Abstract
Introduction
Sorafenib is a multi-kinase inhibitor affecting pathways involved in tumor progression and angiogenesis. We conducted a phase II trial of Sorafenib in platinum-treated extensive stage small cell lung cancer (SCLC) patients to determine the tumor response rate, toxicity and overall survival.
Methods
Patients with histologically confirmed, measurable disease, Zubrod performance status 0–1 and no more than 1 prior platinum based treatment were eligible. Patients were stratified by platinum-sensitivity status: sensitive (progression >90 days after platinum) or refractory (progression during or ≤90 days after platinum). Pts were treated with sorafenib 400mg PO BID continuously on a 28 day cycle.
Results
Of 89 pts registered; 82 were evaluable for toxicity assessment and 83 were evaluable for response There were 4 partial responses seen among the 38 patients in the platinum sensitive stratum, for an estimated response rate of 11% (95% confidence interval: 3% – 25%); and one partial response among the 45 patients in the platinum refractory stratum for an estimated response rate of 2% (95% confidence interval: 0% – 12%). The median overall survival estimates were 5.3 months (95% confidence interval: 3.3–7.5 months) and 6.7 months (95% confidence interval: 6.1–9.1 months) for the platinum-refractory and platinum-sensitive strata respectively. Nineteen patients discontinued treatment due to adverse events or side effects from therapy.
Conclusions
Based on the lack of disease control seen in our trial, further investigation of single-agent sorafenib in the SCLC population is not recommended. Combination trials of Sorafenib and chemotherapy are ongoing.
Keywords: Sorafenib, Platinum Treated, Lung Cancer
INTRODUCTION
Lung cancer will be diagnosed in approximately 186,525 Americans in 2009, and account for 159,390 cancer deaths (1). The lifetime risk of developing lung cancer is 1:13 for men and 1:16 for women. Approximately 15% of these cases will be diagnosed with small cell lung cancer (SCLC), an aggressive malignancy that is usually extensive in two thirds of patients at initial presentation. Patients with extensive stage SCLC are typically treated with platinum-based chemotherapy regimens resulting in high response rates, translating into improved survival and disease palliation. However, despite this initial chemosensitivity of treatment-naive disease, the majority of patients experience a recurrence of disease which is usually drug resistant and lethal.
The activity of new chemotherapeutic agents in previously treated, relapsed SCLC can be distinguished based on the response status to prior platinum-based chemotherapy. Three categories of patients have been described: sensitive, resistant, and refractory. Sensitive refers to patients who had a first-line response that lasted 90 days after treatment was completed. Refractory refers to patients who never responded to first-line therapy or those whose cancer progressed during first-line therapy. Resistant refers to patients who responded initially, but experienced a recurrence within 90 days of completion of their primary therapy. Resistant patients are often grouped together with refractory patients because of their uniformly poor treatment outcomes, with infrequent objective responses and dismal survival. Indeed, clinical trials of salvage chemotherapy for relapsed SCLC often exclude refractory/resistant patients (2). However, a Phase III study conducted at the Royal Marsden Hospital compared oral topotecan to best supportive care for relapsed SCLC. Even in this population of patients “not considered as candidates for standard intravenous therapy,” there was a statistically significant survival benefit to treatment with topotecan (13.9 weeks vs. 25.9 weeks) [3]. Statistical significance for survival was maintained in a subgroup of patients with a short treatment-free interval ≤ 60 days; with median survival of 13.2 weeks vs. 23.3 weeks.
While topotecan offers advantage over best supportive care, improving outcomes in SCLC is certainly an area of great therapeutic need. Therefore, platinum treated, relapsed SCLC patients are a potentially unique group to target and differentiate along sensitivity status when studying agents with unique mechanisms of action.
Sorafenib (BAY 43-9006) is an orally bioavailable, small molecule inhibitor of multiple intracellular and receptor protein kinases involved in signaling pathways that control tumor growth, stromal environment and angiogenesis. Specifically, sorafenib is a potent inhibitor of wild-type and mutant B-raf, VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-β, c-KIT, Flt3 and RET.
Based upon the potential role of inhibition of angiogenic pathways combined with blockade of cell growth pathways, we conducted a Phase II trial of sorafenib in relapsed/refractory SCLC (SWOG 0435).
MATERIALS METHODS
The objectives of the study were to evaluate the efficacy of sorafenib in previously treated, platinum-sensitive and platinum refractory patients with extensive stage small cell lung cancer (E-SCLC). Our primary objective was to assess the objective response rate. Secondary objectives included the assessment of overall survival, progression free survival, as well assessing the qualitative and quantitative toxicities of sorafenib in this patient cohort.
In addition, specimens were collected for the Lung Cancer Specimen Repository Protocol (S9925) for analyses of the relationship between selected markers and patient outcomes. Analysis of tumor and serum for angiogenic markers is ongoing and will be reported separately.
All participating centers were required to have institutional review board approval for the study and all patients gave written informed consent to participate in this study in accordance with institutional and federal guidelines. This study (Clinical Trials Registration Identification Number: NCT00182689) was monitored by the Data and Safety Monitoring Committee of the Southwest Oncology Group.
Eligibility Criteria
Eligible patients had histologically or cytologically confirmed diagnosis of SCLC, with measurable disease per RECIST criteria, Zubrod performance status of 0–1, with adequate hematologic, hepatic and renal function. Patients must have received exactly one prior platinum-based regimen. Patients with asymptomatic, treated brain metastases that did not require either enzyme-inducing anticonvulsants or corticosteroid therapy to control symptoms were eligible. Patients had to be able to tolerate oral medication. Women/men of reproductive potential who entered the study agreed to use an effective contraceptive method.
Treatment
Patients were treated with oral sorafenib 400 mg twice daily for a 28 day cycle. This study used the CTCAE (National Cancer Institute Common Terminology Criteria for Adverse Events) Version 3.0 for toxicity and adverse event reporting. Interruptions and delays of sorafenib therapy were allowed for up to 3 weeks for Grade 3 and 4 toxicities. Dose reduction to 200 mg twice daily was allowed for subsequent cycles after resolution of toxicity to ≤ Grade 2. A second dose reduction to 200 mg daily was allowed. If treatment delay exceeded 3 weeks for toxicity resolution, or Grade 3 or greater toxicities were seen at the lowest dose reduction, the patient was removed from protocol treatment.
Statistical Considerations
The primary endpoint was to evaluate the objective response rate (confirmed and unconfirmed, complete and partial responses per RECIST). Confirmation of response required that repeat studies be performed at least 4 weeks after criteria for response were first met. Duration of response was measured from the date that response criteria were first met until the date of disease progression. Secondary endpoints included progression-free survival and overall survival. Patients were stratified into platinum-sensitive or platinum-refractory groups. Platinum-sensitive disease was defined as an initial response to platinum-based chemotherapy and progression >90 days after the last platinum treatment; whereas platinum-refractory disease was defined as no response to platinum-based chemotherapy or progression during or ≤90 days after the last platinum treatment.
Parallel patient enrollment was implemented for both strata, and a two-stage design proceeded separately for each stratum. Initially, 20 patients were to be accrued to each stratum. If no responses were observed in the first 20 patients, then accrual was to be stopped in that stratum with the conclusion that sorafenib was not promising in the group of patients represented by that stratum. If one or more responses were observed in the first 20, an additional 20 patients were to be accrued to the stratum. Five or more responses out of the total 40 patients in a stratum were considered as evidence warranting further study of sorafenib in that group of patients, providing other factors such as toxicity, progression free survival and overall survival, also appeared favorable. If 4 or fewer responses in 40 patients were observed in a stratum, further study of sorafenib would not be warranted in that setting. In either stratum, the probability of falsely declaring sorafenib as warranting further study was 0.05 (alpha) when the true response rate was 5%; the probability of correctly declaring sorafenib as warranting further study was 0.92 (power) when the true response rate was 20%. Within each stratum, 40 patients would be sufficient to estimate the response rate and the rates of individual toxicities to within ±16% (95% confidence interval) and any toxicity occurring with at least 5% probability would have an 87% chance to be seen at least once.
Overall survival and progression-free survival estimates were calculated using the method of Kaplan-Meier (4), and 95% confidence intervals for their medians were constructed using the method of Brookmeyer-Crowley (5). Exact binomial confidence intervals were calculated for response outcomes.
RESULTS
The study met its accrual goal and 89 patients were registered between July of 2005 and February of 2007. In each stratum, at least one objective response was documented among the first 20 patients enrolled warranting that accrual continued through the second stage. Five patients were ineligible, two due to inadequate hepatic function, one due to inadequate blood coagulation, and two due to no evidence of measurable disease by RECIST criteria. One eligible patient did not receive any protocol treatment due to early disease progression, and was not analyzable for any study endpoint. One additional eligible patient did not have any adverse events assessed due to early death.
Patient Characteristics are noted in Table 1. Median age for the platinum sensitive group was 65 versus 60 in the platinum-refractory group. Two-thirds of patients in the platinum refractory group had multiple lesions in multiple organs compared to approximately half of the patients in the platinum sensitive group. Approximately half of the patients in each group were current smokers.
Table 1.
Patient Characteristics by Platinum Sensitivity
| Platinum sensitive (n=38) | Platinum refractory (n=45) | |||
|---|---|---|---|---|
| AGE | ||||
| Median (Range) | 65 | (48–85) | 60 | (44–80) |
| SEX | ||||
| Males | 20 | 53% | 27 | 60% |
| Females | 18 | 47% | 18 | 40% |
| RACE | ||||
| White | 35 | 92% | 41 | 91% |
| Black | 1 | 3% | 1 | 2% |
| Native American | 1 | 3% | 2 | 4% |
| Multi-Racial | 1 | 3% | 0 | 0% |
| Unknown | 0 | 0% | 1 | 2% |
| SITES OF METASTASES | ||||
| Single organ | 4 | 11% | 7 | 16% |
| Multiple organs | 32 | 84% | 34 | 76% |
| None | 1 | 3% | 3 | 7% |
| Not reported | 1 | 3% | 1 | 2% |
| PERFORMANCE STATUS | ||||
| 0 | 15 | 39% | 15 | 33% |
| 1 | 22 | 58% | 29 | 64% |
| Not reported | 1 | 3% | 1 | 2% |
| SMOKING HISTORY | ||||
| Current | 17 | 45% | 25 | 56% |
| Former | 21 | 55% | 19 | 42% |
| Never | 0 | 0% | 0 | 0% |
| Not reported | 0 | 0% | 1 | 2% |
| WEIGHT LOSS PRIOR 6 MONTH | ||||
| < 5% | 26 | 68% | 31 | 69% |
| 5% to < 10% | 5 | 13% | 6 | 13% |
| 10% to < 20% | 1 | 3% | 5 | 11% |
| ≥ 20% | 1 | 3% | 1 | 2% |
| Not reported | 5 | 13% | 2 | 4% |
The reasons for protocol discontinuation are summarized on Table 2. The majority of patients (67%) were taken off protocol for disease progression. An additional 19 patients (23%) went off study due to adverse events or side effects.
Table 2.
Treatment Summary by Platinum Sensitivity
| TOTAL |
Platinum sensitive |
Platinum refractory |
|
|---|---|---|---|
| REASON OFF TREATMENT | |||
| Adverse Events or side effects | 19 | 8 | 11 |
| Refusal unrelated to adverse events | 4 | 2 | 2 |
| Progression/relapse | 56 | 27 | 29 |
| Death | 1 | 1 | 0 |
| Other - not protocol specified | 3 | 0 | 3 |
Efficacy
Tumor response is summarized in Table 3. There were four partial responses seen among the 38 patients on the platinum sensitive stratum, for an estimated response rate of 11% (95% confidence interval: 3% – 25%); and one partial response among the 45 patients in the platinum refractory stratum, for an estimated response rate of 2% (95% confidence interval: 0% – 12%). Duration of the 4 responses seen in the platinum sensitive stratum was 9, 35, 57 and 274 days. Duration of the single response in the platinum refractory strata was 114 days.
Table 3.
Response by Platinum Sensitivity
| Platinum sensitive |
Platinum refractory |
|||
|---|---|---|---|---|
| Partial Response | 0 | 0% | 1 | 2% |
| Unconfirmed Partial Response | 4 | 11% | 0 | 0% |
| Stable/No Response | 12 | 32% | 13 | 29% |
| Progressive Disease | 17 | 45% | 19 | 42% |
| Symptomatic Deterioration | 3 | 8% | 3 | 7% |
| Assessment Inadequate | 2 | 5% | 9 | 20% |
| Total | 38 | 100% | 45 | 100% |
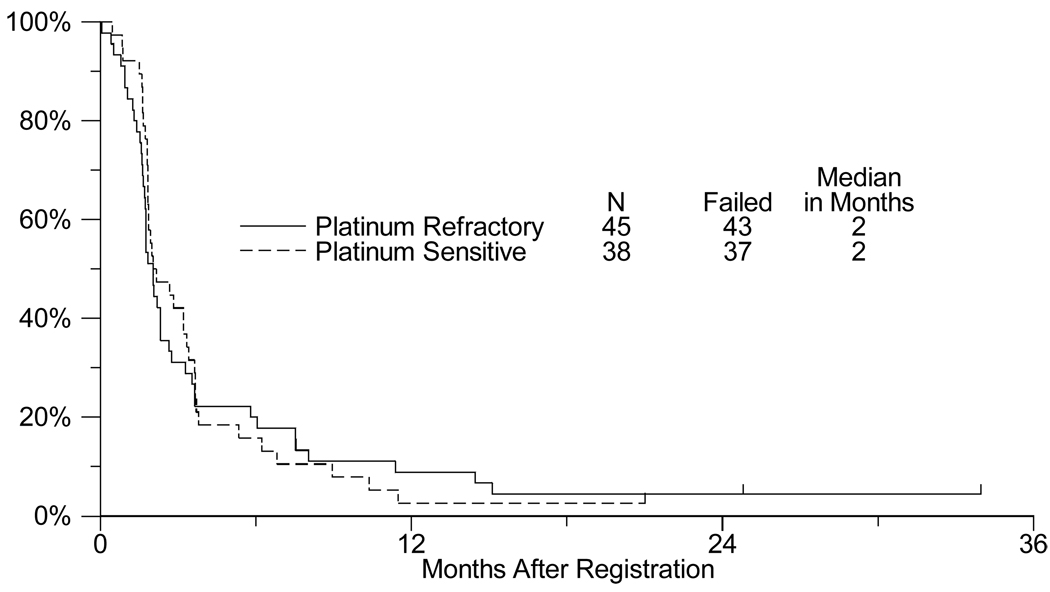
The Kaplan-Meier estimates for progression-free survival (PFS) are presented in Figure 1. The median PFS estimate for the platinum-refractory stratum was 2.0 months (95% confidence interval: 1.7 – 2.2 months), and the estimated median PFS for the platinum sensitive stratum was 2.2 months (95% confidence interval: 1.8 – 3.2 months).
Fig. 1.
Progression-free survival in platinum refractory vs. platinum sensitive patients with extensive stage small cell lung cancer treated with sorafenib
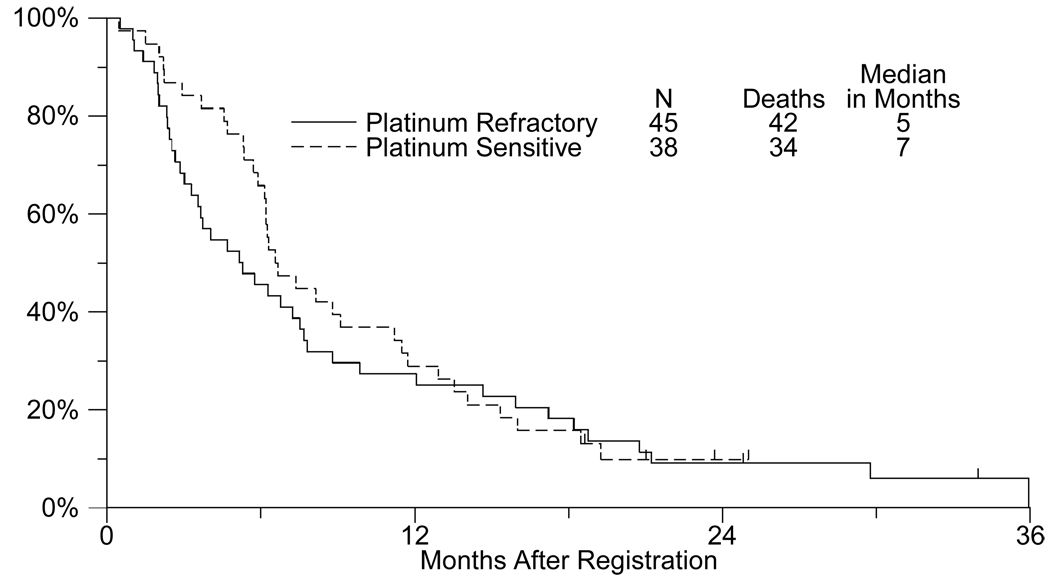
Overall survival is presented in Figure 2. The median overall survival estimates were 5.3 months (95% confidence interval: 3.3–7.5 months) and 6.7 months (95% confidence interval: 6.1–9.1 months) for the platinum-refractory and platinum-sensitive strata respectively.
Fig. 2.
Overall survival in platinum refractory vs. platinum sensitive patients with extensive stage small cell lung cancer treated with sorafenib
Toxicity
Eighty-two patients were assessed for adverse events related to treatment. There was one treatment-related death due to pancreatitis in the platinum-refractory stratum. Two additional patients in the platinum-refractory stratum experienced treatment-related grade 4 adverse events: one due to elevated lipase, and one due to nausea, vomiting, and dehydration. One patient in the platinum-sensitive stratum experienced treatment-related grade 4 adverse events including fatigue, dizziness, and dyspnea. The most common treatment related adverse events exceeding grade 2 were dermatologic (22 patients, none grade 4) and constitutional symptoms (9 patients, one grade 4). A summary table of treatment-related adverse events is presented in Table 4.
Table 4.
Adverse Events by Platinum Sensitivity
| Platinum sensitive (n=38) |
Platinum refractory (n=44) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade |
Grade |
|||||||
| ADVERSE EVENT | 2 | 3 | 4 | 5 | 2 | 3 | 4 | 5 |
| Allergy/immunology | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Blood/Bone Marrow | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Cardiac General | 3 | 3 | 0 | 0 | 3 | 0 | 0 | 0 |
| Coagulation | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Constitutional symptoms | 12 | 4 | 1 | 0 | 15 | 5 | 0 | 0 |
| Dermatology/Skin | 11 | 11 | 0 | 0 | 14 | 11 | 0 | 0 |
| Gastrointestinal | 17 | 2 | 0 | 0 | 16 | 3 | 1 | 0 |
| Hepatobiliary/Pancreas | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Infection | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Metabolic/Laboratory | 2 | 4 | 0 | 0 | 2 | 4 | 1 | 0 |
| Musculoskeletal/Soft Tissue | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Neurology | 6 | 2 | 1 | 0 | 1 | 3 | 0 | 0 |
| Pain | 6 | 3 | 0 | 0 | 3 | 4 | 0 | 0 |
| Pulmonary/Upper Respiratory | 1 | 2 | 1 | 0 | 3 | 1 | 0 | 0 |
| MAXIMUM GRADE ANY ADVERSE EVENT | ||||||||
| Number | 10 | 23 | 1 | 0 | 15 | 20 | 2 | 1 |
DISCUSSION
In this multi-center cooperative group Phase II clinical trial, single agent sorafenib induced an objective response in five patients; one with platinum-refractory disease, and four with platinum sensitive disease. The overall survival of 5.3 months (refractory/resistant) and 6.7months (sensitive), and the overall PFS of 2 months are similar to, or inferior to studies using chemotherapy in this setting. The survival influence of subsequent lines of therapy was not tracked in our trial and would be difficult to interpret in any event due to the limited sample size. Clinical trials of topotecan in sensitive relapsed SCLC have yielded time to progression of approximately 3 months and median survival of 6 to 8 months (2, 6, 7). Topotecan in resistant/refractory disease has yielded median survival of 5–6 months and TTP of about 3 months (3, 8, 9). Other cytotoxic agents show promise for the treatment of relapsed SCLC, particularly amrubicin, a fully synthetic anthracycline, which seems to be active in both sensitive and resistant SCLC. Ongoing Phase III trials are evaluating this agent in either the first-line setting of extensive-stage or relapsed disease (10).
The adverse events and toxicity profile noted in this study are similar to other reported phase II clinical trials of sorafenib. The 23% of patients that were taken off study due to adverse events are comparable to other clinical trials studying sorafenib in patients with hepatocellular and thyroid carcinoma (11, 12).
The five objective responses seen in our trial serve as evidence of therapeutic activity of tyrosine kinase inhibition in SCLC in this small subset of patients. However, even for those responding, this benefit was short-lived. Clearly the majority of patients were quite resistant to sorafenib. We can only speculate on the potential mechanisms of sensitivity and resistance operative in these patients.
Sorafenib was initially identified as an inhibitor of Raf serine/threonine kinase isoforms. At the initiation of this trial, the frequency of activating oncogenic mutation of B-Raf in SCLC was not characterized. In a recent study of 104 human tumor cell lines, only 1 of 34 (2.9%) SCLC cell lines was found to have the T1796A transversion, which confers constitutive kinase activity(13). However, activation of Ras oncogene signaling is considered an important mechanism by which human cancer develops and through which the malignant phenotype is maintained. Raf kinase is involved in the Ras signal transduction pathway regulating several key pathways inducing cellular transformation, including the Raf/Mek/Erk cascade (14). In vivo sorafenib demonstrated activity against several human tumor xenografts both wild type and mutant for Kras (15).
Sorafenib is currently FDA approved for the treatment of advanced renal cell carcinoma and unresectable hepatocellular carcinoma. Cellular signaling mediated by VEGF pathways has been implicated in the molecular pathogenesis of both HCC and RCC (16, 17) and inhibition of VEGFR-2 is felt to be the primary mechanism of antitumor effect for sorafenib in these malignancies. Current evidence suggests that angiogenesis also plays an important role in SCLC growth and regulation. Functional VEFGR-2 and VEGFR-3 has been found in human SCLC cell lines (18). Clinical investigations have correlated elevated pre-treatment serum levels of VEGF and basic fibroblast growth factor to poor response to chemotherapy and shortened survival in SCLC patients (19). In addition to direct inhibition of VEGFR-2, sorafenib may also exert anti-angiogenic effects through inhibition of Raf-1. Recent evidence suggests VEGF and/or basic fibroblast growth factor activation of Raf-1 results in protection from distinct pathways of apoptosis in human endothelial cells (20).
Based on the data discussed above, including the rarity of Raf mutations in SCLC, and the activity of sorafenib in renal cell carcinoma and hepatocellular cancer, one might speculate the objective responses seen in our SCLC population were due to anti-angiogenic effects. Thalidomide, an agent with both immunomodulatory and anti-angiogenic properties has been tested in SCLC. A phase III trial of cisplatin plus etoposide with or without thalidomide in patients with limited or E-SCLC failed to improve survival in 724 randomized patients (21). Clinical trials of tyrosine kinase inhibitors targeting the VEGFR have been explored in SCLC. Cediranib is a potent inhibitor of VEGFR-1, 2 and 3 tyrosine kinases. A Phase II study evaluated cediranib in patients with progressive SCLC following 1 prior platinum based regimen (22). Of 25 treated patients, there was 1 unconfirmed partial response and the trial was terminated for not meeting its predefined efficacy goal. Serial plasma VEGF levels did not correlate with anti-cancer effects in the small cohort of patients. A Phase I trial to assess the safety and tolerability of cediranib in combination with etoposide and cisplatin as first line therapy for SCLC has been completed and had promising efficacy (J. Heymach, personal communication), and the Southwest Oncology Group is planning a Phase II/III trial of this combination. A randomized phase II study using vandetanib, a dual pathway inhibitor of VEGFR and EGFR, as maintenance after objective response to first line platinum based chemotherapy in SCLC, showed no benefit in terms of overall survival or PFS when compared with placebo (23). A Phase I/II trial combining sunitinib with etoposide and cisplatin as first line therapy for extensive stage SCLC is ongoing via the Cancer and Leukemia Group B (CALGB 30504, NCT00453154).
Likewise monoclonal antibodies targeting VEGF pathways have been explored in SCLC trials. Two phase II studies of irinotecan plus platinum combined with bevacizumab in untreated extensive stage SCLC suggested modest improvements in response and survival relative to historical controls, but they did not achieve the outcomes necessary to move on to phase III trials. (24, 25).
In related pathways, up to 70% of SCLC tumors also express cKIT and its ligand SCF resulting in a functional autocrine growth loop (18, 26). Imatinib, an inhibitor of the cKIT tyrosine kinase enzyme was evaluated in a Phase II clinical trial of patients with SCLC and either chemotherapy-naive extensive-stage disease or sensitive relapse (27). There were no objective responses noted in 19 patients. Tumor tissue samples from 4 of the 19 patients (21%) had the KIT receptor (CD117). In two similar Phase II trials, patients with progressive SCLC with c-KIT positive tumors were treated with single agent imatinib. In one trial, no responses were seen in 12 patients and all had progressed by 4 weeks (28). In the other, no objective responses and no confirmed stable disease ≥6 weeks were seen (29). When combined with irinotecan and cisplatin in two Phase I studies, imatinib was found to statistically decrease irinotecan clearance (30). A high incidence of hematologic and gastrointestinal toxicity prohibited dose escalation. Although 5 out of 6 evaluable patients experienced a partial response, the pharmacologic interaction and intolerance precluded further development.
Based on the lack of disease control seen in our trial, further investigation of single-agent sorafenib in the SCLC population is not recommended. The clinical promise of combining chemotherapy with targeted agents in SCLC in some instances, however, has been tempered by safety and pharmacokinetic concerns. Recently, a Phase I trial combining sorafenib with topotecan was suspended due to excessive grades 3 and 4 thrombocytopenia (personal communication DR Leach). However, a phase I/II trial combining sorafenib with cisplatin and etoposide in ES-SCLC is recruiting (NCT00726986). The introduction of novel agents into clinical trials in SCLC and other malignancies will continue to pose many challenges, such as the ability to safely combine with other drugs at active doses, defining therapeutic endpoints and prediction of efficacy based on target expression.
Acknowledgments
Support: This work was supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services: CA32102, CA38926, CA105409, CA35261, CA45807, CA35178, CA76462, CA35431, CA35090, CA67575, CA45560, CA63850, CA86790, CA11083, CA12644, CA67663, CA20319, CA76448, CA76447, CA45808, CA42777, CA27057, CA58861, CA35119, CA04919, CA68183, CA63848, CA45377, CA35176, CA46282, CA46113, CA35128, CA37981, CA58882, CA22433, CA073590
Footnotes
Results presented in part by Dr. Barbara J. Gitlitz, M.D. at the 44nd Annual Meeting of the American Society of Clinical Oncology, May 30–June 3, 2008, Chicago, Illinois
Contributor Information
Barbara J. Gitlitz, University of Southern California, Los Angeles, CA
James Moon, Southwest Oncology Group Statistical Center, Seattle, WA
Bonnie S. Glisson, University of Texas/MD Anderson Cancer Center, Houston, TX
H. Joachim Reimers, St. Louis University, St. Louis, MO
Martin J. Bury, Grand Rapids CCOP, Grand Rapids, MI
Justin D. Floyd, Central Illinois CCOP, Effingham, IL.
Thomas K. Schulz, University of Kansas School of Medicine/Wichita CCOP, Wichita, KS
P. Kothai Sundaram, Columbus CCOP, Westerville, OH
Christopher Ho, University of Southern California, Los Angeles, CA
David R. Gandara, University of California at Davis, Sacramento, CA
REFERENCES
- 1.Jemal A, Siegel R, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25(15):2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24(34):5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statistical Assn. 1958;53:457–481. [Google Scholar]
- 5.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 6.von Pawel J, Gatzemeier U, Pujol JL, et al. Phase II comparator study of oral versus intravenous topotecan in patients with chemosensitive small-cell lung cancer. J Clin Oncol. 2001;19(6):1743–1749. doi: 10.1200/JCO.2001.19.6.1743. [DOI] [PubMed] [Google Scholar]
- 7.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17(2):658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 8.Ardizzoni A, Manegold C, Debruyne C, et al. European organization for research and treatment of cancer (EORTC) 08957 phase II study of topotecan in combination with cisplatin as second-line treatment of refractory and sensitive small cell lung cancer. Clin Cancer Res. 2003;9(1):143–150. [PubMed] [Google Scholar]
- 9.Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative. J Clin Oncol. 1997;15(5):2090–2096. doi: 10.1200/JCO.1997.15.5.2090. [DOI] [PubMed] [Google Scholar]
- 10.Onoda S, Masuda N, Seto T, et al. Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol. 2006;24(34):5448–5453. doi: 10.1200/JCO.2006.08.4145. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(26):4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 12.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27(10):1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masatsugu U, Eisaku T, Osamu N, et al. Mutational analysis of the BRAF gene in human tumor cells. Human Cell. 2008;21(2):13–17. doi: 10.1111/j.1749-0774.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- 14.Chong H, Vikis HG, Guan KL. Mechanisms of regulating the Raf kinase family. Cellular Signalling. 2003;15(5):463–469. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 16.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–880. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Escudier B, Eisen T, Stadler W, et al. Sorafenib in Advanced Clear Cell Renal Cell Carcinoma. N Engl J med. 2007;357(2):203. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 18.Tanno S, Ohsaki Y, Nakanishi K, Toyoshima E, Kikuchi K. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer. 2004;46(1):11–19. doi: 10.1016/j.lungcan.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Salven P, Ruotsalainen T, Mattson K, Joensuu H. High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer. Int J Cancer. 1998;79(2):144–146. doi: 10.1002/(sici)1097-0215(19980417)79:2<144::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301(5629):94–96. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- 21.Lee SM, Woll PJ, Rudd R, et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2009;101(15):1049–1057. doi: 10.1093/jnci/djp200. [DOI] [PubMed] [Google Scholar]
- 22.Ramalingam S, Mack P, Vokes E, et al. Cediranib (AZD2171) for the treatment of recurrent small cell lung cancer (SCLC): A California Consortium phase II study (NCI # 7097) Proc Am Soc Clin Oncol. 2008 abst 7097. [Google Scholar]
- 23.Arnold AM, Smylie M, Ding K, et al. Randomized phase II study of maintenance vandetanib (ZD6474) in small cell lung cancer (SCLC) patients who have a complete or partial response to induction therapy: NCIC CTG BR.20 [2007 ASCO Annual Meeting Proceedings Part I]; J Clin Oncol; p. 7522. [Google Scholar]
- 24.Ready N, Dudek AZ, Wang XF, et al. CALGB 30306: a phase II study of cisplatin (C), irinotecan (I) and bevacizumab (B) for untreated extensive stage small cell lung cancer (ES-SCLC) [2007 ASCO Annual Meeting Proceedings Part I] J Clin Oncol. 2007;25(suppl)(18S):7563. doi: 10.1200/JCO.2011.35.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spigel DR, Hainsworth JD, Burris HA, et al. Irinotecan, carboplatin, and bevacizumab in untreated extensive-stage small-cell lung cancer. J Thorac Oncol. 2007;2(suppl 4)(8):S391. doi: 10.1097/JTO.0b013e3181bbc540. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd KP, Krystal GW. Role of small-molecule kit receptor tyrosine kinase inhibitors in the treatment of small-cell lung cancer. Clin Lung Cancer. 2002;3(3):213–218. doi: 10.3816/clc.2002.n.005. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BE, Fischer T, Fischer B, et al. Phase II study of imatinib in patients with small cell lung cancer. Clin Cancer Res. 2003;9(16 Pt 1):5880–5887. [PubMed] [Google Scholar]
- 28.Krug LM, Crapanzano JP, Azzoli CG, et al. Imatinib mesylate lacks activity in small cell lung carcinoma expressing c-kit protein: a phase II clinical trial. Cancer. 2005;103(10):2128–2131. doi: 10.1002/cncr.21000. [DOI] [PubMed] [Google Scholar]
- 29.Dy GK, Miller AA, Mandrekar SJ, et al. A phase II trial of imatinib (ST1571) in patients with c-kit expressing relapsed small-cell lung cancer: a CALGB and NCCTG study. Ann Oncol. 2005;16(11):1811–1816. doi: 10.1093/annonc/mdi365. [DOI] [PubMed] [Google Scholar]
- 30.Johnson FM, Krug LM, Tran HT, et al. Phase I studies of imatinib mesylate combined with cisplatin and irinotecan in patients with small cell lung carcinoma. Cancer. 2006;106(2):366–374. doi: 10.1002/cncr.21640. [DOI] [PubMed] [Google Scholar]