Abstract
In this prospective cohort study, based on 1,505 mother-infant pairs in rural Bangladesh, we evaluated the associations between early-life exposure to arsenic, cadmium, and lead, assessed via concentrations in maternal and child urine, and children's weights and heights up to age 5 years, during the period 2001–2009. Concurrent and prenatal exposures were evaluated using linear regression analysis, while longitudinal exposure was assessed using mixed-effects linear regression. An inverse association was found between children's weight and height, age-adjusted z scores, and growth velocity at age 5 years and concurrent exposure to cadmium and arsenic. In the longitudinal analysis, multivariable-adjusted attributable differences in children's weight at age 5 years were −0.33 kg (95% confidence interval (CI): −0.60, −0.06) for high (≥95th percentile) arsenic exposure and −0.57 kg (95% CI: −0.88, −0.26) for high cadmium exposure, in comparison with children with the lowest exposure (≤5th percentile). Multivariable-adjusted attributable differences in height were −0.50 cm (95% CI: −1.20, 0.21) for high arsenic exposure and −1.6 cm (95% CI: −2.4, −0.77) for high cadmium exposure. The associations were apparent primarily among girls. The negative effects on children's growth at age 5 years attributable to arsenic and cadmium were of similar magnitude to the difference between girls and boys in terms of weight (−0.67 kg, 95% CI: −0.82, −0.53) and height (−1.3 cm, 95% CI: −1.7, −0.89).
Keywords: arsenic; body height; body weight; cadmium; child, preschool; growth; lead; prenatal exposure delayed effects
Growth restriction in early life is associated with a number of poor health outcomes later in life. In particular, stunting, or height-for-age z score more than 2 standard deviations below the median reference set by the World Health Organization, has been associated with later behavioral problems, cognitive deficits, and increased risk of hypertension and cardiovascular disease (1–5). Underweight, or weight-for-age z score more than 2 standard deviations below the World Health Organization median, is associated with increased risk of death from diseases such as pneumonia, diarrhea, and malaria (6).
The primary risk factor for growth restriction in children is malnutrition. The most important risk factors for stunting and underweight occur during early life, including maternal undernutrition during pregnancy, inadequate breastfeeding, and inappropriate introduction of complementary foods (7). However, other early-life environmental exposures may also play a role in growth restriction (7).
Childhood lead exposure has been associated with stunting in children (8, 9), and studies in animals demonstrate that lead affects growth via toxic effects on bone tissue (10). Both arsenic exposure and cadmium exposure are inversely associated with infant size at birth, and this relationship may vary between boys and girls (11, 12), though less is known about how arsenic and cadmium exposure affect subsequent growth. An inverse association between arsenic exposure and children's height and weight has been reported in relatively small, cross-sectional studies (13–15). However, in a much larger longitudinal study, we found only a weak inverse association between prenatal arsenic exposure and children's growth to age 2 years that was greatly attenuated by adjustment for other risk factors (16). Several small studies have shown that prenatal cadmium exposure was associated with attenuated weight and height in preschool children, though concurrent exposures were not accounted for (17, 18). To our knowledge, no studies to date have considered the independent associations of these toxic metals over time with anthropometric outcomes within the same population.
In the present study, we aimed to evaluate the relationship between both prenatal and concurrent environmental exposure and children's attained weight and height at 5 years, as well as growth velocities from birth to age 5 years and standardized z scores, using a longitudinal study design within a well-characterized mother-child cohort in rural Bangladesh.
MATERIALS AND METHODS
Study area and population
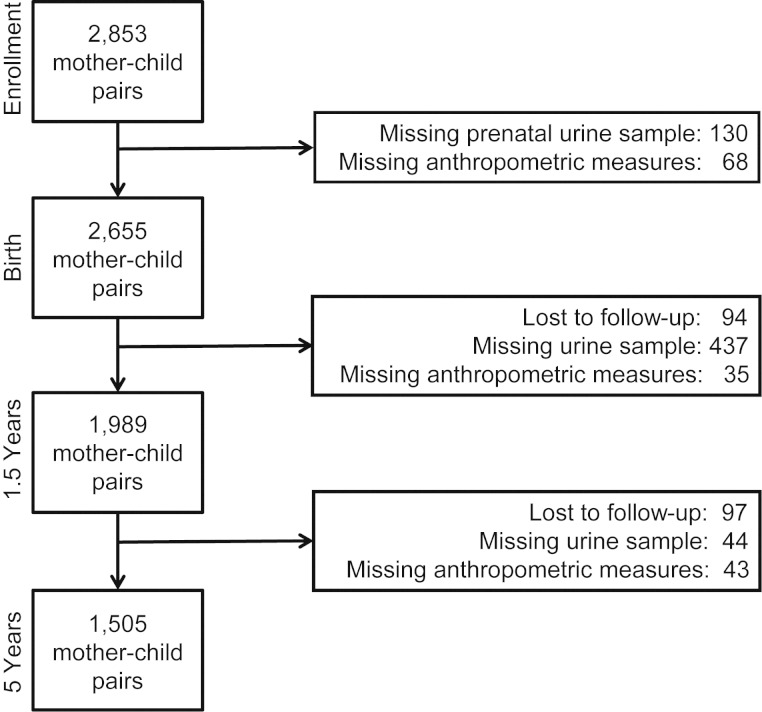
The present study is part of our ongoing research concerning health effects of early-life exposure to toxic agents in Matlab, Bangladesh, a rural area 53 km southeast of Dhaka. This research is nested into a population-based randomized trial of food and micronutrient supplementation, Maternal and Infant Nutrition Interventions in Matlab (MINIMat) (19). Mothers of children born between May 2002 and December 2003 (n = 2,853) were invited to participate in the assessment of environmental contaminants and child health and development (20). Of these mother-child pairs, those who had complete anthropometric data at birth and at ages 1.5 and 5 years, as well as environmental exposure data prenatally and at ages 1.5 and 5 years, were included in this study (n = 1,505; Figure 1). Pairs who were included in the study were similar to excluded pairs in terms of exposures, outcomes, and other demographic characteristics (see Web Table 1, available at http://aje.oxfordjournals.org/).
Figure 1.
Loss to follow-up and exclusion of mother-child pairs within the MINIMat Study, Matlab, Bangladesh, 2007–2009. MINIMat, Maternal and Infant Nutrition Interventions in Matlab.
This study was approved by the Regional Ethical Committee at Karolinska Institutet (Stockholm, Sweden) and the Ethical Review Committee at the International Center for Diarrhoeal Disease Research, Bangladesh (Dhaka, Bangladesh). Informed consent was obtained from the mothers, and all participants were free to refrain from any part of the study.
Anthropometric measurements
Children's weights and lengths/heights were measured at birth, monthly during the first year of life, once every 3 months during the second year of life, and again at a 5-year follow-up visit, according to methods previously described in detail (21, 22). Missing measurements were more common during the first year, because study capacity was still being developed during the first months of the MINIMat follow-up; 41% of measurements were missing at month 1, 20% were missing at month 6, and 5% were missing at month 12.
Socioeconomic status
Data on an extensive list of socioeconomic status (SES) factors at the time of enrollment were extracted from the health and demographic database maintained by the International Centre for Diarrhoeal Disease Research, Bangladesh. SES was defined in terms of assets (on the basis of household ownership of different items), dwelling characteristics, drinking-water source, and household sanitation. Asset scores were standardized as previously described (23, 24).
Exposure assessment
Exposure assessment was based on urinary concentrations of arsenic, cadmium, and lead in spot urine samples collected from pregnant women and their children at ages 1.5 and 5 years (25–27). Urinary arsenic, defined as the total concentration of the metabolites of inorganic arsenic (inorganic arsenic + monomethylarsonic acid (MMA) + dimethylarsinic acid (DMA)), was determined by high-performance liquid chromatography (Agilent 1100 Series system; Agilent Technologies, Waldbronn, Germany) coupled with hydride generation and inductively coupled plasma mass spectrometry (Agilent 7500ce; Agilent Technologies) for all children at both 1.5 and 5 years of age (25, 27, 28) and by hydride generation atomic absorption spectrometry for maternal samples (26). Measurement of cadmium (mass-to-charge ratio, 111) and lead (mass-to-charge ratio, 208) was also performed with inductively coupled plasma mass spectrometry for maternal samples and children's samples at age 5 years (29, 30). The between-assay coefficient of variation was 2.0% for maternal total urinary arsenic (26) and 6.8% and 5.8% for DMA in children's samples at ages 1.5 years (27) and 5 years (25), respectively. For urinary cadmium, the coefficient of variation was 2.9% for maternal samples (12) and 3.3% for children's samples at age 5 years (30). For urinary lead, the coefficient of variation was 11% for maternal samples and 7.3% for children's samples at age 5 years (29). (See the Web Appendix for further description of analytical measurements.)
To compensate for variation in dilution of the urine samples, we adjusted all measurements for the specific gravity of the urine, measured by means of a digital clinical refractometer (EUROMEX RD 712; EUROMEX, Arnhem, the Netherlands) (31). Adjustment by urinary creatinine is problematic in growth studies, particularly those involving arsenic, because urinary creatinine is associated with body size, is influenced by nutritional factors that can also influence arsenic metabolism and growth, and is itself correlated with arsenic metabolism efficiency (31, 32).
Statistical analysis
All statistical analyses were conducted using STATA, version 10 (StataCorp LP, College Station, Texas).
We considered attained weight, height, weight-for-age z score, height-for-age z score, peak weight velocity, and peak height velocity as outcomes. Weight-for-age z score and height-for-age z score were calculated according to standard World Health Organization methods (33). Peak weight velocity and peak height velocity were calculated using all available data from birth to the 5-year visit. The 4-parameter Reed model (34) for children's growth, modified according to the methods described by Simondon et al. (35), was fitted by sex using maximum likelihood estimation and assuming both fixed and random components for all parameters. The first derivatives of the individual fitted growth curves were taken to calculate the maximum weight or height velocity for each child (36).
Given the potential for a nonlinear association between the exposure biomarkers and the outcomes (Figure 2), we tested the crude association between the tertiles of each biomarker and the anthropometric outcomes. We repeated this analysis, adjusting for the following possible confounders: family SES, maternal tobacco-chewing during pregnancy, cooking over fire indoors, maternal educational level (≥5 years or <5 years (the median value)), season of birth (pre–rainy season, rainy season, or post–rainy season), and parity. Possible confounders were defined as being associated with any exposure (Table 1) as well as being associated with children's anthropometric outcomes at age 5 years. Additionally, we included other predictors of the outcomes: child's sex, maternal anthropometric characteristics, and gestational age at birth. The effect of food and micronutrient supplementation on childhood growth has been reported for the full MINIMat cohort elsewhere (22). We also assessed whether adding the 2 different types of food (early and usual) and/or 3 different types of micronutrient supplementation used in the MINIMat supplementation trial (22, 37) to the models changed the associations between environmental exposures and anthropometric outcomes. Because inclusion of these covariates did not change any of the associations (using either this strategy or the modeling strategy outlined below), we did not include these covariates in the final models presented here.
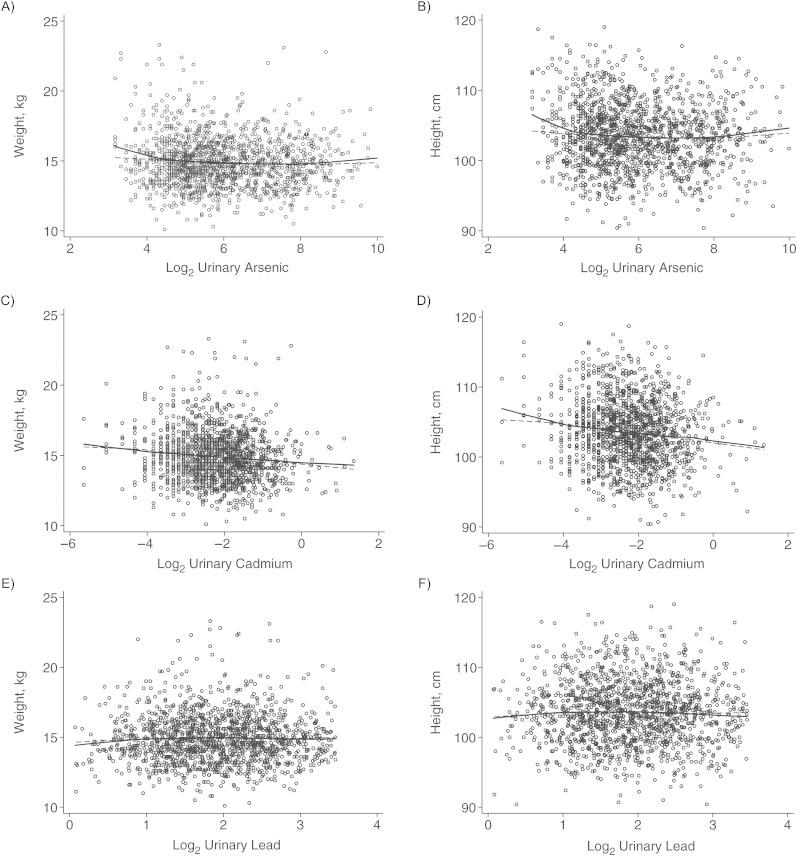
Figure 2.
The relationship between log2-transformed urinary biomarkers of metals exposure and children's weight (left-hand panels) and height (right-hand panels) at age 5 years, Matlab, Bangladesh, 2007–2009. Top row, urinary arsenic; middle row, urinary cadmium; bottom row, urinary lead. In all cases, the solid line represents a LoWeSS (locally weighted scatterplot smoothing) moving-average curve for the raw data represented in the scatterplot. The dashed line represents the fitted curve following log2-transformation of urinary concentration, adjusted for child's sex, family socioeconomic status, season of birth, gestational age at birth, maternal education, maternal height or body mass index (weight (kg)/height (m)2) (as appropriate), maternal tobacco-chewing, indoor cooking without ventilation, and birth order (model 2).
Table 1.
Spearman Correlations Between Biomarkers of Exposure and Predictors of Anthropometric Outcomes at Age 5 Years Among Children in Matlab, Bangladesh, 2007–2009
| Childrena |
Mothersb |
|||||
|---|---|---|---|---|---|---|
| Urinary Arsenic Level, μg/L | Urinary Cadmium Level, μg/L | Urinary Lead Level, μg/L | Urinary Arsenic Level, μg/L | Urinary Cadmium Level, μg/L | Urinary Lead Level, μg/L | |
| Child's urinary cadmium level, μg/L | 0.12** | |||||
| Child's urinary lead level, μg/L | 0.00 | 0.11** | ||||
| Mother's urinary arsenic level, μg/L | 0.45** | −0.01 | −0.02 | |||
| Mother's urinary cadmium level, μg/L | 0.08* | 0.11** | −0.09* | 0.10** | ||
| Mother's urinary lead level, μg/L | −0.04 | 0.02 | 0.14** | −0.03 | 0.11** | |
| Birth orderc | 0.07* | 0.05 | −0.06* | 0.04 | 0.19** | −0.13** |
| Sexd | 0.00 | 0.05 | 0.02 | 0.03 | −0.01 | −0.03 |
| Socioeconomic statuse | −0.12** | −0.16** | 0.06* | −0.12** | −0.16** | 0.19** |
| Post-rainy-season birthf | −0.10** | −0.10** | 0.11** | 0.00 | 0.01 | 0.10** |
| Gestational age at birth, weeks | −0.04 | −0.01 | 0.03 | −0.02 | 0.01 | 0.02 |
| Maternal tobacco-chewingg | 0.03 | 0.01 | 0.02 | 0.05* | 0.03 | −0.09* |
| Indoor open-flame cookingh | −0.01 | −0.02 | −0.02 | −0.01 | 0.09** | 0.01 |
| Maternal educationi | −0.13** | −0.11** | 0.03 | −0.11** | −0.19** | 0.11* |
* P < 0.01; **P < 0.001.
a Concentration measured in children's urine at age 5 years.
b Concentration measured in mothers' urine during early gestation (approximately gestational week 8).
c Birth order or mother's parity (range, 0–7).
d Boys = 0; girls = 1.
e Socioeconomic status was defined on the basis of ownership of various household assets.
f Children born between September and December = 1; all others = 0.
g Mothers were asked postpartum about whether they chewed tobacco, either “jarda” (sweetened tobacco leaves in a mixture of sliced areca nut, calcium carbonate, and a leaf of the piper betel plant) or “gul” (dried and powdered tobacco), during their pregnancy. Tobacco-chewing during pregnancy was classified as either “yes = 1” or “no = 0.”
h Mothers were asked postpartum whether they had cooked food indoors using a flame (not counting gas) in an area that was enclosed on all sides, including the top, for at least 2 weeks during pregnancy.
i In total years of schooling.
We also considered 4 models in which the biomarker concentration was log2-transformed to account for the nonlinear relationship between biomarkers and anthropometric measures (Figure 2). In this analysis, each β coefficient represents the average change in the outcome associated with each doubling of the concentration of the biomarker. In model 1, we examined the crude association between each individual environmental exposure (measured as children's urinary arsenic, urinary cadmium, and urinary lead levels at age 5 years) and each outcome at age 5 years. In model 2, we repeated the analysis, adjusting for possible confounders and predictors of outcome listed above. In model 3, to control for any effects of prenatal environmental exposure, we adjusted the results as in model 2, with the addition of maternal urinary arsenic, urinary cadmium, or urinary lead measured during gestational week 8. In model 4, we evaluated the associations of children's urinary arsenic, urinary cadmium, and urinary lead levels with anthropometric outcomes in the same model, also adjusted as in model 2. After calculating the estimates for all models described here, we used the STATA suest command for “seemingly unrelated estimation,” which provides robust standard errors and adjusts model estimates for intragroup correlation after conducting multiple tests on a set of correlated exposures and outcomes (38). We present the adjusted model estimates. In a subsequent analysis, we added the percentage of MMA, the most toxic arsenic metabolite, into model 2 for the arsenic-only models to test whether efficiency of arsenic metabolism affected these relationships.
We next examined whether prenatal environmental exposures were related to the anthropometric outcomes at age 5 years. We followed the same modeling strategy as outlined above, substituting maternal urinary biomarkers measured at gestational week 8 for children's urinary biomarkers in the models.
To explore the longitudinal relationship between exposure and children's weight and height over time, we used mixed-effects linear regression models with a random intercept and random slope, fitted using maximum likelihood estimation, and including all environmental exposures. Weight and height were regressed separately over time, in terms of months since birth (time = 0). Maternal urinary arsenic, urinary cadmium, and urinary lead were taken as proxy measures for exposure at birth. To allow the effects of environmental exposures to change over time, we included interaction terms for each biomarker and time. To allow for differences between boys and girls and SES to change over time, we included terms for the interaction between these variables and time. We adjusted the results as in model 2 above.
Given evidence that metals may have sex-specific effects (39), we stratified the data by sex and repeated the above analysis, excluding any sex-related terms. Additionally, we lacked data on individual nutritional intake in this study. In order to address the possibility that the observed associations were due to unobserved confounding by nutritional status, we stratified the sample based on quintiles of SES and compared the associations between environmental exposures and weight and height according to SES quintile (highest quintile (rate of stunting at age 5 years: 16%) vs. lowest quintile (rate of stunting at age 5 years: 46%)).
We calculated the multivariable-adjusted difference in weight and height due to environmental exposures by comparing the multivariable-adjusted weight or height of children with urinary arsenic at or above the 95th percentile at age 5 years with that of children with urinary arsenic at or below the 5th percentile. We repeated this procedure for urinary cadmium and urinary lead. For comparison, we also calculated the multivariable-adjusted difference between boys and girls at age 5 years and the multivariable-adjusted difference between the highest and lowest SES quintiles.
RESULTS
Population characteristics
On average, the children were 63 months of age (standard deviation, 1.8 months) at the 5-year visit. At age 5 years, 41% of the children were underweight and 33% were stunted. Table 2 shows in detail the participants’ anthropometric characteristics at age 5 years, according to environmental exposures and demographic information. Detailed descriptions of the anthropometric measures for this cohort at the time of birth (11, 12) and during the first 2 years of life (16) have been reported previously. Children's early-life exposures to arsenic, cadmium, and lead have been explored in detail previously (25, 29, 30). (See Web Table 1 for a summary of the exposure levels for the mother-child pairs included in this study.) Children's and mothers' urinary arsenic, urinary cadmium, and urinary lead levels were weakly but positively correlated, and mothers' and children's urinary arsenic and urinary cadmium levels were inversely associated with SES (Table 1).
Table 2.
Anthropometric Characteristics (Mean and Standard Deviation) at Age 5 Years According to Arsenic, Cadmium, and Lead Exposure and Covariates for Children in Matlab, Bangladesh, 2007–2009
| Variable | No. of Children | Weight, kg | Weight-for-Age z Score | Peak Weight Velocity, kg/month | Height, cm | Height-for-Age z Score | Peak Height Velocity, cm/month |
|---|---|---|---|---|---|---|---|
| All children | 1,505 | 14.9 (1.8) | −1.80 (0.89) | 0.10 (0.03) | 104 (4.6) | −1.59 (0.94) | 0.43 (0.07) |
| Tertile of measured level at age 5 years, μg/L | |||||||
| Urinary arsenic | |||||||
| <35 | 484 | 15.2 (2.1) | −1.68 (0.99) | 0.11 (0.03) | 104 (4.9) | −1.47 (0.99) | 0.43 (0.07) |
| ≥35–<84 | 509 | 14.8 (1.6) | −1.87 (0.85) | 0.09 (0.02) | 103 (4.4) | −1.66 (0.92) | 0.43 (0.06) |
| ≥84 | 512 | 14.8 (1.7) | −1.84 (0.83) | 0.10 (0.02) | 103 (4.5) | −1.62 (0.89) | 0.43 (0.06) |
| Urinary cadmium | |||||||
| <0.16 | 515 | 15.2 (1.8) | −1.68 (0.88) | 0.11 (0.03) | 104 (4.6) | −1.45 (0.94) | 0.44 (0.06) |
| ≥0.16–<0.27 | 480 | 14.9 (1.9) | −1.83 (0.92) | 0.10 (0.03) | 103 (4.5) | −1.62 (0.94) | 0.43 (0.07) |
| ≥0.27 | 510 | 14.7 (1.7) | −1.90 (0.86) | 0.09 (0.02) | 103 (4.7) | −1.69 (0.94) | 0.42 (0.07) |
| Urinary lead | |||||||
| <2.9 | 495 | 14.9 (1.7) | −1.84 (0.87) | 0.10 (0.03) | 104 (4.6) | −1.59 (0.95) | 0.43 (0.07) |
| ≥2.9–<4.6 | 498 | 14.9 (1.9) | −1.78 (0.94) | 0.10 (0.03) | 104 (4.7) | −1.59 (0.94) | 0.43 (0.07) |
| ≥4.6 | 512 | 14.9 (1.7) | −1.78 (0.86) | 0.10 (0.02) | 103 (4.5) | −1.58 (0.94) | 0.43 (0.06) |
| Sex | |||||||
| Boys | 794 | 15.3 (1.8) | −1.73 (0.90) | 0.10 (0.03) | 104 (4.7) | −1.55 (0.97) | 0.43 (0.07) |
| Girls | 711 | 14.6 (1.8) | −1.88 (0.88) | 0.10 (0.03) | 103 (4.5) | −1.63 (0.91) | 0.44 (0.06) |
| Quintile of socioeconomic statusa | |||||||
| 1 (lowest) | 322 | 14.4 (1.5) | −2.04 (0.78) | 0.09 (0.02) | 102 (4.0) | −1.93 (0.83) | 0.41 (0.06) |
| 2 | 341 | 14.5 (1.6) | −2.02 (0.83) | 0.10 (0.02) | 102 (4.5) | −1.81 (0.90) | 0.41 (0.06) |
| 3 | 287 | 14.7 (1.5) | −1.89 (0.80) | 0.10 (0.02) | 103 (4.2) | −1.67 (0.85) | 0.43 (0.06) |
| 4 | 275 | 15.4 (1.9) | −1.59 (0.90) | 0.11 (0.03) | 105 (4.6) | −1.33 (0.94) | 0.44 (0.07) |
| 5 (highest) | 280 | 15.8 (2.2) | −1.37 (0.96) | 0.12 (0.03) | 106 (4.8) | −1.09 (0.95) | 0.46 (0.07) |
| Birth order | |||||||
| Firstborn | 469 | 15.3 (2.1) | −1.62 (0.98) | 0.11 (0.03) | 104 (4.8) | −1.40 (0.99) | 0.45 (0.07) |
| Second-born or more | 1,046 | 14.8 (1.7) | −1.88 (0.84) | 0.10 (0.02) | 103 (4.5) | −1.67 (0.91) | 0.42 (0.06) |
| Season of birth | |||||||
| Pre–rainy seasonb | 383 | 14.9 (4.7) | −1.82 (0.87) | 0.10 (0.03) | 104 (4.4) | −1.53 (0.91) | 0.43 (0.06) |
| Rainy seasonc | 524 | 14.9 (2.0) | −1.83 (0.94) | 0.10 (0.03) | 104 (4.6) | −1.58 (0.94) | 0.43 (0.07) |
| Post–rainy seasond | 598 | 15.0 (1.7) | −1.76 (0.87) | 0.10 (0.02) | 103 (4.8) | −1.62 (0.97) | 0.43 (0.07) |
| Gestational age at birth, weeks | |||||||
| ≤37 | 138 | 15.0 (1.9) | −1.78 (0.97) | 0.11 (0.03) | 104 (4.8) | −1.53 (1.0) | 0.46 (0.07) |
| >37 | 1,367 | 14.9 (1.8) | −1.80 (0.88) | 0.10 (0.03) | 103 (4.6) | −1.59 (0.94) | 0.43 (0.07) |
| Maternal education, years | |||||||
| ≥5 | 806 | 15.2 (2.0) | −1.62 (0.93) | 0.11 (0.03) | 105 (4.8) | −1.37 (0.97) | 0.45 (0.07) |
| <5 | 699 | 14.5 (1.5) | −2.00 (0.80) | 0.09 (0.02) | 102 (4.2) | −1.85 (0.84) | 0.41 (0.06) |
| Tobacco-chewing during pregnancye | |||||||
| Yes | 934 | 14.8 (1.8) | −1.88 (0.88) | 0.10 (0.03) | 103 (4.5) | −1.62 (0.93) | 0.43 (0.06) |
| No | 567 | 15.1 (1.9) | −1.73 (0.90) | 0.10 (0.03) | 104 (4.8) | −1.53 (0.96) | 0.43 (0.07) |
| Indoor open-flame cookingf | |||||||
| Yes | 261 | 14.9 (1.6) | −1.81 (0.81) | 0.10 (0.02) | 103 (4.8) | −1.63 (0.94) | 0.42 (0.07) |
| No | 1,244 | 14.9 (1.9) | −1.80 (0.91) | 0.10 (0.03) | 104 (4.6) | −1.58 (0.94) | 0.43 (0.07) |
a Socioeconomic status was defined on the basis of ownership of various household assets.
b Children born between January and April.
c Children born between May and August.
d Children born between September and December.
e Mothers were asked postpartum about whether they chewed tobacco, either “jarda” (sweetened tobacco leaves in a mixture of sliced areca nut, calcium carbonate, and a leaf of the piper betel plant) or “gul” (dried and powdered tobacco), during their pregnancy. Tobacco-chewing during pregnancy was classified as either “yes = 1” or “no = 0.”
f Mothers were asked postpartum whether they had cooked food indoors using a flame (not counting gas) in an area that was enclosed on all sides, including the top, for at least 2 weeks during pregnancy.
Are concurrent environmental exposures associated with anthropometric outcomes in 5-year-olds?
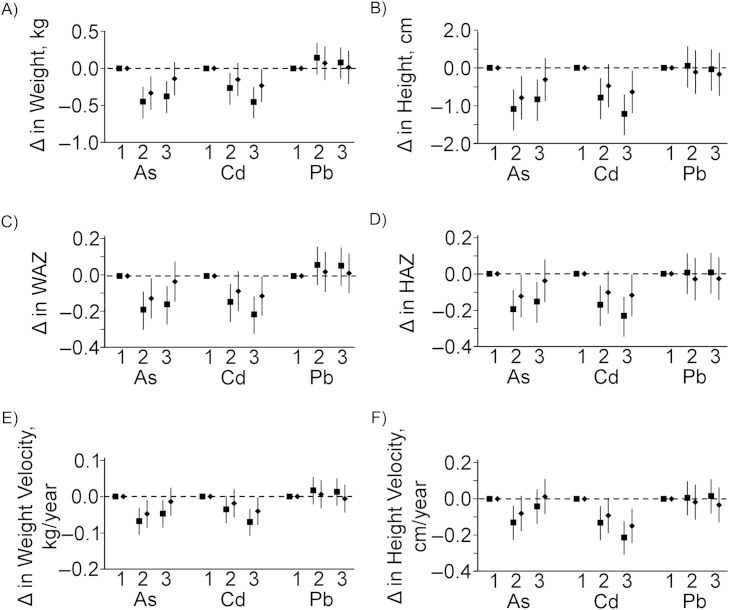
In the tertile analysis (Figure 3), urinary arsenic was inversely associated with both weight and height outcomes. The inverse association was most apparent when comparing the middle tertile with the lowest tertile; in all cases, the association was attenuated in the highest tertile after adjustment. Urinary cadmium was consistently inversely associated with both weight and height outcomes, though not all associations remained statistically significant after adjustment. Urinary lead was not associated with any of the anthropometric outcomes.
Figure 3.
Relationship between urinary arsenic (As), urinary cadmium (Cd), and urinary lead (Pb) concentrations and anthropometric outcomes according to tertiles of exposure, measured in children at age 5 years in Matlab, Bangladesh, 2007–2009. Weight (A), height (B), weight-for-age z score (WAZ) (C), height-for-age z score (HAZ) (D), peak weight velocity (E), and peak height velocity (F) were considered as outcomes. Crude (unadjusted) model estimates are shown as squares. Estimates adjusted for child's sex, family socioeconomic status, season of birth, gestational age at birth, maternal education, maternal height or body mass index (weight (kg)/height (m)2) (as appropriate), maternal tobacco-chewing, indoor cooking without ventilation, and birth order (model 2) are presented as diamonds. Δ indicates “change,” and tertiles are labeled along the x-axis. Vertical lines represent 95% confidence intervals.
In an analysis using log2-transformed data (Figure 4), urinary arsenic was inversely associated with weight, height, weight-for-age z score, height-for-age z score, and weight velocity at age 5 years, although these associations were no longer apparent after adjustment. When the percentage of MMA was included in model 2, we found that the percentage of MMA was positively associated with the anthropometric outcomes (Web Table 2). Urinary cadmium was consistently inversely associated with all anthropometric outcomes. The association was attenuated, though still statistically significant, after adjustment. The association strengthened slightly with the addition of prenatal urinary cadmium to the model (model 3). Urinary lead level was not associated with any of the anthropometric outcomes. In a model accounting for all 3 environmental exposures, urinary cadmium was the only biomarker to remain associated with the anthropometric outcomes.
Figure 4.
Relationship between children's log2-transformed urinary arsenic (As), urinary cadmium (Cd), and urinary lead (Pb) concentrations and anthropometric outcomes at age 5 years (linear regression analysis) in Matlab, Bangladesh, 2007–2009. Weight (A), height (B), weight-for-age z score (WAZ) (C), height-for-age z score (HAZ) (D), peak weight velocity (E), and peak height velocity (F) were considered as outcomes. Crude (unadjusted) associations, as described for model 1 in the Materials and Methods section, are shown as diamonds. Estimates adjusted for child's sex, family socioeconomic status, season of birth, gestational age at birth, maternal education, maternal height or body mass index (weight (kg)/height (m)2) (as appropriate), maternal tobacco-chewing, indoor cooking without ventilation, and birth order (model 2) are shown as squares. Estimates additionally adjusted for log2-transformed maternal urinary As, Cd, or Pb as appropriate (model 3) are shown as triangles. Results from a model jointly estimating the combined effects of urinary As, Cd, and Pb (model 4) are shown as circles. Δ indicates “change.” Vertical lines represent 95% confidence intervals.
Are prenatal environmental exposures associated with anthropometric outcomes in 5-year-olds?
Only maternal urinary cadmium level was inversely associated with the children's anthropometric outcomes, although this relationship disappeared after adjustment (Web Figure 1). None of the maternal biomarkers were associated with the anthropometric outcomes at age 5 years in a model accounting for exposure to all 3 metals or in models also accounting for postnatal exposure.
Are lifelong environmental exposures related to children's growth to age 5 years?
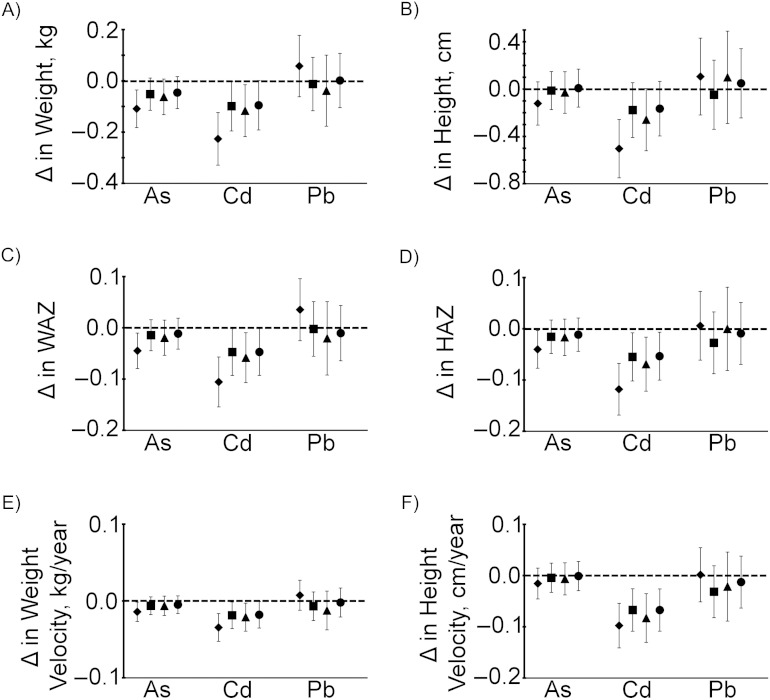
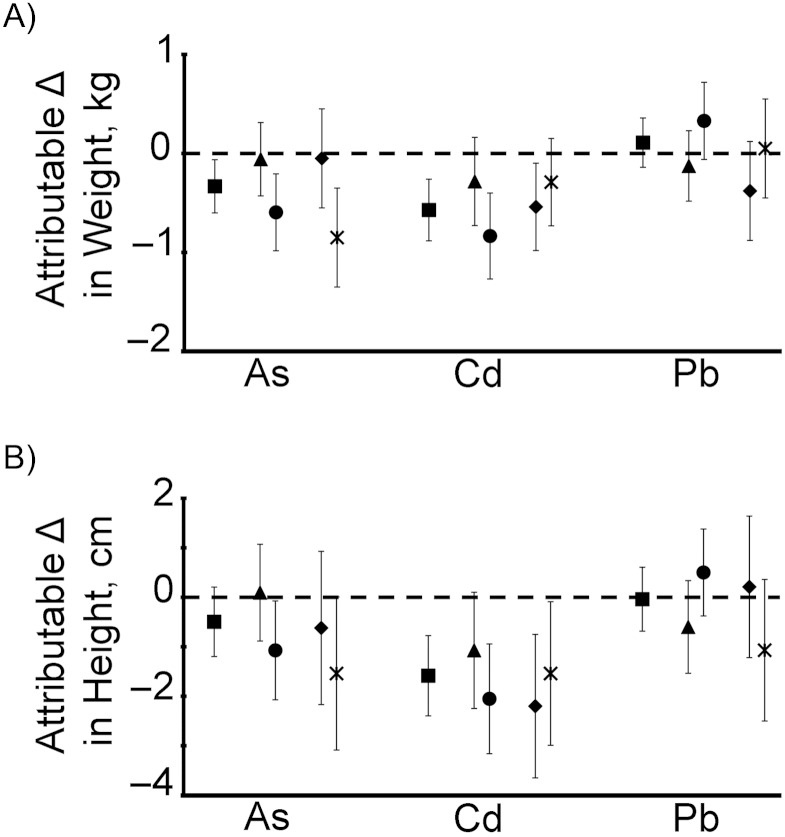
The multivariable-adjusted difference in attained weight at age 5 years between highly exposed (≥95th percentile) and less exposed (≤5th percentile) children was 0.33 kg for arsenic and 0.57 kg for cadmium (Figure 5). The difference in attained height at age 5 years attributable to high cadmium exposure was 1.6 cm, while the difference in attained height attributable to high arsenic exposure was 0.5 cm and not statistically significant (Figure 5). The differences in both weight and height attributable to lead exposure were not statistically significant. For comparison, multivariable-adjusted estimates showed that girls were on average 0.67 kg lighter (95% confidence interval (CI): −0.82, −0.53) and 1.3 cm shorter (95% CI: −1.7, −0.89) than boys at age 5 years and that children with the lowest SES were on average 1.03 kg lighter (95% CI: −1.14, −0.76) and 2.8 cm shorter (95% CI: −3.2, −2.4) than children with the highest SES.
Figure 5.
Differences in children's weights and heights at age 5 years attributable to lifelong arsenic (As), cadmium (Cd), and lead (Pb) exposure, assessed in Matlab, Bangladesh, 2001–2009. The relationship between combined exposure to arsenic, cadmium, and lead over time and the child's weight and length/height from birth to age 5 years was assessed using mixed-effects linear regression and was adjusted for child's sex, family socioeconomic status (SES), season of birth, gestational age at birth, maternal education, maternal height or body mass index (weight (kg)/height (m)2) (as appropriate), maternal tobacco-chewing, indoor cooking without ventilation, and birth order. Multivariable-adjusted attributable differences were calculated by comparing the model-predicted weight (A) or height (B) of children with urinary arsenic, urinary cadmium, and urinary lead levels at or above the 95th percentile at age 5 years with those of children at or below the 5th percentile (shown as squares). The analysis was repeated after stratification for sex and SES, and multivariable-adjusted differences are also shown for boys (triangles), girls (circles), low SES (diamonds), and high SES (X's). Δ indicates “change.” Vertical lines represent 95% confidence intervals.
After stratification by sex, the differences in attained weight and height at age 5 years attributable to high arsenic or high cadmium were apparent only in the girls. After stratification by SES, the differences in attained weight and height attributable to high arsenic exposure were apparent only among those with low SES, while the attributable change due to high cadmium exposure was somewhat attenuated in the high-SES group compared with the low-SES group.
DISCUSSION
In this study, we showed that, among the 3 metals tested, early-life cadmium exposure had the strongest and most consistent inverse association with children's growth in terms of weight and (particularly) height. The inverse association between cadmium and arsenic and children's growth was most apparent among the girls, and these inverse associations persisted even after controlling for the other environmental exposures and relevant factors for children's growth in a longitudinal model. Consistent with our earlier observations of children's size at birth (11), the arsenic dose-effect relationship was nonlinear, with the largest changes occurring within the lower arsenic exposure range and leveling off at higher exposures. While previous cross-sectional studies have shown inverse associations between both cadmium exposure and arsenic exposure during various windows of development and children's height and weight, we showed that concurrent exposures were more strongly correlated with children's size at age 5 years than were prenatal exposures. Surprisingly, children with a higher percentage of MMA, the most toxic arsenic metabolite, had improved anthropometric outcomes. This may indicate that children with the best growth trajectories lack methyl groups readily available for the complete metabolism of arsenic to DMA, since methyl groups are also necessary for building new tissue.
With regard to maternal exposures, cadmium levels of this magnitude are somewhat elevated but not uncommon in other countries (40, 41), particularly in persons who consume greater amounts of rice and vegetables (42). However, the cadmium exposure levels observed among most children in this population were above the median reported for US (43) and German (44) children. The children's rice-based diet is most likely the main source of cadmium exposure (30). Arsenic was markedly elevated in most children (25), in comparison with values reported in German children (44). Exposure to arsenic in this population is due to elevated concentrations of arsenic in tube-well water, as well as elevated concentrations in rice irrigated with arsenic-contaminated water (25). One limitation of this study was that we lacked blood lead measurements for the children; therefore, direct comparisons with exposures in other populations and with the literature on the association between lead and stunting were difficult to make. If the children's blood lead concentrations were similar to their mothers' previously reported values (median, 3.6 μg/dL; range, 1.4–11.5) (29) given their similar urinary lead concentrations, they would be within the range for which associations between lead and stunting have been reported in studies of other populations (8, 45).
The mechanisms by which arsenic and cadmium may affect growth in early life are unclear. The literature on cadmium exposure and osteoporosis suggests that cadmium may be directly toxic to bone in adults, leading to increased risk of osteoporosis (46–48). One recent study showed evidence for increased bone resorption and demineralization in 10-year-old children with exposure to cadmium, suggesting that cadmium may affect bone tissue from an early age (49). Such toxic effects may translate to impaired bone growth in children and, thus, to a reduction in attained height.
Cadmium and arsenic also interfere with the distribution and function of micronutrients. Our studies in this cohort have shown that maternal cadmium exposure was associated with impaired transfer of zinc across the placenta and decreased calcium levels in breast milk (50, 51). While disruption of the transfer of these nutrients in the pregnant and nursing mother could lead to disrupted growth in children, concurrent exposure was more strongly associated with negative outcomes in terms of weight and height than was prenatal exposure. It is plausible that cadmium exposure may affect calcium and zinc distribution and function in children as well.
Arsenic exposure is associated with increased risk of anemia (52). Growth retardation is one potential consequence of anemia in children, and improving iron and zinc status in undernourished children aged 1–4 years improves growth trajectories (53). Both arsenic and cadmium were inversely associated with family SES, a broad indicator of nutritional status (54), and the associations of both markers with anthropometric outcomes were attenuated by the addition of SES and other potential confounders to the model. However, we observed that the inverse association between arsenic exposure and children's growth was apparent only among children of the highest SES, and that the inverse association between cadmium exposure and children's growth was mildly attenuated among children of the highest SES compared with children of the lowest SES. This indicates that our results are not likely to be due to unobserved confounding by nutritional status.
Additionally, cadmium and arsenic have endocrine-disrupting properties, which may disrupt growth in young children, particularly in a sex-specific fashion. Evidence in humans and experimental animals suggests that cadmium disrupts steroidogenesis, particularly in the placenta, and acts as an endocrine-disrupting chemical, capable of mimicking or inhibiting the functions of endogenous estradiol (55). Cadmium exposure in adolescents living in Belgium was associated with decreased height and body mass index, as well as lower circulating levels of estradiol and testosterone in the blood (56, 57). Additionally, in experimental animals, high doses of cadmium have been found to lower plasma concentrations of insulin-like growth factor 1, a hormone critical for childhood growth (58). Arsenic may affect insulin signaling and glucose metabolism (59, 60), eventually leading to glucose intolerance and diabetes in exposed populations. Early disruption of glucose uptake by tissues may plausibly lead to impaired growth. This mechanism may be more pronounced in children with better overall nutrition, potentially explaining our observations of arsenic's effects after stratification by SES.
In conclusion, this study has provided evidence that environmental exposures to cadmium and arsenic during early life may contribute to poor growth. The longitudinal modeling presented here showed that persistent exposure to cadmium in early life led to a cadmium-attributable decrease in height and weight by age 5 years that was of the same magnitude as the differences observed between girls and boys at that age. Arsenic-attributable differences in weight were smaller, though still large enough to prompt concern.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden (Renee M. Gardner, Maria Kippler, Matteo Bottai, Margaretha Grandér, Barbro Nermell, Brita Palm, Marie Vahter); International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh (Fahmida Tofail, Jena Hamadani); and Division of Nutritional Sciences, Cornell University, Ithaca, New York (Kathleen M. Rasmussen).
This work was supported by grants from the Swedish International Development Cooperation Agency, the Swedish Research Council, the European Commission (PHIME (Public Health Impact of Long-term, Low-level Mixed Element Exposure in Susceptible Population Strata) project FOOD-CT-2006-016253), and the Karolinska Institutet. The MINIMat Study was initiated at the International Centre for Diarrhoeal Disease Research, Bangladesh (Principal Investigators: Lars Åke Persson and Shams El Arifeen) with the support of the United Nations Children's Fund; the Swedish International Development Cooperation Agency; the United Kingdom Medical Research Council; the Swedish Research Council; the United Kingdom Department for International Development; the International Centre for Diarrhoeal Disease Research, Bangladesh; the Global Health Research Fund, Japan; the Child Health and Nutrition Research Initiative (Global Forum for Health Research); Uppsala University; and the US Agency for International Development.
We thank the field staff in Matlab for collection of urine samples and data.
All of the authors have read, critiqued, and approved the contents of this article.
Conflict of interest: none declared.
REFERENCES
- 1.Walker SP, Chang SM, Powell CA, et al. Early childhood stunting is associated with poor psychological functioning in late adolescence and effects are reduced by psychosocial stimulation. J Nutr. 2007;137(11):2464–2469. doi: 10.1093/jn/137.11.2464. [DOI] [PubMed] [Google Scholar]
- 2.Sawaya AL, Martins PA, Grillo LP, et al. Long-term effects of early malnutrition on body weight regulation. Nutr Rev. 2004;62(7):S127–S133. doi: 10.1111/j.1753-4887.2004.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 3.Gaskin PS, Walker SP, Forrester TE, et al. Early linear growth retardation and later blood pressure. Eur J Clin Nutr. 2000;54(7):563–567. doi: 10.1038/sj.ejcn.1601057. [DOI] [PubMed] [Google Scholar]
- 4.Walker SP, Grantham-McGregor SM, Powell CA, et al. Effects of growth restriction in early childhood on growth, IQ, and cognition at age 11 to 12 years and the benefits of nutritional supplementation and psychosocial stimulation. J Pediatr. 2000;137(1):36–41. doi: 10.1067/mpd.2000.106227. [DOI] [PubMed] [Google Scholar]
- 5.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378(9799):1325–1338. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 6.Caulfield LE, de Onis M, Blossner M, et al. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80(1):193–198. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 7.Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 8.Ballew C, Khan LK, Kaufmann R, et al. Blood lead concentration and children's anthropometric dimensions in the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. J Pediatr. 1999;134(5):623–630. doi: 10.1016/s0022-3476(99)70250-7. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz J, Angle C, Pitcher H. Relationship between childhood blood lead levels and stature. Pediatrics. 1986;77(3):281–288. [PubMed] [Google Scholar]
- 10.Pounds JG, Long GJ, Rosen JF. Cellular and molecular toxicity of lead in bone. Environ Health Perspect. 1991;91:17–32. doi: 10.1289/ehp.919117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman A, Vahter M, Smith AH, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169(3):304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- 12.Kippler M, Tofail F, Gardner R, et al. Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ Health Perspect. 2011;120(2):284–289. doi: 10.1289/ehp.1103711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasserman GA, Liu X, Parvez F, et al. Water arsenic exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2004;112(13):1329–1333. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe C, Matsui T, Inaoka T, et al. Dermatological and nutritional/growth effects among children living in arsenic-contaminated communities in rural Bangladesh. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42(12):1835–1841. doi: 10.1080/10934520701566850. [DOI] [PubMed] [Google Scholar]
- 15.Minamoto K, Mascie-Taylor CG, Moji K, et al. Arsenic-contaminated water and extent of acute childhood malnutrition (wasting) in rural Bangladesh. Environ Sci. 2005;12(5):283–292. [PubMed] [Google Scholar]
- 16.Saha KK, Hamadani J, Tofail F, et al. Pre- and postnatal arsenic exposure and child growth to age 2 years: a cohort study in rural Bangladesh. Environ Health Perspect. 2012;120(8):1208–1214. doi: 10.1289/ehp.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian LL, Zhao YC, Wang XC, et al. Effects of gestational cadmium exposure on pregnancy outcome and development in the offspring at age 4.5 years. Biol Trace Elem Res. 2009;132(1–3):51–59. doi: 10.1007/s12011-009-8391-0. [DOI] [PubMed] [Google Scholar]
- 18.Lin CM, Doyle P, Wang D, et al. Does prenatal cadmium exposure affect fetal and child growth? Occup Environ Med. 2011;68(9):641–646. doi: 10.1136/oem.2010.059758. [DOI] [PubMed] [Google Scholar]
- 19.Tofail F, Persson LA, El Arifeen S, et al. Effects of prenatal food and micronutrient supplementation on infant development: a randomized trial from the Maternal and Infant Nutrition Interventions, Matlab (MINIMat) study. Am J Clin Nutr. 2008;87(3):704–711. doi: 10.1093/ajcn/87.3.704. [DOI] [PubMed] [Google Scholar]
- 20.Hamadani JD, Grantham-McGregor SM, Tofail F, et al. Pre- and postnatal arsenic exposure and child development at 18 months of age: a cohort study in rural Bangladesh. Int J Epidemiol. 2010;39(5):1206–1216. doi: 10.1093/ije/dyp369. [DOI] [PubMed] [Google Scholar]
- 21.Saha KK, Frongillo EA, Alam DS, et al. Appropriate infant feeding practices result in better growth of infants and young children in rural Bangladesh. Am J Clin Nutr. 2008;87(6):1852–1859. doi: 10.1093/ajcn/87.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan AI, Kabir I, Ekstrom EC, et al. Effects of prenatal food and micronutrient supplementation on child growth from birth to 54 months of age: a randomized trial in Bangladesh. Nutr J. 2011;10:134. doi: 10.1186/1475-2891-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwatkin DR, Rustein S, Johnson K, et al. Socioeconomic Differences in Health, Nutrition, and Population in Bangladesh, HNP/Poverty Thematic Group Working Paper. Washington, DC: The World Bank; 2000. [Google Scholar]
- 24.Saha KK, Frongillo EA, Alam DS, et al. Household food security is associated with infant feeding practices in rural Bangladesh. J Nutr. 2008;138(7):1383–1390. doi: 10.1093/jn/138.7.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner RM, Hamadani J, Grandér M, et al. Persistent exposure to arsenic via drinking water and food in rural Bangladesh affects growth in early childhood: a cohort study. Am J Public Health. 2011;101(suppl 1):S333–S338. doi: 10.2105/AJPH.2010.300025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vahter ME, Li L, Nermell B, et al. Arsenic exposure in pregnancy: a population-based study in Matlab, Bangladesh. J Health Popul Nutr. 2006;24(2):236–245. [PubMed] [Google Scholar]
- 27.Fangstrom B, Hamadani J, Nermell B, et al. Impaired arsenic metabolism in children during weaning. Toxicol Appl Pharmacol. 2009;239(2):208–214. doi: 10.1016/j.taap.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Ekstrom EC, Goessler W, et al. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ Health Perspect. 2008;116(3):315–321. doi: 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergkvist C, Kippler M, Hamadani JD, et al. Assessment of early-life lead exposure in rural Bangladesh. Environ Res. 2010;110(7):718–724. doi: 10.1016/j.envres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Kippler M, Nermell B, Hamadani J, et al. Burden of cadmium in early childhood: longitudinal assessment of urinary cadmium in rural Bangladesh. Toxicol Lett. 2010;198(1):20–25. doi: 10.1016/j.toxlet.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Nermell B, Lindberg AL, Rahman M, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106(2):212–218. doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Basu A, Mitra S, Chung J, et al. Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environ Health Perspect. 2011;119(9):1308–1313. doi: 10.1289/ehp.1003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkey CS, Reed RB. A model for describing normal and abnormal growth in early childhood. Hum Biol. 1987;59(6):973–987. [PubMed] [Google Scholar]
- 35.Simondon KB, Simondon F, Delpeuch F, et al. Comparative study of five growth models applied to weight data from Congolese infants between birth and 13 months of age. Am J Hum Biol. 1992;4(3):327–335. doi: 10.1002/ajhb.1310040308. [DOI] [PubMed] [Google Scholar]
- 36.Hauspie RC, Molinari L. Parametric models for postnatal growth. In: Hauspie RC, Cameron N, Molinari L, editors. Methods in Human Growth Research. Cambridge, United Kingdom: Cambridge University Press; 2004. pp. 205–233. [Google Scholar]
- 37.Persson LA, Arifeen SE, Ekström EC, et al. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: the MINIMat randomized trial. JAMA. 2012;307(19):2050–2059. doi: 10.1001/jama.2012.4061. [DOI] [PubMed] [Google Scholar]
- 38.Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc. 1962;57(298):348–368. [Google Scholar]
- 39.Vahter M, Akesson A, Liden C, et al. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Akesson A, Berglund M, Schutz A, et al. Cadmium exposure in pregnancy and lactation in relation to iron status. Am J Public Health. 2002;92(2):284–287. doi: 10.2105/ajph.92.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McElroy JA, Shafer MM, Hampton JM, et al. Predictors of urinary cadmium levels in adult females. Sci Total Environ. 2007;382(2-3):214–223. doi: 10.1016/j.scitotenv.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Nishijo M, Tawara K, Honda R, et al. Cadmium and nutritional intake in pregnant Japanese women. Toxicol Lett. 2004;148(3):171–176. doi: 10.1016/j.toxlet.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz P, Mumtaz M, Osterloh J, et al. Interpreting NHANES biomonitoring data, cadmium. Toxicol Lett. 2010;198(1):44–48. doi: 10.1016/j.toxlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Schulz C, Angerer J, Ewers U, et al. Revised and new reference values for environmental pollutants in urine or blood of children in Germany derived from the German Environmental Survey on Children 2003–2006 (GerES IV) Int J Hyg Environ Health. 2009;212(6):637–647. doi: 10.1016/j.ijheh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Ignasiak Z, Slawinska T, Rozek K, et al. Lead and growth status of school children living in the copper basin of south-western Poland: differential effects on bone growth. Ann Hum Biol. 2006;33(4):401–414. doi: 10.1080/03014460600730752. [DOI] [PubMed] [Google Scholar]
- 46.Engstrom A, Skerving S, Lidfeldt J, et al. Cadmium-induced bone effect is not mediated via low serum 1,25-dihydroxy vitamin D. Environ Res. 2009;109(2):188–192. doi: 10.1016/j.envres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Akesson A, Bjellerup P, Lundh T, et al. Cadmium-induced effects on bone in a population-based study of women. Environ Health Perspect. 2006;114(6):830–834. doi: 10.1289/ehp.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schutte R, Nawrot TS, Richart T, et al. Bone resorption and environmental exposure to cadmium in women: a population study. Environ Health Perspect. 2008;116(6):777–783. doi: 10.1289/ehp.11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sughis M, Penders J, Haufroid V, et al. Bone resorption and environmental exposure to cadmium in children: a cross-sectional study. Environ Health. 2011;10:104. doi: 10.1186/1476-069X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kippler M, Lonnerdal B, Goessler W, et al. Cadmium interacts with the transport of essential micronutrients in the mammary gland—a study in rural Bangladeshi women. Toxicology. 2009;257(1-2):64–69. doi: 10.1016/j.tox.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Kippler M, Hoque AM, Raqib R, et al. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol Lett. 2010;192(2):162–168. doi: 10.1016/j.toxlet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Heck JE, Chen Y, Grann VR, et al. Arsenic exposure and anemia in Bangladesh: a population-based study. J Occup Environ Med. 2008;50(1):80–87. doi: 10.1097/JOM.0b013e31815ae9d4. [DOI] [PubMed] [Google Scholar]
- 53.Sazawal S, Dhingra U, Dhingra P, et al. Micronutrient fortified milk improves iron status, anemia and growth among children 1–4 years: a double masked, randomized, controlled trial. PloS One. 2010;5(8):e12167. doi: 10.1371/journal.pone.0012167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindstrom E, Hossain MB, Lonnerdal B, et al. Prevalence of anemia and micronutrient deficiencies in early pregnancy in rural Bangladesh, the MINIMat trial. Acta Obstet Gynecol Scand. 2011;90(1):47–56. doi: 10.1111/j.1600-0412.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- 55.Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med (Maywood) 2004;229(5):383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- 56.Dhooge W, den Hond E, Koppen G, et al. Internal exposure to pollutants and sex hormone levels in Flemish male adolescents in a cross-sectional study: associations and dose-response relationships. J Expo Sci Environ Epidemiol. 2011;21(1):106–113. doi: 10.1038/jes.2009.63. [DOI] [PubMed] [Google Scholar]
- 57.Dhooge W, Den Hond E, Koppen G, et al. Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships. Environ Int. 2010;36(4):330–337. doi: 10.1016/j.envint.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Turgut S, Kaptanoglu B, Turgut G, et al. Effects of cadmium and zinc on plasma levels of growth hormone, insulin-like growth factor I, and insulin-like growth factor-binding protein 3. Biol Trace Elem Res. 2005;108(1-3):197–204. doi: 10.1385/BTER:108:1-3:197. [DOI] [PubMed] [Google Scholar]
- 59.Paul DS, Hernandez-Zavala A, Walton FS, et al. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol. 2007;222(3):305–314. doi: 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navas-Acien A, Silbergeld EK, Streeter RA, et al. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect. 2006;114(5):641–648. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.