Summary
The structure of a tendon is an important example of complexity of ECM three-dimensional organization. The extracellular matrix (ECM) is a macromolecular network with both structural and regulatory functions. ECM components belong to four major types of macromolecules: the collagens, elastin, proteoglycans, and noncollagenous glycoproteins. Tendons are made by a fibrous, compact connective tissue that connect muscle to bone designed to transmit forces and withstand tension during muscle contraction. Here we show the ultrastructural features of tendon’s components.
Keywords: tendon, collagen, ultrastructure, extra-cellular matrix
Introduction
The extracellular matrix (ECM) is a macromolecular network with both structural and regulatory functions. Indeed, ECM furnishes mechanical and biochemical signals that cooperate in the integrated control of cell proliferation, survival, migration, and differentiation1–3. ECM components belong to four major types of macromolecules: the collagens, elastin, proteoglycans, and noncollagenous glycoproteins. The most copious proteins in the ECM are members of the collagen family. The collagens are involved in the formation of ECM fibrillar and microfibrillar networks and play a key role in determining tissue-specific mechanical properties. Collagens can be categorized into fibril forming collagens (types I, II, III, V, XI), fibril-associated collagens (FACITs; types IX, XII, XIV, XIX, XX, XXI), hexagonal network-forming collagens (types VIII and X), microfibril-forming collagen (type VI), basement membrane collagen (type IV), anchoring fibril-forming collagen (type VII), transmembrane collagens (types XIII and XVII), and other collagens with peculiar functions (types XV, XVI, XVIII)4–6. Other ECM components that affects tissue mechanical properties are elastin, which is the main constituent of ECM elastic fibers, and the proteoglycans which are composed of a protein core linked to glycosaminoglycan (GAG) side chains7. A different group of ECM components is represented by ECM glycoproteins including laminins and entactin/nidogen which are the major glycoprotein components of the basement membrane1,8, fibronectin which is found as a soluble dimer in plasma and as an insoluble fibrillar polymer of interstitial ECM and basement membranes1,8 and tenascins, which has adhesive and anti-adhesive effects on cell-matrix interactions1,9.
It has been supposed that collagens have a significant role in the evolution of metazoans. Indeed, the fibril-forming collagens may correspond to the most ancient type of collagen in the metazoan and the variety of these fibrils has enhanced during the development of the chordates10.
Tendon’s ultrastructure and ECM
The structure of a tendon is an important example of complexity of ECM three-dimensional organization. Tendons are made by a fibrous, compact connective tissue that connect muscle to bone designed to transmit forces and withstand tension during muscle contraction11,12. The apparently simple tendon’s structure and composition provide both rigidity and flexibility. This ability is based on tendon non-linear, viscous-elastic, anisotropic and heterogeneous mechanical properties11–13.
Similarly to other tissues there is a clear relationship between structure and function of the tendon13. Indeed, different tissue microenvironments provide specific characteristics to the different three-dimensional organization of the ECM since embryogenesis1,3.
The organization of the ECM in the tendon is peculiar as well as within different tendons. The process of tendon’s embryonic structural development differs by location and type of tendon14. In the first stage, for example, tendon progenitor cells originate from the primary germ layer compartments along with progenitors for cartilage and muscle14. This process is different for longer limb tendons as opposed to short trunk tendons14. The dialogues between different cells (fibroblasts, muscle and cartilage cells) or better the dialects between different cells mediated by various signaling cascades dependent on FGFs and TGF-beta are used at decisive stages of development to make specific the phases of induction, organization, aggregation or differentiation of cells14. The structure of the tendon is so complex that it is very difficult to maintain its function in the healing since this process is reparative rather than regenerative in the adult15,16. A tendon consists of 70% of water and 30% of dry mass, which is composed by 60–80% of type I collagen and 2% of elastin. Among collagens, the most abundant component is collagen type I (95%), while type III and type V collagens represent the remaining 5% of the total collagens16. Tendon’s collagen’s is organized in multi-hierarchical structures including fibrils, fibers (primary bundles), fascicles (secondary boundless)15,16 (Fig. 1). The fibrillogenesis in the form of small diameter fibrils begins in embryogenesis and continues after birth with the assembly of collagen type I molecules, followed by linear and lateral growth and collagen interactions with proteins such as other collagens and proteoglycans14. In particular, the linear and lateral growth is determined by a variety of molecules including other collagens (type III, V, XI, XII and XIV) which are expressed in a variable manner. Electron microscopy of longitudinally oriented tendon specimen shows a parallel arrangement of collagen fibrils. The ratio of collagen fibril sizes in human tendon is variable from 1,750 to 600 and 100 A diameter13,17. However, it is difficult to link the size of collagen fibrils with different zone of the tendon17. The collagen type III plays a key role in regulating fibrillogenesis and extensibility of the tendon and its production slowly decreases in the development to reappear in appreciable amount after injury in the healing process14–16.
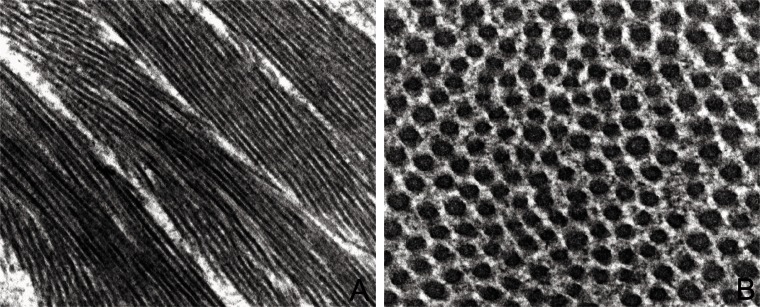
Figure 1.
Ultrastructure features of ECM in tendon. A: Collagen I is the mainly constituent of tendons and creates highly aligned fibrils organized as fibers oriented longitudinally, transversally and also crossing each others, thus conferring a great resistance to different forces (O.M. ×7900). B: Collagen I fibrils are in contact to thin fibrils of collagen V and to proteoglycans and glycoproteins (O.M. ×12500).
Collagen type V and XI are associated to collagen type I and II, respectively and they determine their quantity and quality1,2,4,14. It must be stressed that collagen type V exists in the form of heterotrimers sequestered in the fibrils of abundant collagen type I and in the form of homodimers as thin filament which can act in the ECM as a molecular linker between collagen fibrils or macromolecules depending on their respective distribution in different tissues18,19. The location of collagen type V in the perivascular tissues have made it as a “sequestered antigen” which might be recognized by the immune system in a variety of inflammatory processes and therefore might induce autoimmune diseases3,20 (Fig. 2).
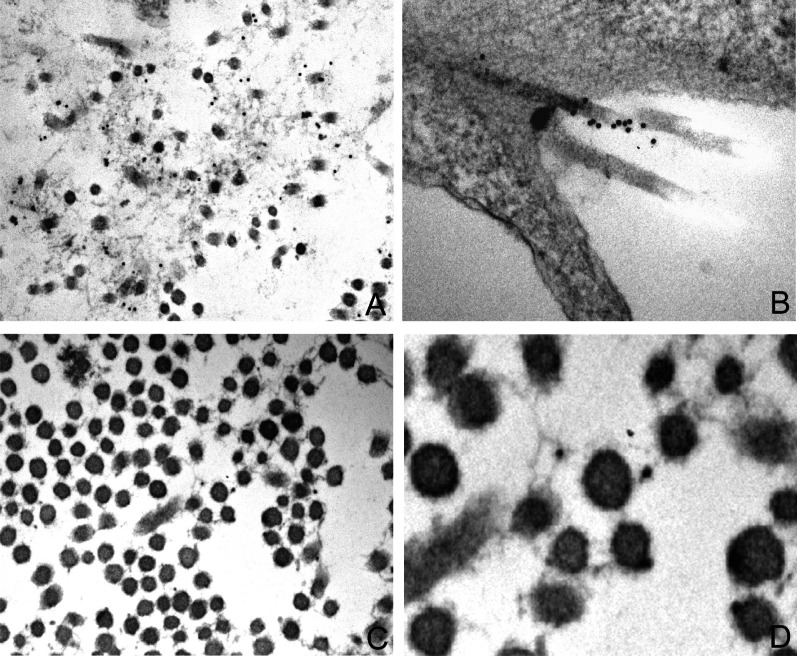
Figure 2.
Immunolocalization of type V collagen in different tissues. Immunostaining with a specific monoclonal antibody against collagen type V and colloidal gold conjugated goat anti-mouse (O.M. × 11500, ×7900, ×18000, × 45000 respectively). A: desmoplasia in breast carcinoma; B: fibroblasts tissue culture; C-D: Degenerative tendon scar
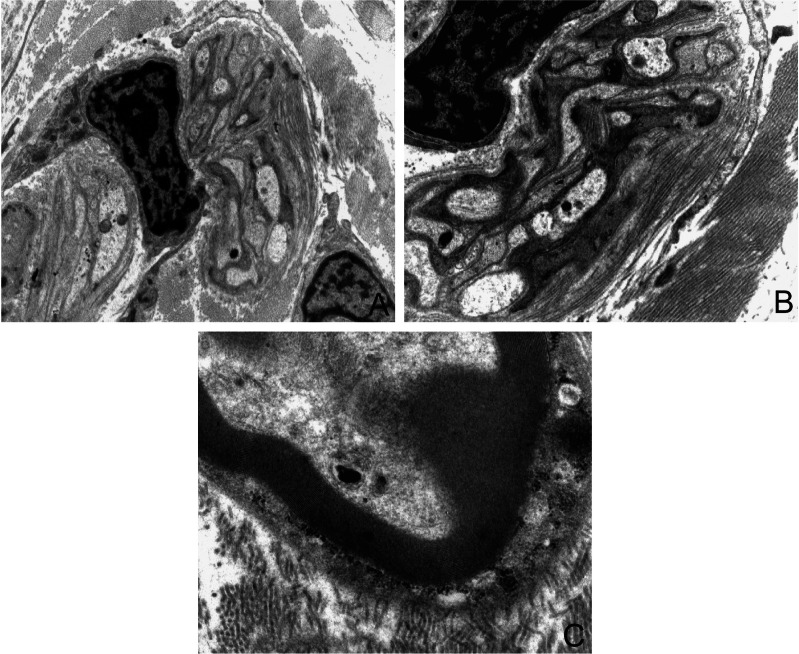
Type XII and XIV collagens are fibril-associated collagens with interrupted triple helices (FACIT)4–6. Tendons in additions to collagen contain proteoglycans, glycosaminoglycans and glycoproteins including fibronectin, trombospondin and tenascin-C immersed in different composition of ECM lying cellular elements represented (90/95%) by tenoblasts and tenocytes13 (Fig. 3). The tenoblasts show a prominent rough endoplasmic reticulum, as well as a developed Golgi complex. The remaining 5–10% of the cells consists of chondrocytes, synovial and vascular cells13.
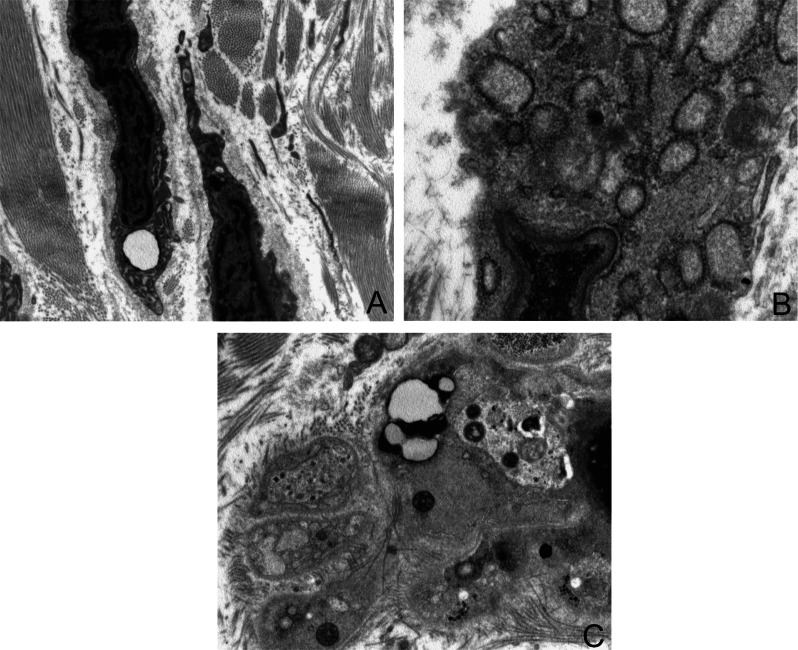
Figure 3.
Ultrastructure features of tenocytes and tenoblasts. The cellular population of tendon is mainly represented by tenocytes: tenocytes are very elongated cells with ovoid and very long nuclei, occupying the entire length of the cell, with a high nucleus-to-cytoplasm ratio. The cellular process are long and thin and in direct contact with extracellular matrix components (A). In the cytoplasm of tenoblasts is often visible a well developed rough endoplasmic reticulum with dilated cisternae (B), few mitochondria, primary and secondary lysosomes, vacuoles, phagosomes and glycogen (C) (O.M. × 3900, ×7900, ×3900).
Tendons are responsible for the connection between bone and muscle at their ends. The junction is called enthesis, which can be divided in fibrous enthesis and fibrocartilaginous enthesis. In tendons, the fibrocartilage is arranged in region compressed against bony or fibrous pulleys21. Fibrocartilage is an avascular tissue whose cells resemble, at the ultrastructural examination, chondrocytes with a prominent rough endoplasmic reticulum, glycogen granules and lipid droplets and intermediate filaments21. The cells synthesize collagens (type I, II, III, V, XII, XII), proteoglycans (aggrecan, versican, decorin, biglycan, lumican, fibromodulin), glycoprotein and in particular tenascin-C by mechanical strain. The tendons is covered by the epitenon, a delicate, loose connective-tissue sheath containing blood and lymphatic vessels, and nerves. The epitenon expands deeply between the tertiary bundles as the endotenon, made by connective tissue surroyunding each fiber13 (Figs. 4 and 5).
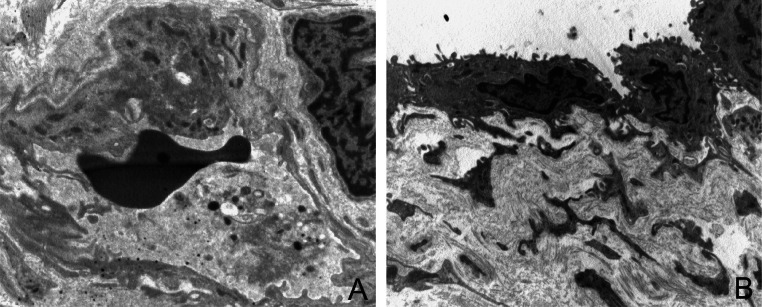
Figure 4.
Ultrastructure features of epitenon. Epitenon is a delicate, loose connective-tissue sheath containing blood and lymphatic vessels. A–B: Endothelial cells show cell membrane protrusion and several granules and pinocytic vesicles in the cytoplasm. Endothelial cells are surrounded by pericytes and show a well constituted basal membrane (O.M. ×4900, ×3900).
Figure 5.
Ultrastructure features of nerves in the epitenon. Unmyelinated (A–B) and myelinated (C) fibers surrounded by cytoplasmic elongation of Schwann cell (O.M. ×3900, ×5500, ×7500).
Acknowledgments
Authors want to express their thanks to Barbara Bulgarini for editorial assistance.
References
- 1.Bosman F, Stamenkovic I. Functional structure and composition of extracellular matrix. J Pathol. 2003;200:423–428. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 2.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signalling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bei R, Masuelli L, Palumbo C, Tresoldi I, Scardino A, Modesti A. Long-lasting tissue inflammatory processes trigger autoimmune responses to extracellular matrix molecules. Int Rev Immunol. 2008;27:137–175. doi: 10.1080/08830180801939280. [DOI] [PubMed] [Google Scholar]
- 4.Gelse K, Poschl E, Aigner T. Collagens-structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris) 2005;53:430–442. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Traub W, Piez KA. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]
- 7.Cattaruzza S, Perris R. Approaching the proteoglycome: molecular interactions of proteoglycans and their functional output. Macromol Biosci. 2006;6:667–680. doi: 10.1002/mabi.200600100. [DOI] [PubMed] [Google Scholar]
- 8.Hohenester E, Engel J. Domain structure and organisation of extracellular matrix proteins. Matrix Biol. 2002;21:115–128. doi: 10.1016/s0945-053x(01)00191-3. [DOI] [PubMed] [Google Scholar]
- 9.Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J Biol Chem. 2005;280:26641–26644. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- 10.Heino J, Huhtala M, Käpylä J, Johnson MS. Evolution of collagen-based adhesion systems. Int J Biochem Cell Biol. 2009;41:341–348. doi: 10.1016/j.biocel.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Aparecida de Aro A, Vidal Bde C, Pimentel ER. Biochemical and anisotropical properties of tendons. Micron. 2012;43:205–214. doi: 10.1016/j.micron.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]
- 13.Franchi M, Trirè A, Quaranta M, Orsini E, Ottani V. Collagen structure of tendon relates to function. Scientific World Journal. 2007;7:404–420. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connizzo BK, Yannascoli SM, Soslowsky LJ. Structure-function relationships of postnatal tendon development: A parallel to healing. Matrix Biol. 2013;32:106–116. doi: 10.1016/j.matbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- 17.Dyer RF, Enna CD. Ultrastructural features of adult human tendon. Cell Tissue Res. 1976;168:247–259. doi: 10.1007/BF00215881. [DOI] [PubMed] [Google Scholar]
- 18.Chanut-Delalande H, Fichard A, Bernocco S, Garrone R, Hulmes DJ, Ruggiero F. Control of heterotypic fibril formation by collagen V is determined by chain stoichiometry. J Biol Chem. 2001;276:24352–24359. doi: 10.1074/jbc.m101182200. [DOI] [PubMed] [Google Scholar]
- 19.Modesti A, Kalebic T, Scarpa S, Togo S, Grotendorst G, Liotta LA, Triche TJ. Type V collagen in human amnion is a 12 nm fibrillar component of the pericellular interstitium. Eur J Cell Biol. 1984;35:246–255. [PubMed] [Google Scholar]
- 20.Dart ML, Jankowska-Gan E, Huang G, Roenneburg DA, Keller MR, Torrealba JR, Rhoads A, Kim B, Bobadilla JL, Haynes LD, Wilkes DS, Burlingham WJ, Greenspan DS. Interleukin-17-dependent autoimmunity to collagen type V in atherosclerosis. Circ Res. 2010;107:1106–1116. doi: 10.1161/CIRCRESAHA.110.221069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin M, Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]