Abstract
The power of the adaptive immune system to identify novel antigens depends on the ability of lymphocytes to create antigen receptors with diverse antigen-binding sites. For immunoglobulins, CDR-H3 lies at the center of the antigen binding site where it often plays a key role in antigen binding. It is created de novo by VDJ rearrangement and is thus the focus for rearrangement-dependent diversity. CDR-H3 is biased for the inclusion of tyrosine. In seeking to identify the mechanisms controlling CDR-H3 amino acid content, we observed that the coding sequence of DH gene segments demonstrate conservation of reading frame-specific sequence motifs, with RF1 enriched for tyrosine and depleted of hydrophobic and charged amino acids. Use of DH RF1 in functional VDJ transcripts is preferred from the earliest stages of B cell development, ‘pushing’ CDR-H3 to include specific categories of tyrosine enriched antigen binding sites. With development and maturation, the composition of the CDR-H3 repertoire appears to be ‘pulled’ into a more refined specific range. Forcing the use of alternative DH reading frames by means of gene targeting alters the expressed repertoire, enriching alternative sequence categories. This change in the repertoire variably affects antibody production and the development of specific B cell subsets.
Keywords: Immunoglobulin, Diversity Gene Segment, Antibody Repertoire, B cell Development
CDR-H3 plays a key role in defining the antigen binding characteristics of its immunoglobulin
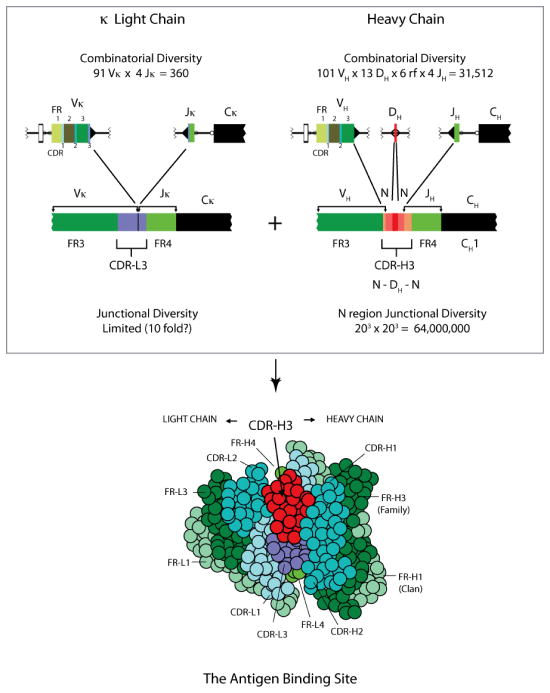
Immunoglobulin (Ig), the B cell antigen receptor (BCR), is a heterodimeric molecule composed of two heavy (H) and two light (L) chains (5–7). Each H and L chain consists of a variable (V) domain, which binds antigen; and one to four constant (C) domains, which carry out the effector function of that chain. Diversity is asymmetrically distributed within the V domain with each V containing of three intervals of hypervariability, termed complementarity determining regions (CDRs), and four intervals of conserved sequence, termed framework regions (FRs) (Figure 1). The four FRs of the H chain and the four FRs of the L chain fold to form the scaffold that brings together the three H chain and three L chain CDRs to create the antigen binding site, as classically defined.
Figure 1. CDR-H3 plays a key role in antigen binding site diversity.
Top. The variable domains of the L and H chains are created by VJ joining, and by VDJ joining and N addition, respectively. Due to the inclusion of a D gene segment, the opportunity to introduce two sets of N nucleotide additions, and the greater flexibility in length and sequence composition, the CDR-H3 interval is the most diverse portion of the pre-immune repertoire [reviewed in (25)]. Bottom. A cartoon of the classic antigen-binding site as seen head-on. Due its central location, most bound antigens will interact with CDR-H3, including its DH component.
Evolutionary comparisons of Ig sequences within and across species have shown that the three dimensional structure of the V domain represents a gradient of diversity. The hydrophobic core of the V domain, which consists of FR2 and FR4, is highly conserved. The sequence of FR1 is also highly conserved (7). FR1s can take three different basic shapes that identify the evolutionary clan of origin (8). V gene segment family identity is determined by the sequence of FR3. There are 16 basic family structures in mouse, and 7 in human (9). The FR3s border the antigen binding site, both supporting and confining the shape of CDR1 and 2. CDRs -H1, -H2, -L1, and -L2, are entirely encoded by their respective V gene segments and are initially limited to germline sequence. CDR- L3 and –H3 are created de novo by VL→JL and VH→DH→JH joining, respectively.
Although there can be great variation in the sequence and size of these five CDRs (CDR-H1, H2, L1, L2 and L3), they form a rather small set of main-chain conformations that are termed canonical structures (10–13). Each such structure is determined by the size of the loop and by the presence of certain residues at key positions in both the loop and framework 4 regions. For example, three canonical structure types have been identified for CDR-H1, four for CDR-H2, five for CDR-L1, one type for CDR-L2, and five types for CDR-L3 (10,11,14). Assuming random assortment, we would expect the repertoire to contain three hundred different combinations of these canonical structures (15). However, only ten of these combinations account for seven-eighths of human and mouse Fab sequences. Thus, by both sequence and structure, the diversity provided by these five germline-encoded CDRs is even more distinctly finite than first appreciated even prior to antigen driven selection
Due to the inclusion of a diversity (D) gene segment and the addition of non-germline encoded nucleotides (N regions) CDR-H3 is by far the most variable of the six CDRs (Figure 1). Enhanced diversity and a central position within the antigen binding site permits CDR-H3 to often play the most critical role in antigen recognition and binding (5,6,16). It is for this reason that CDR-H3 has become a major focus of our studies.
Defining a ‘normal’ range for CDR-H3 diversity
Although highly variable, our comparisons of Ig repertoires between and within species had led us to the hypothesis that young adults might be programmed to express a preferred range of CDR-H3 sequences and structures. The potential diversity of CDR-H3 is so great that it might seem presumptuous to expect that we could use economical methods to identify such conserved features. However, our comparative studies had revealed molecular characteristics of CDR-H3 repertoires that appeared to permit recognition of categorical restrictions in diversity after examination of as few as 100 sequences per developmental stage. These characteristics included V, D, J gene segment usage, the extent of N addition and the length, global amino acid content and average hydrophobicity of CDR-H3 (Figure 2).
Figure 2. Deconstruction and analysis of CDR-H3.
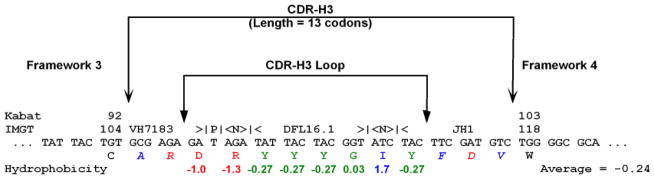
In this hypothetical sequence, the location of CDR-H3, the CDR-H3 loop, and boundaries of FRs 3 and 4 are shown. Kabat and IMGT (5,76) number designations for the TGT codon which marks the terminus of framework 3 and the TGG which marks the beginning of framework 4 are identified. Here the CDR-3 loop has been evaluated for the distribution of individual amino acids and average Kyte-Doolittle hydrophobicity (77,78). Amino acids at the extreme (arginine and isoleucine) have been included to demonstrate the range of the hydrophobicity index. The normalized average hydrophobicity of this CDR-H3 loop is −0.24. This CDR-H3 has also been evaluated for VH, DH, and JH usage, P junctions, N addition, and the length of CDR-H3 in codons. A single palindromic (P) nucleotide flanks the VH sequence. DH DFL16.1 sequence is flanked by three nucleotides of N addition on each side. To facilitate analysis, we have color-coded our data in this and other figures in this application to report relative hydrophobicity. Blue reflects hydrophobicity, green represents neutrality with or without hydrophilicity, and red is used for charge.
Development of the CDR-H3 repertoire is marked by focusing of constraints in length, amino acid utilization, and charge
Construction of CDR-H3 begins early in B cell progenitors. Indeed, the various defined stages of B cell development can be viewed, in part, as transitions through a series of checkpoints that test the assembly and function of CDR-H3 (17–22). In human, the selection during B cell development is associated with a reduction in the mean length of the expressed CDR-H3 repertoire (23), as well as in decreased frequency of highly charged or hydrophobic sequences (24–26).
In order to gain insight into the mechanisms that regulate the antibody repertoire, to determine when during development constraints on CDR-H3 composition are imposed, and to establish the extent to which murine development resembles that of human, we conducted a detailed examination of CDR-H3 repertoire development in BALB/c mice. We used the scheme of Hardy (18) to sort bone marrow B lineage cells into progenitor, immature, and mature B cell fractions (27). We chose to look at RNA message as this is most representative of the expressed, and thus functional, Ig repertoire. We cloned, sequenced, and deconstructed the CDR-H3 component of VH7183DJCμ transcripts (27). Subsequently, we used the same cloning techniques to examine CDR-H3 repertoire development in the spleen, focusing on splenic T1 (Loder) (28), marginal zone (MZ), and follicular (FO) subsets (29–31). We concentrated on the VH7183 family because its germline complement in IgHa alleles had been well-defined (32), it represents a manageable 10% of the active repertoire (33), patterns of VH7183 utilization during ontogeny and development have been well-established (32,34,35), and it contributes to both self and non-self reactivities [reviewed in (7)].
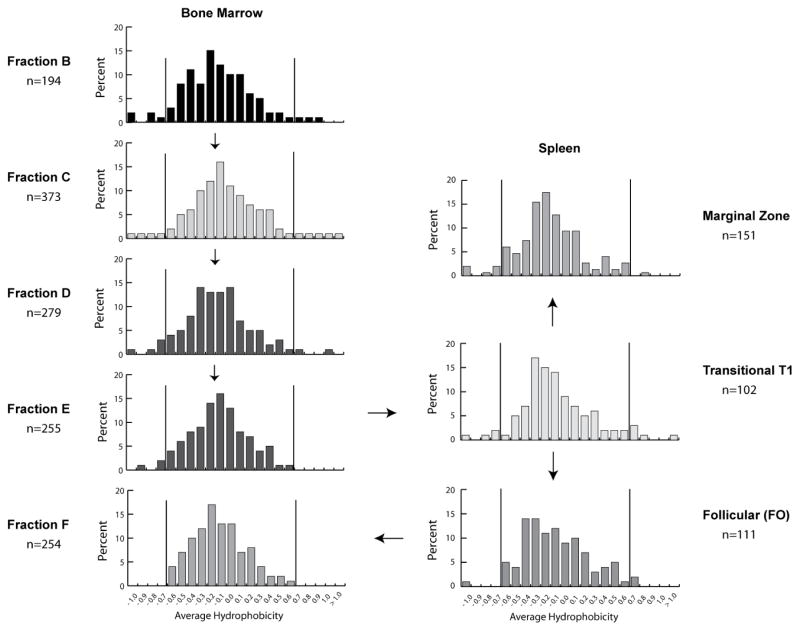
In wild type BALB/c mice, we found that the variance in the studied parameters in both spleen and bone marrow decreased as the developing B cells passed through successive differentiation checkpoints [Figure 3, (23,25,27,36)]. As in our previous studies in human, we found that the distribution of gene segment usage, lengths, global amino acid content, and average hydrophobicity exhibited a specific, controlled distribution at the earliest stage of B cell development evaluated (Fraction B), which is prior to the surface expression of immunoglobulin. We observed a consistent enrichment for tyrosine and glycine in CDR-H3, and an apparent selection against both positively charged and hydrophobic amino acids (Figure 4, bottom). In cross-species studies, we found that the bias for tyrosine and glycine in CDR-H3 was common to all jawed vertebrates [Table 1, (29)].
Figure 3.
Distribution of CDR-H3 charge in VDJCμ transcripts of WT BALB/c mice isolated from phenotypically defined bone marrow and spleen B cell populations as assessed 30 by reference to a normalized Kyte-Doolittle scale (79,80). Prevalence is reported as the percent of the sequenced population of unique, in-frame, open transcripts from each B lineage subset. To facilitate visualization of the change in variance of the distribution, the vertical lines mark the apparent normal boundaries beyond which it appears to be difficult to transition into fraction F.
Figure 4. D-limited mice express polyclonal, altered CDR-H3 repertoires.
Prevalence is reported as the percent of the sequenced population of unique, in-frame, open transcripts from each B lineage fraction. (Right) Distribution of CDR-H3 average hydrophobicity in VDJCμ transcripts from CD19+IgM+IgD+ mature, recirculating bone marrow B cells from homozygous ΔD-DFL, ΔD-DμFS, ΔD-iD, and wild type (wt) mice. The normalized Kyte-Doolittle hydrophobicity scale (78) has been used to calculate average hydrophobicity. To facilitate visualization of the change in distribution, the vertical lines mark the preferred range average hydrophobicity observed in wild-type fraction F [Figure 3, (27)]. (Left) Distribution of amino acids in the CDR-H3 loop as a function of B cell development in the same strains of mice. Amino acids are arranged by polarity from arginine (left) to isoleucine (right). The number of sequences per B cell fraction is shown on the far left.
Table 1.
The representation of amino acids by DH reading frame is non-random and conserved.
| Species | Shark | Mouse | Human | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Gene Segment & Reading Frame (RF) | DH1 | Average Hydropathicity | DFL16.1 | Average Hydropathicity | D3-22 | Average Hydropathicity |
|
| ||||||
| RF by Deletion | ||||||
| RF1 (Hydrophilic) | YYSGY | −0.18 | YYYGSSY | −0.18 | YYYDSSGYYY | −0.28 |
| RF2 (Hydrophobic) | VLNWV | 0.69 | FITTVVA | 0.95 | ITMIVVVIT | 1.17 |
| RF3 (Hydrophobic & Termination) | GTTVG | 0.30 | LLLR**L | 0.86 | VLL***WLLL | 1.18 |
| RF by Inversion | ||||||
| i-RF1 (Charged) | THCST | −0.03 | SYYRSNK | −0.59 | SNNHYYHSN | −0.57 |
| i-RF2 (Hydrophobic) | IPTVV | 0.96 | VATTVVI | 1.02 | VVITTTIIVI | 1.08 |
| i-RF3 (Hydrophobic & Termination) | YPL*Y | 0.21 | *LLP*** | 0.92 | ***PLLS**Y | 0.67 |
Shown are the amino acid sequences of each of the six reading frames for the DH1 from Heterodontus (Litman) (25), DFL16.1 from mouse (26), and D3-22 from human (27). The average hydropathicity of each reading frame has been calculated as described below. In all three species, one reading frame is employed preferentially (RF1 by deletion) such that the HCDR3 is conserved to be slightly hydrophilic and enriched for aromatic amino acids.
Geneticists and evolutionary biologists distinguish between selection at the species level, which reflects reproductive fitness and is thus targeted to the germline in order to be transmitted from parent to child, and selection at the level of the individual cell, which reflects fitness in the local environment of that cell and is typically not transmitted to the child. The former is referred to as natural selection and is the operating principle in the evolution of germline sequence content. The latter is referred to as somatic selection, with changes that may be transmitted from progenitor to daughter cell, but will not be transmitted to the next generation of the species. Thus, the conservation of CDR-H3 tyrosine content suggested to us that CDR-H3 sequence content might be the product of natural selection of germline sequence, as well as the clonal somatic selection that operates in the individual in response to local antigenic exposure. In support of this hypothesis, our comparative studies revealed that the preference for tyrosine in the CDR-H3 repertoire of adult B cells mirrored the preferential use of DH gene segments with tyrosine-enriched RF1s (Figure 5).
Figure 5.

BALB/c DH RF1 amino acid sequences.
These observations led us to the hypothesis that natural selection of the DH germline repertoire might play a key role in controlling the global composition of CDR-H3 and thus of the (final) antibody repertoire.
Genetic control of the CDR-H3 repertoire
In order to test the role of germline control of DH sequence on regulating the composition of CDR-H3 we enlisted the aid of Dr. Klaus Rajewsky to create what we have come to term D-altered, or D-limited, mice (Figure 6). We created a targeting construct that allows the introduction of altered DH sequence into the DH locus at the 5′ position by means of homologous recombination. We introduce loxP sites downstream of the altered DH and just upstream of the JH locus. Either in vivo or in vitro cre-mediated deletion then creates an IgH allele that contains only the altered DH. Although deletion and manipulation of the DH locus have occurred, it must be emphasized that with the rest of the IgH locus is maintained in its normal, germline form. It contains a normal complement of VH, JH, and CH exons. The process of rearrangement occurs in the expected manner at the expected times in development. Following VDJ rearrangement, the amino acid contribution to CDR-H3 of the altered coding sequence of the DH locus is only signature of the gene targeting that remains in the functional allele. The B cell has access to the normal complement of L chains. Class switching to all isotypes is maintained, as well as the potential for somatic hypermutation. Thus, unlike a classic single antibody transgene mutant mouse, altering the sequence of the DH permits progenitor B cells to progress through all the normal checkpoints of B cell development while expressing polyclonal antibody repertoires that differ only by the contribution of the altered DH.
Figure 6.

Generation of a D-limited IgH allele.
Of the thirteen functional DH gene segments per haploid genome in BALB/c mice, twelve are derived from the same evolutionary progenitor and share extensive sequence similarity (Figure 5). This includes a preference for tyrosine in reading frame 1, the preferred reading frame. [The thirteenth DH gene segment, DQ52, does not encode tyrosine, but it contributes to less than five percent of rearrangements.]
To date we have access to four mutant IgH alleles (Figure 7): ΔDQ52, ΔD-DFL, ΔD-D μFS, and ΔD-iD. The first allele available to us, ΔDQ52, was created by our collaborators (37). The DQ52 gene segment has been deleted from this allele, but it retains the other 12 DH elements in germline form. On the other hand the ΔD in ΔD-DFL, ΔD-D μFS, and ΔD-iD reflects the deletion of 12 of the 13 DH gene segments in the IgH locus. We use DFL to signify that DFL16.1 in germline form has remained behind. We use DμFS to identify a DFL16.1 allele that includes two frameshift mutations, one of which places reading frame 1 in-frame with the upstream ATG start site and the other which places a termination codon near the terminus of reading frame 1, as well. Finally, we use the designation iD to identify the allele were the inverted sequence of DSP2.2 has replaced the central coding sequence of DFL16.1 (Figure 8). The ΔD-DFL allele limits the DH locus to the use of DFL16.1, which like the majority of the DH gene segments is enriched for tyrosine in RF1 (Figure 7). Both the ΔD-DFL and ΔDQ52 alleles contain fewer DH gene segments than normal, but the DH that remain(s) encode(s) normal, primarily tyrosine-enriched RF1 sequence. The ΔD-DμFS and ΔD-iD alleles are limited to a single DH, but unlike ΔD-DFL, the only expressed DH is forced to preferentially use an alternative reading frame enriched for valine (ΔD-DμFS) or uses the inverted DSP2.2 reading frame that codes for arginine (ΔD-iD) This review will focus on mice expressing the ΔD-DFL, ΔD-DμFS and ΔD-iD alleles.
Figure 7.
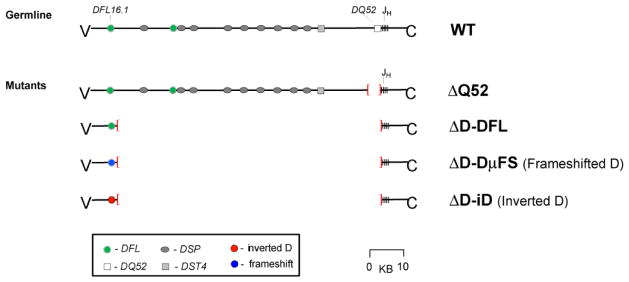
Four D-limited DH alleles.
Figure 8. Mutant and Control DH sequences.

(Top) Germline DFL16.1. (Middle) A Dμ frameshift in DFL16.1 replaces RF1 Y+G amino acids with RF2 V, T, I, and F (DμFS). (Bottom) Replacement of central RF1 Y+G codons with inverted DSP2.2 sequence introduces codons for positively charged R and H as well as polar N in a new RF1.
ΔD-DFL mice create a DFL16.1-specific CDR-H3 repertoire
DFL16.1 normally contributes to approximately 20% of VDJ rearrangements in BALB/c mice. Throughout bone marrow development, ΔD-DFL B lineage cells exhibited the same pattern of CDR-H3 tyrosine and glycine predominance with limited use of hydrophobic or charged amino acids that had been previously observed in wild-type BALB/c mice. In ΔD-DFL mice, the mature, recirculating B cell population (fraction F) pattern of amino acid utilization was equivalent to that observed in wild-type mice (Figure 4). However, ΔD-DFL CDR-H3s contained more neutral amino acids (serine), and fewer charged amino acids (aspartic acid and asparagine) than wild-type. Re-examination of the reading frame 1 sequences of the DH gene segments revealed that of the 13 BALB/c DH, DFL16.1 is the only one that encodes serine. We compared the prevalence of serine, aspartic acid, and asparagine in the ΔD-DFL repertoire to that observed in wild-type DFL16.1-containing sequences and found it to be similar. The nine wild-type BALB/c DSP DH gene segments, which normally constitute the majority of VDJ joins, can be separated into those that encode asparagine and those that encode aspartic acid in RF1. A re-examination of the wild-type CDR-H3 repertoire demonstrated that the relative use of asparagines and aspartic acid in DSP-containing V7183DJCμ transcripts was directly related to whether they used the asparagine-encoding or the aspartic acid-containing members of the DSP family. This asymmetric, DH-specific use of serine, aspartic acid, and asparagine by gene segment was established at the earliest stages of bone marrow B cell development studied (fraction B), and it remained relatively constant at the subsequent stages of B cell development up to and including fraction F.
We then compared the distribution of CDR-H3 length and average hydrophobicity of the ΔD-DFL CDR-H3 repertoire to that of the wild-type repertoire as a whole, as well as to that portion of the wild-type sequences that used DFL16.1, and to the other portion that did not include DFL16.1. DFL16.1 is six nucleotides longer than DSP gene segments, and 12 nucleotides longer than DQ52. Remarkably, DFL16.1 CDR-H3s averaged about two codons more than DSP-containing CDR-H3s and four codons longer than DQ52-containing CDR-H3s. At the earliest stages of B cell development examined, fractions B and C, the average length of ΔD-DFL CDR-H3 sequences was similar to that of the wild-type DFL16.1-containing sequences, but different from both the total wild-type and the non-DFL16.1-containing CDR-H3s. After B cells successfully pass through the step of interaction with surrogate light chain and begin to rearrange one of the light chain loci (fraction D), the average length of the ΔD-DFL CDR-H3 repertoire was similar to both total wild-type and non-DFL16.1 sequences. In contrast, the average ΔD-DFL CDR-H3 length differed from total wild-type and non-DFL16.1 CDR-H3s in the immature B cell (Fraction E) and fraction F populations. By fraction F the average ΔD-DFL CDR-H3 lengths was similar to wild type DFL 16.1. A similar, although not identical, situation occurred with regards to average hydrophobicity. The average hydrophobicity of these loops was similar to total wild-type and to non-DFL16.1-containing CDR-H3 in fractions B, achieved near identity in fraction C, differed in fraction D (p ≤ 0.01), converged toward the wild-type DFL16.1 average in fraction E, and then achieved equivalence with wild-type DFL16.1 in fraction F.
In summary, at early stages of B cell development, the ΔD-DFL repertoire approached the length or hydrophobicity characteristics of the wild-type repertoire as a whole, while still maintaining, or being ‘pushed’ by its germline sequence, to incorporate serine into CDR-H3. Upon successive maturation stages, the ΔD-DFL repertoire increasingly matched the length and hydrophobicity characteristics of that specific component of the wild-type repertoire normally created by the donor DFL16.1 gene segment while remaining distinct from that component of the repertoire that does not include DFL16.1. This suggested a critical role for antigen receptor-driven selection which appears to act by ‘pulling’ the repertoire into a donor DH-specific preferred range.
ΔD-DμFS mice create CDR-H3s enriched for DFL16.1-RF2 sequence
In theory, the inclusion of a D gene segment coupled with random insertion of N nucleotides should produce a CDR-H3 repertoire of random diversity. This permissive role of the D has been referred to as D-diversity (38). In practice, tyrosine and glycine are heavily overrepresented in CDR-H3, comprising 30–40% of the global amino acid content of this hypervariable interval. The tyrosine and glycine content of CDRH-3 reflects preferred use of DH reading frame 1 (25,27,29,39–44). This RF1 preference treats almost two-thirds of all DJ rearrangements with extreme prejudice, which, at first glance, appears quite wasteful. This led to the suggestion that the expression of DH in RF2 might be incompatible with effective antigen recognition and downstream B cell signaling, a concept referred to as Ddisaster (38).
Mechanistically, the preference for RF1 in BALB/c mice has been linked to RF-specific properties and sequence motifs that are shared among twelve of the thirteen DH gene segments. These include a predilection for rearrangement by deletion, the frequent occurrence of stop codons in RF3 which act to reduce the likelihood of creating an open reading frame among VDJ rearrangements that use RF3, a bias towards rearrangement at sites of sequence microhomology between the 5′ end of the JH and the3′ end of the DH which favor rearrangement into RF1, and an ATG start site upstream of RF2 that permits production of a truncated Dμ protein (41,45–48). The DQ52 gene segment is also preferentially rearranged by deletion, and it includes one neutral reading frame (RF1), one hydrophobic reading frame (RF2), and one highly charged reading frame (RF3). However, while RF1 uses glycine, so do RF2 and RF3; and none of the three RFs encode tyrosine. DQ52 also lacks an upstream ATG start site, its 3′ terminus does not share extensive sequence homology with the 5′ termini of the various JH, and it encodes a termination codon at the 5′ end of RF1. This DQ52 gene segment, which contributes to less than 5% of the adult repertoire, demonstrates more random RF usage.
Transgenic studies have shown that the bias against use of RF2 in DFL and DSP gene segments can be released when pre B cells are no longer able to produce membrane bound Dμ protein (49). This suggested that Dμ H chain protein could engage the mechanisms of allelic exclusion, thereby inhibiting subsequent V→DJ rearrangement. However, the extent to which the bias for tyrosine in CDR-H3 reflected genetic control of DH reading frame rearrangement preferences was unclear. Also unclear was whether somatic selection during B cell development would be able to adjust the repertoire to avoid use of RF2-encoded amino acids (38), should the use of RF2 be increased by genetic means.
In order to address the role of DH sequence in regulating RF usage and CDR-H3 amino acid content, we introduced two frameshift mutations into DFL16.1 and then forced the use of this new DH to create the ΔD-DμFS allele [Figure 8, (50)]. The first of the two frameshift mutations placed the Dμ open reading frame ATG in frame with RF2 instead of RF1. The second shifted the region of DH-JH microhomology from RF2 to RF1, and at the same time shifted one of the two TAG termination codons from RF3 to RF1. These two frameshift mutations flipped the normal 3:1 preference for RF1 among progenitor B cells to a 3:1 preference for RF2. This 3:1 ratio was largely maintained throughout B cell development in the bone marrow, including recirculating IgM+IgD+ Fraction F. Thus, germline control of the repertoire again played a deciding role, providing the ‘push’ in the ‘push-pull’ described above. The result was a repertoire that was enriched for valine, as well as other RF2-encoded hydrophobic amino acids, with a compensatory decrease in the use of tyrosine (Figure 4).
Although somatic selection did not have the power to recreate the normal preference for tyrosine, we again witnessed the effect of somatic selection, or antigen receptor-driven ‘pull’, to create a DFL16.1 motif-specific RF1 repertoire. When we compared CDR-H3 composition by reading frame, we found that the ΔD-DμFS RF2 repertoire generally matched that generated by DFL16.1 RF2 in both wild-type and ΔD-DFL mice.
More strikingly, RF1-using CDR-H3s from fraction F B cells in the ΔD-DμFS mice generated a neutral CDR-H3 loop repertoire with a pattern of amino acid usage and an average length that was similar to that obtained from the RF1-generated repertoire from controls. This was unexpected because when we created the second frameshift mutation, we made TAG the penultimate 3′ codon of the DμFS RF1. Use of this RF in a functional sequence thus requires that the mutant DH DμFS undergo a minimum loss of five nucleotides. This was borne out in practice, with productive in-frame VH7183-D-J-Cμ transcripts from ΔD-DμFS fraction B cells losing an average of eight 3′ terminal nucleotides versus an average loss of only four 3′ terminal nucleotides among DFL16.1-containing transcripts from the controls (p <0.001). Because the extent of 5′ loss among the ΔD-DμFS fraction B cells was statistically indistinguishable from controls, the increased loss of 3′ nucleotides led to ΔD-DμFS RF1 contributing, on average, approximately four fewer germ line encoded nucleotides to CDR-H3 (p<0.05). As a result, the average length of CDR-H3 in fraction B was almost one codon shorter than controls. With development, however, the contribution of germline DH sequence increased. This effect was most notable among B cells from fraction F, where the average loss of 3′ nucleotides had dropped from eight to seven; and, more importantly, where the average loss of 5′ nucleotides dropped precipitously from six to two (p <0.05). The effect was to create a mature ΔD-DμFS RF1 repertoire that maintained the same relative contribution of germline DH sequence as wild-type DFL16.1 RF1 in CDR-H3 intervals of the same average length, thus completely compensating for the loss of 3′ tyrosine with the inclusion of a 5′ tyrosine encoded by the 5′ terminus of the DH (Figure 8).
In summary, while the absolute sequence of the RF1-encoded CDR-H3-centric antigen binding site repertoire was not recreated, the global amino acid composition of the repertoire in mature B cells appears to have been ‘pulled’ by antigen receptor-driven selection to match that observed in both wild-type and ΔD-DFL-containing CDR-H3s that use DFL16.1 in RF1. Together, these findings not only emphasized the critical role of germline DH sequence in creating the final range of CDR-H3 diversity, or the ‘push’, they indicated that each DH reading frame creates its own preferred CDR-H3 repertoire and that this repertoire is shaped by the ‘pull’ of somatic selection to fit into a preferred range of lengths, amino acid content, and average hydrophobicity.
Forced use of inverted DH RF1 gene segment sequence creates a CDR-H3 repertoire enriched for arginine and other positively charged amino acids
The ΔD-DμFS allele forced mice to use a reading frame that is expressed less frequently than the RF that is normally preferred, but that still contributes to a significant portion of the CDR-H3s that comprise the normal repertoire. Thus, it remained possible that the negative effect of using this less frequent reading frame was not sufficient to completely engage somatic selection mechanisms. To further test the relative power of somatic versus natural, i.e. germline driven, we created a fourth DH-altered allele, ΔD- iD, where the use of inverted reading frame sequence was forced. The inverted reading frame selected encodes charged amino acids, especially arginine. This reading frame normally contributes to a miniscule portion of the repertoire.
To force use of this reading frame sequence in the context of the normal mechanisms of RF usage, we created an iD DH gene segment wherein replaced the core of DFL16.1 with inverted DSP2.2 in RF1. We preserved 5′ and 3′ terminal nucleotides from the recipient DFL16.1 DH, thus maintaining shared microhomology with VH and JH, respectively. The new iD DH gene segment thus replaced central tyrosine, glycine and serine RF1 codons with arginine, histidine and asparagine codons from inverted RF1(i-RF1) while maintaining the 5′ and 3′ terminal sequences of DFL16.1 [Figure 8, (43,51)].
As in our previous studies, we found that somatic selection could not overcome the global effect of changing DH sequence (Figure 4). In fraction B, 74% of the sequences used the new arginine-enriched RF1, and in fraction F 80% used the same RF. Although DH inversions were more frequent in ΔD-iD B cells than in the wild type or ΔD-DFL controls, their prevalence did not increase with development even though i-RF1 for iD recapitulates the normally preferred tyrosine-enriched sequence of DSP2.2 RF1. Further, we found no evidence of selection for sequences that had undergone extensive exonucleolytic loss or for those with increased N nucleotide content.
The stability of exonucleolytic loss and N region gain created CDR-H3 repertoires whose average length remained unchanged during development. The preservation of iD sequence contributed to a predominance of arginine, asparagine, and histidine at all stages of bone marrow repertoire development examined (Figure 4). Together these amino acids comprised approximately one-third of the amino acids in the CDR-H3 loop, tripling their contribution to the repertoire when compared to controls (p<0.001). Conversely, the contribution of tyrosine and glycine to the loop was halved (p<0.001). Persistence of the charged amino acids was associated with enrichment for CDR-H3 loops with an average normalized Kyte-Doolittle hydrophobicity value of less than −0.700 (Figure 4).
Evidence of somatic selection was still obtained, however; thus conforming to the ‘pull’ of presumed antigen receptor-driven pressures. Although highly charged CDR-H3 loops were retained in the mature ΔD-iD B cell repertoire, highly hydrophobic sequences followed the normal pattern of loss during development (27,44). The end result of the selective loss of these highly hydrophobic intervals in ΔD-iD shifted the average hydrophobicity of the CDRH3 repertoire firmly into the charged range.
Thus, even in this extreme case, the germline sequence of the DH still dictated, or ‘pushed’, the general outline of the CDR-H3 repertoire, with somatic selection apparently focusing, or ‘pulling’ the repertoire into a range that is ‘acceptable’ to the organism.
Role of the CDR-H3 repertoire in controlling B cell development
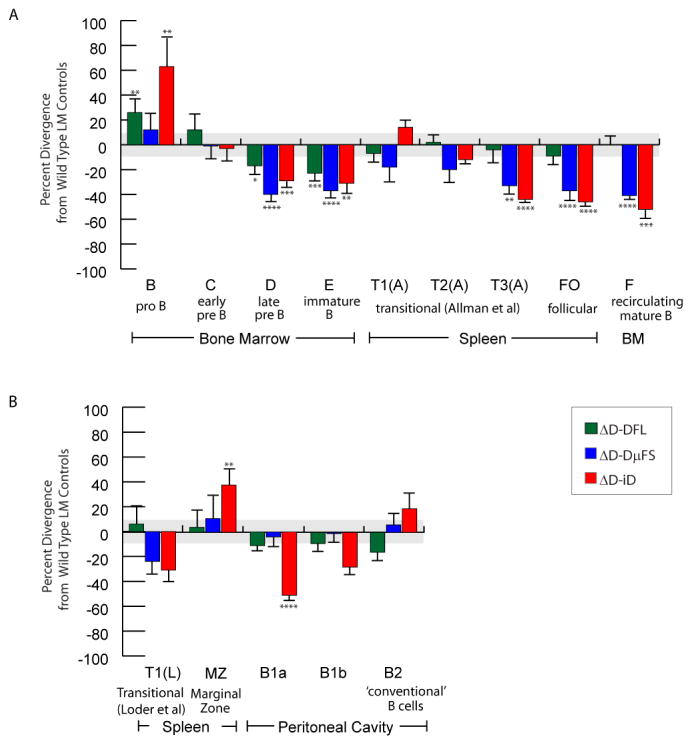
Our evolutionary comparisons had previously indicated evidence of strong natural selection pressure to maintain DH sequence content, and thus by extension, CDR-H3 content. Having found that changes in the repertoire were not a bar to successful B cell production, we then sought to determine whether the change in the repertoire would have an effect on B cell development (43,52,53). We compared the absolute numbers of B cells in our D-altered mice to wild-type controls, focusing on key developmental subsets in the bone marrow, spleen, and peritoneal cavity (44). The effect is most easily visualized by illustrating the percent divergence in absolute numbers of B cells in these key subsets between individual DH mutants and wild-type littermate controls. A graphic depiction of this data (Figure 9) enables a quick view of the pattern of impairment or enhancement in the numbers of B cells in each fraction relative to wild-type.
Figure 9. Divergence in the absolute numbers of B lineage subpopulations from the bone marrow, spleen, and peritoneal cavity of homozygous ΔD-DFL, ΔD-DμFS, and ΔD-iD mice relative to their littermate controls.
A. Percent loss or gain relative to wild type littermate controls in bone marrow fractions B through F; splenic transitional T1 [T(1A)], T2 [T2(A)], and T3 [T3(A)] per Allman et al (81); and splenic mature follicular (FO) B cells. B. Percent loss or gain relative to wild type littermate controls in splenic transitional T1 (T1L) per Loder et al (28); splenic marginal zone (MZ) B cells; CD19 and in both panels the standard peritoneal cavity B1a, B1b, and B2. For the littermate controls, the standard error of the mean of each B lineage subpopulation averaged approximately 11% of the absolute number of cells in each subpopulation (gray area). For ΔD-DFL, ΔD-DμFS, and ΔD-iD, the standard error of the mean is shown as an error bar. ‘*’, p < 0.05; ‘**’, p < 0.01; ‘***’, p < 0.001; and ‘****’, p < 0.0001.
In the bone marrow of our D-limited mice, we observed an increase in the number of fraction B (pro-B cells), equivalence in the number of fraction C (early pre-B cells), and a decrease in both the numbers of fraction D (late pre-B cells) and the numbers of fraction E (immature B cells) irrespective of DH sequence. This suggests that the increase in the pro-B cell population and the decrease in the late pre-B and immature B cell subsets were due to the loss of 87 kb of the DH locus, rather than the change in the sequence of CDR-H3.
However, once released from the bone marrow, the change in the sequence of CDR-H3 had dramatic effects on B cell numbers (43,44,52). In the mice limited to use of a single, normal DFL16.1 gene segment (ΔD-DFL), the numbers of transitional, marginal zone, and follicular cells in the spleen, the numbers of B1a, B1b, and B2 cells in the peritoneal cavity, and the numbers of mature IgM+IgD+ fraction F cells in the bone marrow proved statistically indistinguishable from wild-type littermates. Thus, mice limited to 20% of the normal repertoire were able to populate all the peripheral B cell niches that we examined and to achieve normal numbers of B cells in these niches. In contrast, in the mice that were forced to increase their use of either hydrophobic or positive charged amino acids in CDR-H3, the numbers of conventional splenic follicular and bone marrow mature fraction F cell numbers were nearly halved.
The effect of the change in CDR-H3 content on the marginal zone was of particular interest because FO and MZ B cells appear to have different functions. The follicular subset contains the resting precursors of cells that appear most likely to engage in immune responses to T-dependent antigens. Upon stimulation by antigen, FO cells can give rise to both primary antibody-forming cells and memory B cells of high affinity. In contrast, and based on surface phenotypic criteria, many MZ B cells appear to exist in a semi-activated state, primed to respond to T-cell independent challenges (54–56). However, when given the proper stimuli MZ cells can engage in T-dependent responses.
In our studies, normalization of MZ B cell numbers was observed in mice using the ΔD-D μFS DH allele. In contrast, in mice forced to use arginine-enriched ΔD-iD, marginal zone B cell numbers increased by one third (p<0.01). The increase in MZ B cell numbers in the ΔD-iD mice (Figure 9) suggested to us that the MZ repertoire might be either permissive or even selective for charged sequences. Subsequently we examined the composition of CDR-H3s cloned from wild-type MZ B cells and found that the repertoire is normally enriched for positively charged amino acids [Figure 3, (42)]. These findings provided support for our hypothesis that the increase in MZ cell numbers in ΔD-iD mice simply reflected an increase in the number of B cells bearing BCRs that were acceptable for entry into and survival in this compartment.
Studies from a number of investigators indicate that the marginal zone is often enriched for self- or polyreactive B cells, including those with potentially pathogenic polyreactivity (57–60). A relative increase in the numbers of activated, self-reactive MZ B cells has been seen in several autoreactive states (61–64), however, the functional role of these self-reactive MZ B cells in healthy individuals remains unclear. An excess of charged amino acids in CDR-H3 has been associated with pathogenic self-reactivity, especially to DNA (65–68). In this light, it should be noted that B cells expressing anti-dsDNA reactivity have been shown to be excluded from the follicles (69).
Role of control of the CDR-H3 repertoire on antibody production and on responses to antigen
In general, we found that the greater the divergence from wild-type, the more truncated the immune response. That is, ΔD-DFL mice were the closest in function to wild-type, followed by ΔD-DμFS, whereas ΔD-iD mice were the most divergent (43,44,52). We began by measuring serum immunoglobulin levels. Homozygous wild-type, ΔD-DFL and ΔD-D μFS mice expressed equivalent serum levels of IgM, IgA, and IgG, including the four IgG subclasses. However, while the serum concentration of IgM and IgA was comparable to wild-type, the geometric mean concentration of all four IgG subclasses in the sera of ΔD-iD mice were significantly less than wild-type (p=0.02, p=0.0004, p=0.003, and p=0.0002; respectively).
In wild-type BALB/c mice, intravenous challenge with α (1→3) dextran (DEX) elicits a T-independent response that is dominated by λ1 light chain-bearing antibodies which express a diverse range of antigen binding sites with heterogeneous CDR-H3 sequences (70,71). Seven days after challenge with DEX, we measured the geometric mean of IgM anti-DEX serum levels in homozygous wild-type ΔD-DFL, ΔD-DμFS and ΔD-iD mice and observed a progressive decline. These results suggested a direct correlation between the extent of divergence from the normal repertoire and the divergence of the host response to this T-independent antigen.
In BALB/c, the primary response to the nitrophenylacetyl hapten of NP19-CGG requires T-cell help and contains a large fraction of IgG1λ anti-NP antibodies (72). Among those sequences that have been cloned from this population, many incorporate DFL16.1 in RF1. After primary and secondary intra-peritoneal challenge with NP19-CGG, the anti-NP IgG response in the ΔD-DFL and ΔD-DμFS mice proved indistinguishable from littermate controls. Conversley, the anti-NP response in ΔD-iD, which requires somatic mechanisms to generate DFL16.1-like sequences, was three-fold diminished (43).
In BALB/c, immunization with purified tetanus toxoid elicits a T-dependent response that is dominated by κ light chain bearing antibodies (73). We performed an oral immunization with a recombinant strain of Salmonella that expresses the Tox C fragment of tetanus toxin (TT) (74). Unlike the response to DEX or NP, in some cases repertoire alteration led to an increase in post-challenge titers, in other cases it led to a decrease, and in yet a third category there was no change. For example, the IgM anti-tetanus toxoid response doubled in ΔD-DFL mice (p=0.04); and there was a four-fold increase in the ΔD-DμFS mice (p=0.008). IgM responses in the ΔD-iD mice were similar to wild-type. The total IgG anti-TT response in ΔD-DFL mice was slightly increased, primarily due to a sixteen-fold increase in the IgG3 anti-TT response (p<0.0001). This pattern was reversed in ΔD-DμFS, where the IgG anti-TT titer was sixteen-fold reduced (p=0.004), with a concomitant six-fold reduction in IgG1 (p=0.04) and IgG2a (p=0.03), and a 34-fold decrease in IgG2b. The IgG3 titer proved equivalent to wild-type. In ΔD-iD mice the IgG response was approximately four-fold diminished (p=0.0004). The IgA response in all three D-altered strains proved indistinguishable from wild-type.
To assess the role of the composition of the CDR-H3 repertoire in response to a viral infection, we immunized homozygous ΔD-DFL and ΔD-iD mice with A/Udorn (H3N2) influenza virus. In both sets of mice, the titer against whole influenza virus was equivalent to wild-type controls. After vaccination, both mutant mice were also found to be equivalently protected against homotypic virus when compared to littermate controls. However, when we challenged the mice with the heterologous strain A/pr/8/34 (H1N1); after vaccination with A/Udorn (H3N2), 40% of the ΔD-iD mice died whereas all of the ΔD-DFL and the wild-type littermates survived (p <0.01) [Figure 10, (75)]. From these data we concluded that forcing the global Ig CDR-H3 repertoire to use charged CDR-H3s, a‘disfavored’ category, increases susceptibility to virus infection.
Figure 10. Mortality after challenge with mouse-adapted heterologous influenza virus.
Mice were immunized with influenza strain A/Udorn (H3N2) and then challenged with the heterologous strain A/pr/8/34 (H1N1) at day 0. Values are the percent of mice that remained alive in each group of thirty immunized homozygous wild-type (wt/wt), ten immunized homozygous ΔD-DFL (ΔD-DFL/ΔD-DFL) ten immunized homozygous ΔD-iD (ΔD-iD/ΔD-iD), and ten naïve homozygous wild-type wt/wt mice, respectively, on the given day after challenge.
Nature versus nurture revisited
A central focus of debate among students of the adaptive immune response is the relative contribution of germline content versus somatically generated diversity in regulating lymphocyte function. The final composition this repertoire can be viewed as a compromise between two competing forces. On the one hand, there is pressure to make the repertoire as rich and diverse as possible in order to allow the immune system to recognize novel antigens. On the other hand, the capacity of the genome to encode diversity is distinctly finite and the production of unnecessary or pathogenic antibodies must be avoided. Although at first glance the CDR-H3 component of the antibody repertoire appears random, our findings suggest that even this most diverse portion of the antibody contains a strong element of predetermination. We have shown that alteration of the germline-controlled CDR-H3 repertoire leads to changes in B cell development and antibody production. Still to be examined is the extent to which CDR-H3 control influences immune responses to vaccines and pathogens other than influenza virus, as well as the likelihood of developing diseases of immune function, including autoimmune diseases. These issues are under intense investigation in our laboratories.
Contributor Information
Harry W Schroeder, Jr, Email: hwsj@uab.edu, Division of Clinical Immunology and Rheumatology, Departments of Medicine, Microbiology, and Genetics, University of Alabama at Birmingham, SHEL 176, 1530 3rd Avenue South, Birmingham, AL 35294-2182, TEL: (205) 934-6826, FAX: (205) 975-6352.
Michael Zemlin, Email: Zemlin@med.uni-marburg.de, Department of Pediatrics, Philipps-University Marburg, Baldingerstr. 1, Marburg, Germany, 35043, TEL: (xx49)-6421-5862650, FAX: (xx49)-6421-5868970.
Mohamed Khass, Email: khassm@uab.edu, Department of Microbiology, University of Alabama at Birmingham, SHEL 176, 1530 3rd Avenue South, Birmingham, AL 35294-2182.
Robert L Schelonka, Email: schelonk@ohsu.edu, Division of Neonatology, Department of Pediatrics, University of Oregon Health Sciences Center, 707 S.W., Gaines St. Portland, OR 97239-2998.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual Review of Immunology. 2000;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 3.Alt FW, Baltimore D. Joining of immunoglobulin heavy chain gene segments: Implications from a chromosome with evidence of three D-J heavy fusions. Proc Nat Acad Sci, U S A. 1982;79:4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 5.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of proteins of immunological interest. 5. U.S. Department of Health and Human Services; Bethesda, Maryland: 1991. [Google Scholar]
- 6.Padlan EA. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 7.Kirkham PM, Schroeder HW., Jr Antibody structure and the evolution of immunoglobulin V gene segments. Semin Immunol. 1994;6:347–360. doi: 10.1006/smim.1994.1045. [DOI] [PubMed] [Google Scholar]
- 8.Kirkham PM, Mortari F, Newton JA, Schroeder HW., Jr Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 1992;11:603–609. doi: 10.1002/j.1460-2075.1992.tb05092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giudicelli V, Duroux P, Ginestoux C, Folch G, Jabado-Michaloud J, Chaume D, Lefranc MP. IMGT/LIGM-DB, the IMGT comprehensive database of immunoglobulin and T cell receptor nucleotide sequences. Nucleic Acids Res. 2006;34:D781–D784. doi: 10.1093/nar/gkj088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chothia C, Lesk AM. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987;196:901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- 11.Chothia C, Lesk AM, Tramontano A, Levitt M, Smith-Gill SJ, Air G, Sheriff S, Padlan EA, Davies D, Tulip WR, Colman PM, Spinelli S, Alzari PM, Poljak RJ. Conformations of immunoglobulin hypervariable regions. Nature. 1989;342:877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- 12.Tramontano A, Chothia C, Lesk AM. Framework residue 71 is a major determinant of the position and conformation of the second hypervariable region in the VH domains of immunoglobulins. J Mol Biol. 1990;215:175–182. doi: 10.1016/S0022-2836(05)80102-0. [DOI] [PubMed] [Google Scholar]
- 13.Al Lazikani B, Lesk AM, Chothia C. Standard conformations for the canonical structures of immunoglobulins. J Mol Biol. 1997;273:927–948. doi: 10.1006/jmbi.1997.1354. [DOI] [PubMed] [Google Scholar]
- 14.Chothia C, Lesk AM, Gherardi E, Tomlinson IM, Walter G, Marks JD, Llewelyn MB, Winter G. Structural repertoire of the human VH segments. J Mol Biol. 1992;227:799–817. doi: 10.1016/0022-2836(92)90224-8. [DOI] [PubMed] [Google Scholar]
- 15.Vargas-Madrazo E, Lara-Ochoa F, Almagro JC. Canonical structure repertoire of the antigen-binding site of immunoglobulins suggests strong geometrical restrictions associated to the mechanism of immune recognition.[erratum appears in J Mol Biol 1996 May 24;258(5):893] J Mol Biol. 1995;254:497–504. doi: 10.1006/jmbi.1995.0633. [DOI] [PubMed] [Google Scholar]
- 16.Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13:37–45. doi: 10.1016/s1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 17.Meffre E, Casellas R, Nussenzweig MC. Antibody regulation of B cell development. Nature Immunol. 2000;1:379–385. doi: 10.1038/80816. [DOI] [PubMed] [Google Scholar]
- 18.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 19.Burrows PD, Stephan RP, Wang YH, Lassoued K, Zhang Z, Cooper MD. The transient expression of pre-B cell receptors governs B cell development. Semin Immunol. 2002;14:343–349. doi: 10.1016/s1044-5323(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 20.Keyna U, Beck-Engeser GB, Jongstra J, Applequist SE, Jack HM. Surrogate light chain-dependent selection of Ig heavy chain V regions. J Immunol. 1995;155:5536–5542. [PubMed] [Google Scholar]
- 21.Kline GH, Hartwell L, Beck-Engeser GB, Keyna U, Zaharevitz S, Klinman NR, Jack HM. Pre-B cell receptor-mediated selection of pre-B cells synthesizing functional mu heavy chains. J Immunol. 1998;161:1608–1618. [PubMed] [Google Scholar]
- 22.Martin DA, Bradl H, Collins TJ, Roth E, Jack HM, Wu GE. Selection of Ig mu heavy chains by complementarity-determining region 3 length and amino acid composition. J Immunol. 2003;171:4663–4671. doi: 10.4049/jimmunol.171.9.4663. [DOI] [PubMed] [Google Scholar]
- 23.Shiokawa S, Mortari F, Lima JO, Nunez C, Bertrand FEI, Kirkham PM, Zhu S, Dasanayake AP, Schroeder HW., Jr IgM HCDR3 diversity is constrained by genetic and somatic mechanisms until two months after birth. J Immunol. 1999;162:6060–6070. [PubMed] [Google Scholar]
- 24.Raaphorst FM, Raman CS, Tami J, Fischbach M, Sanz I. Human Ig heavy chain CDR3 regions in adult bone marrow pre-B cells display an adult phenotype of diversity: evidence for structural selection of DH amino acid sequences. Int Immunol. 1997;9:1503–1515. doi: 10.1093/intimm/9.10.1503. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder HW, Jr, Ippolito GC, Shiokawa S. Regulation of the antibody repertoire through control of HCDR3 diversity. Vaccine. 1998;16:1383–1390. doi: 10.1016/s0264-410x(98)00096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov II, Schelonka RL, Zhuang Y, Gartland GL, Zemlin M, Schroeder HW., Jr Development of the expressed immunoglobulin CDR-H3 repertoire is marked by focusing of constraints in length, amino acid utilization, and charge that are first established in early B cell progenitors. J Immunol. 2005;174:7773–7780. doi: 10.4049/jimmunol.174.12.7773. [DOI] [PubMed] [Google Scholar]
- 28.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov II, Link JM, Ippolito GC, Schroeder HW., Jr . Constraints on hydropathicity and sequence composition of HCDR3 are conserved across evolution. In: Zanetti M, Capra JD, editors. The Antibodies. Taylor and Francis Group; London: 2002. pp. 43–67. [Google Scholar]
- 30.Zemlin M, Klinger M, Link J, Zemlin C, Bauer K, Engler JA, Schroeder HW, Jr, Kirkham PM. Expressed murine and human CDR-H3 intervals of equal length exhibit distinct repertoires that differ in their amino acid composition and predicted range of structures. J Mol Biol. 2003;334:733–749. doi: 10.1016/j.jmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 32.Williams GS, Martinez A, Montalbano A, Tang A, Mauhar A, Ogwaro KM, Merz D, Chevillard C, Riblet R, Feeney AJ. Unequal VH gene rearrangement frequency within the large VH7183 gene family is not due to recombination signal sequence variation, and mapping of the genes shows a bias of rearrangement based on chromosomal location. J Immunol. 2001;167:257–263. doi: 10.4049/jimmunol.167.1.257. [DOI] [PubMed] [Google Scholar]
- 33.Viale AC, Coutinho A, Freitas AA. Differential expression of VH gene families in peripheral B cell repertoires of newborn or adult immunoglobulin H chain congenic mice. J Exp Med. 1992;175:1449–1456. doi: 10.1084/jem.175.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huetz F, Carlsson L, Tornberg UC, Holmberg D. V-region directed selection in differentiating B lymphocytes. EMBO J. 1993;12:1819–1826. doi: 10.1002/j.1460-2075.1993.tb05830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall AJ, Wu GE, Paige GJ. Frequency of VH81x usage during B cell development: initial decline in usage is independent of Ig heavy chain cell surface expression. J Immunol. 1996;156:2077–2084. [PubMed] [Google Scholar]
- 36.Schroeder HW, Jr, Zhang L, Philips JB., III Slow, programmed maturation of the immunoglobulin HCDR3 repertoire during the third trimester of fetal life. Blood. 2001;98:2745–2751. doi: 10.1182/blood.v98.9.2745. [DOI] [PubMed] [Google Scholar]
- 37.Nitschke L, Kestler J, Tallone T, Pelkonen S, Pelkonen J. Deletion of the DQ52 element within the Ig heavy chain locus leads to a selective reduction in VDJ recombination and altered D gene usage. J Immunol. 2001;166:2540–2552. doi: 10.4049/jimmunol.166.4.2540. [DOI] [PubMed] [Google Scholar]
- 38.Cohn M. A hypothesis accounting for the paradoxical expression of the D gene segment in the BCR and the TCR. Eur J Immunol. 2008;38:1179–1187. doi: 10.1002/eji.200738089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feeney AJ, Clarke SH, Mosier DE. Specific H chain junctional diversity may be required for non- T15 antibodies to bind phosphorylcholine. J Immunol. 1988;141:1267–1272. [PubMed] [Google Scholar]
- 40.Ichihara Y, Hayashida H, Miyazawa S, Kurosawa Y. Only DFL16, DSP2, and DQ52 gene families exist in mouse immunoglobulin heavy chain diversity gene loci, of which DFL16 and DSP2 originate from the same primordial DH gene. Eur J Immunol. 1989;19:1849–1854. doi: 10.1002/eji.1830191014. [DOI] [PubMed] [Google Scholar]
- 41.Gu H, Forster I, Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VH-D-JH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990;9:2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schelonka RL, Tanner J, Zhuang Y, Gartland GL, Zemlin M, Schroeder HW., Jr Categorical selection of the antibody repertoire in splenic B cells. 2007 doi: 10.1002/eji.200636569. [DOI] [PubMed] [Google Scholar]
- 43.Ippolito GC, Schelonka RL, Zemlin M, Ivanov II, Kobayashi R, Zemlin C, Gartland GL, Nitschke L, Pelkonen J, Fujihashi K, Rajewsky K, Schroeder HW., Jr Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J Exp Med. 2006;203:1567–1578. doi: 10.1084/jem.20052217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schelonka RL, I, Ivanov I, Jung D, Ippolito GC, Nitschke L, Zhuang Y, Gartland GL, Pelkonen J, Alt FW, Rajewsky K, Schroeder HW., Jr A single DH gene segment is sufficient for B cell development and immune function. J Immunol. 2005;175:6624–6632. doi: 10.4049/jimmunol.175.10.6624. [DOI] [PubMed] [Google Scholar]
- 45.Reth MG, Alt FW. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. Nature. 1984;312:418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- 46.Feeney AJ. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990;172:1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feeney AJ. Predominance of VH-D-JH junctions occurring at sites of short sequence homology results in limited junctional diversity in neonatal antibodies. J Immunol. 1992;149:222–229. [PubMed] [Google Scholar]
- 48.Nadel B, Feeney AJ. Influence of coding-end sequence on coding-end processing in V(D)J recombination. J Immunol. 1995;155:4322–4329. [PubMed] [Google Scholar]
- 49.Gu H, Kitamura D, Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991;65:47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- 50.Zemlin M, Schelonka RL, Ippolito GC, Zemlin C, Zhuang Y, Gartland GL, Nitschke L, Pelkonen J, Rajewsky K, Schroeder HW., Jr Regulation of repertoire development through genetic control of DH reading frame preference. J Immunol. 2008;181:8416–8424. doi: 10.4049/jimmunol.181.12.8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ippolito GC, Pelkonen J, Nitschke L, Rajewsky K, Schroeder HW., Jr Antibody repertoire in a mouse with a simplified D(H) locus: the D-limited mouse. Ann N Y Acad Sci. 2003;987:262–265. doi: 10.1111/j.1749-6632.2003.tb06058.x. [DOI] [PubMed] [Google Scholar]
- 52.Schelonka RL, Zemlin M, Kobayashi R, Szalai A, Ippolito GC, Zhuang Y, Gartland GL, Fujihashi K, Rajewsky K, Schroeder HW., Jr Preferential use of DH reading frame 2 alters B cell development and antigen-specific antibody production. J Immunol. 2008;181:8409–8415. doi: 10.4049/jimmunol.181.12.8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zemlin M, Schelonka RL, Ippolito GC, Nitschke L, Pelkonen J, Rajewsky K, Schroeder HW., Jr Forced use of DH RF2 sequence impairs B cell development. In: Kalil J, Cunha-Neto E, Rizzo LV, editors. 13th International Congress of Immunology; Medimond International Proceedings; Bologna. 2007. pp. 507–511. [Google Scholar]
- 54.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J Exp Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory“. Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- 56.Lopes-Carvalho T, Foote J, Kearney JF. Marginal zone B cells in lymphocyte activation and regulation. Curr Opin Immunol. 2005;17:244–250. doi: 10.1016/j.coi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 58.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172:625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 59.Witsch EJ, Cao H, Fukuyama H, Weigert M. Light chain editing generates polyreactive antibodies in chronic graft-versus-host reaction. J Exp Med. 2006;203:1761–1772. doi: 10.1084/jem.20060075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dammers PM, Kroese FG. Recruitment and selection of marginal zone B cells is independent of exogenous antigens. Eur J Immunol. 2005;35:2089–2099. doi: 10.1002/eji.200526118. [DOI] [PubMed] [Google Scholar]
- 61.Duan B, Croker BP, Morel L. Lupus resistance is associated with marginal zone abnormalities in an NZM murine model. Laboratory Investigation. 2007;87:14–28. doi: 10.1038/labinvest.3700497. [DOI] [PubMed] [Google Scholar]
- 62.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogeninduced lupus. J Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 63.Wither JE, Loh C, Lajoie G, Heinrichs S, Cai YC, Vonventi G, MacLeod R. Colocalization of expansion of the splenic marginal zone population with abnormal B cell activation and autoantibody production in B6 mice with an introgressed New Zealand Black chromosome 13 interval. J Immunol. 2005;175:4309–4319. doi: 10.4049/jimmunol.175.7.4309. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J Exp Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barbas SM, Ditzel HJ, Salonen EM, Yang WP, Silverman GJ, Burton DR. Human autoantibody recognition of DNA. Proc Natl Acad Sci U S A. 1995;92:2529–2533. doi: 10.1073/pnas.92.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 68.Li H, Jiang Y, Cao H, Radic M, Prak EL, Weigert M. Regulation of anti-phosphatidylserine antibodies. Immunity. 2003;18:185–192. doi: 10.1016/s1074-7613(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 69.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J Exp Med. 1997;%20, 186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blomberg B, Geckeler WR, Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972;177:178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- 71.Stohrer RC, Kearney JF. Fine idiotype analysis of B cell precursors in the T-dependent and T-independent responses to alpha 1-3 dextran in BALB/c mice. J Exp Med. 1983;158:2081–2094. doi: 10.1084/jem.158.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imanishi-Kari T, Rajnavolgyi E, Takemori T, Jack RS, Rajewsky K. The effect of light chain gene expression on the inheritance of an idiotype associated with primary anti-(4-hydroxy-3- nitrophenyl)acetyl(NP) antibodies. Eur J Immunol. 1979;9:324–331. doi: 10.1002/eji.1830090414. [DOI] [PubMed] [Google Scholar]
- 73.Volk WA, Bizzini B, Snyder RM, Bernhard E, Wagner RR. Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infec Immun. 1984;45:604–609. doi: 10.1128/iai.45.3.604-609.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.VanCott JL, Staats HF, Pascual DW, Roberts M, Chatfield SN, Yamamoto M, Coste M, Carter PB, Kiyono H, McGhee JR. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 75.Nguyen HH, Zemlin M, Vu HL, vanov, Andrasi J, Zemlin C, Schelonka RL, Schroeder HW, Jr, Mestecky J. Heterosubtypic immunity to influenza A virus infection requires a properly diversified antibody repertoire. J Virol. 2007;81:9331–9338. doi: 10.1128/JVI.00751-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lefranc MP. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2003;31:307–310. doi: 10.1093/nar/gkg085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 78.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 79.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 80.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. 595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 81.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]