Abstract
Purpose
To characterize patients’ willingness to donate a biospecimen for future research as part of a breast cancer-related biobank involving a general screening population.
Materials and Methods
We performed a prospective cross-sectional study of 4,217 women aged 21 to 89 years presenting to our facilities for screening mammogram between December 2010 and October 2011. This HIPAA-compliant study was approved by our institutional review board. We collected data on patients’ interest in and actual donation of a biospecimen, motivators and barriers to donating, demographic information, and personal breast cancer risk factors. A multivariate logistic regression analysis was performed to identify patient-level characteristics associated with an increased likelihood to donate.
Results
Mean patient age was 57.8 years (SD 11.1 years). While 66.0% (2785/4217) of patients were willing to donate blood or saliva during their visit, only 56.4% (2378/4217) actually donated. Women with a college education (OR=1.27, p=0.003), older age (OR=1.02, p<0.001), previous breast biopsy (OR=1.23, p=0.012), family history of breast cancer (OR=1.23, p=0.004), or a comorbidity (OR=1.22, p=0.014) were more likely to donate. Asian-American women were significantly less likely to donate (OR=0.74, p=0.005). The major reason for donating was to help all future patients (42.3%) and the major reason for declining donation was privacy concerns (22.3%).
Conclusion
A large proportion of women participating in a breast cancer screening registry are willing to donate blood or saliva to a biobank. Among minority participants, Asian-American women are less likely to donate and further qualitative research is required to identify novel active recruitment strategies to ensure their involvement.
Keywords: biospecimen, biobank, breast cancer, screening, patient willingness
INTRODUCTION
The field of genomics promises an era of personalized medicine, with cancer therapies selected based on patients’ levels of different biomarkers found in their blood and tissue [1,2]. Fulfilling this promise will require large-scale translational research efforts with a large investment in the development of population-based biobanks [3,4]. These tissue repositories linked to electronic personal health information databases are considered essential in discovering genetic associations of cancers [5–7,4,8,9]. In fact, it is believed that the rate-limiting step for genomics-based breakthroughs will not be current genotyping technology, but the availability of biospecimen samples stored in biobanks [10].
In breast cancer, it is well known that BRCA1 and BRCA2 gene mutations account for 2% of malignancies [11]. Yet, studies involving twins suggest that up to 27% of breast cancers can be accounted for by heritable factors, or a ten times greater combined effect than that from currently identified high risk genes [12]. This discrepancy is likely due to the fact that current genetic data is limited almost entirely to Western European and North American Caucasian populations [10]. The few existing breast registries with biobank components, thus far, have also been limited to patients already diagnosed with cancer [13]. In order to fully realize the potential of breast cancer genomics, breast-related biobanks must include a diverse patient population, including a large proportion of minority women and asymptomatic, healthy women [14–18].
While promising, biobanks also come with an array of ethical concerns. Since donated biospecimens may be studied years later, it may not be possible to provide specific information to participants at enrollment regarding how their samples will be used [19]. There may be concern for therapeutic misconception, or a participant’s misguided belief that participation may lead to a cure for themselves or their relatives [20]. Furthermore, it is currently unknown what the motivating factors for and barriers to participation are among healthy women who are eligible for mammographic screening. Prior reports with regards to patients’ willingness to donate to any type of tissue suggest that what influences patients’ decisions to donate or not is specific to the particular disease, patient population, and practice setting [21,22].
Postulated motivating factors include the possibility of personal benefit [23,20] or altruism directed towards family members, future patients, or society as a whole [24,25]. Postulated barriers to donation include a fear of a breach in confidentiality, misuse of information, historical distrust of health care, physical discomfort from a needlestick, and associated lost time or barriers to access [26–29]. Reasons may differ based on race/ethnicity or cultural beliefs [30–33], including a concern for stigma associated with a genetic mutation specific to an ethnic group [34].
The future success and generalizability of breakthroughs based on biobank research rests upon the initial recruitment of a diverse patient population. Understanding patients’ motivations and concerns regarding donation is critical for ensuring a robust informed consent process and developing targeted measures that can alleviate barriers preventing underrepresented women from donation. Therefore, our objective was to characterize patients’ willingness to donate a biospecimen for future genetic research to a breast cancer-related biobank in a general screening population. We aimed to identify personal characteristics of women who are willing to donate a biospecimen for future genetic research, elicit the key motivators for and barriers to donation, and identify factors associated with racial/ethnic disparities with regards to biospecimen donation during a routine screening mammogram visit. We hypothesized that women who donate are motivated by altruism, that the major concern in donating is privacy, and that racial/ethnic minority women are less likely to donate than their Caucasian counterparts.
MATERIALS AND METHODS
Informed Consent
We obtained institutional review board approval for this HIPAA compliant study. Concurrent with this research project, our institution’s breast imaging division underwent a quality improvement initiative to become paperless. Therefore, all patients presenting to our two dedicated breast imaging centers for a screening mammogram were asked to complete their standard-of-care patient history using an electronic tablet (i.e., iPad version 2). At the end of the standard electronic form, patients were presented with a statement about our breast cancer screening registry and asked whether or not they would like to participate. Those who were willing to participate were asked to provide informed consent to allow their electronic data to be used for research purposes. If patients consented to allowing their data to be used, they were then provided with additional electronic questions specific to our study. Those donating a biospecimen were asked to provide a separate written informed consent prior to actual specimen collection. Patients were able to consent to each item (participation in the registry, biobank, or personal electronic health information access) separately or in combination.
Study Population
The ATHENA Breast Health Network is comprised of five UC campuses (UCSF, UCLA, UCSD, UC Davis, and UC Irvine) and aims to capture a large cohort of women from a screening mammogram population. The prospective collection of patient-specific characteristics, clinical data, radiological data, pathologic data, and genetic data promises to revolutionize breast cancer care. UCLA was the first of the five institutions to enroll patients and currently leads patient accrual to the Athena Network. Since the development of a common registry required our breast imaging division to become paperless, the confluence of activities presented a unique opportunity to perform a site-specific evaluation prospectively to explore the motivators and barriers of our general screening population in regards to donation of a biospecimen for future genomic research. All women presenting to our two dedicated breast imaging centers for a routine screening mammogram between December 2010 and October 2011 were invited to participate.
Instrument and Data Collection
The standard-of-care history portion of the electronic survey captured the following patient-level characteristics that were relevant to our study: age, education level, marital status, race/ethnicity, family history of breast cancer, personal history of breast cancer, prior breast biopsy, breast changes at time of mammogram or within the last three months (lump, nipple discharge, pain, other), self-reported health status, and self-reported limitations to regular activities.
Patients consenting to the use of their survey data and who were willing to donate blood or saliva were asked two additional questions about their main motivations and main concerns with regards to donating to the biobank. These additional questions were formulated based on an initial review of the literature. In regards to motivations for donating, participants were able to select one or more of the four closed answer choices: to help future breast cancer patients, to advance scientific knowledge, to help themselves if they develop breast cancer, and/or to help an affected family member. In regards to barriers, participants were able to select one or more of the four closed answer choices: concern for privacy of their genetic data, no self-benefit, too great of a time commitment, or physical discomfort from a needlestick. At the institutional review board’s request, these questions were only provided to those that indicated interest in donating blood or saliva at the completion of the survey. Those not interested in donating were not presented with the targeted questions.
Statistical Analysis
We performed all statistical analyses using STATA version 11 (StataCorp, 2009, College Station, TX). We obtained initial univariate descriptive statistics for the entire study cohort, including their willingness to donate blood or saliva prior to their mammogram and their actual rate of donation after the mammogram. We performed bivariate analyses using the Pearson chi-square test between each patient-level characteristic and actual donation of blood or saliva. We then performed multivariate analyses to identify patient-specific factors associated with an increased theoretical likelihood to donate blood or saliva, as well as for actual donation of blood or saliva. Since our dependent variable (donation of blood or saliva) was dichotomous and we desired to estimate its expected value in relation to specific patient-level characteristics (e.g., race/ethnicity), we used a logistic regression model for our multivariate analysis. Prior to running this model, we tested and accounted for multicollinearity among our independent variables.
RESULTS
Study Population
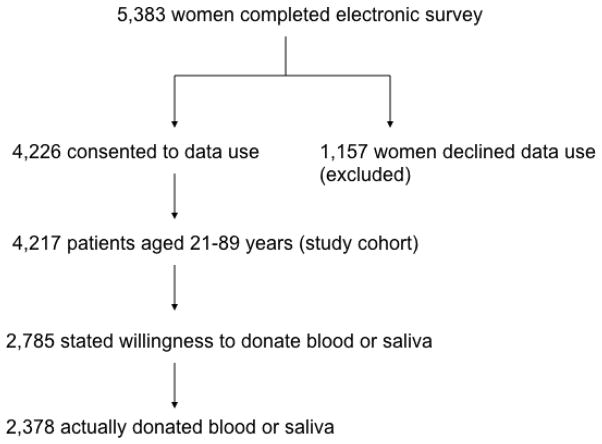
During the study period, 5,385 (26.4%) patients completed the standard-of-care history form using an electronic tablet. Two of these patients were male and thus excluded from our analysis. Of the 5,383 women completing their history forms electronically, 4,226 (78.5%) provided informed consent for allowing their personal health information to be used for research purposes and represent our study cohort. Of the female patients who provided informed consent to allow their data to be used for study purposes, patients aged 21 to 89 years were included in our final analysis (n=4,217). Of these, 66% (2,785/4,217) expressed willingness to donate, but only 56% (2,378/4,217) actually donated. The study cohort is further detailed in Figure 1.
Fig. 1.
Study Cohort
Descriptive Statistics and Bivariate Analysis
The mean patient age was 57.8 years (standard deviation 11.1 years) (see Table 1). Slightly more than three-quarters of our study population (3214/4217, 76.2%) had at least a college-level education, and 35.0% (1473/4217) did not currently live with a partner. In regards to race/ethnicity, 69.0% were Caucasian (2908/4217), 11.9% were Hispanic (502/4217), 10.5% Asian-American (444/4217), 6.2% African-American (261/4217), and 2.4% other (102/4217).
Table 1.
Patient Demographics
| Characteristic | Total Population (n=4217) | Willing to Donate | Not Willing to Donate | P-value for Difference between Groups |
|---|---|---|---|---|
| Mean age (years±SD) | 57.8 (±11.1) | 59.0 (±10.9) | 55.4 (±11.1) | <0.0001#* |
| College education | 3,214 | 2,123 | 1,091 | 0.898 |
| No partner | 1,473 | 981 | 492 | 0.593 |
| Hispanic | 502 | 338 | 164 | 0.618 |
| African-American | 261 | 176 | 85 | 0.624 |
| Asian-American | 444 | 245 | 199 | <0.001* |
| Other Non-Caucasian race/ethnicity | 102 | 71 | 31 | 0.441 |
| Personal history of breast cancer | 431 | 315 | 116 | 0.001* |
| Family history of breast cancer | 1,224 | 874 | 350 | <0.001* |
| Breast biopsy | 1,223 | 876 | 347 | <0.001* |
| Breast symptoms | 967 | 639 | 328 | 0.977 |
| Comorbidity | 1,092 | 791 | 301 | <0.001* |
indicates two-sample t-test p-value; all other p-values are from the Pearson chi-square test;
indicates statistically significant (p<0.05) characteristic. SD = standard deviation.
About one in ten patients (431/4217, 10.2%) had a personal history of breast cancer, 29.0% (1224/4217) had a family history of breast cancer, 29.0% (1223/4217) of women had a breast biopsy during their lifetime, and 23.0% (967/4217) had a breast-specific symptom or complaint at time of screening or within the previous three months. More than one in four patients (1092/4217) reported that they had at least one major comorbidity (e.g., diabetes, hypertension, heart disease, renal disease, liver disease, stroke, or cancer other than breast). Prior to their screening mammogram, 66.0% (2785/4217) of patients were willing to donate blood or saliva during their visit. However, after their mammogram, 56.4% (2378/4217) actually donated blood or saliva to the biobank portion of the registry.
A two-sample independent t-test between mean patient age and actual donation of blood or saliva was statistically significant (p<0.0001). Pearson chi-square tests between all categorical patient-level variables and actual donation demonstrated statistically significant bivariate relationships between actual donation of blood or saliva and Asian-American race (p<0.001), personal history of breast cancer (p=0.001), family history of breast cancer (p<0.001), breast biopsy (p<0.001), and at least one major comorbidity (p<0.001).
Patients willing to donate blood or saliva at pre-mammogram survey were invited to answer additional questions in regards to their major motivations for donating a biospecimen and their major concerns. The major reasons for and barriers to donation are described in Table 2.
Table 2.
Reasons For and Against Blood/Saliva Donation at Mammogram
| Reason for Donating | Number of respondents | Percentage (n=4,217) |
|---|---|---|
| Help future patients | 1784 | 42.3% |
| Advance science | 1460 | 34.6% |
| Help self | 645 | 15.3% |
| Help family member | 577 | 13.7% |
| Reason for Declining | Number of respondents | Percentage (n=4217) |
| Privacy concerns | 939 | 22.3% |
| No personal benefit | 827 | 19.6% |
| Lost time | 576 | 13.7% |
| Needle stick | 395 | 9.4% |
Multivariate Analysis
We summarize the odds ratios (ORs) for the patient-level independent variables from our multiple logistic regression with interest in donation as the dependent variable in Table 3 and from our multiple logistic regression with actual donation as our dependent variable in Table 4. For both the stated interest and actual donation models, older women (p<0.001 for both), Asian-American women (p=0.005 and <0.001, respectively), women with family history (p<0.001 and p=0.004, respectively), and women with at least one comorbid condition were willing to donate (p=0.031 and p=0.014). However, only the actual donation model demonstrated statistical significance for college education (p=0.003) and previous breast biopsy (p=0.012). These two variables approached statistical significance in the stated interest model. In addition, recent breast symptoms were statistically significant in the stated interest model (p=0.031), but no longer significant in the actual donation model (p=0.848).
Table 3.
Multiple Logistic Regression of Patient-Level Variables on Dependent Variable of Stated Interest in Blood or Saliva Donation
| Patient-Level Characteristic | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Increasing age (per year)* | 1.03 | [1.02, 1.04] | <0.001 |
| Any college education | 1.18 | [1.00, 1.39] | 0.052 |
| No partner | 1.00 | [0.87, 1.15] | 0.998 |
| Hispanic | 1.16 | [0.93, 1.43] | 0.186 |
| African-American | 1.00 | [0.75, 1.33] | 0.992 |
| Asian-American* | 0.67 | [0.54, 0.83] | <0.001 |
| Other race | 1.33 | [0.85, 2.06] | 0.209 |
| Personal breast cancer history | 1.04 | [0.80, 1.36] | 0.746 |
| Family history* | 1.42 | [1.22, 1.67] | <0.001 |
| Prior breast biopsy | 1.17 | [0.98, 1.38] | 0.077 |
| Recent breast symptoms* | 1.21 | [1.02, 1.43] | 0.031 |
| Comorbidity* | 1.20 | [1.02, 1.43] | 0.031 |
indicates statistically significant patient-level characteristics. CI = confidence interval.
Table 4.
Multiple Logistic Regression of Patient-Level Variables on Dependent Variable of Actual Blood or Saliva Donation
| Patient-Level Characteristic | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Increasing age* | 1.02 | [1.01, 1.03] | <0.001 |
| Any college education* | 1.27 | [1.08, 1.48] | 0.003 |
| No partner | 0.99 | [0.87, 1.14] | 0.928 |
| Hispanic | 1.02 | [0.83, 1.25] | 0.844 |
| African-American | 0.97 | [0.74, 1.26] | 0.803 |
| Asian-American* | 0.74 | [0.61, 0.92] | 0.005 |
| Other race | 1.41 | [0.93, 2.14] | 0.108 |
| Personal breast cancer history | 0.81 | [0.64, 1.02] | 0.075 |
| Family history* | 1.23 | [1.07, 1.42] | 0.004 |
| Prior breast biopsy* | 1.23 | [1.05, 1.44] | 0.012 |
| Recent breast symptoms | 1.02 | [0.87, 1.19] | 0.848 |
| Comorbidity* | 1.22 | [1.04, 1.42] | 0.014 |
indicates statistically significant patient-level characteristics. CI = confidence interval.
There was no statistical difference in blood or saliva donation between African-American women or Hispanic women versus their Caucasian counterparts in either the stated interest or actual donation models. However, Asian-American women were significantly less likely to donate compared to Caucasian women in both models (p<0.001 and p=0.005, respectively), controlling for all other patient-level variables. Donation was not influenced by whether or not the patient lived with a partner or whether or not the patient had a personal history of breast cancer in either model.
DISCUSSION
To our knowledge, our study is the first to both describe patient willingness to donate blood or saliva during a screening mammogram visit and determine the patient-level characteristics associated with both stated interest in donation and actual donation. We found that a substantial proportion (66%) of women consenting to take part in a large breast screening registry were also willing to donate blood or saliva at their screening mammogram visit. However, actual donation (56.4%) was less than the stated interest in donation. These findings are in line with prior reports of theoretical public willingness to contribute biological samples for research purposes, which ranged between 42% and 90% in general population surveys in both Europe and North America [28,34–38]. However, our study is the first to delineate willingness to donate blood or saliva specifically in a screening mammogram population.
We explored minority participation in research in the context of an ethno-medical model that suggests that individual participation in research is mediated by access to and utilization of healthcare. This model emphasizes maximizing minority research participation in the subset of minority patients who already access and utilize healthcare [39,40]. Thus, among patients who regularly access screening mammogram and have a particular level of health literacy, we found that there was no difference in likelihood to donate blood or saliva among both African-American women and Hispanic women when compared to Caucasian women. This finding, contrary to our hypothesis, differs from previous reports from a US national survey that found African-Americans females as being less willing to provide biospecimens for research purposes [41,42]. However, prior reports were based on national surveys not specific to the breast cancer screening populations. The lack of disparity among Hispanic women in regards to donation is consistent with prior research that suggests Latina women having a high interest in participating in research studies aimed at preventing breast cancer [43].
Interestingly, Asian-American women were statistically less likely to donate blood or saliva compared to Caucasian women with an odds ratio of 0.74 for actual donation. Therefore, the Asian-American screening population likely comprises a racial/ethnic subpopulation that, if undersampled, may bias the generalizability of future discoveries made from biobank-related research. The reasons for Asian-Americans declining donation are currently uncertain, but may stem from cultural preferences and attitudes towards cancer. Further qualitative studies are warranted, including focused groups and patient cognitive interviews to elucidate the specific barriers to donation among this heterogeneous racial/ethnic group.
Older patients and patients with at least some college-level education were more likely to donate blood or saliva, which suggests that their may be a health literacy and/or maturity gap in regards to patient understanding of the potential societal benefits to donation. While women with a personal history of breast biopsy were more likely to donate blood or saliva based on multivariate analysis, the most common reason for donating remained one of altruism – to help all future patients. The motivation of altruism among the screening mammogram population is consistent with prior studies that identified altruism as the major motivating factor for tissue/organ donation in general [22].
The major deterrent for donating blood or saliva in the screening mammogram population was the concern for privacy of information gathered from their biospecimen samples. This finding suggests that researchers need to make a concerted effort to carefully explain the security of patient health information and the impact unforeseen disclosure may have during the informed consent process for donation into a biobank. This also overlaps with prior reports of potential barriers to biospecimen donation, that site a breach in confidentiality, misuse of information by a third party, and historical distrust of health care as major issues [26]. Physical discomfort, such as from a needlestick, was not a major deterrent among consenting participants. However, the negative aspect of this process was likely alleviated by the option to provide a saliva sample in lieu of blood.
There are several limitations to our study. First, the study population consists of a subset of a population-based sample of women presenting to two breast imaging centers affiliated with an academic tertiary medical center. Thus, our results may not be generalizable to the entire US screening populations. However, our cohort encompasses a diverse patient population from a large metropolitan area. Moreover, our study cohort included only those willing to participate in the larger breast screening registry study and those that were literate and fluent in English. Therefore, we do not capture the screening population that decline participation in the registry, non-English speaking patients, and the population of women who do not undergo routine screening mammogram. Nevertheless, this latter population is outside the scope of this study’s purpose, which is to examine willingness to donate blood or saliva in a screening population that already has regular access to care. There were space constraints and limitations in the electronic survey design for additional study questions, so participants were not able to provide open responses and were asked to select between one and four possible closed responses. Finally, only those willing to donate blood or saliva were able to answer targeted questions aimed at elucidating motivators and barriers for participation due to institutional review board concerns.
In summary, our study demonstrates that diverse patient recruitment into a biobank portion of a breast cancer screening registry can be successful in the breast imaging waiting area. Among minority registry participants, there is no disparity in blood or saliva donation among African-American or Hispanic women, but there is a disparity in donation among Asian-American women presenting for screening mammogram. Further qualitative research is required to determine the true concerns and barriers among this minority group. As older women and women with more education are also more likely to donate blood or saliva, the issue of patient maturity and health literacy need to be further addressed in the informed consent process. The focus of future research efforts should be to identify and implement culturally sensitive, active recruitment strategies to ensure that all subpopulations of women understand the implications and potential benefits from contributing biospecimens for future genetic-based analyses.
Acknowledgments
Christoph I. Lee, MD, MSHS received support from the Robert Wood Johnson Foundation Clinical Scholars Program and the National Institutes of Health (NIH)/National Institute for Minority Health and Health Disparities LRP. Carol Mangione, MD, MSPH received support from the UCLA Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under NIH/NIA Grant P30-AG021684 and the NIH/NCATS UCLA CTSI Grant Number UL1TR000124. Arash Naeim, MD, PhD received support from the UC Office of the President and the Safeway Foundation in support of the Athena Breast Network, and by a grant from the California Breast Cancer Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and other funding organizations.
Footnotes
CONFLICTS OF INTEREST
All authors declare no financial conflicts of interest related to this study.
Contributor Information
Christoph I. Lee, Email: LBassett@mednet.ucla.edu.
Mei Leng, Email: MLeng@mednet.ucla.edu.
Sally L. Maliski, Email: smaliski@sonnet.ucla.edu.
Bryan B. Pezeshki, Email: bryanpez@gmail.com.
Colin J. Wells, Email: ColinWells@mednet.ucla.edu.
Carol M. Mangione, Email: cmangione@mednet.ucla.edu.
Arash Naeim, Email: ANaeim@mednet.ucla.edu.
References
- 1.Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research. Nature. 2003;422 (6934):835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- 2.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in medicine: official journal of the American College of Medical Genetics. 2007;9 (10):665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 3.Ozdemir V, Williams-Jones B, Cooper DM, Someya T, Godard B. Mapping translational research in personalized therapeutics: from molecular markers to health policy. Pharmacogenomics. 2007;8 (2):177–185. doi: 10.2217/14622416.8.2.177. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt RE. Biobanking: the foundation of personalized medicine. Curr Opin Oncol. 2011;23 (1):112–119. doi: 10.1097/CCO.0b013e32834161b8. [DOI] [PubMed] [Google Scholar]
- 5.Nelson HD, Huffman LH, Fu R, Harris EL. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(5):362–379. doi: 10.7326/0003-4819-143-5-200509060-00012. 143/5/362 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Guttmacher AE, Collins FS. Genomic medicine--a primer. N Engl J Med. 2002;347 (19):1512–1520. doi: 10.1056/NEJMra012240. [DOI] [PubMed] [Google Scholar]
- 7.Baer AR, Smith ML, Collyar D, Peppercorn J. Issues surrounding biospecimen collection and use in clinical trials. J Oncol Pract. 2010;6 (4):206–209. doi: 10.1200/JOP.777004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury MJ, Millikan R, Little J, Gwinn M. The emergence of epidemiology in the genomics age. Int J Epidemiol. 2004;33 (5):936–944. doi: 10.1093/ije/dyh278. [DOI] [PubMed] [Google Scholar]
- 9.Godard B, Marshall J, Laberge C. Community engagement in genetic research: results of the first public consultation for the Quebec CARTaGENE project. Community genetics. 2007;10 (3):147–158. doi: 10.1159/000101756. [DOI] [PubMed] [Google Scholar]
- 10.Forsti A, Hemminki K. Breast cancer genomics based on biobanks. Methods Mol Biol. 2011;675:375–385. doi: 10.1007/978-1-59745-423-0_23. [DOI] [PubMed] [Google Scholar]
- 11.Syrjakoski K, Vahteristo P, Eerola H, Tamminen A, Kivinummi K, Sarantaus L, Holli K, Blomqvist C, Kallioniemi OP, Kainu T, Nevanlinna H. Population-based study of BRCA1 and BRCA2 mutations in 1035 unselected Finnish breast cancer patients. J Natl Cancer Inst. 2000;92 (18):1529–1531. doi: 10.1093/jnci/92.18.1529. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343 (2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 13.Kaphingst KA, Janoff JM, Harris LN, Emmons KM. Views of female breast cancer patients who donated biologic samples regarding storage and use of samples for genetic research. Clin Genet. 2006;69 (5):393–398. doi: 10.1111/j.1399-0004.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 14.Hobbs SK, Shi G, Homer R, Harsh G, Atlas SW, Bednarski MD. Magnetic resonance image-guided proteomics of human glioblastoma multiforme. J Magn Reson Imaging. 2003;18 (5):530–536. doi: 10.1002/jmri.10395. [DOI] [PubMed] [Google Scholar]
- 15.Yang YS, Guccione S, Bednarski MD. Comparing genomic and histologic correlations to radiographic changes in tumors: a murine SCC VII model study. Acad Radiol. 2003;10 (10):1165–1175. doi: 10.1016/s1076-6332(03)00327-1. [DOI] [PubMed] [Google Scholar]
- 16.Forsti A, Jin Q, Altieri A, Johansson R, Wagner K, Enquist K, Grzybowska E, Pamula J, Pekala W, Hallmans G, Lenner P, Hemminki K. Polymorphisms in the KDR and POSTN genes: association with breast cancer susceptibility and prognosis. Breast cancer research and treatment. 2007;101 (1):83–93. doi: 10.1007/s10549-006-9265-1. [DOI] [PubMed] [Google Scholar]
- 17.Jin Q, Hemminki K, Enquist K, Lenner P, Grzybowska E, Klaes R, Henriksson R, Chen B, Pamula J, Pekala W, Zientek H, Rogozinska-Szczepka J, Utracka-Hutka B, Hallmans G, Forsti A. Vascular endothelial growth factor polymorphisms in relation to breast cancer development and prognosis. Clin Cancer Res. 2005;11 (10):3647–3653. doi: 10.1158/1078-0432.CCR-04-1803. [DOI] [PubMed] [Google Scholar]
- 18.Lei H, Hemminki K, Altieri A, Johansson R, Enquist K, Hallmans G, Lenner P, Forsti A. Promoter polymorphisms in matrix metalloproteinases and their inhibitors: few associations with breast cancer susceptibility and progression. Breast cancer research and treatment. 2007;103 (1):61–69. doi: 10.1007/s10549-006-9345-2. [DOI] [PubMed] [Google Scholar]
- 19.Critchley CR, Nicol D, Otlowski MF, Stranger MJ. Predicting intention to biobank: a national survey. Eur J Public Health. 2010;22 (1):139–144. doi: 10.1093/eurpub/ckq136. [DOI] [PubMed] [Google Scholar]
- 20.Lidz CW, Appelbaum PS, Grisso T, Renaud M. Therapeutic misconception and the appreciation of risks in clinical trials. Soc Sci Med. 2004;58 (9):1689–1697. doi: 10.1016/S0277-9536(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: a survey of public opinions about a large genetic cohort study. Genetics in medicine: official journal of the American College of Medical Genetics. 2008;10 (11):831–839. doi: 10.1097/GIM.0b013e31818bb3ab. [DOI] [PubMed] [Google Scholar]
- 22.Axler RE, Irvine R, Lipworth W, Morrell B, Kerridge IH. Why might people donate tissue for cancer research? Insights from organ/tissue/blood donation and clinical research. Pathobiology. 2008;75 (6):323–329. doi: 10.1159/000164216. [DOI] [PubMed] [Google Scholar]
- 23.Edwards SJ, Lilford RJ, Hewison J. The ethics of randomised controlled trials from the perspectives of patients, the public, and healthcare professionals. BMJ. 1998;317 (7167):1209–1212. doi: 10.1136/bmj.317.7167.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallowfield LJ, Jenkins V, Brennan C, Sawtell M, Moynihan C, Souhami RL. Attitudes of patients to randomised clinical trials of cancer therapy. Eur J Cancer. 1998;34 (10):1554–1559. doi: 10.1016/s0959-8049(98)00193-2. [DOI] [PubMed] [Google Scholar]
- 25.Nurgat ZA, Craig W, Campbell NC, Bissett JD, Cassidy J, Nicolson MC. Patient motivations surrounding participation in phase I and phase II clinical trials of cancer chemotherapy. Br J Cancer. 2005;92 (6):1001–1005. doi: 10.1038/sj.bjc.6602423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paskett ED, DeGraffinreid C, Tatum CM, Margitic SE. The recruitment of African-Americans to cancer prevention and control studies. Prev Med. 1996;25 (5):547–553. doi: 10.1006/pmed.1996.0088. [DOI] [PubMed] [Google Scholar]
- 27.Beskow LM, Burke W, Merz JF, Barr PA, Terry S, Penchaszadeh VB, Gostin LO, Gwinn M, Khoury MJ. Informed consent for population-based research involving genetics. JAMA. 2001;286 (18):2315–2321. doi: 10.1001/jama.286.18.2315. [DOI] [PubMed] [Google Scholar]
- 28.Hoeyer K, Olofsson BO, Mjorndal T, Lynoe N. Informed consent and biobanks: a population-based study of attitudes towards tissue donation for genetic research. Scand J Public Health. 2004;32 (3):224–229. doi: 10.1080/14034940310019506. [DOI] [PubMed] [Google Scholar]
- 29.Hoeyer K, Olofsson BO, Mjorndal T, Lynoe N. The ethics of research using biobanks: reason to question the importance attributed to informed consent. Arch Intern Med. 2005;165 (1):97–100. doi: 10.1001/archinte.165.1.97. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson S, Helgesson G. Potential harms, anonymization, and the right to withdraw consent to biobank research. Eur J Hum Genet. 2005;13 (9):1071–1076. doi: 10.1038/sj.ejhg.5201458. [DOI] [PubMed] [Google Scholar]
- 31.Tu SP, Chen H, Chen A, Lim J, May S, Drescher C. Clinical trials: understanding and perceptions of female Chinese-American cancer patients. Cancer. 2005;104 (12 Suppl):2999–3005. doi: 10.1002/cncr.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen TT, Somkin CP, Ma Y. Participation of Asian-American women in cancer chemoprevention research: physician perspectives. Cancer. 2005;104 (12 Suppl):3006–3014. doi: 10.1002/cncr.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen TT, Somkin CP, Ma Y, Fung LC, Nguyen T. Participation of Asian-American women in cancer treatment research: a pilot study. J Natl Cancer Inst Monogr. 2005;(35):102–105. doi: 10.1093/jncimonographs/lgi046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pentz RD, Billot L, Wendler D. Research on stored biological samples: views of African American and White American cancer patients. Am J Med Genet A. 2006;140 (7):733–739. doi: 10.1002/ajmg.a.31154. [DOI] [PubMed] [Google Scholar]
- 35.Chen DT, Rosenstein DL, Muthappan P, Hilsenbeck SG, Miller FG, Emanuel EJ, Wendler D. Research with stored biological samples: what do research participants want? Arch Intern Med. 2005;165 (6):652–655. doi: 10.1001/archinte.165.6.652. [DOI] [PubMed] [Google Scholar]
- 36.Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Arch Intern Med. 2002;162 (13):1457–1462. doi: 10.1001/archinte.162.13.1457. [DOI] [PubMed] [Google Scholar]
- 37.Nilstun T, Hermeren G. Human tissue samples and ethics--attitudes of the general public in Sweden to biobank research. Med Health Care Philos. 2006;9 (1):81–86. doi: 10.1007/s11019-005-7984-4. [DOI] [PubMed] [Google Scholar]
- 38.Kettis-Lindblad A, Ring L, Viberth E, Hansson MG. Genetic research and donation of tissue samples to biobanks. What do potential sample donors in the Swedish general public think? Eur J Public Health. 2006;16 (4):433–440. doi: 10.1093/eurpub/cki198. [DOI] [PubMed] [Google Scholar]
- 39.Calderon JL, Baker RS, Fabrega H, Conde JG, Hays RD, Fleming E, Norris K. An ethno-medical perspective on research participation: a qualitative pilot study. Med Gen Med. 2006;8 (2):23. [PMC free article] [PubMed] [Google Scholar]
- 40.Gavaler JS, Bonham-Leyba M, Castro CA, Harman SE. The Oklahoma Postmenopausal Women’s Health Study: recruitment and characteristics of American Indian, Asian, Black, Hispanic, and Caucasian women. Alcohol Clin Exp Res. 1999;23 (2):220–223. doi: 10.1111/j.1530-0277.1999.tb04103.x. [DOI] [PubMed] [Google Scholar]
- 41.Moorman PG, Skinner CS, Evans JP, Newman B, Sorenson JR, Calingaert B, Susswein L, Crankshaw TS, Hoyo C, Schildkraut JM. Racial differences in enrolment in a cancer genetics registry. Cancer Epidemiol Biomarkers Prev. 2004;13 (8):1349–1354. [PubMed] [Google Scholar]
- 42.McQuillan GM, Porter KS, Agelli M, Kington R. Consent for genetic research in a general population: the NHANES experience. Genetics in medicine: official journal of the American College of Medical Genetics. 2003;5 (1):35–42. doi: 10.1097/00125817-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Mandelblatt J, Kaufman E, Sheppard VB, Pomeroy J, Kavanaugh J, Canar J, Pallandre L, Cullen J, Huerta E. Breast cancer prevention in community clinics: will low-income Latina patients participate in clinical trials? Prev Med. 2005;40 (6):611–618. doi: 10.1016/j.ypmed.2004.09.004. [DOI] [PubMed] [Google Scholar]