Summary
We studied the effect of spasticity-induced overload on tendons from the gracilis and semitendinosus muscles from cerebral palsy (CP) and healthy subjects (CT) stained with haematoxylineosin, Sirius red and Alcian blue. Vascularity was also characterized using an anti-CD34 antibody.
Light microscopy analysis of haematoxylin-eosin stained sections revealed that the overall structure of tendons was maintained, characterized by parallel and slightly wavy collagen fibers in both CT and CP tendons. However, hypercellularity, cell rounding, increased vascularity and lipoid degeneration were observed in CP samples.
Sirius red stained collagen fibers were more evident in CP tendons, suggesting an increased collagen content induced by spasticity. Alcian blue staining revealed an overall increase of glycosaminoglycans in CP tendons as observed in tendinopathy.
Our results suggest that CP-induced spasticity may be considered as a chronic, persisting and repetitive loading of tendons, inducing ECM remodeling as adaptive response to increased functional demand. At the same time, the evidence of some tendinopathic-like markers in CP tendons suggests that the chronic nature of the CP condition could represent a pathologic condition, possibly leading to a transient weakness of the tissue making it more susceptible to damage from cumulative loading until an overt tendinopathy develops.
Keywords: collagen, extracellular matrix, glycosaminoglycans, spasticity, tendon
Introduction
Tendons are specialized organs that connect muscle to bone and transmit the forces generated in the muscle to bone, resulting in joint movement. They consist of dense regular connective tissue composed by cells, the tenocytes, and extracellular matrix (ECM) primarily containing collagen, proteoglycans and water.
Collagen accounts for the 60–85% of the dry mass and is predominantly type I collagen (COL-I) organized into tensile resistant fibrils, fibers and fiber bundles, providing the major resistance to mechanical forces. Type III collagen (COL-III) is the next most abundant collagen, representing approximately 3% of the total and mostly restricted, in normal tendons, to the endotenon and epitenon. Also type V and VI collagen are present, intercalated into the core of COL-I fibril or forming sheets-like structures, respectively1.
Proteoglycans (PG) are protein/glycosaminoglycans (GAG) complexes in the ECM that trap water and affect the viscoelastic properties of the tissue, helping tendon tissue to resist compressive and tensile forces and allowing collagen fibers to glide over each other. Tendon contains a wide variety of PG, including the large aggregating PG and a variety of small leucine-rich PG2.
Decorin, biglycan, fibromodulin and lumican, all members of the small leucine-rich proteoglycans family, bind to collagen fibrils and actively participate in fibrillogenesis. Aggrecan and versican are two large modular PG providing tendon tissue with a high capacity to resist high compressive and tensile forces. It was shown that exercise leads to changes in PG content3.
The ECM of tendons is characterized by marked regional differences in its composition. In the tensile midtendon, the main ECM components are COL-I and the PG decorin and versican4, 5.
Tenocytes are tendon specialized fibroblasts that respond to mechanical-induced loading with expression of several ECM components6, 7. The ability of tenocytes to sense and respond to load is central to the concept of mechanotransduction and to the subsequent maintenance of tissue homeostasis. It was suggested that mechanotransduction mechanisms are based on integrins that, together with cytoskeleton and tendon cell cilia8, provide a bridge through which forces can be transmitted between inside and outside of the cells, thus influencing tenocyte activity.
Cerebral palsy (CP) is the term to define a range of non-progressive syndromes of posture and motor impairment resulting from an insult to the developing central nervous system. CP is characterized by spasticity, movement disorders, muscle weakness and rigidity9.
Studies in CP patients have shown that spasticity induces relevant modifications of tendons at the molecular level, possibly leading to morpho-functional modifications in order to respond and to adapt to the higher mechanical loading and increased functional demands10,11. This suggests that the knowledge of ECM remodelling in CP patients’ tendon components in relation to mechanical stress is pivotal and may be helpful for monitoring or planning treatment strategies. In this study we aimed at analyzing and characterizing the effect of spasticity-induced overload on tendon structure and ECM components in CP patients.
Materials and methods
Tissues
Tendon fragments were obtained from healthy subjects undergoing surgical procedures to treat anterior cruciate ligament rupture, and used as controls (CT) (n=5) (mean age 12,6 ± 3,7). Tendon fragments from CP patients were obtained during tendon lengthening procedures from the extra tendon removed after their resuture (n=4) (mean age 11,5 ± 5,5). For this study, tendons of semitendinosus and gracilis muscles were used. Samples were collected according to procedures approved by the local ethical committee. Tendon fragments were immediately processed for morphological procedures.
Histochemistry and image analysis
Immediately after surgery, each tendon fragment was fixed in 4% formalin in 0.1M phosphate buffered saline (PBS), pH 7.4, routinely dehydrated, paraffin embedded, and serially cut (thickness 5 μm). Sections were stained with freshly made haematoxylin-eosin to evaluate the cell and tissue morphology.
To obtain specific stain for fibrillary collagen, slides were deparaffinised and immersed for 30 minutes in saturated aqueous picric acid containing 0.1% Sirius red F3BA (Sigma, Milan, Italy).
To obtain specific stain for mucopolysaccharides (GAG and PG), sections were stained with Alcian blue in sodium acetate buffer, pH 5.8 containing different MgCl2 concentration in order to selectively stain different mucopolysaccharides. In particular, in 0.025M MgCl2 all acid mucopolysaccharides stain blue; using 0.3 and 0.65 MgCl2, respectively, sulphated acid mucopolysaccharides and strongly sulphated acid mucopolysaccharides can be observed. The slides were photographed by a digital camera connected to a Nikon Eclipse 80i microscope.
Sections for each tendon and for each stain were analysed in blind by three different operators using a semiquantitative grading scale which assesses various features of the tissue. The variables included in the scale for the analysis of tendon structure are: fiber structure and arrangement, cellularity, rounded nuclei, increased vascularity, lipoid degeneration. A five-point scoring system was used, where 0 indicates normal appearance, 0.5 not completely normal appearance, 1 slightly abnormal appearance, 2 moderately abnormal appearance, and 3 markedly abnormal appearance. The degree of staining was assessed using a similar semiquantitative score based on five point scoring system, where 0 is very faint staining, 0.5 faint staining, 1 moderate staining, 2 strong staining, and 3 very strong staining. The results of the score system are expressed as means.
Immunocytochemistry
Sections were incubated without antigen retrieval with primary monoclonal antibody specific for the vascular endothelial marker CD34 (Dako, Milan, Italy). Immunoreactivity was detected by EnVision (Dako, Milan, Italy). Sections were then counterstained with haematoxylin. Negative controls were obtained omitting the primary antibody.
Results
Tendon structure
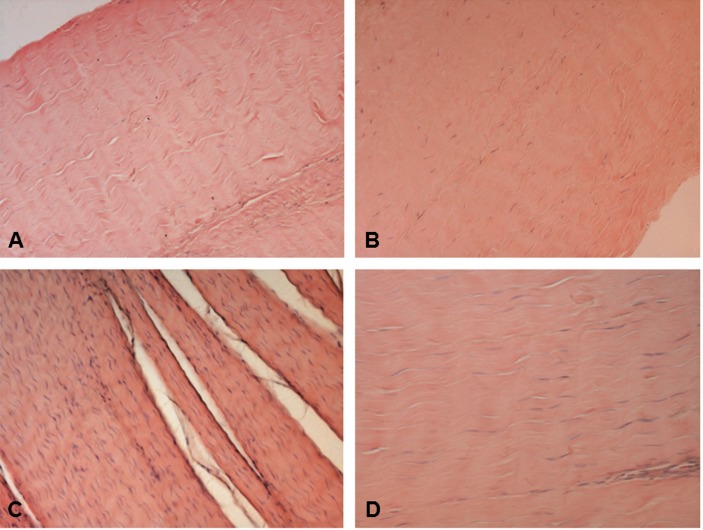
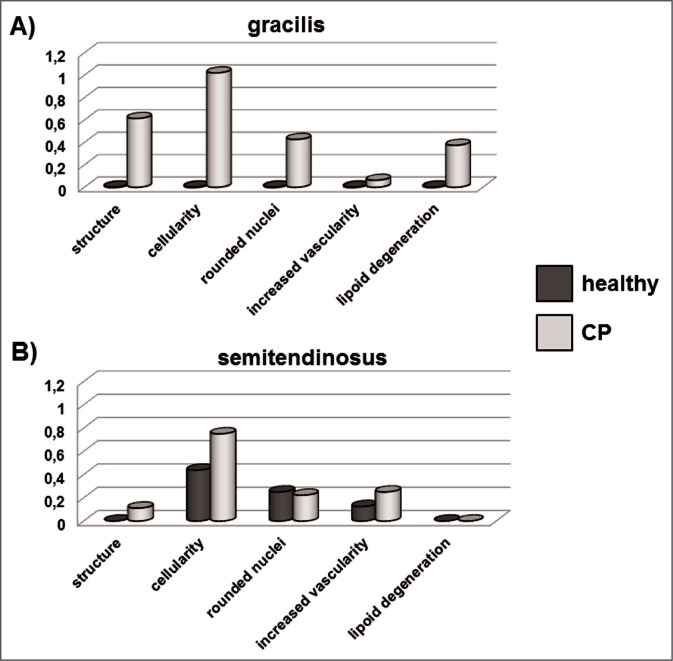
Tendon structure in CT and CP tendons was analyzed in haematoxylin-eosin stained specimens. Light microscopy analysis revealed that in both CT and CP specimens tendon structure was maintained and collagen fibers were ordered and arranged close and parallel to each other with a slightly wavy appearance (Fig. 1). In some CP patients some separation of the fibers was observed and the parallel arrangement was lost. Tenocyte nuclei were flattened, sometimes arranged in rows, but in some CP patients the number of cells resulted higher than in CT tendons, and in some specimens the presence of rounded nuclei was evident, suggesting the presence of some tenoblasts. These changes were more evident in gracilis than in semi-tendinosus tendons, as shown in bar graphs obtained using the semiquantitative score system described in the Materials and methods section (Figs. 2 A, B).
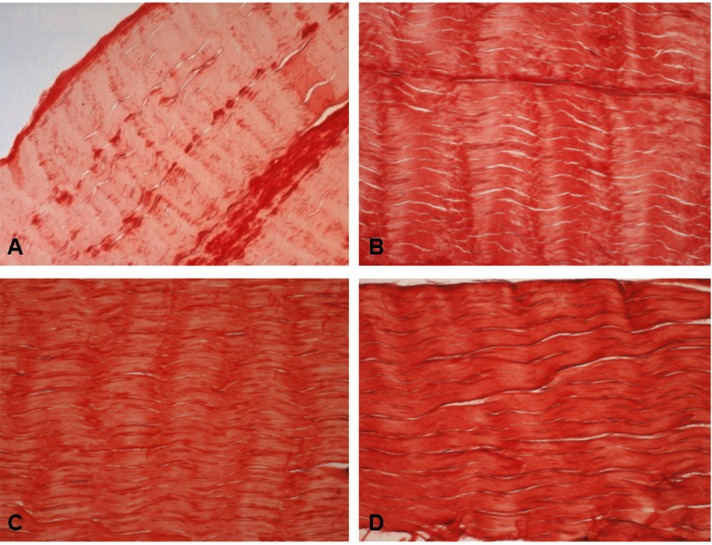
Figure 1.
Haematoxylin-eosin stained longitudinal sections of the mid region of CT (A, B) and CP (C, D) gracilis and semi-tendinosus tendons. Note the closely packed parallel bundles of collagen fibers having an undulating distribution and containing flattened tenocytes. A, C) gracilis tendon. B, D) semitendinosus tendon. Original magnification: 20×.
Figure 2.
Bar graphs showing the assessment by semiquantitative score of the parameters considered for the analysis of tendon structure after haematoxylin and eosin staining of gracilis (A) and semitendinosus (B) tendons.
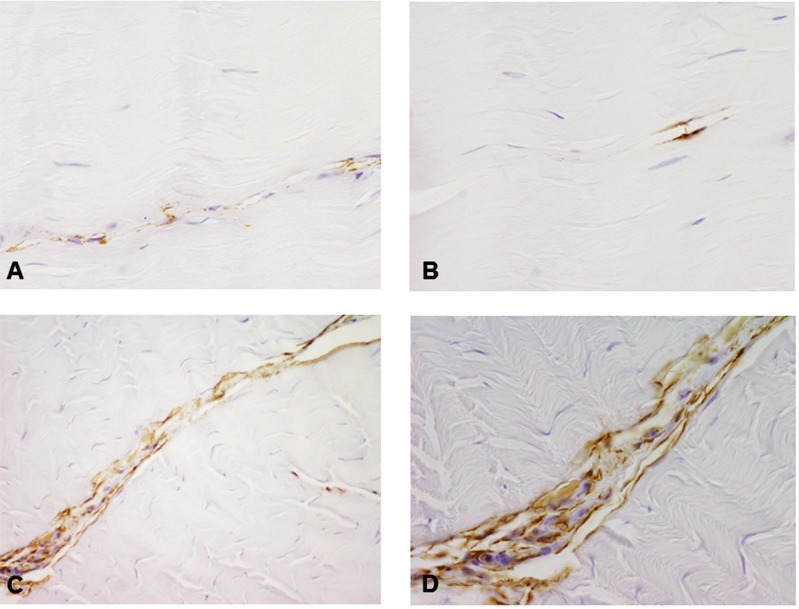
Blood vessels were scarce in both CP and CT specimens, although in some CP tendon increased vascularity was observed (Figs. 2 A, B). This finding was confirmed by immunocytochemistry using a CD34 antibody specific for endothelial cells. CD34-positive vessels resulted more abundant in tendons obtained from CP patients, compared to healthy tendons (Fig. 3).
Figure 3.
Microphotographs showing positive staining of vascular endothelial marker CD34 in CT (A, B) and CP (C, D) tendons. Immunoreactivity is evident in CP tendons. Original magnification: 40×.
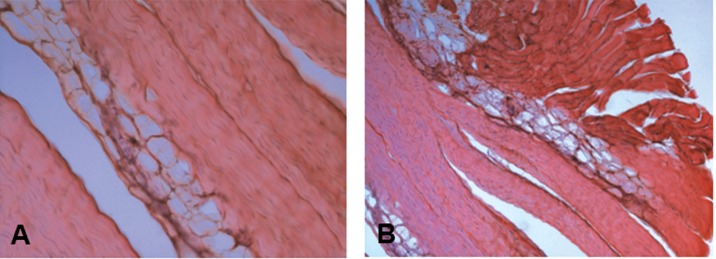
Few CP tendons were characterized by lipoid degeneration, with the evidence of adipocytes accumulation in the tendon between collagen fibers (Fig. 4 A), not only in the mid tendon but also at the level of the myotendineus junction (Fig. 4 B). Lipoid degeneration was observed only in the gracilis tendons obtained from CP patients (Figs. 2 A, B).
Figure 4.
Haematoxylin-eosin stained longitudinal sections of tendons harvested from CP tendons showing lipoid degeneration, characterized by groups of adipocytes located deep in the tendon between the collagen fibers, disrupting the continuity of the collagen fibers and bundles. A) Tendon mid region, original magnification: 20×. B) Myotendinous junction, original magnification: 10×.
Sirius red staining
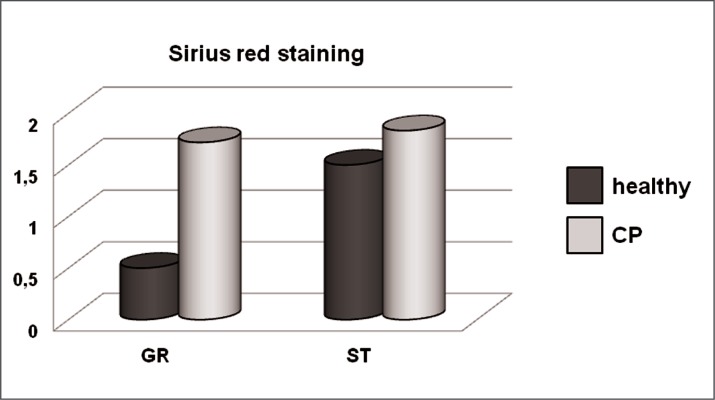
Sirius red stained specimens were observed at the light microscope in order to compare collagen arrangement and content in healthy and CP samples. In the gracilis tendons of CP patients the staining was more intense than in CT, suggesting an accumulation of interstitial collagen induced by spasticity (Figs. 5, 6). By contrast, the analysis of Sirius red stained semitendinosus tendons from CP patients revealed a slight increase of collagen, compared with healthy subjects (Figs. 5, 6).
Figure 5.
Microphotographs showing healthy (CT) (A, B) and CP (C, D) gracilis and semitendinosus tendons stained using Sirius red. Original magnification 20×. A, C: gracilis; B, D: semitendinosus.
Figure 6.
Bar graphs showing the assessment by semiquantitative score of the intensity of Sirius red staining of healthy and CP gracilis and semitendinosus tendons. GR: gracilis. ST: semitendinosus.
Alcian blue staining
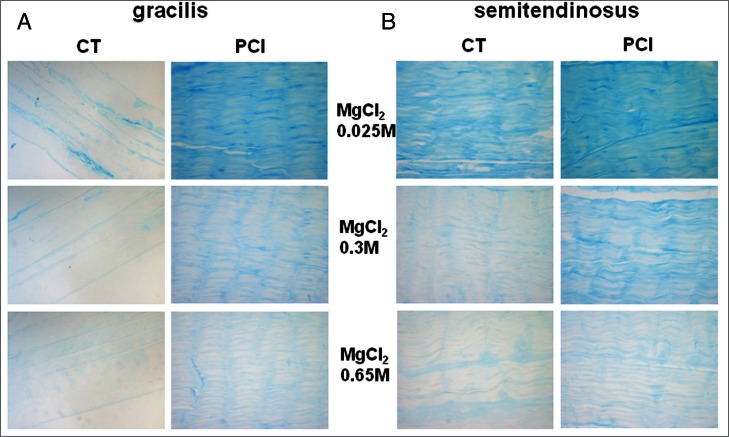
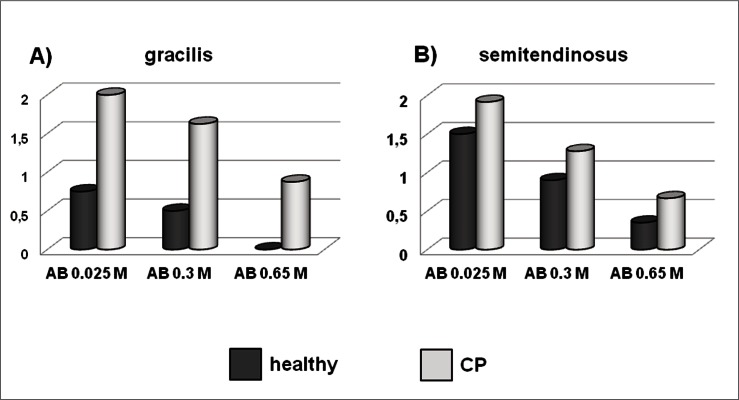
Light microscopy analysis of specimens stained by Alcian blue containing 0.025M MgCl2 revealed a 2.7-folds increase of stain intensity in PC gracilis compared with healthy tendons (Figs. 7, 8), suggesting an increase of the total GAG and PG content induced by spasticity. A similar pattern was observed for the semitendinosus tendons, but the differences between healthy and PC tendons were less marked (Figs. 7, 8). The intensity of the Alcian blue staining containing 0.3 MgCl2 resulted increased in both the gracilis and semitendinosus tendons of CP patients, compared with healthy tendons, suggesting that CP-induced spasticity stimulates the accumulation of sulphated mucopolysaccharides. A similar pattern was observed after staining in Alcian blue containing 0.65 MgCl2, but the differences between healthy and CP tendons were less evident (Figs. 7, 8).
Figure 7.
Microphotographs showing healthy (CT) and CP gracilis (A) and semitendinosus (B) tendons stained using Alcian blue containing different molarities of MgCl2 allowing the detection of differently sulphated GAG. Original magnification 20×.
Figure 8.
Bar graphs showing the assessment by semiquantitative score of the intensity of Alcian blue staining of healthy and CP gracilis (A) and semitendinosus (B) tendons. AB: Alcian blue.
Discussion
The force transmission of the muscle-tendon complex is dependent on the fibrillar arrangement of the tendon and its allowance for absorption and loading of energy. In particular, the tensile strength of the tendon ECM is based on the orientation and density of the collagen fibrils and fibers, and on intra- and inter-molecular cross-links12, since collagen type I is the main component of tendon ECM. Collagen provides the major resistance to mechanical forces1, and it may be expected that its content and organization would be reflected in the mechanical properties of the structure.
The control of ECM composition in response to load is a key aspect of tendon connective tissue, allowing matching of tissue strength to mechanical environment. Tendon ECM turnover is influenced by physical activity13, as well as by degrading matrix metalloproteinases increase with mechanical loading14, 15. In particular, collagen increase was demonstrated both in animal models16 and in humans17, influencing the mechanical properties and the viscoelastic characteristics of the tissue. By contrast, inactivity markedly decreases collagen turnover in tendons and an inverse correlation between MMP-1 gene/protein expression and tensile load occurs14, playing a key role in determining tendon strength.
In a previous study of our laboratory we demonstrated that mRNA levels related to collagen turnover in tendons from CP patients are different than in CP samples compared to normal ones, suggesting that CP induces relevant modifications of tendons at the molecular level, possibly leading to modifications in order to respond to the increased mechanical loading and increased functional demands induced by spasticity11.
Here we aimed at analyzing and characterizing the effect of spasticity-induced overload on tendon structure and ECM components by morphological methods, in order to get new insight in the structure of tendons from CP patients.
Light microscopy analysis of haematoxylin-eosin stained specimens revealed that the overall structure was maintained in CP tendons, characterized by the parallel tight alignment of fibrils and thin tenocytes arranged in rows between collagen fibers. Considering tendon cell morphology, it is possible to distinguish tenocytes, displaying an elongated and thin shape, from rounded and spheroid tenoblasts1. Some CP tendons displayed an increased number of tenocytes, suggesting that hypercellularity may be induced by spasticity. Furthermore, an increased number of tenoblasts with rounded nuclei in CP tendons was observed, particularly in the gracilis tendon. Tenoblasts are sometimes regarded as an activated form of tenocytes in case of intrinsic healing of tendon injuries18.
Interconversion between tenoblasts and tenocytes might occur, and their ratio in tendons play a key role governing the tissue response to various stimuli such as exercise and trauma19. Interestingly, regional variations in cellularity and rounding of the nuclei are morphological features of tendinopathic tendons20.
Compared with muscle, the tendon is a poorly vascularised tissue that relies upon synovial fluid diffusion to provide nutrition. By contrast, hypervascularity was demonstrated in patients with Achilles tendinopathy21 and in a rabbit model of Achilles tendinosis22. We observed increased vascularity in haematoxylin-eosin stained CP tendons, confirmed by immunocytochemistry that revealed more abundant CD34-positive vessels in tendons obtained from CP patients, compared with healthy tendons.
Collagen type I is the main ECM component of the dense connective tissue of tendons, representing the 60% of the dry mass. The analysis of Sirius red stained samples revealed an increased collagen content in CP compared with healthy tendons, suggesting that increased mechanical loading induced by spasticity modifies collagen content in tendons, allowing an adaptive response of tendon to maintain its biomechanical properties. This result is consistent with the previously demonstrated increase of COL-I mRNA levels in CP tendons11.
In addition to collagen fibrils, tendon ECM contains transverse and longitudinal PG filaments, many of them bridge and link between the fibrils. PG filaments were seen orthogonally arranged with respect to the collagen fibrils and this picture was unchanged after application of stress of any level up to breaking point23. PG bridges between collagen fibrils play a role in transmitting and resisting tensile stresses in tendons, contributing to the strength of the tissue. Sometimes axially oriented PG between fibrils were observed, which act as both lubricant and elastic deformation space to facilitate movement among protofibrils23.
In normal tendons, the profile of PG changes with the type of load the tissue is exposed to6. In general, tensional load upregulates decorin, while the large PG aggrecan is upregulated by compression load. Variations in the GAG/PG content have been observed both with age and during disease24.
Our data point to a strong increase of total GAG content exerted by CP-induced mechanical overload on the gracilis tendons. A similar pattern was observed in the semitendinosus, but to a lesser extent. This increase seems sustained in particular by sulphated GAG, as shown by Alcian blue containing 0.3 MgCl2, but also by highly sulphated GAG such as heparin sulphate stained by Alcian blue containing 0.65 MgCl2.
Tendinopathies are classified as degenerative condition characterized by a disrupted collagen matrix, increased cellularity and PG concentration, increased vascularity and lipidic degeneration, but lack of inflammatory cell infiltration25. In vivo tendon loading produced a non inflammatory pathology that was morphologically consistent with what observed in tendinosis26, that can be considered as a non-rupture tendon injury caused by overuse or repetitive motion27.
Data obtained from asymptomatic tendons suggest that tenocyte rounding, proliferation, increased GAG production, in particular hyaluronan and chondroitin sulphate24, may precede collagen tearing28. Our data show that some of these tendinopathic-like markers are observed in tendons from CP patients. The gracilis tendon seems more affected than semitendinosus, although they both assist with knee flexion.
Considered as a whole, our data suggest that CP-induced spasticity may be considered as a chronic and persisting loading of tendons, acting as a repetitive work on them and inducing ECM remodeling as adaptive response to increased functional demand. At the same time, the chronic nature of the condition induced by CP suggests that the differences in the histology features in CP compared with normal tendons represent a pathological condition. Accordingly, CP-induced spasticity could be considered as a repeated strain below the injury threshold, inducing changes in the tendon ECM composition and organization. It is possible that an isolated fibril damage induced by overuse does not cause clinical injury, but is likely that continued loading plays a significant role. The degeneration of the ECM may very likely lead to a transient weakness of the tissue making it more susceptible to damage from cumulative loading and the damage accumulates until an overt pathology consistent with tendinopathy could develop. We feel that our results provide new information on CP-induced modifications on tendons, contributing to understand tendon injury and, possibly, helping to plan interventional treatments.
Acknowledgments
Silvia Celon for her technical support, and the Ariel Foundation for the financial support to conduct this research study.
References
- 1.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 2.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 3.Yoon JH, Brooks R, Jr, Kim YH, Terada M, Halper J. Proteoglycans in chicken gastrocnemius tendons change with exercise. Arch Biochem Biophys. 2003;412:279–286. doi: 10.1016/s0003-9861(03)00064-x. [DOI] [PubMed] [Google Scholar]
- 4.Samiric T, Ilic MZ, Handley CJ. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004;23:27–140. doi: 10.1016/j.matbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Corps AN, Robinson AH, Movin T, Costa ML, Hazleman BL, Riley GP. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology. 2006;45:291–294. doi: 10.1093/rheumatology/kei152. [DOI] [PubMed] [Google Scholar]
- 6.Banes AJ, Horesovsky G, Larson C, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis Cartilage. 1999;7:141–153. doi: 10.1053/joca.1998.0169. [DOI] [PubMed] [Google Scholar]
- 7.Lavagnino M, Arnoczky SP. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J Orthop Res. 2005;23:1211–1218. doi: 10.1016/j.orthres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Lavagnino M, Arnoczky SP, Gardner K. In situ deflection of tendon cell-cilia in response to tensile loading: an in vitro study. J Orthop Res. 2011;29:925–930. doi: 10.1002/jor.21337. [DOI] [PubMed] [Google Scholar]
- 9.Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004;363:1619–1631. doi: 10.1016/S0140-6736(04)16207-7. [DOI] [PubMed] [Google Scholar]
- 10.Gagliano N, Pelillo F, Grizzi F, Picciolini O, Gioia M, Portinaro N. Gene expression profile of extracellular matrix of tendons in cerebral palsy. Dev Med Child Neurol. 2007;49:557–558. doi: 10.1111/j.1469-8749.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 11.Gagliano N, Pelillo F, Chiriva-Internati M, et al. Expression profiling of genes involved in collagen turnover in tendons from cerebral palsy patients. Connect Tissue Res. 2009;50:203–208. doi: 10.1080/03008200802613630. [DOI] [PubMed] [Google Scholar]
- 12.van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 13.Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendons and ligaments II. The relationships of immobilization and exercise on tissue remodeling. Biorheology. 1982;19:397–408. doi: 10.3233/bir-1982-19302. [DOI] [PubMed] [Google Scholar]
- 14.Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–333. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 15.Lavagnino M, Arnoczky SP, Tian T, Vaupel Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res. 2003;44:181–187. doi: 10.1080/03008200390215881. [DOI] [PubMed] [Google Scholar]
- 16.Kovanen V. Effects of ageing and physical training on rat skeletal muscle. An experimental study on the properties of collagen, laminin, and fibre types in muscles serving different functions. Acta Physiol Scand. 1989;577:1–56. [PubMed] [Google Scholar]
- 17.Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson CJ, Ganion LR, Gehlsen GM, Verhoestra B, Roepke JE, Sevier TL. Rat tendon morphologic and functional changes resulting from soft tissue mobilization. Med Sci Sports Exerc. 1997;29:313–319. doi: 10.1097/00005768-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Chuen FS, Chuk CY, Ping WY, Nar WW, Kim HL, Ming CK. Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem. 2004;52:1151–1157. doi: 10.1369/jhc.3A6232.2004. [DOI] [PubMed] [Google Scholar]
- 20.Maffulli N, Longo UG, Franceschi F, Rabitti C, Denaro V. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008;466:1605–1611. doi: 10.1007/s11999-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aström M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995;316:151–164. [PubMed] [Google Scholar]
- 22.Backman C, Boquist L, Fridén J, Lorentzon R, Toolanen G. Chronic achilles paratenonitis with tendinosis: an experimental model in the rabbit. J Orthop Res. 1990;8:541–547. doi: 10.1002/jor.1100080410. [DOI] [PubMed] [Google Scholar]
- 23.Cribb AM, Scott JE. Tendon response to tensile stress: an ultrastructural investigation of collagen: proteoglycan interactions in stressed tendon. J Anat. 1995;187:423–428. [PMC free article] [PubMed] [Google Scholar]
- 24.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1994;53:367–376. doi: 10.1136/ard.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley G. Tendinopathy-from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4:82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 26.Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 27.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology. 2004;43:131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- 28.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]