Abstract
Epithelial integrity in metazoan organs is maintained through the regulated proliferation and differentiation of organ-specific stem and progenitor cells. Although the epithelia of organs such as the intestine regenerate constantly and thus remain continuously proliferative1, other organs, such as the mammalian urinary bladder, shift from near-quiescence to a highly proliferative state in response to epithelial injury2–4. The cellular and molecular mechanisms underlying this injury-induced mode of regenerative response are poorly defined. Here we show in mice that the proliferative response to bacterial infection or chemical injury within the bladder is regulated by signal feedback between basal cells of the urothelium and the stromal cells that underlie them. We demonstrate that these basal cells include stem cells capable of regenerating all cell types within the urothelium, and are marked by expression of the secreted protein signal Sonic hedgehog (Shh). On injury, Shh expression in these basal cells increases and elicits increased stromal expression of Wnt protein signals, which in turn stimulate the proliferation of both urothelial and stromal cells. The heightened activity of this signal feedback circuit and the associated increase in cell proliferation appear to be required for restoration of urothelial function and, in the case of bacterial injury, may help clear and prevent further spread of infection. Our findings provide a conceptual framework for injury-induced epithelial regeneration in endodermal organs, and may provide a basis for understanding the roles of signalling pathways in cancer growth and metastasis.
The multi-layered bladder epithelium consists of a lumenal layer of fully differentiated, usually binucleate umbrella cells5, which overlie intermediate cells with limited proliferative potential, and long-term label-retaining basal cells able to produce large colonies on culture in vitro6. These urothelial layers are separated by a basement membrane from the lamina propria, a thin layer of fibroblast-like stromal cells, and submucosal, smooth muscle and serous layers. Infections of the urinary tract, occurring in 10% of women annually7, are a common cause of injury to the bladder, and can be modelled in mice by infection with uropathogenic bacteria isolated from patients2,3,8.
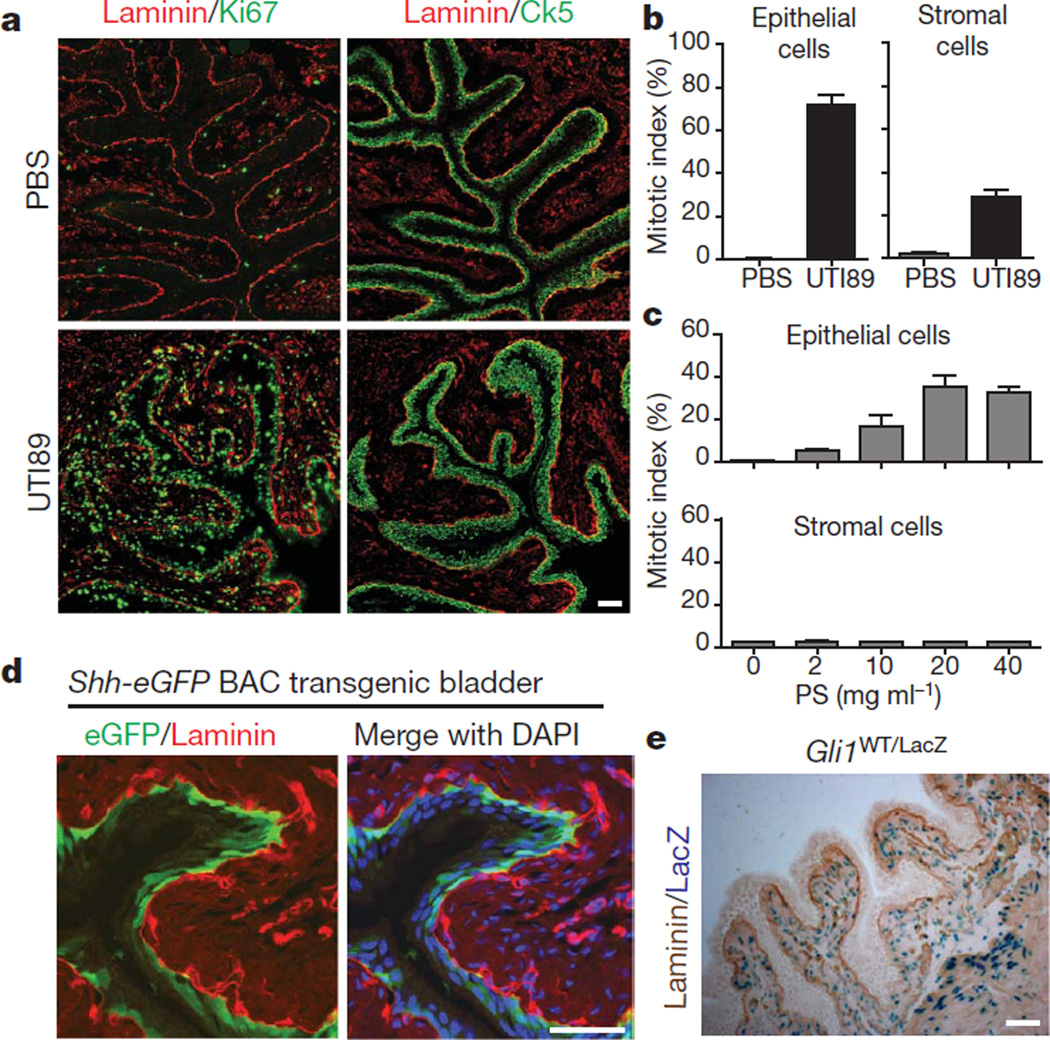
To establish baseline parameters of the bladder regenerative response, we induced injury in female mice by transurethral instillation of UTI89 (Supplementary Figs 1, 2a, 3a, b), a uropathogenic strain of Escherichia coli, or of the injurious compound protamine sulphate (PS). We found that expression of the proliferative marker Ki67 increased from near zero to 72% of epithelial and 28% of stromal cells within 24 h of UTI89 infection (Fig. 1a, b).Thenumber of urothelial cell layers and total cells expressing the basal cell marker cytokeratin 5 (Ck5, also known as Krt5) was markedly expanded (Supplementary Fig. 3c, d), suggesting that injury-induced proliferation occurs primarily in basal urothelial cells. Instillation of increasing PS concentrations also induced Ki67 expression, reaching a plateau of ~35% at 20 mg ml−1 PS and above (Fig. 1c and Supplementary Fig. 4); Ck5-positive cell number increased more modestly with PS injury. Interestingly, stromal cells showed no increased proliferation, even at PS concentrations that saturate the epithelial response (Fig. 1c).
Figure 1. Injury-induced proliferation and Hedgehog signalling in the bladder.
a, UTI89 instillation induces proliferation of basal epithelial and stromal cells of the bladder. Ki67, Ck5 and laminin immunostaining highlight proliferation, basal epithelial cells and the basement membrane, respectively, in bladders 24 h after instillation of UTI89. Adjacent sections were 10 mm apart. b, Quantification of epithelial and stromal cell proliferation in response to bacterial injury. Ki67-positive cells are shown as a per cent of total 4′,6-diamidino-2-phenylindole (DAPI)-staining nuclei. c, Quantification of epithelial and stromal cell proliferation in response to chemical injury. Ki67-positive cells are shown as a per cent of total DAPI-staining nuclei 24 h after instillation of the indicated concentrations of PS. Note the absence of a proliferative response in the stroma. For panels b and c, data are from 3 bladders, 2 sections each, and are shown as mean ± s.e.m.; numerical data are in Supplementary Tables 1 and 2, respectively. d, Expression of eGFP in basal epithelial cells from a Shh-eGFP BAC transgenic mouse. e, Gli1-LacZ expression in the stromal compartment. Bladder sections from Gli1LacZ/WT mice were co-stained with X-gal and anti-laminin. Scale bars in panels a, d and e represent 50 µm.
Shh is expressed in and has a role in development of urogenital sinus derivatives, including bladder and prostate9–11. In the adult urothelium, we found that Shh is expressed primarily in Ck5-positive basal cells, as indicated by immunostaining and GFP expression in a Shh-GFP BAC transgenic strain (Fig. 1d and Supplementary Fig. 5a). Hedgehog (Hh) pathway activity, indicated by a reporter Gli1-LacZ strain12 (Supplementary Fig. 6a, b), is restricted to stromal, submucosal and muscle layers, outside the urothelium(Fig. 1e), and depends on the Shh signal, as indicated by reduced Gli1 and Ptc (also known as Ptch1) expression on treatment with a Shh-blocking antibody (Supplementary Fig. 5b, c).
Shh and Gli1 mRNA levels both increased in response to injury (Supplementary Fig. 5d and Supplementary Table 3), and Shh protein expression extended to multiple layers of Ki67-positive epithelial cells, including basal cells and Ck5-positive basal-like cells that result from injury-induced proliferation (Supplementary Fig. 5e). Shh response was augmented by injury, requiring only four instead of twenty hours of X-gal staining for detection in Gli1-LacZ reporter mice, but nevertheless remained confined to the stromal compartment (Supplementary Fig. 5f).
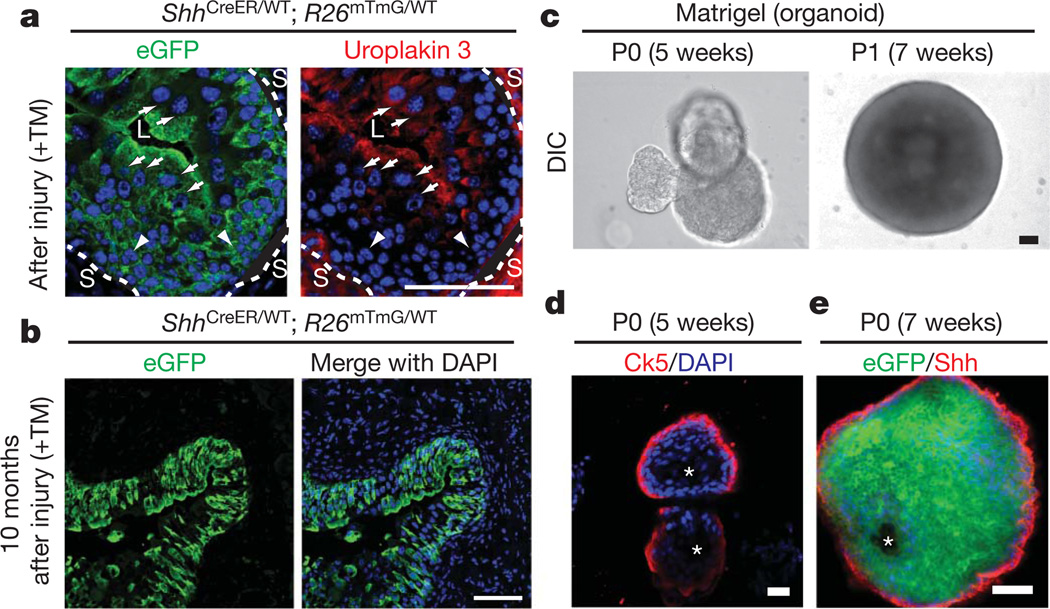
Basal cells have been suggested to function as stem cells in many epithelia including bladder and prostate6,13; we marked Shh-expressing cells in vivo using a CreER tamoxifen-dependent site-specific recombinase expressed under the control of the Shh promoter (ShhCreER)14 in combination with R26mTmG, a Cre-sensitive bi-fluorescent reporter15. In ShhCreER/WT; R26mTmG/WT mice, the membrane-associated tomato fluorescent protein (mT) is expressed until tamoxifen (TM) injection (Supplementary Figs 2b, 7a), after which membrane-associated GFP (mG) marks Shh-expressing Ck5-positive cells in basal urothelium (Supplementary Fig. 8a). With three rounds of bacterial injury and recovery after TM injection (Supplementary Fig. 2b), mG labelled all or most urothelial cells including Ck5-positive basal cells, intermediate cells, and Ck5-negative luminal umbrella cells marked by expression of uroplakin 3 (ref. 16; Fig. 2a and Supplementary Figs 7c, 8a), indicating multipotency of Shh-expressing cells. With reducedTMtreatment and a single round of bacterial injury, we observed less extensive marking in isolated, vertically coherent patches that included basal, intermediate and luminal cells (data not shown); overall regeneration thus appears to result from the combined activation of many local urothelial units.
Figure 2. Shh-expressing basal cells repopulate the urothelium and form organoids in vitro.
a, eGFP-marked Shh-expressing cells generate luminal cells positive for uroplakin 3. ShhCreER/WT; R26mTmG/WT mice were treated with TM and subjected to three rounds of injury. Uroplakin-3-positive and negative cells are denoted by white arrows and arrowheads, respectively. Dotted lines demarcate urothelium and stroma (L, lumen; S, stroma). b, Long-term regenerative capacity of eGFP-marked Shh-expressing cells. After TM injection and seven rounds of injury over 10 months, mG expression in bladder from ShhCreER/WT; R26mTmG/WT mice marks most urothelial cells. See Supplementary Fig. 2b for experimental schemes. c, Extended culture of Shh-expressing cells in Matrigel. Single eGFP-positive bladder cells isolated from TM-injected ShhCreER/WT; R26mTmG/WT mice and cultured in Matrigel for 5 weeks formed organoids. Dissociated cells from primary culture (P0) organoids also generated new organoids on subsequent passage (P1) in Matrigel culture. DIC, differential interference contrast. d, Confocal analysis of a bladder organoid. The organoid has multiple layers of epithelial cells with Ck5-expressing cells in the outer layer that contacts the extracellular matrix, and inner cells that line a luminal space and do not express Ck5. e, A section through the wall of an organoid grown in Matrigel and immunostained for Shh. Note eGFP expression in all cells, indicative of Shh expression in the cell initially cultured, but loss of Shh immunostaining from cells that are not in the outer layer. Scale bars represent 50 µm; asterisks denote organoid lumen.
We found similar labelling in the majority of the urotheliumin mice carrying marked Shh-expressing cells and subjected to seven cycles of bacterial infection and recovery over a period of 10 months (Fig. 2b and Supplementary Fig. 8b). This persistence through lengthy and repeated periods of intense proliferation suggests that Shh-expressing cells have a capacity for self-renewal. Similarly marked cells traced through a 10-month period without injury produced less extensive labelling that nevertheless included Ck5-positive and Ck5-negative cells (Supplementary Fig. 8c, d), indicating that Shh-expressing cells also participate in regular homeostatic turnover in the absence of injury.
Similar experiments using Gli1-CreER17 to mark Shh-responsive stromal cells revealed that mG expression marks most cells of the lamina propria, and no urothelial cells (Supplementary Figs 7b, 9a, b). We also timed TM administration such that cells were marked during the proliferative response to injury, and noted no injury-induced plasticity with regard to segregation of Shh and Gli1 expression within epithelial and stromal compartments, respectively (Supplementary Fig. 10a–c).
EnhancedGFP(eGFP)-positive cells isolated by fluorescence-activated cell sorting (FACS) from the bladders of TM-injected ShhCreER/WT; R26mTmG/WT mice (WT, wild type; Supplementary Figs 2c, 11a, b) formed spheres within 2 weeks of culture in suspension or in Matrigel (Supplementary Fig. 11a–d). Approximately 5–6%of isolated cells formed primary spheres in culture, with most of the remaining cells dying rapidly, probably as a result of stress resulting from the isolation procedure; about 30–40% of cells within primary spheres were able to form secondary spheres in subsequent cultures (Supplementary Fig. 11e, f). After 5–7 weeks of culture in Matrigel, single Shh-expressing cells formed cyst-like organoids 700–1,000 µm in diameter that resemble the bladder in containing multiple layers of epithelial cells with Ck5- and Shh-expressing cells in the outer layer that contacts the extracellular matrix, and inner cells that line a luminal space and express neither Ck5 nor Shh (Fig. 2c–e, Supplementary Fig. 11g, i and Supplementary Movie 1). Single cells from these organoids were capable of self-renewing by generating new organoids in subsequent cultures (Fig. 2c, Supplementary Fig. 11h, j and Supplementary Movie 2). Our in vivo and in vitro evidence thus indicates that Shh-expressing basal urothelial cells include multipotent stem cells that are capable of self-renewal and differentiation.
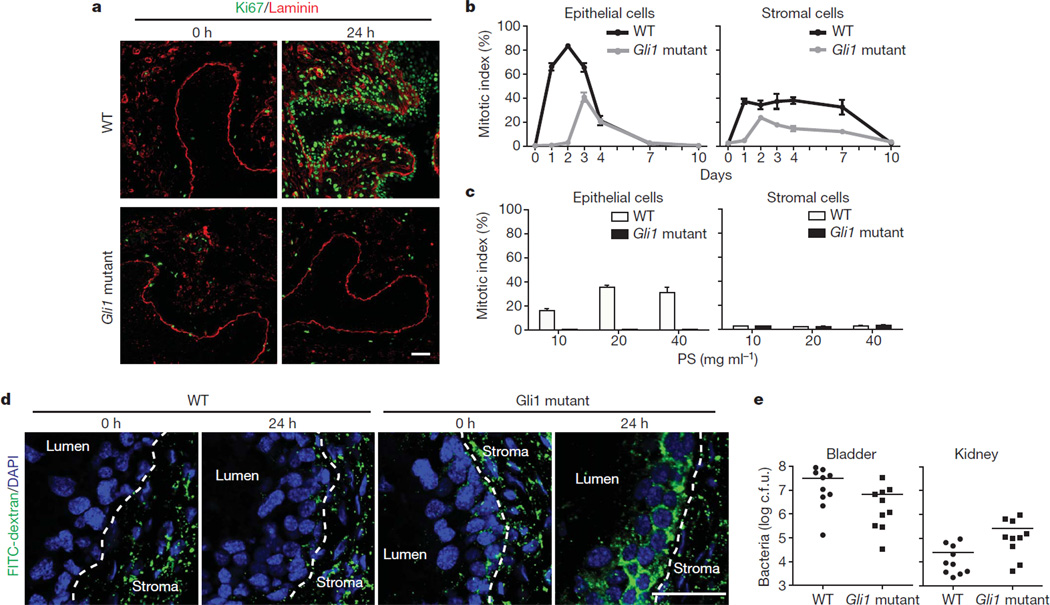
The Gli1 member of the Gli family of transcriptional effectors that mediate transcriptional response to Hh signalling (Supplementary Fig. 6a, b), although not essential for viability or fertility12, can contribute significantly to pathway activity. For example, the incidence of medulloblastoma in Ptc+/− mice is reduced ~10-fold on deletion of the Gli1 gene18. We found that epithelial proliferation induced by UTI89 instillation was nearly absent in Gli1 mutant bladders at the 24–48 h peak of wild-type proliferation, with no additional layers of basal-like cells; a later peak in proliferation was ~1/2 of the wild-type maximum (Fig. 3a, b and Supplementary Fig. 12a). Proliferation of stromal cells was also affected in Gli1 mutants, with a similar delay and decrease in Ki67 expression (Fig. 3a, b). Injection of a Shh-blocking antibody reduced expression of pathway targets Gli1 and Ptc (Supplementary Fig. 5b, c), and correspondingly reduced proliferative responses in both epithelial and stromal compartments (Supplementary Fig. 12b–d and Supplementary Table 5). The response to chemical injury was also markedly reduced in Gli1 mutants (Fig. 3c and Supplementary Fig. 13a, b), and these results together indicate that stromal Gli1-mediated response to the epithelial Shh signal promotes proliferative activity in response to both chemical and bacterial injury.
Figure 3. Gli1 mediates injury-induced proliferation, restoration of urothelial integrity and reduction of infectious spread.
a, Gli1 loss delays and attenuates proliferative response to bacterial injury. Bladders from wild-type or homozygous Gli1 mutant mice were analysed at the indicated times after UTI89 instillation by immunostaining for Ki67 and laminin. b, Quantification of Gli1 effect on epithelial and stromal cell proliferation. Ki67-positive cells are shown as a per cent of total DAPI-staining nuclei at the indicated times after UTI89 instillation in wild-type and Gli1 mutant bladders. c, Gli1 loss blocks the proliferative response of epithelial cells to chemical injury. Ki67-positive cells are shown as a per cent of total DAPI-staining nuclei 24 h after instillation of the indicated concentrations of PS. In panels b and c, data are shown as mean ± s.e.m. from 3 bladders, 2 sections each, and numerical data are shown in Supplementary Tables 4 and 6, respectively. d, Paracellular permeability in injured bladders from Gli1 mutant mice. Bladders from wild-type and Gli1 homozygotes were instilled with UTI89 and analysed at the times indicated after infection, with FITC-dextran instillation preceding bladder collection by 1.5 h. Dotted lines demarcate the border between urothelium and stroma. Note that normal reduced levels of paracellular permeability are restored by 24 h in wild-type but not Gli1 homozygotes. e, Infectious spread to kidneys is enhanced by Gli1 loss. Bacterial titres 24 h after UTI89 instillation were lower in bladders from Gli1 homozygotes as compared to wild-type (6.6 ± 3.27 × 106 versus 3.2 ± 1.03 ± 107 colony-forming units (c.f.u.); P < 0.05). In contrast, bacterial titres were significantly higher in kidneys from Gli1 homozygotes as compared to wild-type (2.59 ± 1.0 × 105 versus 2.55 ± 1.0 × 104; P < 0.05). Data are presented as mean ± s.e.m. (10 mice), and significance was calculated by an unpaired Student’s t-test. Scale bars represent 50 µm.
We tested the role of proliferation in restoring urothelial integrity by instilling fluorescein isothiocyanate (FITC)-conjugated dextran after UTI89-mediated injury in wild-type and Gli1 mutants. At 6 h after infection, by which time umbrella cells have exfoliated8, wild-type and mutant bladders both showed penetration of FITC-dextran into interstitial spaces of the urothelium (not shown); at 24 h, however, wild-type but not mutant bladders had re-established exclusion of FITC-dextran from extracellular spaces (Fig. 3d).
We also found that, although bacterial titres were somewhat lower in the bladders of infected Gli1 mutant mice (Fig. 3e), the kidneys of mutant mice contained more than tenfold higher numbers of bacteria (Fig. 3e). These findings indicate that in addition to helping restore epithelial integrity, rapid proliferation of urothelial cells during normal regeneration may help reduce the risk of bacterial spread from the bladder to the kidneys, perhaps by competing for adhesive interactions that otherwise might aid in bacterial ascent via the ureters to the kidneys19.
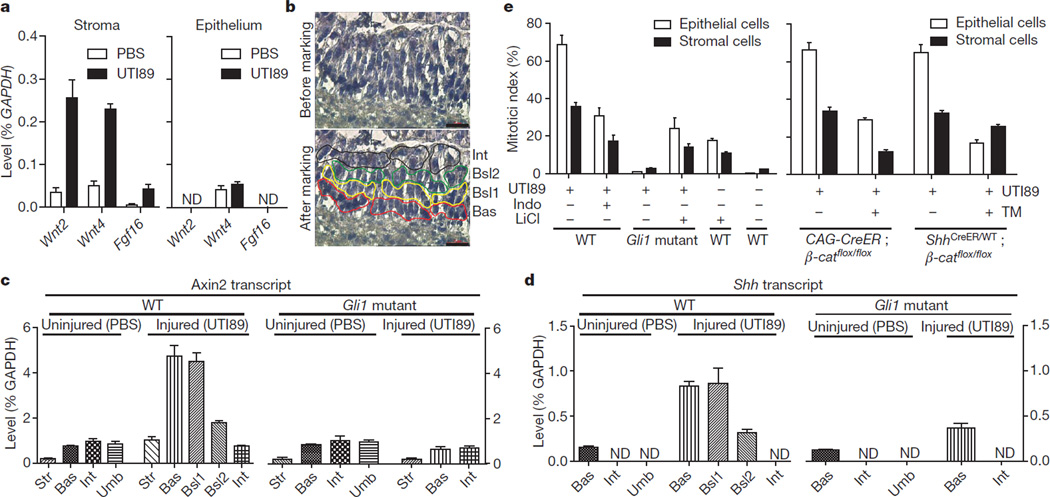
The requirement for stromal Gli1 in mediating proliferative response to epithelial injury suggested the possibility of Shh/Gli1-dependent transcription of secreted signals within the stroma. From gene expression profiles (data not shown) and quantitative polymerase chain reaction with reverse transcription (RT–PCR) with RNA from injured and uninjured wild-type or Gli1 mutant bladders, we found that Wnt2, Wnt4 and Fgf16 showed significant injury- and Gli1- dependent responses (Supplementary Fig. 14a, b). With RNA isolated from stromal and epithelial compartments using laser capture micro-dissection (LCM; Supplementary Fig. 15a, b), we found that levels of Wnt2, Wnt4 and Fgf16 transcripts increased with injury only in the stroma (Fig. 4a).
Figure 4. Hedgehog-induced expression of stromal Wnt signals mediates urothelial and stromal proliferation.
a, Wnt2, Wnt4 and Fgf16 expression in microdissected epithelium or stroma. Stromal expression of Wnt2, Wnt4 and Fgf16 increased significantly on injury. Although Wnt4 RNA was detected in the epithelium, this expression did not increase on injury. ND, not detected. b, Laser capture microdissection of urothelial cell layers in regenerating bladder. Red, yellow, green and black lines illustrate selection and outlining of cells in basal (Bas), basal-like 1 (Bsl1), basal-like 2 (Bsl2), and intermediate (Int) layers, respectively, before microdissection. c, d, Axin2 and Shh expression in microdissected cell layers from wild-type and Gli1 mutant bladders 24 h after instillation of UTI89 or PBS. c, Expression of Axin2 increased 5.5 fold in stroma (P < 0.05), and 6.3 fold in basal (P < 0.05), 5.9 fold in basal-like 1 (P < 0.05), and 2.3 fold in basal-like 2 (P < 0.01) as compared to uninjured basal cells. Expression of Axin2 did not change significantly in basal and intermediate cells of injured Gli1 homozygous mutants as compared to basal and intermediate cells of uninjured Gli1 mutant bladder. d, Expression of Shh in cells increased 5.5 fold in basal (P < 0.01), 5.7 fold in basal-like 1 (P < 0.05), and 2.1 fold in basal-like 2 (P < 0.05) as compared to uninjured basal cells. Expression of Shh in Gli1 homozygous mutants increased 3 fold (P < 0.05) in injured as compared to uninjured basal epithelial cells. Data are presented as mean ± s.e.m., and significance was calculated by a paired Student’s t-test. Str, stroma; Umb, umbrella cells. e, Modulation of Wnt pathway activity in regenerative response to bacterial injury. Left, pharmacological reduction (indomethacin (Indo)) or augmentation (LiCl) of the Wnt signal response respectively decreases or increases bladder proliferation, with or without bacterial injury, as shown, in mice of the indicated genotype (see also Supplementary Fig. 17b, c, d). Right, tamoxifen-induced inactivation of β-catenin decreases proliferation in epithelium and stroma (CAG-CreER) or in epithelium (ShhCreER). Data are presented as mean ± s.e.m. from 3 bladders, 2 sections each (see also Supplementary Fig. 18e, f).
For further analysis we isolated basal, intermediate and umbrella cell layers from uninjured wild-type or Gli1 mutant bladders (Supplementary Fig. 15c, d); as umbrella cells were absent from regenerating bladders owing to bacterially induced exfoliation, we isolated a single lumenal layer of intermediate cells, a single layer of Ck5-positive basal cells and two layers of Ck5-positive basal-like cells (basal-like1 and basal-like2) from wild type (Fig. 4b and Supplementary Fig. 15e) or, from Gli1 homozygous mutants, intermediate cells and a single layer of basal cells (Supplementary Fig. 15f). With RNA from these microdissected cell layers we found that transcription of Axin2, a universal indicator of Wnt signal response20,21, increased markedly in stromal cells and in basal and basal-like1 cells, to a lesser extent in the second basal-like2 layer, and not at all in the intermediate cell layer (Fig. 4c). No increase in Axin2 levels could be seen in stromal or urothelial layers of injured, Gli1-mutant bladders (Fig. 4c). Increased Wnt response thus occurs in stromal cells and in urothelial cell layers in closest proximity to the stromal source of Wnt signals.
Shh expression also increased in basal and basal-like cells (Fig. 4d and Supplementary Fig. 16), indicating stromal Gli1-mediated positive feedback on epithelial Shh expression. Notably, however, Shh expression in basal cells also increased somewhat in the Gli1 mutant, indicating an injury-induced effect on Shh expression that does not require Gli1-mediated feedback from the stroma.
We tested pharmacological modulators of Wnt signalling, and found that indomethacin treatment reduced Axin2 transcripts twofold in the bladder (Supplementary Fig. 17a), indicating a reduction in Wnt response20, and correspondingly suppressed UTI89-induced proliferation in the epithelium and stroma (Fig. 4e and Supplementary Fig. 17b). We also found that LiCl22 increased Axin2 transcripts (Supplementary Fig. 17a), and correspondingly induced proliferation in the epithelium and stroma of uninjured wild-type mice or substantially rescued the proliferative response of Gli1 mutant mice (Fig. 4e and Supplementary Fig. 17c, d). We also observed a marked enhancement of the proliferative response to instillation of 2 mgml−1 PS, from ~5% to 30% in the epithelium and from 0 to ~15% in the stroma (Supplementary Fig. 18a, b), in mice heterozygous for the Apcmin mutation, which show constitutive Wnt pathway activity due to reduced dosage of a negative Wnt response regulator23 (Supplementary Fig. 18c).
We inactivated the Wnt response with a homozygous conditional allele of the essential Wnt pathway component b-catenin (also known as Ctnnb1), and found that ablation in TM-injected mice by the ubiquitously expressed CAG-CreER reduced Axin2 expression in injured bladder (Supplementary Fig. 18d) and correspondingly reduced injury-induced proliferation in both epithelial and stromal compartments (Fig. 4e and Supplementary Fig. 18e). In contrast, the proliferation defect produced in ShhCreER/WT; β-cateninflox/flox mice was restricted to basal epithelium (Fig. 4e and Supplementary Fig. 18f). Our pharmacological and genetic data indicate a role for Wnt pathway activity within basal epithelial and stromal cells in the activation of proliferative responses, with the potential role and importance of Fgf or other Hh-induced stromal signals remaining to be explored.
Our findings, summarized schematically in Supplementary Fig. 19, reveal an essential contribution by Hh and Wnt signals acting across the epithelial–stromal boundary during bladder regeneration, and are reminiscent of the well-studied Drosophila embryonic segment, in which Hh and Wingless signalling across the parasegment boundary are essential for segmental patterning during development. Interestingly, however, Hh/Wnt feedback signalling in the Drosophila segment operates to specify future pattern within an undifferentiated epithelium, whereas in the bladder it functions to maintain differentiated structures in a mature organ.
Surprisingly, despite its dispensability for normal development, Gli1 contributes significantly to bladder regeneration, thus providing a useful tool to demonstrate a role of regenerative proliferation not only in restoring urothelial integrity but also in preventing bacterial spread to the kidneys. Further studies will be required to determine whether Gli1 also contributes to the regeneration of other organs in which Hh signalling has a role11,24; such flexibility in Gli1 transcriptional output would be consistent with the apparent flexibility of Gli1 in mediating qualitatively distinct responses to bacterial and chemical bladder injury, which differ markedly in the presence or absence of stromal cell proliferation.
As many as 10% of women experience infections of the urinary tract in a year7,25, including cystitis and pyelonephritis, and ~26% of urinary tract infections recur within six months26. Recent work has demonstrated that intracellular reservoirs of bacteria can form in umbrella cells or in transitional cells3, and exfoliation of infected cells harbouring intracellular bacterial reservoirs induced by PS or other agents has been suggested as potentially beneficial in clearing these infections. Our current data suggest that it may also be worth exploring the effect of more directly activating the Wnt signalling pathway by treating with LiCl; on the other hand use of drugs such as indomethacin, which inhibit the regenerative response in the urothelium, may be contraindicated.
Another clinical arena in which our findings may be relevant is the growth and dissemination of cancers in which Hh ligand production in primary cells of the tumour triggers pathway activity in tumour stroma, which then expands and supports the growth of primary cells within the tumour27,28. This tumour–stromal interaction might now be viewed as the growth-enhancing activation of a feedback circuit normally triggered by injury. Tumour–stromal interactions are also critical in metastasis, and the preferential colonization of particular tissues by individual tumour types might be determined by the competence of target tissues to respond to eliciting signals from the tumour cells, similar to the epithelial–stromal interaction noted here in bladder regeneration. In this connection it is interesting to note that tumours of the prostate, which like the bladder is a derivative of the urogenital sinus and also requires Hh pathway activity for its regeneration11, metastasize most commonly to bone, lung and liver29, a preference very similar to that of bladder tumours, which metastasize most commonly to liver, lung and bone30. Further work will be required to identify regenerative signals in other organs and to determine how important their activities may be in cancer growth and metastasis.
METHODS
Mice
Gli1LacZ/WT heterozygotes12 from our colony were interbred to generate Gli1LacZ/LacZ homozygotes. Heterozygous and wild-type littermates were used as controls. Female mice between 6 and 10 weeks of age were used for all injury experiments. Shh-GFP BAC strain was obtained from the GENSAT project at Rockefeller University. For lineage tracing experiments, ShhCreER/WT, or Gli1CreER/WT mice14,17 were crossed with R26mTmG/mTmG strain15 to obtain ShhCreER/WT; R26mTmG/WT, or Gli1CreER/WT; R26mTmG/WT. ShhCreER/WT or CAG-CreER were crossed with β-cateninflox/flox mice to obtain ShhCreER/WT; β-cateninflox/WT or CAG-CreER; β-cateninflox/WT. Resulting mice were crossed with β-cateninflox/flox mice to obtain ShhCreER/WT; β-cateninflox/flox or CAG-CreER; β-cateninflox/flox. All mouse strains except as otherwise indicated were obtained from Jackson Laboratories. All bladder instillation procedures were performed under isoflurane anaesthesia, which was administered in a fume hood with a standard vaporizer (J. B. Baulch and Associates). All procedures were performed under a protocol approved by the Administrative Panel on Laboratory Animal Care at Stanford University.
Bacterial and chemical injury
For bacterial injury, a uropathogenic E. coli(UPEC) strain, UTI89, was grown for 16 h in a static culture, and inoculated via transurethral instillation of anaesthetized female mice at a concentration of 107 c.f.u. in 50 µl as previously described2. Animals were maintained after infection for the indicated period of time or urine was collected after 4 h to confirm infection. For chemical injury, 50 µl of PS (Sigma) solution in PBS at 2 mg ml−1, 10 mg ml−1, 20 ml ml−1 or 40 mg ml−1 was delivered transurethrally as indicated. Bladders were collected and analysed at distinct time-points after instillation.
Lineage tracing studies
For fate mapping of Shh-expressing cells before bacterial injury, ShhCreER/WT; R26mTmG/WT or Gli1CreER/WT; R26mTmG/WT mouse strains were injected intraperitoneally with 4mg of TM (per 30 g body weight) daily for three consecutive days. Seven days after the last TM injection, mice were subjected to three transurethral instillations of UTI89 with 10-day intervals after the first and second infections, and 15 days after the third. Mice were killed and bladders then dissected for further analysis. For labelling of Shh- or Gli1-expressing cells before injury, mice were injected with TM (4 mg per 30 g body weight) for 3 days and analysed 5 days after the last injection. For lineage tracing of Shh or Gli1 expressing cells during injury, ShhCreER/WT; R26mTmG/WT or Gli1CreER/WT; R26mTmG/WT mice were injected intraperitoneally with 4 mg of TM (per 30 g body weight) for 5 consecutive days, starting 2 days before infections to allow enough time for tamoxifen to be absorbed by the bladder. Six days after the last injection of TM, the entire procedure was repeated. Tissue sections were prepared 6 days after final injection of TM for further analysis. For long-term lineage tracing, ShhCreER/WT; R26mTmG/WT mouse strains were injected intraperitoneally with 4 mg of TM(per 30 g bodyweight) daily for 3 consecutive days. Seven days after the last TM injection, mice were subjected to seven transurethral instillations of UTI89, twice a month for 2 months and once a month for next 3 months. Five months after the last instillation, mice were killed and bladders then dissected for further analysis. For long-term lineage tracing experiments to study homeostasis, ShhCreER/WT; R26mTmG/WT mouse strains were injected with 4mg of TM (per 30 g body weight) daily for 3 consecutive days, and bladders were analysed 10 months after the last TM injection.
In vitro culture of Shh-expressing cells
To isolate Shh-expressing cells, ShhCreER/WT; R26mTmG/WT mouse strains were injected intraperitoneally with 4 mg of TM (per 30 g body weight) daily for 3 consecutive days. Three days after TM injection, bladders were collected, inverted and inflated as described previously31. Inverted bladders were incubated in 0.25% Trypsin-EDTA containing 500 U ml−1 collagenase for 2 h at 37 °C. Tissues were then minced and, after the lysis of red blood cells, a single-cell suspension was obtained by 10 min of trituration, followed by filtration through 40-µm cell strainers. Cells were sorted using a FACS AriaII cytometer (BD Biosciences), and analysis of flow cytometry data was performed using FlowJo Software (Treestar). Sorted cells were cultured in Ultra Low attachment plates (Corning) for suspension culture in media containing 1:1 mixture of conditioned media and DMEM(Invitrogen) supplemented with 50 ng ml−1 EGF (PeproTech). Conditioned medium was prepared by growing 90% confluent V79 lung fibroblast-derived cells (ATCC) in DMEM with 5% FBS for 24 h. For Matrigel culture, a single-cell suspension was mixed with 200 µl of ice-cold Matrigel (reduced growth factors; BD Bioscience), layered onto 12-mm Transwell clear filters, and allowed to solidify at 37 °C. Pre-warmed growth medium was added above and below the gel and cells were grown until the time of analysis. For passaging organoids in Matrigel culture, organoids were released from Matrigel matrix by depolymerizing Matrigel using MatriSphere Cell Recovery Solution (BD Bioscience) according to the manufacturer’s instructions. Organoids were then dissociated into single cells by incubating with 0.25% Trypsin-EDTA for 30 min, followed by 5 min of trituration. Cells were then seeded in Matrigel as described earlier.
Antibody injection
Mice were injected intraperitoneally with either anti-mouse Shh antibody or isotype control (5E1 and 6B3, respectively; Developmental Studies Hybridoma Bank) daily for 7 days. Mice received 10 mg kg−1 body weight of antibody for the first 3 days and 5 mg kg−1 body weight thereafter. Bladders were dissected on the last day of injection to isolate RNA for qRT–PCR analysis. For assays of proliferation, mice were injected with 10 mg antibody per kg body weight daily for 3 days, and 5 mg antibody per kg body weight thereafter. Mice were infected with UTI89 on the fourth day of antibody injection.
Microscopy and laser capture microdissection
All images were obtained using a Zeiss LSM510 inverted confocalmicroscope and prepared for publication with Zeiss LSM 5 Image Browser software and Adobe Photoshop CS3. Three-dimensional reconstructions of confocal images were generated using Imaris software (Bitplane Scientific Software). For LCM, bladder sections were prepared using an LCM staining kit (Ambion) and a Leica LMD6000 Laser Microdissection Microscope.
Quantitative RT–PCR
For qRT–PCR, bladders were frozen in liquid nitrogen and total RNAs isolated from frozen bladder tissue using RNeasy Plus Mini (Qiagen). qRT–PCR was performed using iScript one-step RT–PCR kit with SYBR Green and the Bio-Rad iCycler (BioRad). Assays were performed on bladders from six animals, and all values normalized to the GAPDH internal control, which does not vary on injury (data not shown). For material isolated by LCM, total RNA was prepared using RNAqueous-Micro RNA isolation kit (Ambion).
Indomethacin and LiCl treatment
Mice were injected with 2.5 mgkg−1 of body weight with indomethacin or DMSO vehicle control every 12 h throughout the experiment20. UTI89 was instilled 36 h after initial indomethacin injection, and bladders were analysed on the third day, 60 h after the initial indomethacin injection. For LiCl treatment, wild-type or Gli1LacZ/LacZ mice received either 200 mg kg−1 of body weight of LiCl in 100 µl of deionized water or 100 µl of deionized water as a vehicle control every day for 3 days by oral gavage. At the time of oral gavage, mice were also injected transurethrally with 40 mg kg−1 of body weight of LiCl. UTI89 infection was performed at 30 h after initial LiCl treatment, and bladders were analysed on the third day, 54 h after initial LiCl treatment.
Conditional ablation of β-catenin
ShhCreER/WT; β-cateninflox/flox or CAG-CreER; β-cateninflox/flox female mice were injected intraperitoneally with 4 mg of TM (per 30 g body weight) daily for 3 consecutive days. For CAG-CreER; β-cateninflox/flox, bladder was inoculated with UTI89 the day after the last injection of TM, and bladders were collected 24 h after infection. For ShhCreER/WT; β-cateninflox/flox, bladder was inoculated with UTI89 3 days after the last injection of TM, and collected 24 h after infection.
In vivo permeability assay
10 mg ml−1 of FITC-dextran (10000MW, Invitrogen) in a 50 ml volume of PBS32 was injected transurethrally into the bladder lumen 1.5 h before collection of bladders at the indicated time points. Bladder sections were made and analysed for FITC.
Bacterial titration and analysis of urine
Urine from infected bladders was collected 6 h after UTI89 infection, and slides were prepared and stained for analysis using Cytospin and Hema3 staining kit (Fisher). Kidneys and bladders were dissected from wild-type littermate controls or Gli1LacZ/LacZ mice 24 h after infection. Tissues were homogenized in 1 ml of PBS, and bacterial titres were determined by microtitre-plate dilution on LB plates2.
X-gal histochemistry and immunofluorescence analysis
Bladders were dissected and embedded in OCT compound for snap freezing (Tissue-Tek). Frozen blocks were sectioned at 10-µm intervals using a Microm cryostat. For X-gal staining, frozen sections were fixed in 0.2% glutaraldehyde in PBS containing 5mM EGTA and 2 mM MgCl2 for 30 min at 4 °C. After washing twice with PBS containing 2 mM MgCl2, sections were incubated with 1 mg ml−1 of X-gal solution in PBS containing 0.02% NP40, 0.01% deocycholic acid, 2 mM MgCl2, 5 mM EGTA, 5 mM C6FeK3N6, 5 mM C6FeK4N6 for 4 h to overnight. Stained sections were counterstained with eosin solution (Sigma). For immunostaining, frozen tissue sections were fixed in 4% of paraformaldehyde for 30 min at 4 °C. After washing three times with PBS, tissue sections were blocked in 2% goat serum in PBS containing 0.25% Trion X-100 for 1 h, incubated with the following primary antibodies diluted in blocking solution overnight at 4 °C in a humidified chamber: rat anti-Shh (R&D, 1:200); rabbit anti-Ki67 (Abcam, 1:500); rabbit anti-Ck5 (Abcam, 1:500); chicken anti-laminin (Abcam, 1:300); mouse anti-uroplakin 3 (Fitzgerald). Sections were washed three times with PBS containing 0.25% Triton X-100, incubated with DAPI and appropriate Alexa fluoro 488, 594, or 633 conjugated secondary antibodies diluted 1:1,000 in blocking solution for 2 h at 22 °C, washed again three times, and mounted on slides with Prolong Gold mounting reagent (Invitrogen). For immunostaining of organoids, Matrigel plugs were removed from the filter supports of Transwell plates, washed with PBS, and incubated with MatriSphere Cell Recovery Solution (BD Bioscience) for 20 min to partially dissolve the Matrigel. Subsequently, the gel-embedded bladder organoids were fixed in 4% paraformaldehyde for 15 min at 4 °C, followed by washing three times with PBS. Fixed organoids then were permeablized in 0.25% Triton X-100 for 30 min, followed by incubation in blocking solution of 2% goat serum in PBS containing 0.25% Triton X-100 for 1 h. Organoids were then incubated with primary antibodies diluted in blocking solution for 2 days at 4 °C in a humidified chamber. After three washes, bladder organoids were incubated with secondary antibody overnight in 4 °C, followed by three washes and mounting on Coverwell chamber (Grace Bio-Lab) with Prolong Gold mounting reagent (Invitrogen).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software v.5. All data are presented as mean ± s.e.m., and two group comparisons were done with a two-tailed Student’s t-test. A value of P < 0.05 was taken as statistically significant.
Supplementary Material
Acknowledgements
We thank A. Oro and J. Brooks for their critical reading of the manuscript, and the Stanford Center for Digestive Diseases for help with laser capture microdissection. This research was supported in part by grants from the Department of Defense and from the National Institutes of Health (P.A.B.) and a Pathway to Independence Award (K99/R00) to I.U.M. P.A.B. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions K.S. and P.A.B. conceived ideas and experimental design. K.S. performed the experiments. N.G. aided in immunohistochemical analysis, J.L. and J.K. helped withmouse strains, A.L. assisted with in vitro cell culture studies, L.Q. performed the genotyping of experimental mice, and I.U.M. helped analyse data. K.S. and P.A.B wrote the manuscript.
The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 2.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nature Protocols. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. USA. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks RM. Themammalian urinary bladder: an accommodating organ. Biol. Rev. Camb. Philos. Soc. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Labelretaining cells of the bladder: candidate urothelial stem cells. Am. J. Physiol. Renal Physiol. 2008;294:F1415–F1421. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- 7.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. North. Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 8.Klumpp DJ, et al. Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect. Immun. 2006;74:5106–5113. doi: 10.1128/IAI.00376-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev. Biol. 1999;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi R, et al. Molecular analysis of coordinated bladderand urogenital organ formation by Hedgehog signaling. Development. 2007;134:525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- 11.Karhadkar SS, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 12.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 13.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc. Natl Acad. Sci. USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global doublefluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 16.Wu XR, Sun TT. Molecular cloning of a 47 kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J. Cell Sci. 1993;106:31–43. doi: 10.1242/jcs.106.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive Hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Kimura H, Stephen D, Joyner A, Curran T. Gli1 is important for medulloblastoma formation in Ptc1+/− mice. Oncogene. 2005;24:4026–4036. doi: 10.1038/sj.onc.1208567. [DOI] [PubMed] [Google Scholar]
- 19.Hagberg L, et al. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 24.Fendrich V, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol. Clin. North Am. 2008;35:1–12. doi: 10.1016/j.ucl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am. J. Public Health. 1990;80:331–333. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yauch RL, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 28.Tian H, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc. Natl Acad. Sci. USA. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bubendorf L, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 30.Wallmeroth A, et al. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): an autopsy study on 367 patients. Urol. Int. 1999;62:69–75. doi: 10.1159/000030361. [DOI] [PubMed] [Google Scholar]
- 31.Kurzrock EA, Lieu DK, deGraffenried LA, Isseroff RR. Rat urothelium: improved techniques for serial cultivation, expansion, freezing and reconstitution onto acellular matrix. J. Urol. 2005;173:281–285. doi: 10.1097/01.ju.0000141585.17953.fa. [DOI] [PubMed] [Google Scholar]
- 32.Ibla JC, Khoury J. Methods to assess tissue permeability. Methods Mol. Biol. 2006;341:111–117. doi: 10.1385/1-59745-113-4:111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.