Summary
Sensory plasticity related to reproductive state, hormonal profiles, and experience is widespread among vertebrates, including humans [1–5]. Improvements in audio-vocal coupling that heighten the detection of conspecifics are part of the reproductive strategy of many non-mammalian vertebrates [6, 7]. While seasonal changes in hearing are known [7–11], molecular mechanisms determining this form of adult sensory plasticity remain elusive. Among both non-mammals [12] and mammals [13, 14], large-conductance, calcium-activated potassium (BK) channels underlie a primary outward current having a predominant influence on frequency tuning in auditory hair cells [12]. We now report an example from fish showing that increased BK channel abundance can improve an individual’s ability to hear vocalizations during the breeding season. Pharmacological manipulations targeting BK channels, together with measures of BK transcript abundance, can explain the seasonal enhancement of auditory hair cell sensitivity to the frequency content of calls. Plasticity in ion channel expression is a simple, evolutionarily labile solution for sculpting sensory bandwidth to maximize the detection of conspecific signals during reproductive cycles.
Results and Discussion
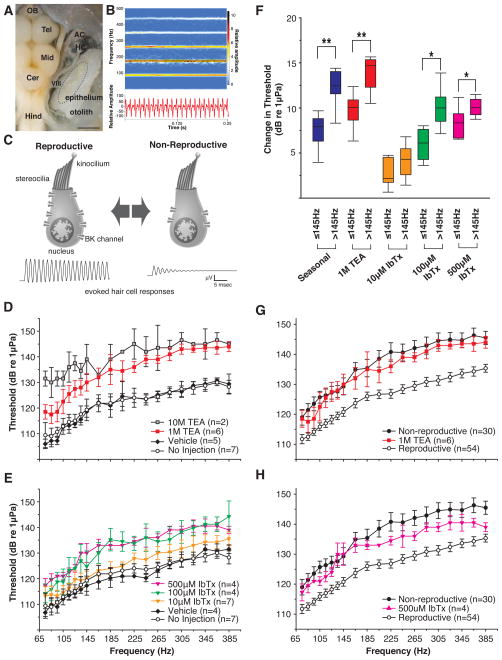
Midshipman fish (Porichthys notatus) migrate annually from deep waters in winter to the intertidal zone in summer, where males nest under rocks and vocalize to attract females [15, 16]. As males and females transition between non-reproductive and reproductive phenotypes, they display improved frequency encoding by the hair cell epithelium of the saccule, the division of the inner ear used for hearing in this and many fish species (Figure 1A) [17, 18]. Both sexes exhibit decreases in hair cell response thresholds of ~10dB during the reproductive season [17, 18]. This plasticity occurs across the frequency spectrum of vocalizations, but especially within upper harmonics (Figure 1B) that transmit best in their shallow water breeding environment [15, 19]. The ~10dB shift would improve the range of signal detection by ~1 meter, based on the distance over which natural calls attenuate by a similar magnitude in their habitat of closely spaced nests [19].
Figure 1.
Frequency encoding by peripheral auditory system. (A) Dorsal view of brain and inner ear. AC, anterior semicircular canal ampulla; Cer, cerebellum; HC, horizontal canal ampulla; Hind, hindbrain; Mid, midbrain; Tel, telencephalon; VIII, eight cranial nerve. Portions of semicircular canals were removed to better visualize auditory division of the inner ear, the saccule and its hair cell epithelium (circled). Scale bar is 1.5 mm. (B) Spectrogram (top) and waveform (bottom) of a representative midshipman advertisement vocalization (‘hum’) with energy concentrated at the fundamental (~100Hz) and first two upper harmonics (~200–300 Hz). See Supplemental Video S1 for video and audio of a ‘humming’ male in an artificial nest. (C) Schematic summarizing proposed role of slo1, BK abundance in seasonal auditory plasticity [also see 12]. Increased expression leads to robust encoding of higher frequencies in upper harmonics of advertisement calls. (D–H) Potassium channels, BK, and auditory hair cell thresholds. (D) TEA significantly increased auditory thresholds in males compared to controls. Since both sexes exhibit similar plasticity [17], we used the more available males (also in E–H) [15]. (E) 100 and 500 μM IbTx significantly increased thresholds compared to controls. (F) Seasonal and pharmacologically-induced changes in thresholds were greatest at frequencies above 145Hz. Only 10 μM IbTx had no significant difference in its effect at frequencies above or below 145Hz. (Seasonal data from [17]; * P < 0.01, ** P < 0.0001). Both (G) TEA and (H) IbTx iontophoresis into the saccule of reproductive males shifted thresholds to be significantly different from non-manipulated reproductive animals, but not different from non-reproductive animals. Data for reproductive and non-reproductive animals include males and females from [17]. (D–E, G–H) Data plotted as mean threshold ± 95% confidence intervals. See Supplemental Figure S1 for further information on micro-CT 3D reconstructions on saccule volume and frequency-dependence of toxin effects.
Pharmacological reduction of BK currents replicates natural range of seasonal hair cell plasticity
Frequency tuning by hair cells in non-mammals depends on electrical resonance, with increased BK abundance driving elevated resonant frequency [12]. Given the essential role of BK currents in hair cell tuning [12], we hypothesized that reducing BK channel availability could increase hair cell response thresholds in fish exhibiting a reproductive auditory phenotype, thus inducing a non-reproductive phenotype (Figure 1C). Evoked saccular potentials, a measure of auditory hair cell resonance in fish [20], were recorded as previously [17] from the saccule following iontophoretic injection of a potassium channel antagonist or corresponding vehicle into the saccule, or no injection (non-perturbed control). Each compound was dissolved in an artificial endolymph solution based on ionic composition of endolymph in a closely related toadfish, a species in the same family [21]. Antagonist concentrations here and below reflect electrode concentrations and not the substantively lower effective concentration following diffusion into the saccular endolymph (see Supplemental Experimental Procedures and Figure S1A–C for discussion of injection methods, and estimates of maximum antagonist concentrations within the saccule based on saccule volume determined with micro-CT imaging and 3D reconstruction). Animals in reproductive condition were collected during the summer breeding season from nest sites, whereas non-reproductive animals were collected from offshore sites during non-breeding months (see Supplemental Experimental Procedures) [15, 17]. As in prior studies, multiple characters were used to confirm reproductive state including visual inspection of gonad size and the enlarged, highly vascularized vocal swim bladder muscle of nest-building males, a secondary sex characteristic induced by elevated plasma androgen levels just prior to and during reproductive months (see Supplemental Experimental Procedures for measures) [15, 17].
We first confirmed that thresholds are potassium channel-dependent by making iontophoretic injections of the broad-spectrum potassium channel antagonist tetraethylammonium chloride (TEA, Sigma) into one saccule of reproductive males. Given the lack of sex-specific patterns of seasonal shifts in frequency encoding by the saccule [17], we used males for all neurophysiology experiments given their greater year-round availability [15, 17]. TEA transformed frequency encoding from reproductive into non-reproductive phenotypes, with thresholds increasing more with higher frequency for 1 M TEA (Figure 1D, main effect: P < 0.0001, frequency effect: P < 0.003) (see Supplemental Experimental Procedures for all statistical tests). Both 1 M and 10 M TEA pipette concentrations produced significant threshold increases (P < 0.0001) compared to vehicle and no injection controls (Figure 1D); control groups did not differ (P = 0.95). 10 M TEA had a significantly greater effect than 1 M TEA (P = 0.0072). TEA-dependent shifts were reversible, with thresholds returning to levels comparable to non-injection controls (i.e., the reproductive phenotype) in approximately 1 h (Figure S1D).
Once a general potassium channel mechanism was established with TEA, we selectively blocked BK channels via iontophoresis of the BK-specific antagonist iberiotoxin (IbTx, Tocris, Figure 1E) [22]. Like TEA, IbTx increased thresholds in a frequency-dependent manner (main effect: P < 0.0001, frequency effect: P < 0.0001) at 100 μM (P = 0.0005) and 500 μM (P = 0.0003), but not at a 10 μM (P = 0.13) pipette concentration compared to vehicle and non-injected controls; control groups did not differ (P = 0.98). 100 μM and 500 μM IbTx effects were similar (Figure 1E, P = 0.99) and neither was different from 1 M TEA (P = 0.17, 0.12, respectively). These experiments showed that a BK-specific blocker closely mimicked the natural seasonal transformation in frequency encoding (Figure 1F–H).
BK channel antagonists replicate frequency dependence of seasonal hair cell plasticity
Seasonal hair cell plasticity in midshipman is frequency-dependent with threshold differences greatest at frequencies above 145 Hz (Figure 1F), overlapping the upper harmonics of male calls (Figure 1B) [17]. BK channel antagonists produced similar frequency-dependent effects in reproductive individuals (Figure S1E and F). As with reproductive to non-reproductive transformations, 1 M TEA (P < 0.0001), and 100 μM IbTx (P = 0.0002) and 500 μM IbTx (P = 0.006) pipette concentrations, produced threshold shifts compared to vehicle controls that were greater at frequencies above 145 Hz (Figure 1D–F). Both 1 M TEA (Figure 1G) and 500 μM IbTx (Figure 1H) produced shifts significantly different from reproductive (TEA, P = 0.0002; IbTx, P < 0.05), but not from non-reproductive (TEA, P = 0.56; IbTx, P = 0.27), phenotypes. These findings further demonstrated that selective blocking of BK channels mimicked seasonal transformations in frequency encoding by auditory hair cells.
High, 10 M pipette doses of the broad-spectrum K channel antagonist TEA were able to induce changes in threshold beyond the natural range of plasticity (Figure 1D). Threshold shifts were larger at frequencies below 145 Hz, compared to those above 145 Hz (Figure S1E). This pattern was the reverse of the threshold-frequency relationship observed for the natural seasonal variation and IbTx’s effects (Figure S1F). The results suggest that while BK channels contribute to thresholds across the natural range of plasticity, other TEA-sensitive K channels [23] contribute to sculpting hair cell thresholds [12] but not substantially to seasonal variation.
Duplicate BK channel transcripts are upregulated in hair cells when thresholds are lowest
Since fluctuations in hair cell thresholds could be largely accounted for by levels of BK activity, we hypothesized that BK abundance would exhibit seasonal variation. The highly conserved pore-forming α-subunits of BK channels are encoded by duplicate slo1 genes in midshipman and other fishes [24]. BK expression, revealed by an antibody raised to a conserved region encoded by slo1a and slo1b (Figure S2A and B), was robust in hair cells and weak, if not entirely absent, in nearby ganglion cells (Figure 2A). Thus, as described below, measures of slo1a and slo1b transcript abundance in RNA isolated from whole saccules largely, if not entirely, represent transcript abundance contributed by hair cells. BK immunoreactivity was concentrated at apical neck and basal surfaces (small arrows, Figure 2A insert), which contrasts with frogs [25] and birds [26] that show BK channels expressed in an apical (low) to basal (high) gradient. Preadsorption controls showed no immunolabeling of hair cells (Figure S2C–E), indicating that the pattern of BK immunoreactivity is specific. Future paired patch recordings of focal membrane currents [25] may clarify subcellular distribution of BK channels within midshipman hair cells.
Figure 2.
BK potassium channel expression in auditory epithelium (saccule). (A) BK immunoreactivity in saccular hair cells, but not adjacent ganglion cells; apical surface at top. Controls (not shown) included no primary antibody and primary antibody preadsorption. Scale bar is 50 μm. Inset: Photographic exposure was adjusted so the no primary controls produced no visual signal. BK immunoreactivity was still concentrated at the apical neck and basal (small arrows) surfaces, but lacked dense label near the very apical end, indicative of cuticular plate staining artifact in the larger image in A. Scale bar is 12.5 μm for inset. Depth of basal surface varies with hair cell length along epithelium [44]. See Supplemental Figure S2 for further information on BK antibody. (B) Normalized mRNA levels significantly upregulated for slo1a (n = 4) and slo1b (n = 5) in reproductive animals compared to non-reproductives (n = 5). * P < 0.05, ** P < 0.0001 Data plotted as mean normalized expression level ± standard error on the mean (SEM).
Quantitative real time polymerase chain reaction (qPCR) showed that both slo1a (P = 0.017) and slo1b (P = 0.0008) mRNA transcript expression were significantly upregulated in the saccule of reproductive relative to non-reproductive males (Figure 2B). These qPCR results are of a similar magnitude to the reproductive state-dependent difference in transcript abundance previously observed in a pilot study of females [personal communication, K. N. Rohmann]. Hence, slo1 abundance is predictive of the seasonal phenotype of hair cell physiology (Figure 1C). Although we did not quantify BK protein levels, BK α-subunit transcript levels are correlated with protein expression in chick cochlear hair cells [27]. BK abundance could influence apoptotic-dependent events [28], thereby modifying hair cell abundance that varies seasonally in the midshipman saccule [29]. Although increased hair cell number could contribute to increased slo1 abundance, the magnitude of seasonal variation in slo1 transcript levels (Figure 2B) far exceeds that of saccular hair cell density [29]. As we show below, slo1 transcript levels alone can account for individual patterns of auditory tuning.
BK slo1a and slo1b abundance varies with hair cell thresholds between individuals
To establish that BK transcript abundance can account for individual physiological phenotypes, we investigated slo1 expression in fish from which auditory threshold tuning curves were previously reported [17] (see Supplemental Experimental Procedures for detailed explanation of statistical analyses). Only females were used here due to the greater availability of females for these experiments. The use of females in this subset of experiments, as of males for neurophysiology (see earlier comment), should not impact our results because seasonal differences in both physiology [17] and gene expression (see above) are not sexually dimorphic. Figure 3 shows each threshold tuning curve color coded according to ordinal ranking of slo1a and slo1b normalized transcript expression levels across all individuals. While normalized slo1 levels were used for statistical analyses, expression levels were ranked to more clearly illustrate the results. In some cases, more than one tuning curve was collected from an individual and all curves are presented. The slo1a and slo1b transcript levels did not correlate with one another (P = 0.79). Both slo1a (P = 0.01) and slo1b (P = 0.03) accounted for significant variability in thresholds among non-reproductive animals; hair cell epithelia with the highest slo1a (Figure 3A) and slo1b (Figure 3B) transcript abundances generally had the lowest thresholds. In contrast, only slo1b abundance (P = 0.02) accounted for threshold variability among reproductive individuals (slo1a, P = 0.37). Accounting for seasonal identity, the expression level of slo1b (P = 0.03), but not slo1a (P = 0.32), maintained a significant relationship to threshold, implying an enhanced role for slo1b in determining individual auditory hair cell threshold.
Figure 3.
Potassium channel, BK, expression varies with hair cell thresholds. (A) slo1a and (B) slo1b expression levels were ordinally ranked across all individuals including 6 non-reproductive (circles) and 11 reproductive (squares) animals. Ranked expression levels were then assigned to a color map with the lowest expression level (#1) shown as blue up to the highest expression level (#17) shown as red. While normalized expression levels were used for statistical analyses, the ranked color map was chosen to better illustrate the results in a four dimensional data space. One or more sets of thresholds were recorded per individual (example: left and right saccule, caudal and rostral positions along the same saccule): 14 non-reproductive and 23 reproductive [shaded background] animals. Adjacent recordings with similar length tick marks come from the same animal; adjacent recordings in which the tick marks differ in length come from two different animals. Only females were used here due to greater sample size of epithelia; physiology data for these fish from [17].
These results, together with our study showing tissue-specific expression and alternative splicing [24], support subfunctionalization as a mechanism favoring the evolution and retention of slo1 co-orthologues. Unlike different muscle types that present a clear case of tissue-specific subfunctionalization via loss of either slo1a or slo1b expression [24], the subfunctionalization of slo1 genes in saccular epithelium is revealed in divergent coupling to seasonal changes in hair cell physiology rather than in the pattern of expression. This suggests that even within tissues in which both slo1 genes are expressed, these genes can undergo differential regulation. Further experiments will be needed to determine if differences in slo1a and slo1b coding regions produce channels with functionally distinct properties such as gating kinetics and calcium and voltage sensitivity, as observed for different slo1 splice variants in tetrapods [12]. These and related mechanisms, in turn, may be sensitive to hormonal influences that have been linked to changes in auditory frequency sensitivity in non-mammals and humans [30–33].
BK channel expression is one mechanism that could explain shifts in frequency-dependent hair cell sensitivity, whether tuning is dependent on electrical resonance as in fish and other non-mammals or on mechanical adaptations as in mammals [12] where BK currents are still a primary outward current in many inner [13, 34, 35] and outer [14, 36] hair cells. BK currents underlie both the alternating and direct current components of hair cell membrane potentials [37]. Thus, a single cell type can encode both fine (frequency) and gross (duration) temporal structure of acoustic signals. In non-mammals, increased BK expression correlates with higher electrical resonant frequency [12] and larger amplitude BK currents [38] (Figure 1C). In our experiments, pharmacologically reducing BK currents had the greatest effects at higher frequencies where hair cells expressing more BK channels would be more susceptible to antagonist effects. Within the range of frequencies studied, BK channels are a primary source of large amplitude, rapid outward current in fishes and other vertebrates, including mammals [12–14, 39]. Variation in BK channel abundance may thus be a common mechanism for regulating high frequency auditory encoding across vertebrate taxa.
Reproductive imperatives rely on mechanisms enabling the successful performance of mating and related behaviors, including those dependent on acoustic communication [1–3, 6, 7, 40–42]. Here, we show that changes in the abundance of a single family of potassium channels can regulate auditory receptor sensitivity that enhances frequency encoding of vocalizations by the peripheral auditory system during the breeding season. Though our studies focused on BK channels as a central node of hair cell intrinsic properties, our results do not exclude complementary changes in other determinants of hair cell resonance such as BK channel beta subunits and calcium and other potassium channels [12]. Plasticity in frequency encoding by hair cells over the time span of seasonal shifts investigated here, as well as over longer evolutionary time scales for species isolation and sexual selection [6, 42], may depend on the same deeply conserved molecular mechanisms. Ion channels may thus be selected as evolutionarily dissociable units sculpting mechanisms of stimulus filtering that bias the detection and encoding of behaviorally relevant stimuli.
Experimental Procedures
For detailed experimental procedures, please see Supplemental Experimental Procedures.
Animals
Midshipman fish have two male morphs. Type I males build nests and acoustically court females; type II males only sneak-spawn [16]. Because comparable seasonal auditory hair cell plasticity has been demonstrated in type I males and females [17], both type I males and females were used throughout this study based on availability. Type I males were used in the physiology and qPCR studies presented in Figures 1 and 2. Saccular epithelia for comparison of slo1 expression with saccular physiology (see Figure 3) were taken from 17 females used in a previous physiological study [17]. The Institutional Animal Care and Use Committee at Cornell University approved all methods.
Immunohistochemistry
A midshipman specific BK channel antibody was generated. Peptide synthesis and rabbit polyclonal antibody production was performed by Pocono Rabbit Farm and Laboratory (Canadensis, PA). Fluorescent immunohistochemistry was performed on sectioned saccular epithelia immersion fixed in 4% paraformaldehyde.
Real-Time PCR
Methods were adapted from those previously used in our laboratory [43]. Relative absolute (standard curve method) quantitative real-time PCR (qPCR) was conducted on cDNA derived from ear RNA using gene specific primer pairs using an Applied Biosystems Viia7 Real-Time PCR System. Primers were made by Integrated DNA Technologies (Coralville, IA, USA) and designed using their web-based primer design tools.
Statistical Analyses
All statistical analyses were performed using JMP 9 pro (S.A.S. Institute Inc., Cary, NC, USA). Physiology data were analyzed as described previously [17]. An ANCOVA was used to compare frequency dependence of threshold changes occurring both naturally and by different pharmacologic manipulations. qPCR data were analyzed with an ANOVA as described previously [43]. Please see the Supplemental Experimental Procedures for a detailed explanation of statistical analyses conducted for each set of experiments.
Supplementary Material
Highlights.
BK channel antagonists replicate natural non-reproductive auditory physiology.
BK channel transcript abundance changes seasonally in auditory epithelium.
BK channel transcript abundance correlates with individual auditory physiology.
Acknowledgments
We thank B. Chagnaud, D. Deitcher, R. Genova, B. Land, M. Marchaterre, H. Menninger, A. Rice and Cornell University Statistical Consulting Unit for technical assistance; M. Riccio of the Cornell Institute of Biotechnology, Ithaca, NY for the micro-CT scans, 3D reconstructions and saccule morphometry; N. Place, E. Adkins-Regan, H. Greene for references; M. Nelson for Figure 1C; R. Baker, P. Fuchs, D. McCobb, 3 anonymous reviewers and especially R. Hoy for comments on earlier versions of the manuscript. Support from NIH training grant (GM007469, KNR), individual NRSA (DC009941, KNR) and research grant (DC00092, AHB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hau M, Perfito N, Moore IT. Timing of breeding in tropical birds: Mechanisms and evolutionary implications. Ornitologia Neotropical. 2008;19:39–59. [Google Scholar]
- 2.Migaud H, Davie A, Taylor JF. Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J Fish Biol. 2010;76:27–68. doi: 10.1111/j.1095-8649.2009.02500.x. [DOI] [PubMed] [Google Scholar]
- 3.Nelson RJ. An Introduction to Behavioral Endocrinology. 4. Sunderland, MA: Sinauer Associates; 2011. [Google Scholar]
- 4.Miranda JA, Liu RC. Dissecting natural sensory plasticity: Hormones and experience in a maternal context. Hear Res. 2009;252:21–28. doi: 10.1016/j.heares.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walpurger V, Pietrowsky R, Kirschbaum C, Wolf OT. Effects of the menstrual cycle on auditory event-related potentials. Horm Behav. 2004;46:600–606. doi: 10.1016/j.yhbeh.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Ryan MJ, Perrill SA, Wilczynski W. Auditory tuning and call frequency predict population-based mating preferences in the cricket frog, Acris crepitans. Am Nat. 1992;139:1370–1383. [Google Scholar]
- 7.Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. J Neurosci. 2003;23:1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillery CM. Seasonality of two midbrain auditory responses in the treefrog, Hyla chrysoscelis. Copeia. 1984;1984:844–852. [Google Scholar]
- 9.Walkowiak W. The coding of auditory signals in the torus semicircularis of the fire-bellied toad and the grass frog: Responses to simple stimuli and to conspecific calls. J Comp Physiol [A] 1980;138:131–148. [Google Scholar]
- 10.Miranda J, Wilczynski W. Female reproductive state influences the auditory midbrain response. J Comp Physiol [A] 2009;195:341–349. doi: 10.1007/s00359-008-0410-7. [DOI] [PubMed] [Google Scholar]
- 11.Gall MD, Salameh TS, Lucas JR. Songbird frequency selectivity and temporal resolution vary with sex and season. Proc R Soc Lond B Biol Sci. 2013:280. doi: 10.1098/rspb.2012.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- 13.Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- 14.Wersinger E, McLean WJ, Fuchs PA, Pyott SJ. BK channels mediate cholinergic inhibition of high frequency cochlear hair cells. PLoS ONE. 2010;5:e13836. doi: 10.1371/journal.pone.0013836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bass AH. Shaping brain sexuality. Am Sci. 1996;84:352–363. [Google Scholar]
- 16.Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- 17.Rohmann KN, Bass AH. Seasonal plasticity of auditory hair cell frequency sensitivity correlates with plasma steroid levels in vocal fish. J Exp Biol. 2011;214:1931–1942. doi: 10.1242/jeb.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sisneros JA. Seasonal plasticity of auditory saccular sensitivity in the vocal plainfin midshipman fish, Porichthys notatus. J Neurophysiol. 2009;102:1121–1131. doi: 10.1152/jn.00236.2009. [DOI] [PubMed] [Google Scholar]
- 19.Bass AH, Clark CW. The physical acoustics of underwater sound communication. In: Simmons AM, Fay RR, Popper AN, editors. Spring handbook of auditory research: Acoustic Communication. New York: Springer; 2003. pp. 15–64. [Google Scholar]
- 20.Furukawa T, Ishii Y. Neurophysiological studies on hearing in goldfish. J Neurophysiol. 1967;30:1377–1403. doi: 10.1152/jn.1967.30.6.1377. [DOI] [PubMed] [Google Scholar]
- 21.Ghanem TA, Breneman KD, Rabbitt RD, Brown HM. Ionic composition of endolymph and perilymph in the inner ear of the oyster toadfish, Opsanus tau. Biol Bull. 2008;214:83–90. doi: 10.2307/25066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- 23.Kaczmarek LK, Levitan IB. Neuromodulation: The Biochemical Control of Neuronal Excitability. New York, NY: Oxford Uiversity Press, Inc; 1987. [Google Scholar]
- 24.Rohmann KN, Deitcher DL, Bass AH. Calcium-activated potassium (BK) channels are encoded by duplicate slo1 genes in teleost fishes. Mol Biol Evol. 2009;26:1509–1521. doi: 10.1093/molbev/msp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts W, Jacobs R, Hudspeth A. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samaranayake H, Saunders JC, Greene MI, Navaratnam DS. Ca2+ and K+ (BK) channels in chick hair cells are clustered and colocalized with apical–basal and tonotopic gradients. J Physiol. 2004;560:13–20. doi: 10.1113/jphysiol.2004.069856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Atkin GM, Morales MM, Liu LQ, Tong M, Duncan RK. Developmental expression of BK channels in chick cochlear hair cells. BMC Dev Biol. 2009;9:67. doi: 10.1186/1471-213X-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokolowski B, Orchard S, Harvey M, Sridhar S, Sakai Y. Conserved BK channel-protein interactions reveal signals relevant to cell death and survival. PLoS ONE. 2011;6:e28532. doi: 10.1371/journal.pone.0028532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffin AB, Mohr RA, Sisneros JA. Saccular-specific hair cell addition correlates with reproductive state-dependent changes in the auditory saccular sensitivity of a vocal fish. J Neurosci. 2012;32:1366–1376. doi: 10.1523/JNEUROSCI.4928-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caras ML, Brenowitz E, Rubel EW. Peripheral auditory processing changes seasonally in Gambel’s white-crowned sparrow. J Comp Physiol [A] 2010;196:581–599. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- 32.Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. 2006;126:10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- 33.Guimaraes P, Frisina ST, Mapes F, Tadros SF, Frisina DR, Frisina RD. Progestin negatively affects hearing in aged women. Proc Natl Acad Sci U S A. 2006;103:14246–14249. doi: 10.1073/pnas.0606891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skinner LJ, Enee V, Beurg M, Jung HH, Ryan AF, Hafidi A, Aran JM, Dulon D. Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the Guinea pig cochlea. J Neurophysiol. 2003;90:320–332. doi: 10.1152/jn.01155.2002. [DOI] [PubMed] [Google Scholar]
- 35.Oliver D, Taberner AM, Thurm H, Sausbier M, Arntz C, Ruth P, Fakler B, Liberman MC. The role of BKCa channels in electrical signal encoding in the mammalian auditory periphery. J Neurosci. 2006;26:6181–6189. doi: 10.1523/JNEUROSCI.1047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, et al. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci U S A. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell IJ, Sellick PM. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Art JJ, Crawford AC, Fettiplace R. Electrical resonance and membrane currents in turtle cochlear hair cells. Hear Res. 1986;22:31–36. doi: 10.1016/0378-5955(86)90073-0. [DOI] [PubMed] [Google Scholar]
- 39.Steinacker A, Romero A. Characterization of voltage-gated and calcium-activated potassium currents in toadfish saccular hair cells. Brain Res. 1991;556:22–32. doi: 10.1016/0006-8993(91)90543-5. [DOI] [PubMed] [Google Scholar]
- 40.Brenowitz EA. Plasticity of the adult avian song control system. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. xvi. New York: Cambridge University Press; 2008. p. 550. [Google Scholar]
- 41.Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. New York: Academic Press; 2009. [Google Scholar]
- 42.Gerhardt HC. Recognition of spectral patterns in the green treefrog - neurobiology and evolution. Exp Biol. 1986;45:167–178. [PubMed] [Google Scholar]
- 43.Arterbery AS, Deitcher DL, Bass AH. Corticosteroid receptor expression in a teleost fish that displays alternative male reproductive tactics. Gen Comp Endocrinol. 2010;165:83–90. doi: 10.1016/j.ygcen.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanford PJ, Platt C, Popper AN. Structure and function in the saccule of the goldfish (Carassius auratus): a model of diversity in the non-amniote ear. Hear Res. 2000;143:1–13. doi: 10.1016/s0378-5955(00)00015-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.