Abstract
Evidence to substantiate recommendations for restriction of caffeinated or acidic beverages as self-management for lower urinary tract symptoms (LUTS) is limited. We examined longitudinal and acute associations between beverage intake and LUTS in the Boston Area Community Health (BACH) cohort (n = 4,145) between 2002 and 2010. Multivariable models tested associations between baseline intakes and progression of LUTS at 5-year follow-up, between follow-up intakes and International Prostate Symptom Scores at follow-up, and between 5-year intake changes and LUTS progression. Greater coffee or total caffeine intake at baseline increased the odds of LUTS progression in men (coffee: >2 cups/day vs. none, odds ratio = 2.09, 95% confidence interval: 1.29, 3.40, P-trend = 0.01; caffeine: P-trend < 0.001), particularly storage symptoms. Women who increased coffee intake by at least 2 servings/day during follow-up (compared with categories of decreased or unchanged intakes) had 64% higher odds of progression of urgency (P = 0.003). Women with recently increased soda intake, particularly caffeinated diet soda, had higher symptom scores, urgency, and LUTS progression. Citrus juice intake was associated with 50% lower odds of LUTS progression in men (P = 0.02). Findings support recommendations to limit caffeinated beverage intake for LUTS, and in men, they suggest benefits of citrus juice consumption. Further clinical research is warranted, particularly of the precise role of sodas containing artificial sweeteners in bladder sensations and urological function.
Keywords: beverages; bladder outlet obstruction; carbonated beverages; coffee; lower urinary tract symptoms; urinary bladder, overactive
Lower urinary tract symptoms (LUTS) are prevalent in approximately 1 of 5 adults, and it is estimated that they will affect 2.3 billion persons worldwide by the year 2018 (1–4). LUTS include storage problems such as urgency, frequency, or nocturia and voiding problems such as weak or hesitant urinary stream or failure to empty the bladder completely. LUTS are strongly associated with diminished health-related quality of life, mental health, and work productivity (1, 5, 6). Although pharmacological treatments are available, behavioral modifications are primary treatment strategies to help manage symptoms.
Dietary advice for LUTS management often includes avoidance of certain types of foods or beverages that can irritate the bladder. In particular, suggestions to avoid caffeinated, carbonated, or acidic beverages are common (7, 8). It is plausible that these components of beverages have direct effects on the bladder and systemic effects that could contribute to LUTS, both soon after consumption and over the long term with habitual intake. In experiments in rodents, caffeine, artificial sweeteners, and ascorbic and citric acids (often used as preservatives in carbonated beverages) increased bladder pressure and detrusor muscle contraction (9–11).
Nevertheless, scientific evidence specifying the roles of certain beverages in LUTS development among men and women is limited. Most clinical studies included small numbers of subjects. For example, in a study of 12 men and women with overactive bladder symptoms, caffeine intake at a moderate dose of 4.5 mg/kg promoted urgency and frequency (12). In a crossover trial of 20 nonsymptomatic adults, artificially sweetened carbonated beverages led to urgency symptoms, regardless of caffeine content; however, the volume of fluid consumed was not considered in the study (13). To analyze these associations in larger populations, observational epidemiologic studies are useful. In an epidemiologic study in the United Kingdom, carbonated beverage intake was associated with 1-year onset of urgency symptoms in women but not men. There were no associations for coffee, tea, or fruit juice in either sex (14, 15).
Prospective studies are generally preferred to help establish temporal relationships between exposures and outcomes because cross-sectional associations could be due to reverse causation (i.e., LUTS cause changes in beverage intake behaviors). However, LUTS remission is common (2, 16, 17), and the temporal relationship between certain exposures, such as beverage intake, and LUTS onset might be brief. That is, LUTS could occur shortly after consumption, and it is possible that a proximate association would be missed in a longitudinal study.
The objective of this analysis was to examine relationships between beverage consumption and LUTS in a population-based sample of men and women. The Boston Area Community Health Survey (BACH) had the distinct advantage of conducting a qualitative substudy alongside quantitative data collection, with participants asked to openly discuss their management strategies for urological symptoms. Many participants mentioned generally altering intake of fluids to help manage symptoms. However, they did not consistently alter relative intakes of specific beverages, including coffee, soda, or juice (18). Thus, BACH is a unique resource to examine whether caffeinated, carbonated, and other beverages contribute to LUTS over and above total fluid intake. In this article, we use the quantitative BACH survey data to separately examine longitudinal and acute relationships between beverage intakes and LUTS, by analyzing 1) average beverage consumption over the year before baseline and progression of LUTS approximately 5 years later, 2) recent beverage intakes and the presence of LUTS during the follow-up interview, and 3) changes in intake levels and LUTS progression between baseline and follow-up.
MATERIALS AND METHODS
BACH is a prospective cohort study designed to assess the prevalence and determinants of urological symptoms. A random stratified cluster sample design was used to recruit Boston residents, 30–79 years of age, from 3 racial/ethnic groups. Study design details have been published (19). Participants provided written informed consent. The New England Research Institutes' Institutional Review Board approved the study.
Of the 5,502 participants (2,301 men, 3,201 women) who completed in-person interviews at baseline (2002–2005), 4,145 (1,610 men; 2,534 women) completed follow-up assessment approximately 5 years later (2006–2010). The mean (standard deviation) time to follow-up was 4.8 (0.6) years. Of those lost to follow-up, 348 were ineligible (deceased, incarcerated, or medically incapable), 350 refused, 655 were not reached, and 5 had incomplete follow-up data. Those lost to follow-up were more likely to be Hispanic, more than 70 years old, and male and to have lower socioeconomic status, but there were no significant differences in LUTS presence.
To separately evaluate longitudinal and acute associations between beverage intake and LUTS, 3 distinct analyses were conducted among participants who completed follow-up. Table 1 describes the beverage intake measures, operational definitions of LUTS, and sample sizes for each analysis.
Table 1.
Approach Used to Assess Longitudinal and Acute Associations Between Intake of Beverage Types and LUTS in Analysis of the Boston Area Community Health Survey, 2002–2010
| Association Examined | Longitudinal (After ∼5 Years) | Acute (Cross-Sectional) |
Intake Changes (Longitudinal)a |
|---|---|---|---|
| Study time points (years) used | Baseline (2002–2005) and follow-up (2006–2010) | Follow-up (2006–2010) | Baseline (2002–2005) and follow-up (2006–2010) |
| Beverage intake assessment | Baseline | Follow-up | Change from baseline to follow-up |
| Time reference | Usual beverage intake over the previous year | Average daily intake in previous 7 days | Average daily intake in previous 7 days |
| Measurement tool | Self-administered validated food frequency questionnaireb (46, 47) | In-person interview | In-person interview |
| Question format | “How many glasses of these beverages do you drink per day or per week?” | “Think about the past 7 days. How many servings of [item] did you drink each day? A serving is one 8 oz. glass or cup.” | “Think about the past 7 days. How many servings of [item] did you drink each day? A serving is one 8 oz. glass or cup.” |
| Response format | Categorical responses to “How often” (ranging from “never or less than once per month” to “2+ per day”) and “How much,” (ranging from 1 to ≥5, with a serving size of glass used for juice, bottles or cans for soda, and cup for tea/coffee) | Open numeric field | Open numeric field |
| Operationalized measure | Servings per day | ||
| LUTS assessment | Baseline and follow-up | Follow-up | Baseline and follow-up |
| Measurement tools | |||
| Operationalized measures | Progression of LUTS from baseline to follow-up (yes/no), defined as IPSS increase of: | Continuous IPSS at follow-up for: | Progression of LUTS from baseline to follow-up (yes/no), defined as IPSS increase of: |
|
|
|
|
| Exclusions from analysis |
|
None |
|
| Sample sizes available for analysis | |||
| Men | 1,101 | 1,610 | 1,608 |
| Women | 1,727 | 2,534 | 2,532 |
Abbreviations: IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms.
a Analyses were conducted as secondary analyses only for total coffee and total soda, because the questions for other or more specific beverage types were not available or comparable between baseline and follow-up, in either the in-person interview or the food frequency questionnaire.
b In addition to usual beverage intake, the baseline food frequency questionnaire was used to derive total caffeine intake from chocolate, coffee, green/black/iced tea, and cola.
In analysis of acute (i.e., temporally proximate) associations, the BACH follow-up interview assessment of beverages was used, rather than the follow-up food frequency questionnaire (FFQ), because the interview assessed recent intake and captured the caffeinated/noncaffeinated status of coffee, tea, and soda. However, the interview did not identify artificially sweetened sodas or juice variety. Thus, we obtained information on 100% citrus juice and soda type (diet vs. nondiet) from the follow-up FFQ. Additional analyses of soda were restricted to participants who had reported drinking diet and nondiet varieties; a 5-level exposure variable was created, which combined information on diet/nondiet and caffeinated/noncaffeinated sodas, with nondrinkers of soda used as the reference group.
Analyses of intake changes were secondary analyses, conducted by using the BACH interview assessments of recent consumption of coffee and soda only. Caffeinated content of coffee and soda was not assessed in the baseline interview; therefore, individual responses to caffeinated and decaffeinated/noncaffeinated coffee and soda at follow-up were summed to calculate changes in total intakes.
Measurement of LUTS
During the in-home interviews, LUTS were assessed by the International Prostate Symptom Score (IPSS) (20–22). The IPSS was originally developed and validated for benign prostatic hyperplasia in men (20) but has been validated (23) and repeatedly shown to capture LUTS in women (1, 21, 24–26). Total IPSS ranges from 0 to 35; the presence of moderate to severe total LUTS is identified by IPSS ≥8. The total score consists of subscores for the voiding symptoms of incomplete bladder emptying, intermittency, weak urinary stream, and hesitancy and for the storage symptoms of frequency, urgency, and nocturia. Because nocturia can be distinct in etiology from other storage symptoms and can be directly affected by stimulatory effects of caffeine, our analysis of the IPSS storage subscore excluded nocturia, thereby focusing on daytime frequency and urgency. Urgency was of particular interest because it is a hallmark storage symptom and might be less influenced by volume or timing of fluid intake than are nocturia or frequency symptoms (27).
Statistical analysis
Logistic regression was used to calculate odds ratios and 95% confidence intervals for dichotomous outcomes of progression (yes/no). Generalized linear models were used to obtain β (standard error) estimates for continuous symptom scores. For each aim, separate multivariable models were created to analyze the association between intake of each beverage type and LUTS outcome by sex. For inclusion in the models, we considered the following variables, which are suspected or established risk factors for LUTS or are correlated to LUTS: age; total energy intake; total nonalcoholic fluid intake; alcohol intake; waist circumference; physical activity; race/ethnicity; diabetes; urinary tract infections; cigarette smoking; bladder surgery; vitamin C supplement use; and use of diuretic, α-blocking (men only), 5-α reductase–inhibiting (men only), antispasmodic, anticholinergic, or tricyclic antidepressant medications. In models for women, we also considered surgery for urinary incontinence and menopausal status. Final multivariable models were created with consideration of variables' statistical significance and their effects on estimates of the association for the beverage of interest. Multiple imputation methods were used to impute values for variables with missing values, except variables derived from the FFQ. All statistical tests were 2 sided at α = 0.05 and were conducted in SUDAAN v.10.0.1 (RTI International, Research Triangle Park, North Carolina) or R v.2.14.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) with Zelig v3.5.5 (http://CRAN.R-project.org/package=Zelig).
RESULTS
At baseline, drinkers of more than 2 servings/day of cola were more likely to have diabetes and a larger waist circumference and be non-Hispanic black, whereas coffee drinkers were more commonly white (Table 2). Among the subsample who reported whether they typically drank diet or nondiet soda, 64.6% of men and 54.8% of women drank nondiet sodas.
Table 2.
Characteristics of Men and Women in the Longitudinal Analysis Boston Area Community Health Survey Subsample, Overall and by Consumption of Coffee, Cola, and Citrus Juice at Baseline (2002–2005)
| Characteristic | Overall | Average No. of 8-oz. Servings/Day |
|||||
|---|---|---|---|---|---|---|---|
| Coffee |
All Cola-Type Soda |
Orange/Grapefruit Juice |
|||||
| 0 | >2.0 | 0 | >2.0 | 0 | >1.0 | ||
| Men | |||||||
| No. of participants | 1,101 | 285 | 165 | 262 | 102 | 150 | 146 |
| Mean age, years | 49.9 | 49.3 | 52.5 | 52.1 | 46.1 | 50.9 | 48.6 |
| Mean waist circumference, cm | 97.7 | 98.5 | 99.6 | 95.6 | 104.0 | 98.2 | 97.5 |
| Physical activity, % | |||||||
| Low | 30 | 35 | 27 | 29 | 26 | 37 | 27 |
| Medium | 46 | 44 | 53 | 48 | 48 | 45 | 45 |
| High | 24 | 21 | 20 | 23 | 27 | 18 | 28 |
| Cigarette smoking, % | |||||||
| Never | 39 | 51 | 23 | 42 | 33 | 29 | 48 |
| Past | 31 | 25 | 31 | 32 | 29 | 31 | 25 |
| Current | 30 | 25 | 46 | 26 | 38 | 40 | 27 |
| Diabetes, % | 12 | 12 | 13 | 9 | 19 | 17 | 8 |
| Alcoholic drinks/day, % | |||||||
| None | 32 | 36 | 35 | 32 | 45 | 30 | 37 |
| 0.1–0.9 | 37 | 37 | 34 | 37 | 32 | 37 | 38 |
| ≥1 | 31 | 27 | 32 | 31 | 23 | 33 | 25 |
| Race/ethnicity, % | |||||||
| Black | 28 | 37 | 12 | 29 | 39 | 22 | 36 |
| Hispanic | 26 | 19 | 19 | 24 | 22 | 25 | 28 |
| White | 47 | 44 | 69 | 47 | 39 | 53 | 36 |
| Women | |||||||
| No. of participants | 1,727 | 461 | 196 | 537 | 115 | 372 | 142 |
| Mean age, years | 51.3 | 49.8 | 53.0 | 52.6 | 48.7 | 52.9 | 51.0 |
| Mean waist circumference, cm | 92.7 | 92.6 | 93.5 | 88.5 | 98.7 | 93.7 | 93.0 |
| Physical activity, % | |||||||
| Low | 33 | 31 | 41 | 32 | 37 | 38 | 37 |
| Medium | 51 | 54 | 46 | 51 | 53 | 47 | 55 |
| High | 16 | 15 | 13 | 17 | 10 | 16 | 9 |
| Cigarette smoking, % | |||||||
| Never | 51 | 58 | 34 | 50 | 42 | 40 | 58 |
| Past | 28 | 27 | 31 | 32 | 21 | 37 | 23 |
| Current | 21 | 16 | 35 | 17 | 37 | 23 | 19 |
| Diabetes, % | 12 | 11 | 12 | 9 | 17 | 14 | 10 |
| Alcoholic drinks/day, % | |||||||
| None | 47 | 50 | 51 | 43 | 55 | 46 | 57 |
| 0.1–0.9 | 41 | 40 | 37 | 45 | 31 | 43 | 30 |
| ≥1 | 12 | 10 | 13 | 13 | 14 | 11 | 13 |
| Race/ethnicity, % | |||||||
| Black | 32 | 43 | 15 | 26 | 44 | 27 | 39 |
| Hispanic | 26 | 19 | 26 | 25 | 22 | 18 | 39 |
| White | 41 | 38 | 59 | 49 | 34 | 55 | 22 |
The prevalence and progression of LUTS at 5-year follow-up were similar by sex (Table 3) and baseline FFQ completion status. Progression of LUTS occurred among 23% of participants, with a mean 6.1-point (standard error, 0.2) increase in IPSS. Storage symptoms were most common, particularly nocturia (34% of participants), frequency (21%), and urgency (15%).
Table 3.
Presence and Progression of LUTS at Follow-up (2006–2010) in the Boston Area Community Health Survey
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| IPSS (Sub)Score, Mean (SE) | Moderate to Severe LUTS Present, % | Change in IPSS,a Mean (SE) | Progression,a % | IPSS (Sub)Score, Mean (SE) | Moderate to Severe LUTS Present, % | Change in IPSS,a Mean (SE) | Progression,a % | |
| LUTS (total IPSS) | 4.7 (0.1) | 20.9 | 0.4 (0.1) | 22.5 | 4.9 (0.1) | 22.0 | 0.0 (0.1) | 22.7 |
| Voiding (subscore) | 1.8 (0.1) | 14.1 | 0.1 (0.1) | 20.0 | 1.5 (0.1) | 10.8 | 0.0 (0.1) | 15.4 |
| Storage (frequency/urgency subscore) | 1.7 (0.1) | 25.1 | 0.0 (0.1) | 18.9 | 2.0 (0.1) | 31.5 | 0.0 (0.1) | 23.2 |
Abbreviations: IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; SE, standard error.
a Among those with baseline food frequency questionnaire data available.
Longitudinal associations (baseline beverage intakes and LUTS progression)
Among women, baseline beverage intakes were generally not associated with progression in total IPSS, voiding, or frequency/urgency scores at follow-up (Table 4). However, progression of urgency symptoms was more likely for women drinking more than 2 cups/day of coffee versus none (adjusted odds ratio (OR) = 1.53, 95% confidence interval (CI): 1.02, 2.29). Women drinking 0.1–1 cup/day of diet cola-type sodas had 1.29 times the odds of reporting LUTS, particularly voiding symptoms, but the findings were marginally statistically significant (P = 0.048 LUTS, P = 0.053 voiding), and there was no association with greater intake levels.
Table 4.
Baseline (2002–2005) Beverage Intake and Progression of LUTS, Voiding, and Daytime Storage (Frequency and/or Urgency) Symptoms at 5-Year Follow-Up (2006–2010) in the Boston Area Community Health Survey: Multivariable-Adjusted Odds Ratios and 95% Confidence Intervalsa
| Beverage Type and Quantity (No. of 8-oz. Servings/day) | Men |
Women |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LUTS (Total IPSS) (n = 1,100) |
Voiding Symptoms (n = 1,099) |
Storage Symptoms (n = 1,086) |
LUTS (Total IPSS) (n = 1,725) |
Voiding Symptoms (n = 1,724) |
Storage Symptoms (n = 1,678) |
|||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Coffee (caffeinated) | ||||||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 0.1–2.0 | 1.41 | 0.98, 2.04 | 0.99 | 0.68, 1.42 | 1.34 | 0.91, 1.98 | 1.09 | 0.83, 1.45 | 1.14 | 0.82, 1.59 | 0.96 | 0.73, 1.27 |
| >2.0 | 2.09 | 1.29, 3.40** | 1.17 | 0.71, 1.93 | 1.72 | 1.01, 2.94* | 0.94 | 0.61, 1.43 | 1.12 | 0.69, 1.83 | 1.01 | 0.66, 1.53 |
| Tea (caffeinated black or green) | ||||||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 0.1–1.0 | 1.31 | 0.95, 1.81 | 1.21 | 0.86, 1.71 | 1.27 | 0.90, 1.81 | 1.08 | 0.83, 1.40 | 0.94 | 0.69, 1.27 | 0.90 | 0.69, 1.17 |
| >1.0 | 1.34 | 0.81, 2.20 | 1.41 | 0.84, 2.35 | 1.24 | 0.72, 2.11 | 0.67 | 0.45, 1.01 | 0.64 | 0.40, 1.02 | 0.78 | 0.53, 1.15 |
| Total cola-type carbonated sodas | ||||||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 0.1–1.0 | 1.01 | 0.71, 1.46 | 1.14 | 0.77, 1.69 | 0.85 | 0.58, 1.26 | 1.18 | 0.90, 1.54 | 1.24 | 0.90, 1.70 | 0.89 | 0.68, 1.17 |
| >1.0 | 1.12 | 0.71, 1.76 | 1.36 | 0.83, 2.22 | 1.24 | 0.77, 2.01 | 1.29 | 0.89, 1.86 | 1.17 | 0.76, 1.80 | 1.09 | 0.75, 1.57 |
| Nondiet cola-type carbonated sodas | ||||||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 0.1–1.0 | 0.98 | 0.70, 1.36 | 1.00 | 0.70, 1.42 | 0.99 | 0.69, 1.41 | 1.02 | 0.79, 1.32 | 1.17 | 0.88, 1.57 | 0.93 | 0.72, 1.20 |
| >1.0 | 1.26 | 0.75, 2.12 | 1.75 | 1.04, 2.96* | 1.31 | 0.77, 2.23 | 1.00 | 0.62, 1.62 | 0.94 | 0.54, 1.66 | 0.91 | 0.56, 1.48 |
| Diet cola-type carbonated sodas | ||||||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 0.1–1.0 | 1.07 | 0.77, 1.48 | 1.21 | 0.85, 1.71 | 0.94 | 0.66, 1.34 | 1.29 | 1.00, 1.67* | 1.35 | 1.00, 1.82 | 1.04 | 0.80, 1.36 |
| >1.0 | 1.18 | 0.69, 2.03 | 1.36 | 0.77, 2.43 | 1.17 | 0.65, 2.09 | 1.02 | 0.61, 1.69 | 1.33 | 0.75, 2.37 | 1.08 | 0.65, 1.79 |
| Citrus juice | ||||||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 0.1–1.0 | 0.67 | 0.44, 1.00* | 0.80 | 0.52, 1.23 | 0.64 | 0.41, 0.99* | 1.13 | 0.85, 1.51 | 1.22 | 0.87, 1.71 | 1.05 | 0.79, 1.41 |
| >1.0 | 0.50 | 0.28, 0.88* | 0.77 | 0.42, 1.40 | 0.51 | 0.28, 0.94* | 1.16 | 0.72, 1.87 | 1.40 | 0.81, 2.39 | 1.03 | 0.64, 1.66 |
| Total nonalcoholic fluids | ||||||||||||
| <8.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 8.0–10.9 | 0.94 | 0.65, 1.38 | 1.18 | 0.80, 1.74 | 0.79 | 0.53, 1.18 | 0.80 | 0.60, 1.08 | 1.06 | 0.75, 1.50 | 0.84 | 0.63, 1.14 |
| 11.0–12.9 | 0.94 | 0.61, 1.45 | 1.05 | 0.66, 1.66 | 0.75 | 0.46, 1.21 | 0.89 | 0.64, 1.23 | 0.93 | 0.62, 1.39 | 0.84 | 0.60, 1.17 |
| >13.0 | 1.14 | 0.74, 1.77 | 1.26 | 0.80, 1.97 | 1.00 | 0.62, 1.61 | 0.87 | 0.61, 1.24 | 1.16 | 0.77, 1.73 | 0.95 | 0.67, 1.35 |
Abbreviations: CI, confidence interval; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; OR, odds ratio.
* P < 0.05; ** P < 0.01.
a From multivariable logistic regression models adjusted for age, total energy intake (kcal/day), physical activity level (low, medium, high), and race/ethnicity. Models for women additionally included smoking status (never, former, current) and, for storage symptoms, history of urinary tract infection.
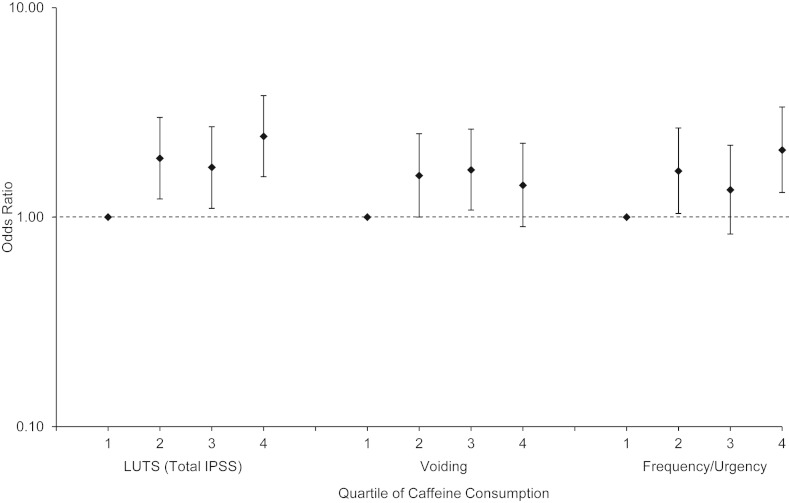
Men who consumed more than 2 cups/day of coffee were more likely to develop progression in LUTS (adjusted OR = 2.09, 95% CI: 1.29, 3.40, P = 0.003), particularly storage symptoms. There were no associations with caffeinated tea or diet cola. Men who drank more than 1 cup/day of regular, nondiet cola had 1.75 times the odds of developing progression in voiding symptoms. The total amount of caffeine ingested per day from foods and beverages was significantly associated with LUTS progression in men (Figure 1) but not in women (data not shown).
Figure 1.
Baseline (2002–2005) caffeine intake and progression of lower urinary tract symptoms (LUTS) at 5-year follow-up (2006–2010) among men from the Boston Area Community Health Survey: multivariable-adjusted log odds ratios with 95% confidence intervals (bar set at reference value of 1.0). Quartiles of average daily caffeine intake were as follows: first quartile, 0–48.0 mg; second quartile, 48.1–163.9 mg; third quartile, 164.0–339.9 mg; and fourth quartile, ≥340.0 mg. IPSS, International Prostate Symptom Score.
Among men only, greater citrus juice consumption was associated with decreased odds of LUTS progression (P-trend = 0.06). Men who drank more than 1 cup/day of citrus juice were less likely to have LUTS progression (OR = 0.50, 95% CI: 0.28, 0.88, P = 0.02), particularly frequency/urgency symptoms, than were nondrinkers.
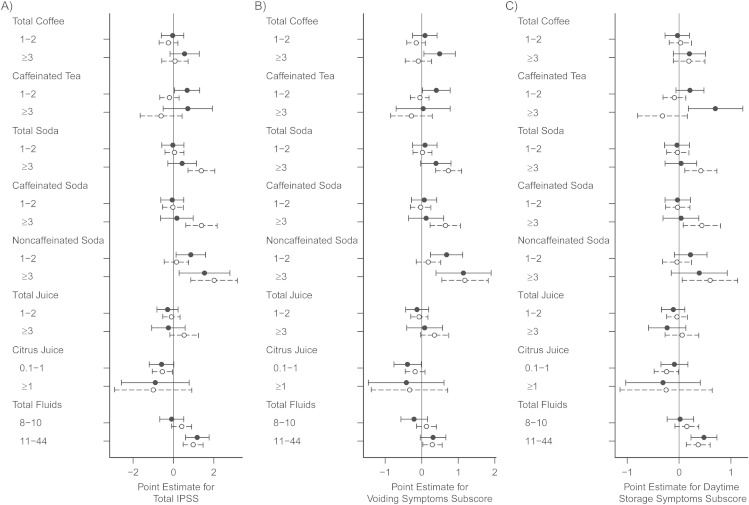
Acute associations (beverage intakes and LUTS at follow-up)
Total fluid intake in the previous week was associated with IPSS at follow-up. After adjustment for total fluids and other confounders, intake of more than 2 cups/day of coffee or caffeinated tea in the previous week was associated with worse voiding or total symptom scores in men but not in women (Figure 2). For example, the multivariable-adjusted storage symptoms score for men who recently drank more than 2 cups/day of caffeinated tea was 0.7 (95% CI: 0.2, 1.2) points higher than that of men who drank no caffeinated tea (P = 0.008). Results were similar for total coffee when separated into caffeinated or decaffeinated categories (not shown); decaffeinated herbal tea was not analyzed given qualitative evidence that some men drank herbal tea because of their urological symptoms (18). When all caffeinated beverages (coffee, tea, soda) were summed, men who drank more than 2 cups/day in the previous week were also more likely to report urgency symptoms (adjusted OR = 2.06, 95% CI: 1.23, 3.43).
Figure 2.
Recent beverage intakes (category of servings/day) and International Prostate Symptom Scores at follow-up (2006–2010) in 1,610 men (•) and 2,534 women (○) in the Boston Area Community Health Survey. β estimates and 95% confidence intervals are shown for A) total International Prostate Symptom Score, B) voiding symptoms subscore, and C) daytime storage (frequency, urgency, or both) subscore in association with recent beverage consumption. Recent intake referred to the previous 7 days for all beverages except citrus (orange or grapefruit) juice, for which intake was estimated from the subsample of respondents with follow-up food frequency questionnaire data (982 men; 1,623 women). For specific beverage types, the reference group was nondrinkers of that beverage. For total fluids, the reference group was drinkers of ≤7 servings/day of all beverages. Values were obtained from generalized linear multivariable models with adjustment for age, race/ethnicity, total fluid intake, urinary tract infection, antispasmodic or anticholinergic medication use, physical activity, and waist circumference.
Although coffee was not associated with the IPSS in women, women who drank more than 2 cups/day of coffee or caffeinated soda in the previous week were more likely to have urgency symptoms (coffee: OR = 1.54, 95% CI: 1.08, 2.18; soda: OR = 1.50, 95% CI: 1.01, 2.21). Soda was associated with IPSS (Figure 2) regardless of caffeine content. In exploratory analysis, caffeinated diet soda had the strongest associations with LUTS, particularly storage symptoms (e.g., for >1 cup/day vs. none: storage score β = 0.6, 95% CI: 0.1, 1.1; total IPSS β = 1.2, 95% CI: 0.1, 2.3), as compared with caffeinated nondiet (β = 0.1, 95% CI: −0.3, 0.5), noncaffeinated diet, or noncaffeinated nondiet soda. When women were classified into one type of diet/caffeine soda combination and compared with nondrinkers of soda, those drinking more than 1 cup/day of caffeinated diet soda in the previous week were the only group with a statistically significantly higher storage score (β = 0.4, 95% CI: 0.1, 0.7). Among men, associations were slightly stronger for noncaffeinated regular (nondiet) soda than for other diet/caffeine combinations of soda (not shown).
Total juice intake was not associated with LUTS. However, 1 cup/day of citrus juice was associated with lower IPSS for both men and women (β = −0.6, men P = 0.06, women P = 0.03).
Longitudinal changes in beverage intake and LUTS progression
Compared with those who did not change their coffee intake or changed it by 1 serving/day, men and women who increased coffee consumption by at least 2 servings/day between baseline and follow-up had 60%–80% higher odds of progression of storage symptoms (Table 5). Progression of urgency specifically (data not shown) was also associated with increased coffee intake of at least 2 servings/day in women (OR = 1.42, 95% CI: 1.03, 1.98, P = 0.04) and men (OR = 1.56, 95% CI: 1.04, 2.35, P = 0.03). Of note, for men, higher odds of storage or voiding progression were also observed with decreased coffee intake. However, this trend did not appear for urgency progression; only increased intake (described above), not decreased intake (OR = 1.06, 95% CI: 0.70, 1.60, P = 0.8) was associated with urgency progression. Women who increased soda consumption by at least 2 servings/day had 60% higher odds of LUTS progression.
Table 5.
Changes in Coffee or Soda Intake and Progression of LUTS, Voiding, and Daytime Storage Symptoms (Frequency, Urgency, or Both) Between Baseline (2002–2005) and 5-Year Follow-up (2006–2010) in the Boston Area Community Health Survey: Multivariable-Adjusted Odds Ratios and 95% Confidence Intervalsa
| Beverage Intake: Change by 2 or More 8-oz. Servings/dayb | Men |
Women |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | LUTS (Total IPSS) |
Voiding Symptoms |
Storage Symptoms |
n | LUTS (Total IPSS) |
Voiding Symptoms |
Storage Symptoms |
|||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| Coffee (total) | ||||||||||||||
| No | 1,291 | 1.00 | 1.00 | 1.00 | 2,099 | 1.00 | 1.00 | 1.00 | ||||||
| Decreased intake | 166 | 1.29 | 0.89, 1.86 | 1.79 | 1.23, 2.59** | 1.51 | 1.03, 2.22* | 231 | 0.91 | 0.66, 1.27 | 1.21 | 0.86, 1.72 | 1.05 | 0.75, 1.46 |
| Increased intake | 151 | 1.61 | 1.10, 2.34** | 1.55 | 1.03, 2.33* | 1.80 | 1.22, 2.67** | 203 | 1.32 | 0.95, 1.82 | 1.18 | 0.81, 1.72 | 1.64 | 1.18, 2.27** |
| Soda (total) | ||||||||||||||
| No | 1,167 | 1.00 | 1.00 | 1.00 | 1,948 | 1.00 | 1.00 | 1.00 | ||||||
| Decreased intake | 266 | 1.09 | 0.80, 1.51 | 1.21 | 0.86, 1.69 | 0.94 | 0.67, 1.33 | 349 | 0.93 | 0.70, 1.23 | 1.13 | 0.83, 1.54 | 1.17 | 0.89, 1.54 |
| Increased intake | 175 | 1.37 | 0.95, 1.96 | 1.19 | 0.80, 1.76 | 1.02 | 0.68, 1.51 | 236 | 1.59 | 1.18, 2.15** | 1.58 | 1.13, 2.22** | 1.40 | 1.02, 1.91* |
Abbreviations: CI, confidence interval; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; OR, odds ratio.
* P < 0.05; **P ≤ 0.01.
a From multivariable logistic regression models adjusted for age, physical activity level (low, medium, high), and race/ethnicity. Models for women additionally included smoking status (never, former, current) and, for storage symptoms, history of urinary tract infection. Analyses of changes in beverage consumption were conducted only for total coffee and total soda, because the question wording for other beverage types or subclasses was not available or did not allow comparable measures between baseline and follow-up. The number in each category of beverage intake change refers to the number in the analysis of LUTS (IPSS total score).
b The reference group includes participants who reported no change or change by 1 serving/day (increase or decrease).
DISCUSSION
In this observational study, coffee and caffeine intake were associated with LUTS progression, greater symptom scores, and urgency in men. In women, increases in coffee consumption between baseline and follow-up were associated with progression of urgency symptoms, although baseline coffee intake itself was not. Women who drank more soda at follow-up, particularly caffeinated diet soda, or increased their soda intake over time, were more likely to report a variety of LUTS or progression of symptoms. Consumption of orange or grapefruit juice was associated with protection from LUTS progression in men.
A strength of this study was the use of various measures to examine longitudinal relationships with habitual beverage intake and changes in intake levels, as well as short-term relationships between recent beverage intakes and recent symptoms. LUTS can be a seemingly constant problem in some persons, but most studies, including BACH, show remission and progression in symptoms over time (2, 16, 17). Thus, analyses of LUTS as a chronic condition, using longitudinal data with long time spans between assessment measures, might miss acute associations that exist in a temporally brief causal pathway. Certain lifestyle behaviors (e.g., physical activity, smoking) and medical factors (e.g., depression) could contribute to LUTS over the long term (28, 29). Other factors can lead to LUTS relatively quickly, through immediate effects on nervous system activity (30) or as chemical irritants to the bladder. It is plausible that components of beverages have both direct effects on the bladder and systemic effects that contribute to LUTS. Thus, our multimode analyses provided different yet complementary information.
The finding that total fluid intake was associated with LUTS acutely, but not longitudinally, is evidence of the validity of our analysis of proximate associations. There is little reason to believe that long-term average fluid intake levels lead to pathophysiological changes causing LUTS. However, drinking large amounts of fluids acutely causes greater frequency, urgency, and voiding volumes (18, 31), as we observed. Given that reverse causation is possible in a cross-sectional analysis, we considered results of the BACH qualitative interviews and did not analyze beverage types that participants mentioned while discussing symptom management: herbal tea (participants generally increased consumption to improve health) and water (some increased water intake to improve health, whereas others decreased intake to avoid frequent urination) (18). Overall, the nature of the acute associations observed in the present analysis does not implicate reverse causation. One exception is noncaffeinated soda consumption among men, which was associated with LUTS acutely, whereas caffeinated soda consumption was not. It is possible that men with LUTS chose noncaffeinated soda to avoid caffeine.
Interestingly, in BACH qualitative interviews, participants with LUTS generally did not mention avoidance of caffeine. Similarly, in a small study in the United Kingdom, men with LUTS were unaware of caffeine's possible effects and consumed large amounts of caffeinated drinks; only 13% of men with LUTS believed caffeine affected their symptoms (32). Caffeine has long been anecdotally implicated in contributing to LUTS, but data supporting the link have been limited. Our results are consistent with the findings of small clinical studies in which caffeine intake led to detrusor instability, urgency, and frequency (12, 33). Caffeine could act by directly stimulating the detrusor muscle via increased sympathetic nervous activity, increased production of nitric oxide, or diuretic effects (34–37).
It should be noted that our analysis of recent intake showed similar findings for caffeinated and decaffeinated coffee, even after adjustment for total fluid intake and other confounding factors. Changes in total coffee intake also showed similar associations between increased or decreased intakes and LUTS (with the exception of urgency symptoms, which were associated only with increased total coffee intake). In a clinical crossover trial that substituted decaffeinated for caffeinated coffee, no difference in detrusor overactivity was found (31). It is possible that other components of coffee also contribute to LUTS (in one small trial, decaffeinated coffee increased sympathetic nervous activity in nonhabitual drinkers), but most studies have attributed such effects to caffeine (37, 38).
Our finding that carbonated sodas were associated with urgency and worse symptom scores in women is consistent with a prior epidemiologic study of 1-year incidence of urgency symptoms in women in the United Kingdom. Dallosso et al. (14) found that those who drank soda at least once per day, versus those who drank soda less than once per week, were 70% more likely to develop urgency than were nondrinkers, but specific types of sodas were not assessed; thus the reason for the association was unclear. Carbonated beverages (i.e., sodas, soft drinks) commonly have various components suspected to contribute to LUTS: caffeine, artificial sweeteners or high levels of sugar, colorants, and preservatives (e.g., citric acid) (10, 11). Our data, which showed the strongest associations for caffeinated diet sodas, support findings from a small clinical crossover trial that caffeine and artificial sweeteners together could contribute to LUTS in women (13). In experiments in rodents, low concentrations of artificial sweeteners increased detrusor muscle contraction via modulation of L-type calcium channels (10). Similar results were found for low levels of ascorbic acid and citric acid, common soda preservatives (11). Given small effect sizes, the authors noted that ascorbic or citric acids alone are unlikely to have effects on normal bladder contraction; however, when combined with artificial sweeteners and other constituents of popular sodas, the contractile response was considerably greater (11).
Citrus juice was of particular interest in our analysis because of the contrasting possibilities of adverse acidic effects on the bladder and beneficial effects on systemic inflammation and oxidative stress (39, 40). Orange juice is a primary dietary source of vitamin C and contains various beneficial compounds. Analyses of baseline BACH data showed that high doses of supplemental vitamin C were associated with greater odds of LUTS, but similar associations were not found for vitamin C from dietary sources such as juice (41, 42). Orange juice reduces inflammation and oxidative stress and improves endothelial function (39, 40). These effects could help prevent benign prostatic hyperplasia, a common cause of LUTS in men. Thus, a systemic pathway toward improved health and prevention of LUTS with habitual intake over time is consistent with our longitudinal findings in men, which were not apparent in women.
Acute associations for citrus juice and LUTS were observed in both men and women, but these were generally weaker and statistically significant only for moderate citrus juice intake. The sample size for citrus juice analysis was limited to those who returned the follow-up FFQ, which lowered power and could result in nonresponse bias, as well as residual confounding by healthy lifestyle (43).
Limitations of this study include possible nonresponse bias to the FFQ or follow-up and confounding by unmeasured factors that are associated with beverage intakes and LUTS. Although the FFQ and interview provided complementary information, the baseline FFQ was limited by lack of data on noncola sodas (e.g., lemon-lime, ginger ale), and the BACH interview did not assess artificially sweetened sodas. Thus, comparing results of the longitudinal and proximate analyses is difficult. Furthermore, differences in the format of questions asked at baseline and follow-up limited analyses of changes in beverage intake to total coffee or total soda.
The focus of our longitudinal analysis was on progression of symptoms for clinical relevance; however, many participants reported symptom remission, which was not specifically taken into account. Our definition of progression was based on a clinical study of patient-reported outcomes, where a 3-point change in IPSS was perceived by men as a global worsening of their condition (44). Thresholds for meaningful IPSS changes have not been established in women, although prior studies support the use of the same threshold, given similar scores and associations with quality of life (25, 26). Additional research, particularly studies with clinical assessments repeatedly measured over follow-up, is needed to verify our findings.
In summary, the present study adds evidence that not only the volume but also the types of beverages consumed can be associated with urological symptoms. A novel finding was that benefits of orange and grapefruit juice could extend to LUTS, particularly for men. Thus, more rigorous evidence is needed to substantiate recommendations to limit intake of acidic beverages that are generally healthful and commonly enjoyed, such as 100% orange juice. Although coffee has been associated with benefits for other health outcomes (45), our data support recommendations to limit caffeinated beverage intake for LUTS. Further clinical research is warranted on sodas containing various artificial flavorings, sweeteners, and preservatives, to determine their precise role in bladder sensations and urological function.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, New England Research Institutes, Watertown, Massachusetts (Nancy N. Maserejian, Carrie G. Wager, Teresa M. Curto, John B. McKinlay); Department of Nutrition and Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Edward L. Giovannucci); Department of Medicine, Channing Division of Network Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston Massachusetts (Edward L. Giovannucci); Division of Urology, School of Medicine, Southern Illinois University, Springfield, Illinois (Kevin T. McVary).
This research was supported by grant R21DK081844 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
The authors thank statisticians Peter Shrader and Gretchen Chiu for assistance with data analysis.
Conflict of interest: none declared.
REFERENCES
- 1.Kupelian V, Wei JT, O'Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166(21):2381–2387. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 2.Wennberg AL, Molander U, Fall M, et al. A longitudinal population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in women. Eur Urol. 2009;55(4):783–791. doi: 10.1016/j.eururo.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Martin SA, Haren MT, Marshall VR, et al. Prevalence and factors associated with uncomplicated storage and voiding lower urinary tract symptoms in community-dwelling Australian men. World J Urol. 2011;29(2):179–184. doi: 10.1007/s00345-010-0605-8. [DOI] [PubMed] [Google Scholar]
- 4.Irwin DE, Kopp ZS, Agatep B, et al. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Wein AJ, Tubaro A, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103(suppl 3):4–11. doi: 10.1111/j.1464-410X.2009.08371.x. [DOI] [PubMed] [Google Scholar]
- 6.Sexton CC, Coyne KS, Vats V, et al. Impact of overactive bladder on work productivity in the United States: results from EpiLUTS. Am J Manag Care. 2009;15(4 suppl):S98–S107. [PubMed] [Google Scholar]
- 7.Cardozo L. Systematic review of overactive bladder therapy in females. Can Urol Assoc J. 2011;5(5 suppl 2):S139–S142. doi: 10.5489/cuaj.11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukacz ES, Sampselle C, Gray M, et al. A healthy bladder: a consensus statement. Int J Clin Pract. 2011;65(10):1026–1036. doi: 10.1111/j.1742-1241.2011.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JG, Wein AJ, Levin RM. The effect of caffeine on the contractile response of the rabbit urinary bladder to field stimulation. Gen Pharmacol. 1993;24(4):1007–1011. doi: 10.1016/0306-3623(93)90180-6. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta J, Elliott RA, Doshani A, et al. Enhancement of rat bladder contraction by artificial sweeteners via increased extracellular Ca2+ influx. Toxicol Appl Pharmacol. 2006;217(2):216–224. doi: 10.1016/j.taap.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta J, Elliott RA, Tincello DG. Modification of rat detrusor muscle contraction by ascorbic acid and citric acid involving enhanced neurotransmitter release and Ca2+ influx. Neurourol Urodyn. 2009;28(6):542–548. doi: 10.1002/nau.20701. [DOI] [PubMed] [Google Scholar]
- 12.Lohsiriwat S, Hirunsai M, Chaiyaprasithi B. Effect of caffeine on bladder function in patients with overactive bladder symptoms. Urol Ann. 2011;3(1):14–18. doi: 10.4103/0974-7796.75862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartwright R, Srikrishna S, Cardozo L, et al. Does Diet Coke cause overactive bladder? A 4-way crossover trial, investigating the effects of carbonated soft drinks on overactive bladder symptoms in normal volunteers. Poster presented at the 37th Annual Meeting of the International Continence Society Annual Meeting, Rotterdam, Holland, August 22, 2007. [Google Scholar]
- 14.Dallosso HM, McGrother CW, Matthews RJ, et al. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int. 2003;92(1):69–77. doi: 10.1046/j.1464-410x.2003.04271.x. [DOI] [PubMed] [Google Scholar]
- 15.Dallosso HM, Matthews RJ, McGrother CW, et al. The association of diet and other lifestyle factors with the onset of overactive bladder: a longitudinal study in men. Public Health Nutr. 2004;7(7):885–891. doi: 10.1079/phn2004627. [DOI] [PubMed] [Google Scholar]
- 16.Moller LA, Lose G, Jorgensen T. Incidence and remission rates of lower urinary tract symptoms at one year in women aged 40–60: longitudinal study. BMJ. 2000;320(7247):1429–1432. doi: 10.1136/bmj.320.7247.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link CL, McKinlay JB. Progression and remission of urologic symptoms: longitudinal results from the Boston Area Community Health (BACH) Survey. J Urol. 2011;185(4 suppl):e25. [Google Scholar]
- 18.Elstad EA, Maserejian NN, McKinlay JB, et al. Fluid manipulation among individuals with lower urinary tract symptoms: a mixed methods study. J Clin Nurs. 2011;20(1–2):156–165. doi: 10.1111/j.1365-2702.2010.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) survey. Eur Urol. 2007;52(2):389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 21.Boyle P, Robertson C, Mazzetta C, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92(4):409–414. doi: 10.1046/j.1464-410x.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 22.Chai TC, Belville WD, McGuire EJ, et al. Specificity of the American Urological Association voiding symptom index: comparison of unselected and selected samples of both sexes. J Urol. 1993;150(5 Pt 2):1710–1713. doi: 10.1016/s0022-5347(17)35874-3. [DOI] [PubMed] [Google Scholar]
- 23.Okamura K, Nojiri Y, Osuga Y, et al. Psychometric analysis of international prostate symptom score for female lower urinary tract symptoms. Urology. 2009;73(6):1199–1202. doi: 10.1016/j.urology.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 24.Boyle P, Robertson C, Mazzetta C, et al. The relationship between lower urinary tract symptoms and health status: the UrEpik study. BJU Int. 2003;92(6):575–580. doi: 10.1046/j.1464-410x.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 25.Scarpero HM, Fiske J, Xue X, et al. American Urological Association Symptom Index for lower urinary tract symptoms in women: correlation with degree of bother and impact on quality of life. Urology. 2003;61(6):1118–1122. doi: 10.1016/s0090-4295(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 26.Terai A, Matsui Y, Ichioka K, et al. Comparative analysis of lower urinary tract symptoms and bother in both sexes. Urology. 2004;63(3):487–491. doi: 10.1016/j.urology.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 27.Abrams P, Chapple CR, Junemann KP, et al. Urinary urgency: a review of its assessment as the key symptom of the overactive bladder syndrome. World J Urol. 2012;30(3):385–392. doi: 10.1007/s00345-011-0742-8. [DOI] [PubMed] [Google Scholar]
- 28.Maserejian NN, Kupelian V, Miyasato G, et al. Are physical activity, smoking and alcohol consumption associated with lower urinary tract symptoms in men or women? Results from a population based observational study. J Urol. 2012;188(2):490–495. doi: 10.1016/j.juro.2012.03.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SY, Woo J, Leung JC, et al. Depressive symptoms and lifestyle factors as risk factors of lower urinary tract symptoms in Southern Chinese men: a prospective study. Aging Male. 2010;13(2):113–119. doi: 10.3109/13685530903440432. [DOI] [PubMed] [Google Scholar]
- 30.McVary KT, Rademaker A, Lloyd GL, et al. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174(4 Pt 1):1327–1433. doi: 10.1097/01.ju.0000173072.73702.64. [DOI] [PubMed] [Google Scholar]
- 31.Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol. 2005;174(1):187–189. doi: 10.1097/01.ju.0000162020.10447.31. [DOI] [PubMed] [Google Scholar]
- 32.Brown CT, O'Flynn E, van der Meulen J, et al. Towards a self-management intervention programme for men with LUTS: the role of caffeinated drinks [abstract] BJU Int. 2002;90(suppl 1):37. [Google Scholar]
- 33.Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96(1):85–89. doi: 10.1016/s0029-7844(00)00808-5. [DOI] [PubMed] [Google Scholar]
- 34.Creighton SM, Stanton SL. Caffeine: does it affect your bladder? Br J Urol. 1990;66(6):613–614. doi: 10.1111/j.1464-410x.1990.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 35.Birder LA, Apodaca G, De Groat WC, et al. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275(2 Pt 2):F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- 36.Birder LA, Nealen ML, Kiss S, et al. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22(18):8063–8070. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Echeverri D, Montes FR, Cabrera M, et al. Caffeine's vascular mechanisms of action. Int J Vasc Med. 2010;(2010):834060. doi: 10.1155/2010/834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corti R, Binggeli C, Sudano I, et al. Coffee acutely increases sympathetic nerve activity and blood pressure independently of caffeine content: role of habitual versus nonhabitual drinking. Circulation. 2002;106(23):2935–2940. doi: 10.1161/01.cir.0000046228.97025.3a. [DOI] [PubMed] [Google Scholar]
- 39.Buscemi S, Rosafio G, Arcoleo G, et al. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr. 2012;95(5):1089–1095. doi: 10.3945/ajcn.111.031088. [DOI] [PubMed] [Google Scholar]
- 40.Ghanim H, Sia CL, Upadhyay M, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91(4):940–949. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maserejian NN, Giovannucci EL, McVary KT, et al. Intakes of vitamins and minerals in relation to urinary incontinence, voiding, and storage symptoms in women: a cross-sectional analysis from the Boston Area Community Health survey. Eur Urol. 2011;59(6):1039–1047. doi: 10.1016/j.eururo.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maserejian NN, Giovannucci EL, McVary KT, et al. Dietary, but not supplemental, intakes of carotenoids and vitamin C are associated with decreased odds of lower urinary tract symptoms in men. J Nutr. 2011;141(2):267–273. doi: 10.3945/jn.110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Lloyd B, Yang M, et al. Impact of orange juice consumption on macronutrient and energy intakes and body composition in the US population. Public Health Nutr. 2012;15(12):2220–2227. doi: 10.1017/S1368980012000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154(5):1770–1774. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 45.Freedman ND, Park Y, Abnet CC, et al. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366(20):1891–1904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Block G, Hartman AM, Dresser CM, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 47.Block G, Wakimoto P, Jensen C, et al. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006;3(3):A77. [PMC free article] [PubMed] [Google Scholar]
- 48.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Urology. 2003;61(1):37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]