Abstract
Purpose
The purpose of this study was to compare heart rate variability (HRV) in low risk preterm infants to one infant diagnosed with intraventricular hemorrhage (IVH).
Method
A case study design was used to compare HRV of one subject diagnosed with IVH to a convenience sample of 38 low-risk preterm infants at 30 and 31 post-menstrual weeks of age. Heart periods were recorded for 300-s with the infant in an active sleep state. Heart rate variability was quantified by spectral analysis. A confidence interval comparison of the total spectral components (0.02–2.0 Hz), high frequency components (0.20–2.0 Hz), and the low frequency components (0.02–0.20 Hz) was conducted.
Findings
At 30 weeks’ post-menstrual age, 10-days following diagnosis with a grade-III IVH, the low frequency components were above the 90th percentile. One week later, at 31 weeks, the low frequency components had decreased to the 27th percentile range and the total and high frequency components were at or below the 25th percentile range of the confidence intervals for the low-risk preterm infants.
Discussion
The neurobehavioral organization of preterm infants is limited, due to prematurity and the cumulative effect of medical complications (such as IVH). This study has implications for the use of HRV in the identification of infants diagnosed with IVH.
Keywords: Premature Infant, Heart Rate Variability, Intraventricular Hemorrhage
IVH remains a significant problem for preterm infants, potentially leading to increased length of hospital stay, overall long-term healthcare costs, long-term neurologic impairment, and decreased survival (Inder et al., 2005; Ward & Beachy, 2003). Early identification of infants at-risk for IVH is therefore needed in order to reduce costs (Chang, Lin, Lin, & Yeh, 2000) and long-term impairments (Vohr, O’Shea & Wright, 2003; Vollmer et al, 2003). The purpose of this study was to investigate heart rate variability (HRV) as a tool for early identification of infants at-risk for IVH by comparing normative changes in HRV (obtained by spectral analysis) to that of one infant diagnosed with IVH.
Management begins in the perinatal period, with the prevention of preterm birth, birth asphyxia, and birth trauma. Prompt resuscitation at birth minimizes hypoxemia and hypercarbia, which can alter cerebral autoregulation during the initiation of an intraventricular or periventricular bleed (Blackburn, 1998; Bassan et al., 2006). Activities that increase intracranial pressure or cause wide swings in pressure are minimized in the first 72 hours of life—if possible. Specific interventions to reduce or prevent risk of increased pressure include avoiding hypoxic events, rapid alterations in cerebral blood flow, and systemic blood pressure. These include positioning the head in the midline and with the head of the bed elevated; avoiding tight bands around the head; avoiding rapid infusion for volume expansion; frequent monitoring of blood pressure; suctioning of the endotracheal tube only when needed; maintaining the infant within the neutral thermal range; avoiding interventions that cause crying, such as frequent venipunctures; a lot of manipulation or handling; using analgesics for stressful procedures; and avoiding interventions that cause hypoxia (Blackburn, 1998). Early initiations of such measures, however, require identification of increased intracranial pressure.
More than 90% of infants with increased intracranial pressure secondary to IVH are identified by head ultrasound within the first 72 hours after birth, with 50% identified during the first 24 hours after birth (Blackburn, 1998). However, because the signs and symptoms of IVH vary widely and are sometimes subtle, a referral for head ultrasound frequently does not occur until after a catastrophic bleed has been identified. Volpe (1987) has described three clinical syndromes: silent, catastrophic, and saltatory (Blackburn, 1998). Most infants are at the silent end of this continuum, where there are no clinical signs. The catastrophic syndrome involves major hemorrhages that evolve rapidly over minutes to hours. Clinical findings include stupor progressing to coma, and respiratory distress progressing to apnea, seizures, decerebrate posturing, fixation of pupils, and flaccid quadriparesis. This is associated with a decreasing hematocrit, bulging anterior fontanelle, hypotension, bradycardia, and hypoglycemia. The saltatory pattern is associated with small hemorrhages that develop over hours to days. Signs and symptoms are subtle and irregular. Some clinical signs may include hypotonia, abnormal eye movements or positions, and an unexplained decrease in hematocrit by 10% or more (Blackburn, 1998).
Specific measures of HRV may be a potentially useful tool for identifying increased intracranial pressure when other clinical signs are not present. Hanna and colleagues (2000) investigated the relationship between HRV and IVH, and reported that IVH was consistently correlated with diminished measures of HRV, length of hospital stay, and neurodevelopmental function (Hanna et al, 2000). Intraventricular hemorrhage was highly correlated with lower measures of RMDS (Root Mean Square Deviation) or a pattern of HRV that reflects parasympathetic control of HRV (like the high frequency components obtained by spectral analysis) (see Table 1). Compared to infants with IVH, low-risk infants had greater parasympathetic control of HRV and had shorter lengths of stay in the hospital (Hanna et al, 2000). The correlation between IVH and HRV was, however, performed approximately 8 weeks after birth without clarification of how much time had incurred since diagnosis of the IVH. The purpose of this case study was therefore to compare HRV (using spectral analysis) in low-risk preterm infants to that of one infant more recently diagnosed with IVH or within 10-days of diagnosis.
Table 1.
A Description of Spectral Frequencies for Heart Rate Variability
| Name | Frequency Range (Hz) | Control |
|---|---|---|
| Total | 0.02–2.0 | An estimate of the overall variability in the entire signal. |
| Low | 0.02–0.20 | An estimate of primarily sym pathetic control of HRV. |
| High | 0.20–2.0 | Occur in response to respirations and an estimate of parasympathetic control of HRV (De Rogalski Landrot, 2007; Kimura et al, 1996; Metsala, Siimes, & Valimaki, 1995). |
Case Study
This case study was conducted as part of a larger study entitled, Heart Rate Variability and Learning in the Premature Infant. Following Institutional Review Board approval, mothers were asked to give informed consent for their infants to participate. Infant Girl A is one infant (out of the 38 infants enrolled) diagnosed with IVH.
Infant A was born to a 21 year-old Caucasian, multiparous mother (G 6 P 4105) who had graduated from high school. Admission history to labor and delivery revealed suspected sepsis and spontaneous rupture of membranes for >48 hours before birth. The mother was treated with clindamycin < 8 hours before a spontaneous (vertex presentation) vaginal delivery.
Infant A was delivered at 28 weeks’ gestation (confirmed by a 19-week ultrasound) and weighed 1350 grams with apgar scores of 3, 7, and 6 at 1, 5, and 10 minutes. During immediate care following birth, Infant A was intubated and taken to the NICU with respiratory distress and suspected sepsis. By day 1 of life, a grade III IVH was diagnosed and described as a slight left ventricular bleed. The IVH progressed to both ventricles by the fifth day.
Care at 30 and 31 weeks’ post-menstrual age consisted of HAL with lipid supplementation for nutrition. airway assistance by room air at 30 weeks, and nasal cannula at 31 weeks of age. Medications administered during the weeks of testing consisted of caffeine, ampicillin, gentamicin, and Infasurf. No surgeries were performed until after the test sessions were completed, when a VP shunt was placed.
Method
Procedure
The current study compared the HRV of low-risk preterm infants (in the larger study), to one infant diagnosed with IVH (Infant A) at 30 and 31 weeks’ post-menstrual age. Study procedures occurred during weekly test sessions and were performed for all infants while they remained in their assigned incubator beds. Recordings of infants' HRV were initiated at least 15 minutes following a meal and once they were determined to be in an active sleep state of the sleep-wake cycle (Thoman, 1990). Recordings of the ECG lasted 300-s and occurred at approximately the same time of day for each infant (between 10 AM–12 PM) in an attempt to control for potential circadian influences on heart rate patterns and movements (Arduini et. al., 1995).
Cardiac data collection system
The electrocardiogram signal (ECG) was sampled at equal intervals for 300-s at a rate of 500 Hz . The signal was transferred using ECG electrodes attached in the standard three-lead manner (via an RS232 interface and software) from an Agilent Neonatal monitor (Model #1092A) to a Dell Inspiron 8100 laptop. A Matlab program was used to filter or smooth the signal by removing noise components and baseline wander. The filtered signal was then passed through a QRS detection algorithm (also implemented in Matlab), resulting in a time series of R-R intervals, or heart periods.
Spectral analysis of heart periods
Spectral analysis of the heart periods was performed off-line using a Lomb procedure (Lomb, 1976). The total or entire spectrum (0.02–2.0 Hz) was examined except for very low frequencies (0.0–0.02 Hz), since these components are typically produced by slow-trend artifacts (or noise). The high frequency components (0.20–2.0 Hz)—occurring in response to respirations and controlled only by the parasympathetic nervous system. The low frequency components (0.02–0.20 Hz) reflecting a mix of sympathetic and parasympathetic control (Akselrod, 1981; Chatow, Davidson, Reishman, & Akselrod, 1995; Pomeranz et al., 1985; Reed, Ohel, David, & Porges, 1999).
Sleep-wake criteria
Criteria for use with preterm infants (Thoman, 1990) was employed to detect an active sleep state during each test session and was confirmed post-hoc by also evaluating infants’ range of short-term heart rate variability (Nijhuis et al., 1982). Infants were determined to be in an active sleep state if: 1) respirations were irregular, 2) no body movement was present, 3) eyes were closed, and 4) heart rate variability ≤5 beats per minute (performed post-hoc). Interrater reliability in state detection was maintained at ≥ 90% agreement.
Findings
Low-risk preterm infants
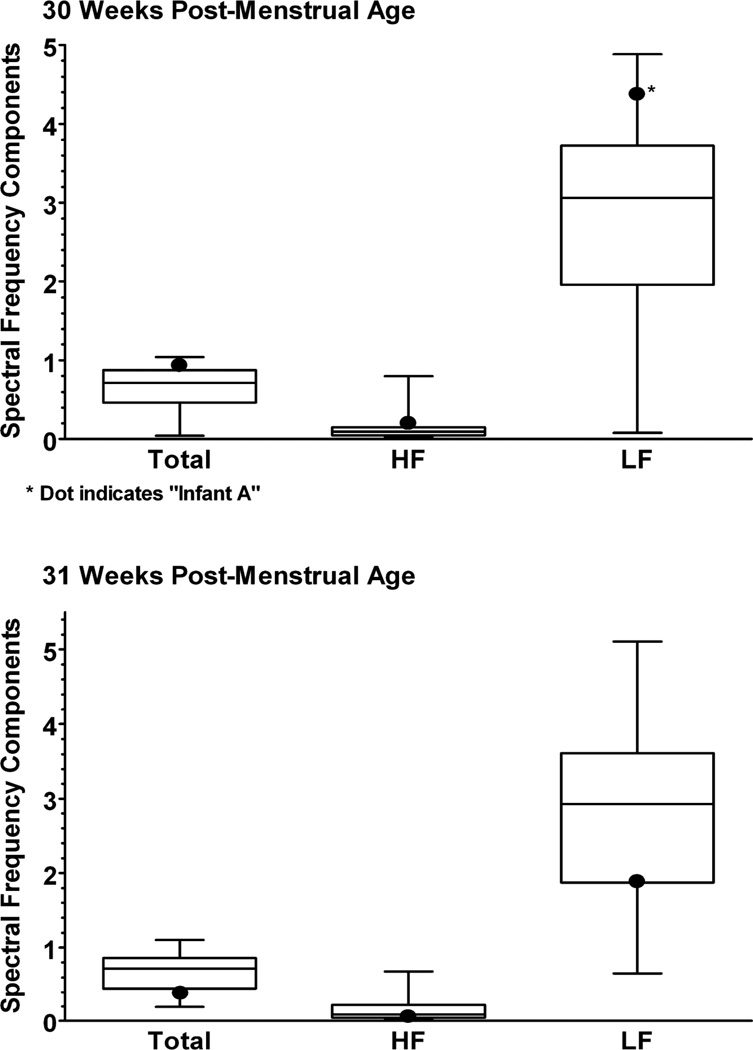
The overall mean, range and standard deviation for the low-risk subjects at 30 weeks post-menstrual age was as follows: low frequency (M=2.84, sd=1.25, range=0.08–4.88); high frequency (M=0.18, sd=0.23, range=0.03–0.80); total spectrum (M=0.68, sd=0.26, range=0.04–1.04). The overall mean, range and standard deviation for the low-risk subjects at 31 weeks’ post-menstrual age was as follows: low frequency (M=2.76, sd=1.10, range=0.65–5.10); high frequency (M=0.18, sd=0.22, range=0.0.02–0.68); total spectrum (M=0.66, sd=0.25, range=0.19–1.10).
Infant A
At 30 weeks for infant A, the low (4.37 Hz) frequency value was at the 91st percentile. Total (0.97 Hz) and high (0.20 Hz) frequency values were the 86th and 82nd percentile, respectively. Percentiles divide the data into 100 equal parts, each representing one percent of all the values. For infant A at 31 weeks, the low (1.86 Hz) frequency and total (0.38 Hz) values were at the 27th and 19th percentiles, respectively. The high (0.04 Hz) frequency value had similarly decreased to the 23th percentile (see Figure 1).
Figure 1. Heart Rate Variability at 30 and 31 Weeks of Age.
All outcomes (total, high, and low frequency components) were above the median at 30 weeks’ post-menstrual age and below the median at 31 weeks’ post-menstrual age.
Note. Total= Total range of frequency components; HF= High frequency range of components; LF= Low frequency range of components.
Discussion
Within 10-days of diagnosis with a grade-III IVH, infant A exhibited measures of HRV above the 50th percentile range of the low-risk subjects. One week later (31 weeks’ post-menstrual age), the infant continued to exhibit measures of HRV outside the 50th percentile range; however, they were then in the lower 25th percentile. While case studies are limited by generalizability (Luck, Jackson, & Usher, 2006), these findings substantiate reported lower high frequency component values 8 weeks following birth in infants with IVH (Hanna et al., 2000). These findings, suggest the need for longitudinal descriptions of how changes in HRV occur for infants diagnosed with IVH.
Previous research describing normative trends in HRV suggests there is a general shift from no change in the high frequency components (prior to 28 weeks) to an increase in high frequency components between 31 to 40 post-menstrual weeks (Chatow et al., 1995; Clairambault, Curzi-Dascalova, Kauffmann, Medigue, & Leffler, 1992; Sahni et al., 2000). The current case study, with its trend of relative decrease in high frequency components and increase in low frequency components between 30–31 weeks’ post-menstrual age, demonstrates the usefulness of normative descriptions during this time period.
Findings reported here at 31 weeks’ post-menstrual age (or 17 days following diagnosis of IVH), are further supported by work conducted by Porges and colleagues, who used spectral analysis of HRV as a tool for differentiating between low and high risk status in the newborn and preterm infant (DiPietro, O'Brien Caughy, Cusson & Fox, 1994; Doussard-Roosevelt, McClenny, & Porges, 2001; Fox, & Porges, 1985; Porges, Doussard Roosevelt, Portales, & Suess, 1994). Porges et al. demonstrated significant relationships between high frequency components of HRV and developmental outcomes, suggesting that lower values are related to greater risk status of the infant (greater days hospitalized/on respiratory support, less weight gain, and lower Bayley scores at 1-year of age) (DiPietro et al., 1994; Doussard-Roosevelt et al., 2001; Fox, & Porges, 1985; Porges et al., 1994).
Additional factors potentially impacting the study outcome were also investigated. Previous critiques of studies involving preterm infants have included calls to address the impact of varied levels of respiratory support, and medications provided, (Philbin & Klass, 2000). Post-hoc analyses of the normative data provided by low-risk subjects revealed that none of these factors significantly interacted with the primary outcome variable (HRV).
Clinical Implications
The vast majority of infants do not display any behavioral changes prior to significant intracranial bleeds (Blackburn, 1998). As caretakers of preterm infants, however, we can educate ourselves to detect the subtle changes in behavior that may precede the development of intraventricular hemorrhage. These changes may include hypotonia, abnormal eye movements or positions, and/or an unexplained decrease in hematocrit by 10% or more (Blackburn, 1998). Beyond this, with increased involvement of parents at the bedside, providing educational information has become a necessary component of any infant’s care. It is therefore essential that methods to limit increased intracranial pressure are taught and explanations for these actions provided (see Table 2).
Table 2.
Information Useful for Parents of Infants Diagnosed with IVH
| • Keep head positioned midline and slightly elevated |
| • Avoid tight head bands |
| • Keep from extreme temperatures |
| • Avoid crying |
| • Avoid frequent handling |
The ability to identify increased risk for IVH, along with the possibility of intervening sooner to decrease negative outcomes, is of vital importance to those providing care for preterm infants, but new tools are needed. This case study provides important supportive evidence that measures of HRV may prove useful in detecting IVH earlier. HRV was measured within 10 days of diagnosis of IVH with comparisons to low-risk preterm infants revealing substantial differences. While future studies are needed to increase confidence in the findings, studies like the one reported here highlight the fragile status of the preterm infant. Case study designs, fortunately, allow the researcher to begin exploring situations (like IVH) that do not come about frequently (Luck et al., 2006).
Acknowledgments
The material was funded by the National Institute of Health (NIH/NINR P20 NR07791, NIH/NCRR MO1 RR00082)
Contributor Information
Charlene A. Krueger, University of Florida, Assistant Professor Gainesville Florida.
Elizabeth A. Gyland, University of Florida, Doctoral Student Gainesville, Florida Wolfson Children’s Hospital Jacksonville, Florida.
Douglas Theriaque, University of Florida, Statistician General Clinical Research Center Gainesville, Florida.
References
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, Cohen RJ. Power spectral analysis of heart rate fluctuation: A quantitative probe of beat to beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Arduini D, Rizzo G, Romanini C. Fetal behavioral states and behavioral transitions in normal and compromised fetuses. In: Lecanuet JP, Fifer W, Krasnegor N, Smotherman W, editors. Fetal development: A psychobiological perspective. Hillsdale, NJ: Erlbaum; 1995. [Google Scholar]
- Bassan H, Feldman H, Limperopoulos C, Benson c, Ringer S, Veracruz E, Soul J, Volpe J, du Plessis A. Periventricular hemorrhagic infarction: Risk factors and neonatal outcome. Pediatric Neurology. 2006;35:85–92. doi: 10.1016/j.pediatrneurol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Blackburn ST. Assessment and management of neurologic dysfunction. In: Kenner C, Lott JW, Flandermeyer AA, editors. Comprehensive Neonatal Nursing. A Physiologic Perspective. Philadephia: WBSaunders Company; 1998. pp. 564–607. [Google Scholar]
- Chang SC, Lin CH, Lin JJ, Yeh TF. Mortality, morbility, length and cost of hospitalization in very-low-birth-weight infants in the era of National Health Insurance in Taiwan: a medical center’s experience. Acta Paediatr Taiwan. 2000;41(6):308–312. [PubMed] [Google Scholar]
- Chatow U, Davidson S, Reichman B, Akselrod S. Development and maturation of the autonomic nervous system in premature and term infants using spectral analysis of heart rate fluctuations. Pediatric Research. 1995;37:294–302. doi: 10.1203/00006450-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Clairambault J, Curzi-Dascalova L, Kauffmann F, Medigue C, Leffler C. Heart rate variability in normal sleeping full-term and preterm neonates. Early Human Development. 1992;28:169–183. doi: 10.1016/0378-3782(92)90111-s. [DOI] [PubMed] [Google Scholar]
- De Rogalski Landrot I, Roche F, Pichot V, teyssier G, Gaspoz J-M, Barthelemy JC, Patural H. Autonomic nervous system activity in premature and fullterm infants from theoretical term to 7 years. Autonomic Neuroscience. 2007;136(1–2):105–109. doi: 10.1016/j.autneu.2007.04.008. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, O'Brien Caughy M, Cusson R, Fox NA. Cardiorespiratory functioning of preterm infants: Stability and risk associations for measures of heart rate variability and oxygen saturation. Developmental Psychobiology. 1994;27:137–152. doi: 10.1002/dev.420270302. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt J, McClenny B, Porges S. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Developmental Psychobiology. 2001;38:56–66. [PubMed] [Google Scholar]
- Fox N, Porges S. The relationship between neonatal heart period patterns and developmental outcome. Child Development. 1985;56:28–37. [PubMed] [Google Scholar]
- Hanna BD, Nelson MN, White-Traut RC, Silvestri JM, Vasan U, Meleedy Rey P, Patel MK, Comiskey E. Biology of the Neonate. 2000;77:147–155. doi: 10.1159/000014209. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura K, Watanabe T, Murotsuki J, Suzuki T, Yano M, et al. Power spectral analysis for autonomic influences in heart rate and blood pressure variability in fetal lambs. American Journal of Physiology. 1996;271:H1333–H1339. doi: 10.1152/ajpheart.1996.271.4.H1333. [DOI] [PubMed] [Google Scholar]
- Metsala T, Siimes A, Valimaki I. The effect of change in sympatho-vagal balance on heart rate and blood pressure variability in the foetal lamb. Acta Physiologica Scandinavica. 1995;154(2):85–92. doi: 10.1111/j.1748-1716.1995.tb09890.x. [DOI] [PubMed] [Google Scholar]
- Lomb NR. Least-squares frequency analysis of unequally spaced out data. Astrophysics and Space Science. 1976;39:447–462. [Google Scholar]
- Luck L, Jackson D, Usher K. Case study: A bridge across the paradigms. Nursing Inquiry. 2006;13(2):103–109. doi: 10.1111/j.1440-1800.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- Nijhuis J, Prechtl H, Martin C, Bots R. Are there behavioural states in the human fetus? Early Human Development. 1982;6 doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- Philbin MK, Klass P. Hearing and behavioral responses to sound in full-term newborns. Journal of Perinatology. 2000;20(8):S68–S76. doi: 10.1038/sj.jp.7200441. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Macauley R, Caudil M, Kutz I, Adam D, Gordon D, Kilborn K. Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology. 1985;17:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Porges S, Doussard-Roosevelt J, Portales A, Suess P. Cardiac vagal tone: Stability and relation to difficulties in infants and 3-year-olds. Developmental Psychobiology. 1994;27:289–300. doi: 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Reed SF, Ohel G, David R, Porges SW. A neural explanation of fetal heart rate patterns: a test of the Polyvagal theory. Developmental Psychobiology. 1999;35:108–118. [PubMed] [Google Scholar]
- Sahni R, Schulze K, Kashyap S, hira-Kist K, Fifer W, Myers M. Maturational changes in heart rate and heart rate variability in low birth weight infants. Developmental Psychobiology. 2000;37:73–81. doi: 10.1002/1098-2302(200009)37:2<73::aid-dev2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Thoman E. Sleeping and waking states in infancy: A functional perspective. Neuroscience and Behavioural Reviews. 1990;14:93–107. doi: 10.1016/s0149-7634(05)80165-4. [DOI] [PubMed] [Google Scholar]
- Vohr B, O'Shea M, Wright L. Longitudinal multicenter follow-up of high-risk infants: Why, who, when, and what to assess. Seminars in Perinatology. 2003;27(4):333–342. doi: 10.1016/s0146-0005(03)00045-4. [DOI] [PubMed] [Google Scholar]
- Vollmer B, Roth S, Baudin S, Stewart AL, Neville BGR, Wyatt JS. Predictors of long term outcome in very preterm infants: gestational age versus neonatal cranial ultrasounds. Pediatrics. 2003;112:1108–1114. doi: 10.1542/peds.112.5.1108. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the Newborn. 2nd ed. Philadelphia: W.B. Saunders Company; 1987. pp. 311–361. [Google Scholar]
- Ward RM, Beachy JC. Neonatal complications following preterm birth. BJOG: an International Journal of Obstetrics and Gynaecology. 2003;110:8–16. doi: 10.1016/s1470-0328(03)00012-0. [DOI] [PubMed] [Google Scholar]