 |
| KBIT-2 nonverbal | 0.00 | 0.6 | −2.5, 3.7 | −0.1 | −3.2, 3.1 | 2.6 | −0.6, 5.9 | 0.11 |
| Folate | ||||||||
| WRAML2 | 0.00 | 0.0 | −0.9, 0.8 | 0.4 | −0.5, 1.4 | 0.5 | −0.4, 1.4 | 0.21 |
| KBIT-2 verbal | 0.00 | 0.7 | −1.7, 3.0 | 1.2 | −1.3, 3.7 | −0.2 | −2.7, 2.3) | 0.93 |
| KBIT-2 nonverbal | 0.00 | −1.7 | −4.9, 1.4 | −1.2 | −4.5, 2.1 | −0.6 | −3.9, 2.7 | 0.85 |
| Second Trimester | ||||||||
| Choline | ||||||||
| WRAML2 | 0.00 | 0.6 | −0.3, 1.4 | 0.7 | −0.2, 1.5 | 1.4 | 0.5, 2.4 | 0.003 |
| KBIT-2 verbal | 0.00 | 0.4 | −2.0, 2.9 | 1.1 | −1.3, 3.6 | 0.9 | −1.7, 3.5 | 0.43 |
| KBIT-2 nonverbal | 0.00 | 1.6 | −1.6, 4.8 | 1.3 | −1.8, 4.5 | 3.5 | 0.1, 6.9 | 0.06 |
| Vitamin B12 | ||||||||
| WRAML2 | 0.00 | 0.3 | −0.6, 1.3 | −0.2 | −1.2, 0.8 | 0.2 | −0.8, 1.3 | 0.86 |
| KBIT-2 verbal | 0.00 | −1.3 | −3.9, 1.4 | −1.5 | −4.3, 1.2 | −1.2 | −4.1, 1.7 | 0.51 |
| KBIT-2 nonverbal | 0.00 | 1.1 | −2.3, 4.5 | 1.0 | −2.6, 4.6 | −0.1 | −3.8, 3.7 | 0.81 |
| Betaine | ||||||||
| WRAML2 | 0.00 | 0.4 | −0.5, 1.3 | −0.1 | −1.0, 0.8 | 0.5 | −0.5, 1.4 | 0.46 |
| KBIT-2 verbal | 0.00 | 0.1 | −2.4, 2.6 | 1.0 | −1.5, 3.5 | 1.2 | −1.4, 3.8 | 0.29 |
| KBIT-2 nonverbal | 0.00 | −0.9 | −4.1, 2.4 | −0.7 | −4.0, 2.6 | 1.1 | −2.3, 4.5 | 0.37 |
| Folate | ||||||||
| WRAML2 | 0.00 | −0.5 | −1.4, 0.4 | −0.6 | −1.5, 0.3 | −0.7 | −1.6, 0.3 | 0.11 |
| KBIT-2 verbal | 0.00 | 2.5 | 0.0, 4.9 | 0.1 | −2.4, 2.5 | −0.4 | −3.0, 2.1 | 0.95 |
| KBIT-2 nonverbal | 0.00 | −0.6 | −3.9, 2.6 | −2.8 | −6.1, 0.4 | −1.4 | −4.7, 2.0 | 0.22 |
Abbreviations: CI, confidence interval; HOME, Home Observation for Measurement of the Environment; KBIT-2, Kaufman Brief Intelligence Test, Second Edition; WRAML2, Wide Range Assessment of Memory and Learning, Second Edition (Design and Picture Memory subtests).
a Results were adjusted for maternal age, race/ethnicity, education, parity, smoking, intakes of choline, vitamin B12, betaine, and folate, fish, and energy during pregnancy, and KBIT-2 score; paternal education; HOME score; and child's sex and age at the 7-year visit.
b The β coefficient represents the mean difference in score from the lowest quartile (referent).
DISCUSSION
In this prospective cohort study, higher maternal second-trimester choline intake was associated with modestly higher child memory score at age 7 years as measured by the WRAML2 Design and Picture Memory subtests. First-trimester choline intake was also positively, albeit more weakly, associated with age 7 WRAML2 score. There was a suggestive positive association between second-trimester choline intake and nonverbal KBIT-2 score as well. However, intakes of vitamin B12, betaine, and folate were not associated with scores on any of the cognitive tests.
Our finding that maternal gestational choline intake was positively associated with child visual memory is consistent with robust data from animal models (3). For example, offspring of rat dams supplemented with choline in mid- to late gestation (days 11–17 or 18) performed better on the Morris water maze (6) and 12-arm radial maze (4) tests. Choline intake may alter brain function through formation of acetylcholine affecting cholinergic transmission (2) or changes in DNA methylation leading to differentiation and apototosis of neurons. In mice, choline supplementation of pregnant dams from days 12–17 of gestation caused differences in gene-specific methylation and protein expression in the fetal hippocampus, the region of the brain associated with memory (7). These changes in the brain lasted through at least age 24 months and were associated with increased cell proliferation and decreased apoptosis in fetal hippocampi (8, 9, 28, 29). Moreover, these cellular results were consistent in rats, in vivo and in vitro, and, interestingly, in cultured human neuroblastoma cells (9, 29–32).
We found a mean adjusted difference of 1.4 points in WRAML2 score between the highest and lowest quartiles of second-trimester choline intake. Unlike the usual IQ test with a mean score of 100 points, the mean for this test is about 17 points. The adjusted difference we found is approximately one-third of the standard deviation in this study population (4.4 points). Yasik et al. (33) found a similar order of magnitude (2.0 points) of difference in WRAML2 Design and Picture Memory scores between children and adolescents with posttraumatic stress disorder and those without the disorder. Previous studies have found that better working memory is associated with superior scholastic skills, including arithmetic, reading, and writing, and general academic achievement in school-aged children (34–36). Therefore, while the difference in memory in our study based on choline intake was modest and did not translate into overall IQ, it may be relevant in terms of the academic potential of the participants.
We found a stronger association of child memory with second-trimester choline intake than with first-trimester intake. This finding may suggest a stronger effect of choline on brain formation in midgestation than early in pregnancy. Most developmental animal studies focus on choline supplementation in mid- to late gestation, when cholinergic neurons in the forebrain undergo final mitotic division (37). However, previous animal studies did not directly compare effects of choline in early gestation and midgestation on memory (3). It is possible that the differences across gestational age are due to chance.
Our finding of a positive association between gestational choline intake and child memory is novel in humans. Signore et al. (10) found that serum choline in umbilical cord blood was not associated with child IQ score. However, the participants in that study came from a disadvantaged inner-city population, in which other factors may have influenced cognition more dramatically than diet. In contrast, the participants in our study were predominantly well-educated and of relatively higher socioeconomic status, so it is possible that we were better able to study the subtle association between diet and cognition in this population. Another difference is that Signore et al. examined serum levels, which may be influenced by other internal factors in the body, whereas we evaluated dietary intake. It is possible that the serum choline results reflected recent choline intake rather than long-term intake, although, as Signore et al. noted (10), women tended to have consistently high or low choline intakes across multiple time points during pregnancy. In addition, Signore et al. did not adjust for other potential confounders, such as maternal intake of fish or other methyl donors during pregnancy, parity, or paternal education (10), although in our study population there was little evidence of confounding by these factors. Finally, the full IQ test does not specifically measure visuospatial memory, which was the domain affected by choline intake in animal models. In a previous Project Viva analysis, we did not find meaningful associations between maternal choline intake and PPVT-III or WRAVMA scores at age 3 years, but these tests do not specifically assess visuospatial memory (11). Signore et al. isolated memory components of the IQ test and still did not find an association between choline and these outcomes (e.g., a 1-unit increase in cord serum choline z score was associated with a mean difference of 0.18 points on the block design subtest; P = 0.22) (10), but in our study we were able to use the WRAML2 Design and Picture Memory subtests, which are probably more representative of the domains affected by choline in animal models. This distinction may explain why we found an association in our study despite the previous null findings.
The association between second-trimester choline intake and WRAML2 score was stronger in males, although the P value for interaction was well above 0.05. Men may be more susceptible to choline deficiency than premenopausal women, because estrogen promotes de novo choline production through the phosphatidylethanolamine N-methyltransferase pathway (38). Because of differences in choline metabolism, the adequate intake levels set for choline in the United States by the Food and Nutrition Board of the Institute of Medicine are higher for men than for women, although they are identical for male and female children (39). Few animal studies of gestational choline and offspring cognition have considered effect modification by sex, but Williams et al. (40) found a greater effect of in utero choline supplementation on cholinergic neural cell size and memory tests in male rats. Since power was limited for stratified analysis in our study and these findings were post hoc, we suggest that future studies consider differences by sex to confirm or refute our results.
Our findings that vitamin B12 and folate were not associated with child cognition differed from some previous studies showing positive associations between gestational intake of these nutrients and child cognition (11, 13–15, 41). These studies used different cognitive tests, which could explain the differences in our results. We previously found that first-trimester maternal folate intake was associated with a modestly higher PPVT-III score (1.3 points for each 600-µg/day increment (95% CI: 0.1, 3.1)) at age 3 years, which may be explained by differences in cognitive domains tested at ages 3 and 7 years (11). The differences in our findings at age 7 years from other studies may also be due to the fact that our participants, unlike participants in other studies, were generally folate-replete and had adequate vitamin B12 intake. For example, in our cohort, only 4 women (0.5%) did not meet the Recommended Daily Allowance for vitamin B12 of 2.6 µg/day in the first trimester of pregnancy, while in the Pune study, nearly half of the women had low plasma B12 levels (13). It is plausible that folate, vitamin B12, and other methyl donors are also important for brain development and cognition but that beyond the high level of these nutrients in our study due to fortification and supplementation, there is no additional benefit for child cognition.
Our analysis had several potential limitations. First, dietary intake is always difficult to measure in epidemiologic studies, since intake of nutrients is continuous and constantly changing. The study visits did not occur at the precise end of the first or second trimester for every woman, which may have added some measurement error in the timing of intake. In addition, conducting the analysis by quartile of nutrient intake introduces imprecision in the exposure, reduces power, and could bias multivariable-adjusted results. However, we prospectively collected dietary data using a modified FFQ calibrated for use during pregnancy (19) that was similar to FFQs shown in previous studies to validly measure choline and other methyl donor nutrients (20, 21). After adjustment for total energy intake, the FFQ should accurately rank individual intakes of nutrients, and the quartiles should discriminate between women with very high and very low intakes. Second, there was potential for error in measurement of the cognitive domains, but the tests were administered by trained research assistants, and any error in the dependent variable would probably have caused reduced precision in our confidence intervals, making our findings conservative. Third, we only had data on dietary intake from the first and second trimesters of pregnancy, not on third-trimester, postnatal, or child diet. We may have missed associations if intake during the third trimester is important in cognitive development in the offspring. Fourth, there was the potential for unmeasured confounding, particularly from maternal and paternal memory in the WRAML2 analysis. However, we measured many environmental, sociodemographic, and biological covariates, especially parental education, maternal KBIT-2 score, and HOME score, that did not substantially attenuate the associations between choline and child memory.
Loss to follow-up is another concern, given that more than half of the original cohort was not included in this analysis and that those lost to follow-up were more likely to be racial/ethnic minorities and of lower socioeconomic status. However, choline intake did not differ according to follow-up, results were similar when we limited our analysis to underrepresented racial and socioeconomic groups, and associations were robust despite adjustment for confounding. Finally, our finding of a positive association between gestational choline intake and age 7 WRAML2 score may have been due to chance, since we examined many associations. However, these findings are consistent with the robust animal literature showing a causal relationship between gestational choline intake and offspring memory and performance (3). Other strengths of this study include its prospective design, detailed dietary information, and large sample size.
In conclusion, higher maternal gestational intake of choline, but not intake of other methyl donors, was associated with modestly better child memory at age 7 years as measured by WRAML2 score. We also found a suggestive positive association of maternal second-trimester choline intake with KBIT-2 nonverbal score. In the future, investigators should examine this association in other studies and assess benefits and risks of choline intake in multiple populations before we can recommend choline supplementation in pregnancy.
ACKNOWLEDGMENTS
Author affiliations: Departments of Nutrition and Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Caroline E. Boeke, Eduardo Villamor); Channing Division of Network Medicine, Brigham and Women's Hospital, Boston, Massachusetts (Caroline E. Boeke); Obesity Prevention Program, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts (Matthew W. Gillman, Sheryl L. Rifas-Shiman, Emily Oken); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Michael D. Hughes); and Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan (Eduardo Villamor).
This work was supported by grants from the National Institutes of Health (grants K24 HL 068041, K24 HD 069408, R01 HD 034568, R01 ES 016314, and T32 CA 09001).
This paper was presented as an abstract at the Developmental Origins of Health and Disease 7th World Congress, Portland, Oregon, September 18–21, 2011. Dr. Caroline Boeke received the Junior Investigator Clinical Research Excellence Award based on the abstract and presentation.
Conflict of interest: none declared.
REFERENCES
- 1.Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr. 2009;89(2):673S–677S. doi: 10.3945/ajcn.2008.26811D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6(1):48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- 3.McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30(5):696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res Dev Brain Res. 1999;118(1-2):51–59. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 5.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27(4):385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 6.Mellott TJ, Williams CL, Meck WH, et al. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18(3):545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- 7.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20(1):43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albright CD, Tsai AY, Friedrich CB, et al. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999;113(1-2):13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 9.Albright CD, Friedrich CB, Brown EC, et al. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res. 1999;115(2):123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 10.Signore C, Ueland PM, Troendle J, et al. Choline concentrations in human maternal and cord blood and intelligence at 5 y of age. Am J Clin Nutr. 2008;87(4):896–902. doi: 10.1093/ajcn/87.4.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villamor E, Rifas-Shiman SL, Gillman MW, et al. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr Perinat Epidemiol. 2012;26(4):328–335. doi: 10.1111/j.1365-3016.2012.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poly C, Massaro JM, Seshadri S, et al. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am J Clin Nutr. 2011;94(6):1584–1591. doi: 10.3945/ajcn.110.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhate V, Deshpande S, Bhat D, et al. Vitamin B12 status of pregnant Indian women and cognitive function in their 9-year-old children. Food Nutr Bull. 2008;29(4):249–254. doi: 10.1177/156482650802900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veena SR, Krishnaveni GV, Srinivasan K, et al. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J Nutr. 2010;140(5):1014–1022. doi: 10.3945/jn.109.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Rio Garcia C, Torres-Sanchez L, Chen J, et al. Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): their impact on child neurodevelopment. Nutr Neurosci. 2009;12(1):13–20. doi: 10.1179/147683009X388913. [DOI] [PubMed] [Google Scholar]
- 16.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, et al. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144(2):240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, et al. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14(10):754–762. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Cho E, Zeisel SH, Jacques P, et al. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83(4):905–911. doi: 10.1093/ajcn/83.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verkleij-Hagoort AC, de Vries JH, Stegers MP, et al. Validation of the assessment of folate and vitamin B12 intake in women of reproductive age: the method of triads. Eur J Clin Nutr. 2007;61(5):610–615. doi: 10.1038/sj.ejcn.1602581. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 23.Adams W, Sheslow D. WRAML2: Wide Range Assessment of Memory and Learning, Second Edition. Wilmington, DE: Wide Range, Inc; 2003. [Google Scholar]
- 24.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition. Minneapolis, MN: American Guidance Service, Inc; 2004. [Google Scholar]
- 25.Oken E, Radesky JS, Wright RO, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167(10):1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donahue SM, Rifas-Shiman SL, Olsen SF, et al. Associations of maternal prenatal dietary intake of n-3 and n-6 fatty acids with maternal and umbilical cord blood levels. Prostaglandins Leukot Essent Fatty Acids. 2009;80(5-6):289–296. doi: 10.1016/j.plefa.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman PK, Reardon DC, Cougle J. The quality of the caregiving environment and child developmental outcomes associated with maternal history of abortion using the NLSY data. J Child Psychol Psychiatry. 2002;43(6):743–757. doi: 10.1111/1469-7610.00095. [DOI] [PubMed] [Google Scholar]
- 28.Albright CD, Siwek DF, Craciunescu CN, et al. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr Neurosci. 2003;6(2):129–134. doi: 10.1080/1028415031000084418. [DOI] [PubMed] [Google Scholar]
- 29.Craciunescu CN, Albright CD, Mar MH, et al. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133(11):3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niculescu MD, Craciunescu CN, Zeisel SH. Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Res Mol Brain Res. 2005;134(2):309–322. doi: 10.1016/j.molbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Yen CL, Mar MH, Meeker RB, et al. Choline deficiency induces apoptosis in primary cultures of fetal neurons. FASEB J. 2001;15(10):1704–1710. doi: 10.1096/fj.00-0800com. [DOI] [PubMed] [Google Scholar]
- 32.Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89(5):1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasik AE, Saigh PA, Oberfield RA, et al. Posttraumatic stress disorder: memory and learning performance in children and adolescents. Biol Psychiatry. 2007;61(3):382–388. doi: 10.1016/j.biopsych.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Andersson U. Working memory as a predictor of written arithmetical skills in children: the importance of central executive functions. Br J Educ Psychol. 2008;78(2):181–203. doi: 10.1348/000709907X209854. [DOI] [PubMed] [Google Scholar]
- 35.Gathercole SE, Brown L, Pickering SJ. Working memory assessments at school entry as longitudinal 109 predictors of National Curriculum attainment levels. Educ Child Psychol. 2003;20(3):109–122. [Google Scholar]
- 36.Henry L, MacLean M. Relationships between working memory, expressive vocabulary and arithmetical reasoning in children with and without intellectual disabilities. Educ Child Psychol. 2003;20(3):51–64. [Google Scholar]
- 37.Semba K, Fibiger HC. Time of origin of cholinergic neurons in the rat basal forebrain. J Comp Neurol. 1988;269(1):87–95. doi: 10.1002/cne.902690107. [DOI] [PubMed] [Google Scholar]
- 38.Fischer LM, daCosta KA, Kwock L, et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85(5):1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. Food and Nutrition Board, Institute of Medicine. [PubMed] [Google Scholar]
- 40.Williams CL, Meck WH, Heyer DD, et al. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794(2):225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- 41.Julvez J, Fortuny J, Mendez M, et al. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr Perinat Epidemiol. 2009;23(3):199–206. doi: 10.1111/j.1365-3016.2009.01032.x. [DOI] [PubMed] [Google Scholar]