Abstract
Bile acids are signaling molecules that activate nuclear receptors, such as farnesoid X receptor, pregnane X receptor, constitutive androstane receptor, and vitamin D receptor, and play a critical role in the regulation of lipid, glucose, energy, and drug metabolism. These xenobiotic/endobiotic-sensing nuclear receptors regulate phase I oxidation, phase II conjugation, and phase III transport in bile acid and drug metabolism in the digestive system. Integration of bile acid metabolism with drug metabolism controls absorption, transport, and metabolism of nutrients and drugs to maintain metabolic homeostasis and also protects against liver injury, inflammation, and related metabolic diseases, such as nonalcoholic fatty liver disease, diabetes, and obesity. Bile-acid–based drugs targeting nuclear receptors are in clinical trials for treating cholestatic liver diseases and fatty liver disease.
Keywords: Nuclear receptors, bile acid synthesis, cholestasis, drug metabolism
Introduction
Bile acids are physiological detergents that play important roles in facilitating hepatobiliary secretion of endogenous and xenobiotic metabolites as well as intestinal absorption of dietary fats, fat-soluble vitamins, and drugs (Chiang, 2009). Bile acids also are signaling molecules that regulate liver metabolism (Lefebvre et al., 2009) and drug disposition (Eloranta and Kullak-Ublick, 2005). Therefore, maintaining bile acid homeostasis is important for liver metabolic function. Abnormal bile acid metabolism has been associated with cholestatic liver diseases, fatty liver disease (FLD), dyslipidemia, diabetes, obesity, and cardiovascular disease (CVD) (Chiang, 2009; Claudel et al., 2011; Porez et al., 2012).
Nuclear receptors are a group of ligand-activated transcriptional factors. Upon ligand binding and activation, nuclear receptors regulate the transcription of genes involved in various biological processes, ranging from development, differentiation, and metabolism (Mangelsdorf et al., 1995). The ligands for nuclear receptors are usually lipophilic molecules, such as hormones, endogenous metabolites, or xenobiotics. Farnesoid X receptor (FXR; NR1H4) was the first nuclear receptor identified as a bile acid receptor and was shown to regulate the transcription of key genes in bile acid metabolism, from synthesis and transport to detoxification (Makishima et al., 1999). Subsequently, three closely related xenobiotic nuclear receptors in the NR1I subfamily, pregnane X receptor (PXR; NR1I2) (Staudinger et al., 2001), vitamin D receptor (VDR; NR1I1) (Makishima et al., 2002), and constitutive androstane receptor (CAR; NR1I3) (Guo et al., 2003), were shown to be activated by bile acids or bile acid metabolites. These xenobiotic nuclear receptors play critical roles in regulation of the transcription of genes involved in phase I oxidation, phase II conjugation, and phase III transport in drug metabolism and also bile acid metabolism (Figure 1). More-recent studies have uncovered that activation of CAR and PXR also affect glucose and energy metabolism (Wada et al., 2009; Gao and Xie, 2012). Therefore, drug and bile acid metabolism cross-talk to regulate lipid, glucose, and energy metabolism and significantly affect inflammatory and metabolic diseases, such as nonalcoholic fatty liver disease (NAFLD), diabetes, and obesity. FXR, PXR, CAR, and VDR are promising therapeutic targets for the treatment of liver and metabolic diseases (Lefebvre et al., 2009; Claudel et al., 2011; Porez et al., 2012). This review will summarize recent advances in our understanding of the roles of bile-acid–activated nuclear receptors in regulating bile acid homeostasis as well as the therapeutic potential of bile acids and their derivatives for the treatment of metabolic and liver diseases, including cholestasis, NAFLD, diabetes, and obesity.
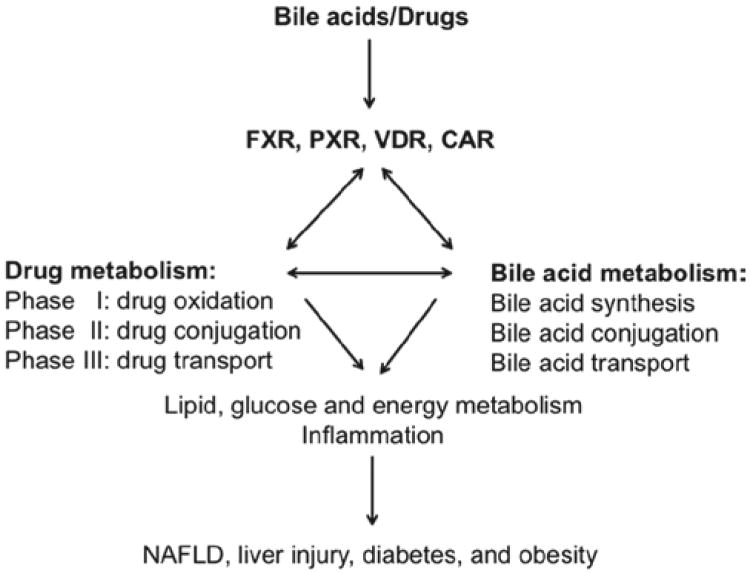
Figure 1.
Roles of FXR, PXR, VDR, and CAR in regulation of drug and bile acid metabolism. Bile acids are endogenous ligands of FXR, PXR, and VDR. Bile acids also activate CAR by an indirect mechanism. These four bile-acid–activated nuclear receptors play a critical role in the regulation of phase I hydroxylation (oxidation), phase II conjugation, and phase III transport in both drug and bile acid metabolism and coordinately regulate lipid, glucose, and energy metabolism and inflammation. Bile acid metabolism plays a key role in maintaining metabolic homeostasis and preventing NAFLD, diabetes, and obesity.
Bile acid metabolism
Similar to drug metabolism, bile acid metabolism includes phase I bile acid synthesis in the liver, phase II bile acid conjugation in the liver and intestine, and phase III bile acid transport in the liver, kidney, and intestine. Bile acid synthesis is a complicated pathway involving three hydroxylation reactions catalyzed by cytochrome P450 (CYP) monooxygenases, epimerization, isomerization, and oxidative cleavage reactions.
Phase I: bile acid synthesis
Bile acids are synthesized from cholesterol solely in the liver (Chiang, 1998, 2009; Russell and Setchell, 1992). Cholic acid (CA) and chenodeoxycholic acid (CDCA) are the two major primary bile acids synthesized in human livers (Figure 2A). There are two bile acid biosynthetic pathways: the classic pathway and the alternative pathway. In humans, the classic pathway accounts for synthesis of more than 90% of total bile acid synthesis, producing CA and CDCA in approximately equal amounts. Cholesterol 7α-hydroxylase (CYP7A1) is a microsomal cytochrome p450 enzyme that catalyzes the first and rate-limiting step in the classic bile acid biosynthetic pathway. Microsomal sterol 12α-hydroxylase (CYP8B1) is required for synthesis of CA. Therefore, CYP7A1 regulates the overall rate of bile acid synthesis, whereas CYP8B1 regulates the ratio of CA to CDCA in the bile acid pool. Mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes steroid oxidation in the classic pathways and initiates the alternative pathway, which mainly produces CDCA. Oxysterol 7α-hydroxylase (CYP7B1) is a nonspecific enzyme that metabolizes steroid intermediates in macrophages and other tissues to produce 7α-hydroxylated metabolites that may be used for the synthesis of bile acids in the liver. There are other hydroxylases, such as microsomal steroid 25-hydroxylase and a brain-specific steroid, 24-hydroxylase (CYP46A1), that are involved in bile acid metabolism.
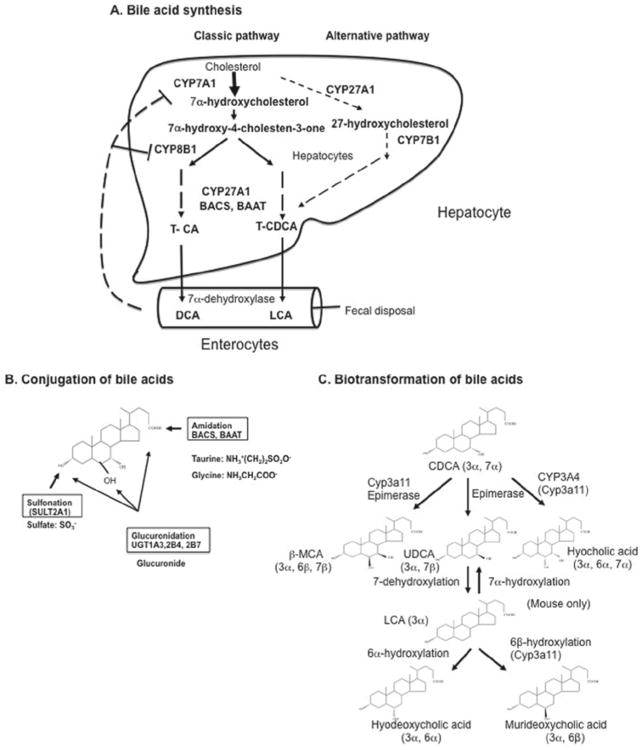
Figure 2.
Bile acid synthesis, conjugation, and biotransformation. Bile acid synthesis in the liver converts cholesterol to bile acids through the classic and alternative pathways. (A) Bile acid synthesis. The classic pathway involves 17 enzymes located in the endoplasmic reticulum, cytosol, mitochondria, and peroxisomes. Four CYP monooxygenases (CYP7A1, CYP8B1, CYP27A1 and CYP7B) are involved in hydroxylation reactions. In the classic pathway, a steroid nucleus undergoes hydroxylation, isomerization, and epimerization first, then oxidative cleavage of the side chain by CYP27A1, and cleavage of the 3-C side chain by peroxisomal β oxidation. CYP7A1 is the first and rate-limiting enzyme in the classic pathway, which synthesizes two primary bile acids (CA and CDCA) in the human liver. CYP8B1 is required for synthesis of CA. CYP27A1 catalyzes side-chain oxidation reactions. The alternative pathway is initiated by CYP27A1, followed by CYP7B1, and then further metabolized as the classic pathway to produce CDCA. (B) Conjugation of bile acids. Amidation (BACS and BAAT), sulfation (SULT2A1), and glucuronidation (UGT1A1, 2B4, and 2B7) at specific positions by specific enzymes are shown. (C) Biotransformation of bile acids. Secondary bile acids CDCA and LCA can be converted to more hydrophilic bile acids in the hepatocytes and intestine. Hydroxylation (by human CYP3A4 or mouse Cyp3a11), epimerization, and dehydroxylation interconverts CDCA to UDCA to LCA as well as other bile acids.
Phase II: bile acid conjugation
After synthesis, most bile acids are immediately conjugated to amino acids (glycine or taurine) through amidation at the carboxyl group by bile acid coenzyme A synthase (BACS) and bile acid amino acid transferase (BAAT) (Figure 2B). In humans, the ratio of glycine to taurine conjugates is approximately 3 to 1, whereas in mice most bile acids (95%) are taurine conjugated and the remaining are free bile acids. Bile acids also are conjugated to sulfate at the 3-hydroxyl group by sulfotransferase (SULT2A1). Some bile acids are glucuronidated at 3, 6, and 24 positions by UDP-glucuronosyl N-transferases (UGT1A1, 2B4, and 2B7). Conjugation of bile acids increases ionization and solubility at acidic pH, prevents Ca2+ precipitation, minimizes passive absorption, and prevents cleavage by pancreatic enzymes in the intestine. Like drug conjugation, bile acid conjugation greatly prepares bile acids for efficient transport and detoxification. In the intestine, some conjugated bile acids are deconjugated, and, subsequently, 7α-dehydroxylase activity in bacterial flora converts CA and CDCA to secondary bile acids, such as deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. These secondary bile acids are highly insoluble and toxic and may cause cholestatic liver injury or promote colon cancer. DCA is reabsorbed in the colon, whereas most LCA is excreted into feces. A small amount (1–4%) of LCA is circulated to the liver and rapidly conjugated by sulfation and amidation to form diconjugated LCA, which is excreted into bile. Sulfation by SULT2A1 is the major pathway for detoxification of hydrophobic bile acids in humans (Hofmann, 2004).
Bile acids undergo biotransformation in the liver and intestine (Figure 2C). In mice, Cyp3a11 (a homolog of human CYP3A4) plays a key role in metabolism of LCA and CDCA to a highly soluble and nontoxic bile acid (β-muricholic acid), which is epimerized to α-muricholic acid. Cyp3a11 also hydroxylates CDCA to hyocholic acid. CDCA is epimerized to ursodeoxycholic acid (UDCA), a highly soluble, nontoxic bile acid. UDCA levels are high in bears and mice, but low in humans. UDCA can be converted to LCA. In mice, but not humans, LCA can be converted to UDCA, hyodeoxycholic acid, and murideoxycholic acid. In humans, the bile acid pool consists of CA, CDCA, and DCA in an approximate ratio of 40:40:20 and is highly hydrophobic. In contrast, the mouse bile acid pool consists mostly of CA and muricholic acids (MCAs; 95%) and is highly hydrophilic.
Phase III: bile acid transport and enterohepatic circulation of bile acids
Bile formation is essential for the elimination of toxic metabolites and drugs/xenobiotics from the liver (Trauner and Boyer, 2003). Bile secretion is also the major route for excretion of endogenous compounds and metabolites, such as drugs, phospholipids, cholesterol, and bilirubin. Bile acids are major components in bile and play a pivotal role in bile formation. Conjugated bile acids are secreted into bile through specific transporters located in the canalicular membrane and are stored in the gallbladder. After each meal, bile acids are released from the gallbladder into the intestine, efficiently reabsorbed in the ileum, and transported back to the liver by portal circulation. The transport of bile acids between the liver and intestine is referred to as enterohepatic circulation of bile acids (Figure 2A). This physiological pathway not only inhibits bile acid synthesis, but also plays a major role in absorption and transport of nutrients from the intestine to the liver for metabolism and distribution to other organs and tissues. In humans, enterohepatic circulation of bile acids is highly efficient; most bile acids (95%) are reabsorbed in the ileum and only 5% of bile acids are excreted into feces and are replenished by de novo synthesis. Bile acids are recycled 4 to 12 times a day. The bile acid pool size is defined as the total amount of bile acids circulating in the enterohepatic circulation.
The enterohepatic circulation of bile acids is a process that involves several bile acid and drug transporters (Trauner and Boyer, 2003). Hepatocytes are polarized epithelial cells with basolateral (sinusoidal) and apical (canalicular) membrane domains (Figure 3). Hepatocytes take up bile acids through the basolateral membrane, which is in direct contact with the portal blood, and excrete bile acids at the canalicular membrane into the bile (Boyer, 1980). Because biliary bile acid concentration is significantly higher in bile than in hepatocytes, canalicular bile acid transport is an active transport process requiring energy provided by hydrolysis of adenosine triphosphate (ATP) or cotransportation with other molecules. Efflux of bile acids to bile is the rate-limiting step in bile formation and provides the driving force for bile fow. Several members of the ATP-binding cassette (ABC) transporter family are responsible for transporting bile acids and other charged compounds across the canalicular membrane against their concentration gradients. The bile salt export pump (BSEP; ABCB11), originally identified as the sister of P-glycoprotein, is the principal bile acid efflux transporter at the canalicular membrane (Childs et al., 1995). Mutations in the BSEP gene were first identified in patients with progressive familial intrahepatic cholestasis subtype 2 (PFIC-2) (Strautnieks et al., 1997). The multidrug resistance associated protein (ABCC2, Mrp2) effluxes a wide range of conjugated metabolites, including bile acids, bilirubin, and drugs (Kullak-Ublick et al., 2000). Phospholipids are excreted by the phospholipid flippase, MDR2 (ABCB4) (Smit et al., 1993). Biliary cholesterol secretion by ABCG5/G8 heterodimer transporters requires MDR2 (Langheim et al., 2005). Bile acids, phospholipids, and cholesterol form mixed micelles in bile to increase cholesterol solubility and reduce bile acid toxicity in the bile duct. Bile acids are stored in the gallbladder. After a meal, cholecystokinine secreted by the intestine causes the gallbladder to contract and release bile into the digestive tract for absorption of dietary fats, fat-soluble vitamins, and drugs. Most bile acids are reabsorbed in the ileum by the apical sodium-dependent bile acid transporter (ASBT), located in the brush border membrane. Once absorbed, bile acids bind the ileum bile-acid–binding protein (I-BABP) and are transported to the basolateral membrane for secretion into portal blood by heterodimeric organic solute transporters (OSTα and OSTβ) (Dawson et al., 2005). NPC1L1 is the dietary cholesterol absorption transporter. Bile acids, especially CA, facilitate absorption of fats, cholesterol, and lipid-soluble vitamins into enterocytes. ABCG5/G8 transporter in the apical membrane of enterocytes effluxes plant sterols and cholesterol. Mutations of ABCG5/G8 genes have been identified in sitosterolemia patients (Berge et al., 2000; Lee et al., 2001).
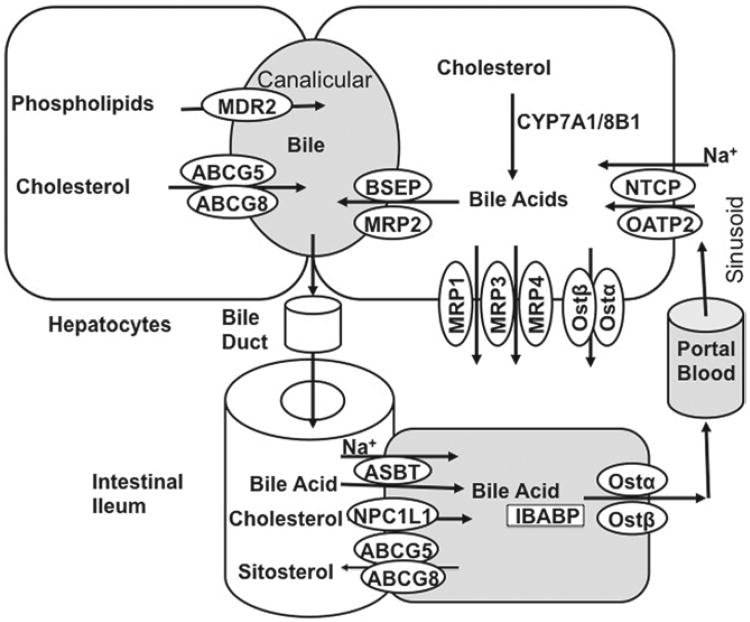
Figure 3.
Enterohepatic circulation of bile acids. Bile acids are synthesized from cholesterol in hepatocytes. Bile acids are secreted into the gallbladder by BSEP and MRP2. Phospholipids are transported by MDR2, and cholesterol is transported by ABCG5/G8 transporters into bile. In the gallbladder, bile acids, phospholipids, and cholesterol form mixed micelles to solubilize cholesterol and reduce bile acid toxicity. After meal intake, the gallbladder releases bile into the small intestine, where bile acids facilitate the absorption of dietary lipids and vitamins. At the terminal ileum, most of the bile acids are reabsorbed by ASBT into the enterocytes and are secreted into the portal circulation by the basolateral bile acid transporters, Ostα/Ostβ. At the basolateral membrane of the hepatocytes, bile acids are taken up by the NTCP transporter for resecretion into the gallbladder, whereas Ostα/Ostβ and MRPs are responsible for basolateral bile acid efflux.
In hepatocytes, Na+-dependent taurocholate cotransport peptide (NTCP), located in the sinusoidal membrane, takes up most of the reabsorbed bile acids. Sodium-independent organic anion transporters (OATPs) also uptake bile acids into hepatocytes. The sinusoidal membranes also express bile acid efflux transporters, including OSTα/OSTβ and MRP3/MRP4, which serve to efflux bile acids into the blood circulation. These sinusoidal bile acid efflux transporters are induced during cholestasis and may play critical roles in the protection of liver injury when bile acids are accumulated abnormally in hepatocytes.
Bile-acid–activated nuclear receptors
Bile acid homeostasis is maintained by a combined balance of bile acid synthesis, metabolism, and transport. Bile acids activate several nuclear receptors to regulate bile acid homeostasis. These nuclear receptors act as sensors of bile acid levels in hepatocytes and enterocytes and coordinately regulate transcription of a network of genes in bile acid synthesis, conjugation, and transport. In chronic liver diseases, such as cholestasis, high levels of hepatic bile acids activate these nuclear receptors to increase bile acid metabolism to protect against liver injury.
Bile acids directly activate three nuclear receptors (FXR, PXR, and VDR). FXR and PXR are highly expressed in tissues that are exposed to bile acids, including liver and intestine, whereas VDR is widely expressed in most tissues. Among all bile acids, CDCA is the most efficacious ligand of FXR, with a half-maximal effective concetration (EC50) of ∼10 μM, whereas one of the secondary bile acids, LCA, is the most potent activator of PXR and VDR, with an EC50 of ∼100 nM. Bile acids do not bind or activate CAR. FXR is a master regulator of bile acid metabolism by regulating several key genes involved in bile acid synthesis, conjugation, and transport. PXR, CAR, and VDR all play roles in the three phases of drug and bile acid metabolism in the liver and intestine. FXR, PXR, VDR, and CAR target genes identified in bile acid metabolism are shown in Table 1.
Table 1. Nuclear receptor target genes in bile acid and drug metabolism.
| NR | Ligand | Target Gene | Tissue | Regulation | Function |
|---|---|---|---|---|---|
| FXR | CDCA | CYP7A1 | Liver | Down | Encodes the rate-limiting enzyme in classic bile acid synthetic pathway |
| CA | |||||
| DCA | |||||
| LCA | BSEP | Liver | Up | Rate-limiting step in canalicular bile acid transport into the gallbladder | |
| GW4064 | |||||
| INT-747 | |||||
| NTCP | Liver | Down | Basolateral bile acid uptake into the hepatocytes | ||
| OSTαOSTβ | Liver Intestine | Up | Basolateral bile acid secretion into the portal blood | ||
| I-BABP | Intestine | Up | Intracellular bile acid transport | ||
| FGF15/19 | Liver Intestine | Up | Bile acid synthesis inhibition | ||
| SHP | Liver | Up | Bile acid synthesis inhibition | ||
| PXR | Rifampicin (human) PCN (mouse) |
CYP3A, CYP1, CYP2 isoforms | Liver Intestine | Up | Phase I bile acid and drug metabolism |
| LCA John's wort |
SULT2A1, UGT1A | Liver Intestine | Up | Phase II bile acid and xenobiotic detoxification | |
| MRP1,MRP2, MRP3, MRP4 OATP1A4, MDR1 | Liver, Intestine Kidney | Up | Canalicular or basolateral bile acid and drug conjugates transport | ||
| CYP7A1 | Liver | Down | Bile acid synthesis | ||
| FGF15/19 | Intestine | Up | Bile acid synthesis inhibition | ||
| CAR | Phenobarbital TCPOBOP | CYP2 isoforms, CYP3A, SULT2A, UGT1A1 | Liver Intestine | Up | Phase I/II bile acid and drug detoxification |
| Androstanol | MRP2/MRP3/MRP4 | Liver | Up | Canalicular or basolateral bile acid and drug conjugates transport | |
| VDR | 1α, 25-OH VD3 LCA | CYP3A | Intestine | Up | Phase I bile acid and drug metabolism |
| SULT2A1 | Intestine | Up | Bile acid conjugation | ||
| CYP7A1 | Liver | Down | Bile acid synthesis | ||
| FGF15 | Intestine | Up | Bile acid synthesis inhibiton |
The role of FXR in regulation of bile acid homeostasis
The first identified FXR target gene involved in bile acid metabolism is I-BABP (Grober et al., 1999). FXR/RXR heterodimer binds to an inverted repeat in the I-BABP gene promoter and stimulates I-BABP gene transcription in response to bile acid treatment. It is well established that the CYP7A1 gene is repressed by bile acids, a mechanism that allows the liver to decrease bile acid synthesis in response to an increase in bile acid levels and thus maintains a constant bile acid pool (Chiang, 1998). FXR is the key mediator in this bile acid feedback repression mechanism. It was first discovered that hepatic FXR induced a negative nuclear receptor, small heterodimer partner (SHP), which interacts with and represses the transcriptional activator, liver related homolog 1 (LRH-1), that binds to the CYP7A1 gene promoter, thus inhibiting CYP7A1 gene transcription (Goodwin et al., 2000). However, bile acids and FXR agonists still are able to inhibit CYP7A1 in SHP-deficient mice, suggesting that a SHP-independent pathway also exists (Makishima et al., 2002, Wang et al., 2002). More recently, FXR was shown to induce intestinal fibroblast growth factor 15 (FGF15), which acts as an endocrine factor to repress CYP7A1 gene transcription in hepatocytes (Inagaki et al., 2005). Direct infusion of recombinant FGF15 into mouse blood circulation or overexpression of FGF15 in mouse liver by an adenovirus expression vector caused marked repression of CYP7A1 messenger RNA (mRNA) expression. FGF15 activates the cell-surface receptor, FGFR4, leading to activation of extracellular signal-realted kinase (ERK)1/2 signaling to inhibit CYP7A1, but the exact molecular mechanism of FGF15 repression of CYP7A1 has yet to be elucidated (Song et al., 2009). The identification of an intestine-initiated bile acid feedback mechanism is consistent with a long-observed phenomenon that intraduodenal, but not intravenous, infusion of taurocholic acid repressed CYP7A1 mRNA expression in rats (Pandak et al., 1991). In mice lacking functional Ostα/Ostβ heterodimer, bile acid transportation to the liver was reduced and bile acids were accumulated in the intestine. Interestingly, these mice showed increased intestinal FGF15 expression and reduced liver CYP7A1 mRNA and total bile acid pool (Rao et al., 2008). Further, intestine-specific FXR knockout, but not liver-specific FXR knockout, prevented GW4064 (an FXR agonist) repression of liver CYP7A1 gene expression in mice (Kim et al., 2007; Kong et al., 2012). These studies collectively suggest that intestinal FXR plays a dominant role in mediating bile acid feedback repression of bile acid synthesis. FGF19, the human ortholog of mouse FGF15, has been shown to repress CYP7A1 expression and bile acid synthesis in human hepatocytes (Song et al., 2009). FGF19 mRNA and protein are detectable in human liver and hepatocytes (Song et al., 2009; Schaap et al., 2009). FGF19 protein is also present in human blood circulation (Lundasen et al., 2006). It is likely that bile acid accumulation in the human liver may induce the FXR/FGF19 pathway to repress CYP7A1 in an autocrine manner.
In addition to its role in mediating bile acid inhibition of bile acid synthesis, FGF15 was shown to stimulate gallbladder refilling (Choi et al., 2006). Both fgf15−/− mice and fgfr4−/− mice had depleted gallbladder volume, which was rapidly reversed by FGF15 or FGF19 treatment. Because FGF15 does not seem to be expressed in mouse hepatocytes or gallbladder, it is likely that intestinal bile acids may play a role in FGF15-regulated gallbladder refilling in mice. On the other hand, FGF19 is highly expressed in human gallbladder and biliary tract, and human bile contains high levels of secreted FGF19, indicating a potentially important function of FGF19 in the biliary tract (Zweers et al., 2012). Currently, the importance of FGF15 signaling in hepatobiliary transport of bile acids and other organic compounds remains to be determined. Beyond bile acid metabolism, FGF15 has been shown to repress hepatic lipo- and gluconeogenesis as well as to stimulate hepatic glycogen and protein synthesis (Potthof et al., 2011; Bhatnagar et al., 2009; Kir et al., 2011). A recent study showed that FGF19 treatment ameliorated hepatic lipid disorders in mice lacking FXR (Miyata et al., 2011).
FXR also plays a key role in regulation of bile acid transport in the enterohepatic system. It was first demonstrated that bile acid activation of FXR induced BSEP (Ananthanarayanan et al., 2001) and MRP2 in hepatocytes (Kast et al., 2002). On the other hand, FXR “indirectly” repressed NTCP by induction of SHP (Denson et al., 2001). Thus, activation of liver FXR protects hepatocytes from toxic bile acid accumulation by stimulating bile acid secretion at the canalicular membrane and limiting bile acid uptake from the portal circulation. FXR/RXR heterodimer binds to the OSTα and OSTβ gene promoters and induces their expression in the sinusoidal membrane to efflux bile acids to systemic circulation (Lee et al., 2006) and, subsequently, are eliminated by renal excretion. Several basolateral drug transporters (MRP1, MRP3, and MRP4) have substrate specificity for bile acid conjugates (Trauner and Boyer, 2003; Hirohashi et al., 2000; Zelcer et al., 2003; Kullak-Ublick et al., 2000). These MRPs are induced during cholestasis in mice and humans (Zollner et al., 2007; Chai et al., 2011). Stimulation of basolateral bile acid efflux in cholestasis and primary biliary cirrhosis may be an important protective response against cholestatic liver injury (Mennone et al., 2006; Marschall et al., 2006; Zollner et al., 2006; Boyer et al., 2006).
In the intestine, FXR is involved in limiting canalicular bile acid uptake and basolateral bile acid secretion. Mouse and human, but not rat, brush border ASBT is repressed by bile acids (Neimark et al., 2004; Chen et al., 2003; Arrese et al., 1998). ASBT is positively regulated by the RAR/RXR heterodimer and LRH that bind to its gene promoter (Chen et al., 2003; Neimark et al., 2004). It was hypothesized that FXR-mediated induction of SHP might repress RAR or LRH transactivation of ASBT transcription. A more-recent study provides additional evidence that FGF15 may play a role in mediating bile acid repression of ASBT (Sinha et al., 2008). FXR induces the major basolateral bile acid efflux transporters, OSTα and OSTβ (Lee et al., 2006). Thus, increased bile acid levels in enterocytes may activate FXR to limit bile acid absorption and promote basolateral efflux of bile acids for subsequent biliary or renal excretion.
Given the role of FXR in regulating the bile acid transport system, numerous studies have been carried out to evaluate the role of FXR in regulating bile acid homeostasis and detoxification in Fxr-null mice under bile duct ligation (BDL), bile acid loading, or drug-induced intrahepatic cholestasis (Wagner et al., 2003, 2011; Cui et al., 2009). Mice lacking FXR showed elevated CYP7A1 mRNA expression and enlarged bile acid pool size, but otherwise were phenotypically normal (Kok et al., 2003; Sinal et al., 2000). When fed a cholic-acid–containing diet, Fxr-null mice showed more-severe hepatotoxicity, which was accompanied by the lack of CYP7A1 repression and induction of FXR-regulated transporters. Similarly, Fxr-null mice were more susceptible to bile-acid– induced liver injury after BDL, despite induction of the PXR-regulated bile acid detoxification network.
The role of PXR, CAR, and VDR in regulation of bile acid metabolism and toxicity
A potential role of PXR in regulating bile acid synthesis was first implicated by the observation that treating rodents with pregnenolone 16α-carbonitrile (PCN), a PXR agonist, repressed liver CYP7A1 activity (Mason and Boyd, 1978; Stahlberg, 1995), which provides a molecular basis for cross-talk between bile acids and drug metabolism. A regulatory role of PXR in bile acid synthesis was subsequently confirmed by studies showing that PCN repressed CYP7A1 mRNA expression and biliary bile acid secretion, but failed to do so in PXR-deficient mice (Staudinger et al., 2001). In human hepatocytes and liver cell line models, two groups showed that rifampicin-activated PXR repressed human CYP7A1 gene transcription by inhibiting the nuclear receptor, hepatocyte nuclear factor 4 alpha (HNF4α), and coactivator peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) complex that is required for CYP7A1 gene transcription (Li and Chiang, 2005; Bhalla et al., 2004). More recently, activation of PXR in the intestine was shown to induce FGF15 expression, and a PXR response element was identified in the promoter of the FGF15 gene (Wistuba et al., 2007). These results suggest that drug or bile acid activation of intestinal PXR may mediate inhibition of CYP7A1 and bile acid synthesis. Whether PXR induces FGF19 in human hepatocytes remains to be determined. PXR induction of intestinal FGF19 was recently associated with promotion of colon tumor growth, implicating a link between PXR activation and anticancer drug resistance (Wang et al., 2011). Interestingly, a recent study showed that mice lacking PXR fed a lithogenic diet had higher susceptibility to development of cholesterol gallstones, which is associated with decreased Cyp7a1 gene expression, reduced biliary bile acid secretion, and higher intestinal FGF15 expression (He et al., 2011).
The hydroxylation of bile acids is mediated mainly by CYP3A enzymes that are induced upon PXR activation (Staudinger et al., 2001). In addition, the bile acid conjugation enzymes, SULT2A1 and UGTs, the canalicular transporter, MRP2, and the basolateral transporter, OATP2, are also induced by PXR activation (Kliewer and Willson, 2002). Therefore, LCA activation of PXR is clearly an adaptive protection to reduce bile acid toxicity during cholestasis. Indeed, PXR target genes involved in bile acid detoxification were induced in mice treated with LCA or BDL, whereas mice lacking PXR were more susceptible to hepatotoxicity caused by LCA treatment or BDL, supporting the notion of adaptive activation of the PXR-signaling network to reduce bile acid toxicity during cholestasis (Staudinger et al., 2001; Stedman et al., 2005). In addition, numerous studies have collectively demonstrated that activation of PXR protects against bile-acid–induced liver injury in experimental cholestasis models (Stedman et al., 2005). When phenobarbital and 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP)-treated FXR and PXR knockout (KO) mice were administered CA, they were protected from bile acid toxicity and CAR target genes Ctp2b, Cyp3a, Mrp2, Ugt1a1, and Gsta were induced, suggesting that FXR, PXR, and CAR protect against hepatic bile acid toxicity in a complementary manner (Guo et al., 2003). In humans, rifampicin has been used to reduce pruritus associated with cholestasis (Hofmann, 2002). The pathophysiological cause of pruritus is not clear, though it may be related to high tissue and serum bile acid accumulation. Thus, PXR repression of bile acid synthesis and promotion of bile acid detoxification may provide benefits in reducing bile acid cytotoxicity in cholestasis. The effectiveness of rifampicin treatment in reducing pruritus has been demonstrated in several clinical studies, but the degree of relief varied among individuals (Hofmann, 2002). However, hepatotoxicity has been reported in patients treated with rifampicin, suggesting that the development of an improved PXR agonist is needed for treating pruritus associated with cholestasis.
Similar to PXR, CAR is constitutively active and is considered to be another master regulator of drug metabolism in the liver (Stanley et al., 2006). CAR and PXR recognize the same xenobiotic response elements in target gene promoters and thus regulate an overlapping set of target genes in xenobiotic and bile acid metabolism and detoxification (Table 1). One study reports that activation of CAR also repressed CYP7A1 gene transcription in hepatocytes (Miao et al., 2006). Activation of CAR is beneficial for protecting against bile acid toxicity during cholestasis in mice (Beilke et al., 2009a, 2009b; Saini et al., 2004, Stedman et al., 2005, Guo et al., 2003). There is no evidence that bile acids bind or activate CAR. One study showed that mice lacking CAR had a higher degree of liver injury than wild-type (WT) mice during LCA- or BDL-induced cholestasis, and such liver damage was aggravated in CAR/PXR double KO mice, suggesting that PXR and CAR may play overlapping, but also complementary, roles in bile acid detoxification (Stedman et al., 2005). Because PXR is thought to be the major mediator of CYP3A induction in bile acid hydroxylation, CAR is thought to play a central role in inducing LCA sulfation, because the resistance of CAR transgenic mice to LCA toxicity was associated with increased LCA sulfation independent of CYP3A induction (Saini et al., 2004).
VDR is primarily activated by 1α,25-dihydroxyvitamin D3. Recent studies suggest that besides its well-known role in regulating calcium homeostasis, VDR also regulates drug metabolism, bile acid metabolism, and inflammation (Nagpal et al., 2005). 1α,25-dihydroxyvitamin D3, acting through VDR, was first shown to induce CYP3A4, CYP2B, and CYP2C in intestinal cells, suggesting a role for VDR in drug and bile acid metabolism (Schmiedlin-Ren et al., 2001; Thummel et al., 2001). VDR mainly acts as an intestinal bile acid sensor and protects the gut from bile acid toxicity. In addition to VDR induction of CYP3A, later studies identified that activation of VDR also induced SULT2A1 and thus simultaneously stimulated bile acid sulfation (Chatterjee et al., 2005). Further, MRP3 and ASBT, two bile acid transporters, have been shown to be direct VDR targets in the intestine (McCarthy et al., 2005; Chen et al., 2006). Induction of MRP3 in the intestine is thought to stimulate excretion of conjugated and unconjugated bile acids, whereas stimulation of ASBT may facilitate bile acid absorption and transport to the liver. Because high levels of LCA in the gut are thought to promote colon cancer, accumulation of LCA in the intestine may activate VDR to induce CYP3A, which metabolizes LCA to less-toxic intermediates for excretion. Treating primary human hepatocytes with 1α,25-dihydroxyvitamin D3 induces known VDR target genes, including CYP3A, CYP2B, and CYP2C (Drocourt et al., 2002). VDR mRNA and protein were detected at low levels in primary human hepatocytes (Han et al., 2010). Because it was shown that cholestasis could result in a significant elevation of LCA levels in the liver and LCA is a potent endogenous ligand for VDR, studies were thus carried out to investigate the effect and mechanism of VDR activation on hepatic bile acid synthesis. It was first shown that treating primary human hepatocytes with 1α,25-dihydroxyvitamin D3 repressed CYP7A1 mRNA expression (Han and Chiang, 2009). Mechanistic studies suggest that VDR may interact with HNF4 and compete for PGC-1α in transactivation of the CYP7A1 gene. Nuclear VDR repression of CYP7A1 gene transcription is further augmented by a cell-surface VDR-initiated, cell-signaling pathway that led to activation of ERK signaling (Han et al., 2010). Another recent study reported that mice lacking VDR had elevated hepatic CYP7A1 gene expression and enlarged bile acid pool size, whereas 1α,25-dihydroxyvitamin D3 treatment repressed hepatic CYP7A1 gene expression in mice (Schmidt et al., 2010). It was shown that vdr−/− mice expressed lower levels of FGF15 in the intestine, whereas 1α,25-dihydroxyvitamin D3 treatment increased intestinal FGF15 in WT mice. A VDR-binding site was identified in the FGF15 promoter (Schmidt et al., 2010). Further, 1α,25-dihydroxyvitamin D3 failed to repress CYP7A1 in fgf15−/− mice, supporting that activation of intestinal VDR represses hepatic CYP7A1 by FGF15 signaling in mice. Taken together, studies conducted in primary human hepatocytes and mouse models provide evidence that VDR represses hepatic CYP7A1 gene transcription and bile acid synthesis.
Despite the proposed role of VDR activation in the repression of bile acid synthesis and activation of bile acid detoxification, existing studies on potential effects of VDR ligands in the protection of cholestatic liver injury are still very limited. One study showed that 1α, 25-dihydroxyvitamin D3 treatment did not alter hepatic or serum bile acid levels in mice after BDL, suggesting a minimal role of VDR in modulating bile acid metabolism in cholestasis (Ogura et al., 2009). However, this study showed that VDR activation in BDL mice reduced proinflammatory cytokine expression and release, consistent with the known role of VDR in immunity. These results suggest that the anti-inflammatory properties of VDR could provide some benefits during cholestasis. The role of VDR in regulating innate and adaptive immunity is reviewed in detail elsewhere (Nagpal et al., 2005). 1α,25-dihydroxyvitamin D3 treatment generally reduces hepatic and serum bile acids, which is accompanied by increased renal MRP2, MRP3, and MRP4 mRNA expression and increased renal bile acid secretion (Nishida et al., 2009). However, this study showed that in mice fed a chow diet, 1α,25-dihydroxyvitamin D3 treatment markedly increased hepatic CYP7A1 mRNA without altering total bile acid pool size or serum bile acid concentration. Further studies are needed to evaluate the potential effects of pharmacological activation of VDR in modulating bile acid metabolism and cholestasis liver injury.
Conclusion
This review summarizes the current knowledge of the roles of major bile acid and xenobiotic-sensing nuclear receptors in the regulation of bile acid metabolism and detoxification. The identification of these regulatory mechanisms provided the molecular basis for developing bile acid receptor-based therapies to treat human liver diseases. Several potent FXR agonists have been tested in clinical trials for treating cholestasis (Pellicciari et al., 2004; Rizzo et al., 2005). In addition, FXR agonists also show great promise for the treatment of FLD, diabetes, and CVDs as a result of the roles of FXR in regulating lipid and glucose metabolism (Fiorucci et al., 2010; Mencarelli and Fiorucci, 2010; Porez et al., 2012). PXR and CAR are also attractive drug targets for treating cholestasis, based on their key function in the activation of the bile acid detoxification network. Recent studies indicated that these xenobiotic sensors play important roles in many other physiological aspects, including lipid metabolism and inflammation (Modica et al., 2009). Future advances in the field are needed to improve our understanding of the control of bile acids over metabolism, which is also critical in developing effective nuclear receptor modulators for the treatment of liver diseases.
Acknowledgments
Declaration of interest: This study was supported by NIH grants DK44442 and DK58379.
References
- Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- Arrese M, Trauner M, Sacchiero RJ, Crossman MW, Shneider BL. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology. 1998;28:1081–1087. doi: 10.1002/hep.510280424. [DOI] [PubMed] [Google Scholar]
- Beilke LD, Aleksunes LM, Holland RD, Besselsen DG, Beger RD, Klaassen CD, et al. Constitutive androstane receptor-mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice. Drug Metab Dispos. 2009a;37:1035–1045. doi: 10.1124/dmd.108.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilke LD, Aleksunes LM, Olson ER, Besselsen DG, Klaassen CD, Dvorak K, et al. Decreased apoptosis during CAR-mediated hepatoprotection against lithocholic acid-induced liver injury in mice. Toxicol Lett. 2009b;188:38–44. doi: 10.1016/j.toxlet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- Bhalla S, Ozalp C, Fang S, Xiang L, Kemper JK. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha. Functional implications in hepatic cholesterol and glucose metabolism. J Biol Chem. 2004;279:45139–45147. doi: 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284:10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL. New concepts of mechanisms of hepatocyte bile formation. Physiol Rev. 1980;60:303–326. doi: 10.1152/physrev.1980.60.2.303. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, et al. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1124–G1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- Chai J, Luo D, Wu X, Wang H, He Y, Li Q, et al. Changes of organic anion transporter MRP4 and related nuclear receptors in human obstructive cholestasis. J Gastrointest Surg. 2011;15:996–1004. doi: 10.1007/s11605-011-1473-2. [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Echchgadda I, Song CS. Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. Methods Enzymol. 2005;400:165–191. doi: 10.1016/S0076-6879(05)00010-8. [DOI] [PubMed] [Google Scholar]
- Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278:19909–19916. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen F, Liu S, Glaeser H, Dawson PA, Hofmann AF, et al. Transactivation of rat apical sodium-dependent bile acid transporter and increased bile acid transport by 1alpha,25-dihydroxyvitamin D3 via the vitamin D receptor. Mol Pharmacol. 2006;69:1913–1923. doi: 10.1124/mol.105.020792. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:D176–D193. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs S, Yeh RL, Georges E, Ling V. Identification of a sister gene to P-glycoprotein. Cancer Res. 1995;55:2029–2034. [PubMed] [Google Scholar]
- Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, et al. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- Claudel T, Zollner G, Wagner M, Trauner M. Role of nuclear receptors for bile acid metabolism, bile secretion, cholestasis, and gallstone disease. Biochim Biophys Acta. 2011;1812:867–878. doi: 10.1016/j.bbadis.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci. 2009;110:47–60. doi: 10.1093/toxsci/kfp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, et al. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277:25125–25132. doi: 10.1074/jbc.M201323200. [DOI] [PubMed] [Google Scholar]
- Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Cipriani S, Baldelli F, Mencarelli A. Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog Lipid Res. 2010;49:171–185. doi: 10.1016/j.plipres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Gao J, Xie W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol Sci. 2012;33:552–558. doi: 10.1016/j.tips.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Grober J, Zaghini I, Fujii H, Jones SA, Kliewer SA, Willson TM, et al. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid × receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem. 1999;274:29749–29754. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- Han S, Chiang JY. Mechanism of vitamin D receptor inhibition of cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes. Drug Metab Dispos. 2009;37:469–478. doi: 10.1124/dmd.108.025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol. 2010;24:1151–1164. doi: 10.1210/me.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Nishida S, Xu M, Makishima M, Xie W. PXR prevents cholesterol gallstone disease by regulating biosynthesis and transport of bile salts. Gastroenterology. 2011;140:2095–2106. doi: 10.1053/j.gastro.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J Biol Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. Rifampicin and treatment of cholestatic pruritus. Gut. 2002;51:756–757. doi: 10.1136/gut.51.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev. 2004;36:703–722. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Willson TM. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J Lipid Res. 2002;43:359–364. [PubMed] [Google Scholar]
- Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, Stellaard F, et al. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem. 2003;278:41930–41937. doi: 10.1074/jbc.M306309200. [DOI] [PubMed] [Google Scholar]
- Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis. 2000;20:273–292. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- Langheim S, Yu L, von Bergmann K, Lutjohann D, Xu F, Hobbs HH, et al. ABCG5 and ABCG8 require MDR2 for secretion of cholesterol into bile. J Lipid Res. 2005;46:1732–1738. doi: 10.1194/jlr.M500115-JLR200. [DOI] [PubMed] [Google Scholar]
- Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47:201–214. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2005;288:G74–G84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fbroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall HU, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, et al. Fxr(-/-) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- Mason JI, Boyd GS. The suppressive effect of the catatoxic steroid, pregnenolone-16alpha-carbonitrile, on liver microsomal cholesterol-7alpha-hydroxlyase. Steroids. 1978;31:849–854. doi: 10.1016/s0039-128x(78)80048-8. [DOI] [PubMed] [Google Scholar]
- McCarthy TC, Li X, Sinal CJ. Vitamin D receptor-dependent regulation of colon multidrug resistance-associated protein 3 gene expression by bile acids. J Biol Chem. 2005;280:23232–23242. doi: 10.1074/jbc.M411520200. [DOI] [PubMed] [Google Scholar]
- Mencarelli A, Fiorucci S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J Cell Mol Med. 2010;14:79–92. doi: 10.1111/j.1582-4934.2009.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, et al. Mrp4-/- mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43:1013–1021. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- Miao J, Fang S, Bae Y, Kemper JK. Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J Biol Chem. 2006;281:14537–14546. doi: 10.1074/jbc.M510713200. [DOI] [PubMed] [Google Scholar]
- Miyata M, Sakaida Y, Matsuzawa H, Yoshinari K, Yamazoe Y. Fibroblast growth factor 19 treatment ameliorates disruption of hepatic lipid metabolism in farnesoid X receptor (Fxr)-null mice. Biol Pharm Bull. 2011;34:1885–1889. doi: 10.1248/bpb.34.1885. [DOI] [PubMed] [Google Scholar]
- Modica S, Bellafante E, Moschetta A. Master regulation of bile acid and xenobiotic metabolism via the FXR, PXR and CAR trio. Front Biosci. 2009;14:4719–4745. doi: 10.2741/3563. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- Neimark E, Chen F, Li X, Shneider BL. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 2004;40:149–156. doi: 10.1002/hep.20295. [DOI] [PubMed] [Google Scholar]
- Nishida S, Ozeki J, Makishima M. Modulation of bile acid metabolism by 1alpha-hydroxyvitamin D3 administration in mice. Drug Metab Dispos. 2009;37:2037–2044. doi: 10.1124/dmd.109.027334. [DOI] [PubMed] [Google Scholar]
- Ogura M, Nishida S, Ishizawa M, Sakurai K, Shimizu M, Matsuo S, et al. Vitamin D3 modulates the expression of bile acid regulatory genes and represses inflammation in bile duct-ligated mice. J Pharmacol Exp Ter. 2009;328:564–570. doi: 10.1124/jpet.108.145987. [DOI] [PubMed] [Google Scholar]
- Pandak WM, Li YC, Chiang JY, Studer EJ, Gurley EC, Heuman DM, et al. Regulation of cholesterol 7 alpha-hydroxylase mRNA and transcriptional activity by taurocholate and cholesterol in the chronic biliary diverted rat. J Biol Chem. 1991;266:3416–3421. [PubMed] [Google Scholar]
- Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559–4569. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease: Tematic Review Series: New Lipid and Lipoprotein Targets for the Treatment of Cardiometabolic Diseases. J Lipid Res. 2012;53:1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–3896. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G, Renga B, Mencarelli A, Pellicciari R, Fiorucci S. Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:289–303. doi: 10.2174/1568008054863781. [DOI] [PubMed] [Google Scholar]
- Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry. 1992;31:4737–4749. doi: 10.1021/bi00135a001. [DOI] [PubMed] [Google Scholar]
- Saini SP, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, et al. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol. 2004;65:292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fbroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem. 2010;285:14486–14494. doi: 10.1074/jbc.M110.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, Watkins PB. Induction of CYP3A4 by 1 alpha,25-dihydroxyvitamin D3 is human cell line-specific and is unlikely to involve pregnane X receptor. Drug Metab Dispos. 2001;29:1446–1453. [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Sinha J, Chen F, Miloh T, Burns RC, Yu Z, Shneider BL. Beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2008;295:G996–G1003. doi: 10.1152/ajpgi.90343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fbroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlberg D. Effects of pregnenolone-16 alpha-carbonitrile on the metabolism of cholesterol in rat liver microsomes. Lipids. 1995;30:361–364. doi: 10.1007/BF02536046. [DOI] [PubMed] [Google Scholar]
- Stanley LA, Horsburgh BC, Ross J, Scheer N, Wolf CR. PXR and CAR: nuclear receptors which play a pivotal role in drug disposition and chemical toxicity. Drug Metab Rev. 2006;38:515–597. doi: 10.1080/03602530600786232. [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A. 2005;102:2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strautnieks SS, Kagalwalla AF, Tanner MS, Knisely AS, Bull L, Freimer N, et al. Identification of a locus for progressive familial intrahepatic cholestasis PFIC2 on chromosome 2q24. Am J Hum Genet. 1997;61:630–633. doi: 10.1086/515501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, et al. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60:1399–1406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- Wada T, Gao J, Xie W. PXR and CAR in energy metabolism. Trends Endocrinol Metab. 2009;20:273–279. doi: 10.1016/j.tem.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023–1034. doi: 10.1002/hep.24148. [DOI] [PubMed] [Google Scholar]
- Wang H, Venkatesh M, Li H, Goetz R, Mukherjee S, Biswas A, et al. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest. 2011;121:3220–3232. doi: 10.1172/JCI41514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, et al. Redundant pathways for negative feedback regulation of bile Acid production. Dev Cell. 2002;2:721–731. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- Wistuba W, Gnewuch C, Liebisch G, Schmitz G, Langmann T. Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by PXR. World J Gastroenterol. 2007;13:4230–4235. doi: 10.3748/wjg.v13.i31.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, et al. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4) Biochem J. 2003;371:361–367. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Fickert P, Silbert D, Gumhold J, Zatloukal K, et al. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int. 2007;27:920–929. doi: 10.1111/j.1478-3231.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, et al. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- Zweers SJ, Booij KA, Komuta M, Roskams T, Gouma DJ, Jansen PL, et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology. 2012;55:575–583. doi: 10.1002/hep.24702. [DOI] [PubMed] [Google Scholar]