Abstract
Activation of type 1 angiotensin (AT1) receptors causes hypertension, leading to progressive kidney injury. AT1 receptors are expressed on immune cells, and previous studies have identified a role for immune cells in angiotensin II–dependent hypertension. We, therefore, examined the role of AT1 receptors on immune cells in the pathogenesis of hypertension by generating bone marrow chimeras with wild-type donors or donors lacking AT1A receptors (BMKO). The 2 groups had virtually identical blood pressures at baseline, suggesting that AT1 receptors on immune cells do not make a unique contribution to the determination of baseline blood pressure. By contrast, in response to chronic angiotensin II infusion, the BMKOs had an augmented hypertensive response, suggesting a protective effect of AT1 receptors on immune cells with respect to blood pressure elevation. The BMKOs had 50% more albuminuria after 4 weeks of angiotensin II–dependent hypertension. Angiotensin II–induced pathological injury to the kidney was similar in the experimental groups. However, there was exaggerated renal expression of the macrophage chemokine monocyte chemoattractant protein 1 in the BMKO group, leading to persistent accumulation of macrophages in the kidney. This enhanced mononuclear cell infiltration into the BMKO kidneys was associated with exaggerated renal expression of the vasoactive mediators interleukin-1β and interleukin-6. Thus, in angiotensin II-induced hypertension, bone marrow-derived AT1 receptors limited mononuclear cell accumulation in the kidney and mitigated the chronic hypertensive response, possibly through the regulation of vasoactive cytokines.
Keywords: angiotensin II, hypertension, inflammation, kidney diseases, lymphocytes
Activation of the renin-angiotensin system (RAS) promotes blood pressure elevation leading to progressive kidney injury.1,2 Most of the effects of angiotensin (Ang) II to increase blood pressure are mediated through type 1 Ang (AT1) receptors.3,4 Accordingly, in human patients with hypertension, treatment with AT1 receptor blockers (ARBs) lowers blood pressure and slows the progression of chronic kidney disease.5–7
Because ARB therapy slows the progression of chronic kidney disease but does not completely prevent this complication in hypertensive patients, determining precisely which tissue pools of AT1 receptors drive blood pressure elevation and end-organ damage will be paramount to the design of potent targeted therapies to protect from renal and cardiovascular morbidity in hypertension. Recent studies point to a role for the immune system in Ang II–dependent hypertension and its complications. For example, blockade of inflammatory responses can blunt the chronic hypertensive response to Ang II, leading to reduced cardiac hypertrophy.8 Moreover, lymphocyte deficiency during Ang II infusion mitigates blood pressure elevation and vascular dysfunction.9 However, whether these injurious effects of Ang II in hypertension are mediated through direct activation of AT1 receptors on immune cells or through activation of Ang receptors on other intermediary cell lineages requires elucidation.
Existing evidence indicates that AT1 receptors on immune cells play a complex role in inflammatory tissue damage.10–14 Nevertheless, on the basis of the aforementioned protective effects of lymphocyte suppression or deficiency in the setting of hypertension, we hypothesized that activation of AT1 receptors on immune cells would exacerbate Ang II–dependent hypertension. To directly test this hypothesis, we eliminated immune responses mediated by AT1 receptors by generating AT1 receptor–deficient bone marrow chimeras (BMKOs) and wild-type (WT) controls (BMWTs).
Methods
Animals
Mice with a targeted deletion of the AT1A receptor gene locus (Agtr1a) were generated as described previously.3 Agtr1a+/− 129/ SvEv mice were generated through repeated backcrosses for >12 generations. Successive intercrosses then yielded the Agtr1a+/+ and Agtr1a−/− 129/SvEv littermates that acted as bone marrow donors in our experiments. WT 129/SvEv mice serving as the bone marrow recipients were purchased from Taconic. All of the animal studies were approved by the Durham Veterans' Affairs Medical Center Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. These studies used 2- to 4-month–old male mice.
Bone Marrow Cell Transplantation
Bone marrow cells were obtained by flushing the femurs and tibias of donor Agtr1a+/+ or Agtr1a−/− 129/SvEv mice with RPMI 1640 medium supplemented with 10% fetal calf serum, as described.15 After counting, cells were then resuspended at a concentration of 107 cells per milliliter. Recipient 129/SvEv mice were lethally irradiated with 10.5 Gy using a cesium irradiator.16 Within 4 hours after irradiation, the mice were injected via the retro-orbital venous sinus with 2× 106 cells.15–17 Eight weeks were allowed for bone marrow engraftment before initiating the protocol for blood pressure measurement.
Model of Ang II–Induced Hypertension
After bone marrow engraftment, the recipients of Agtr1a+/+ (BMWT) or Agtr1a−/− (BMKO) bone marrow underwent left nephrectomy followed 1 week later by implantation of a pressure-sensing catheter (TA11PA-C10, Transoma Medical) via the left common carotid artery, as described previously.18 Allowing 7 days for reestablishment of diurnal blood pressure variation, baseline blood pressure measurements were recorded for 3 days continuously by radiotelemetry (Transoma) in conscious unrestrained animals. Then, an osmotic mini-pump (ALZET model 2004, Durect) was implanted subcutaneously to infuse Ang II (1000 ng/kg per day; Sigma-Aldrich; n≥11 mice per group) or saline (n=4 to 5 mice per group) continuously for 28 days, as described previously.19 Blood pressure measurements continued for 3 weeks of saline or Ang II infusion. On day 25, the mice were placed in metabolic cages, and urine was collected for 24 hours. Urinary concentrations of albumin, prostaglandin E2, and prostacyclin were measured in individual samples using specific ELISAs for mouse albumin (Exocell), prostaglandin E metabolite (Cayman Chemical Company), and prostaglandin F1α, respectively, as described previously.20 Creatinine concentrations were measured with a picric acid-based method using a kit (Exocell). Albumin excretion is expressed as micrograms per milligram of creatinine.
Histopathologic Analysis
After 28 days of saline or Ang II infusion, the kidneys were harvested, weighed, and fixed in formalin, sectioned, and stained with Masson trichrome. All of the tissues were examined by a pathologist (P.R.) without knowledge of the experimental groups. The pathological abnormalities in the kidney were graded on the basis of the presence and severity of component abnormalities, including glomerulosclerosis, epithelial reactivity, chronic inflammation, tubular casts, fibrosis, and vascular injury. Grading for each component was performed using a semiquantitative scale, as described previously,21,22 where 0 represented no abnormality and where 1, 2, 3, and 4 represented mild, moderate, moderately severe, and severe abnormalities, respectively. The total injury score for each kidney was a summation of these component injury scores.
To assess macrophage and T-lymphocyte infiltration in the kidneys, sections were stained with anti-F4/80 (#MCA497G, Serotec) and anti-CD3 (clone SP7, Laboratory Vision) antibodies, respectively, per the manufacturer's instructions. On each section, 20 randomly selected fields were then scored in a blinded fashion for the presence or absence of macrophages (≥5 in field) or T cells. Because T lymphocytes were clustered in the kidney around the vasculature, blood vessels were identified on each kidney section, and the severity of perivascular T-cell infiltrates was also scored in a blinded fashion on the basis of a previously established method23 by assigning vessels to the following quartiles: (1) mild, indicating 0 to 4 infiltrating T cells; (2) moderate, indicating infiltrates containing 5 to 10 T cells; (3) moderately severe, indicating infiltrates containing 11 to 20 T cells; and (4) severe, indicating infiltrates with >20 T cells.
Quantification of Inflammatory Gene Expression
Kidneys were harvested, and total RNA was isolated by using the RNeasy Mini Kit, as described above. RNA expression levels in the kidney were then determined for interferon-γ (IFN-γ), tumor necrosis factor-α, transforming growth factor-β (TGF-β), interleukin-1α, interleukin-1β, interleukin-6, monocyte chemoattractant protein 1, and CCL5 (regulated on activation, normal T expressed and secreted) using TaqMan primers (Applied Biosystems) in real-time PCR, as described above.
Quantitation of Macrophage Migration
The chemotaxis procedure was adapted from previously published methods.13,24 Peritoneal macrophages were harvested from mice 4 days after peritoneal injection of 3% thioglycollate. The peritoneal macrophages were suspended in DMEM without FBS13 and loaded at a density of 5 × 105 cells per well (200 μL) into the upper chamber of a Transwell chemotaxis plate (catalog No. 3421, Corning-Costar Corp) with a 5-μm pore polycarbonate filter insert. The lower chamber of each well contained 500 μL of prewarmed chemotaxis buffer with the indicated chemokine concentrations. Monocyte chemoattractant protein 1 (MCP-1) was obtained from R&D Systems. PD123319 was obtained from Sigma. The plate was incubated for 2.5 hours at 37°C in a 5% CO2 atmosphere. The filter was then stained for 1 hour with 0.5% crystal violet solution and then destained with water, after which cells remaining on the upper surface of the filter were removed mechanically. The filters with the stained cells adhering to the lower surface were dried overnight. The filters then underwent vortexing in 200 μL of 40% acetic acid. The degree of chemotaxis was quantitated as the absorbance of the acetic acid solution at 562 nm on an Emax Precision Microplate Reader (Molecular Devices) using the Softmax Pro 4.8 software. Migratory activity is reported as the fold increase in chemotaxis compared with cells incubated without chemoattrac-tants. All of the conditions were run with samples from 6 mice.
Results
Generation of AT1A Receptor–Deficient Bone Marrow Chimeras
To characterize Ang II–dependent hypertension in animals lacking AT1 receptors in immune cells alone and in WT controls, we lethally irradiated 129/SvEv mice and the same day transplanted them with bone marrow from syngeneic 129/SvEv donors that were either WT or deficient in the dominant murine AT1 receptor isoform, AT1A. At the conclusion of the experiment 14 weeks later, we performed real-time PCR for the Agtr1a gene starting with splenocyte RNA to confirm bone marrow engraftment in the recipients. Compared with recipients of WT bone marrow (BMWT), recipients of AT1A receptor-deficient bone marrow (BMKO) had a 98% reduction in AT1A receptor expression (0.02±0.00 versus 1.00±0.07 arbitrary units [au]; P<0.00001), thus confirming deletion of the AT1A receptor from immune cells in these animals. We also confirmed AT1A receptor deletion by PCR analysis of genomic DNA in the recipient splenocytes. At the DNA level, Agtr1a expression could not be detected in the splenocytes of the BMKO group (Figure S1, available in the online Data Supplement, at http://hyper.ahajournals.org). Despite this difference in Agtr1a expression, the spleen weights were similar between the BMWT and BMKO groups (3.1±0.2 versus 3.2±0.1 mg/g of body weight; P value not significant), as were the total splenic mononuclear cell numbers (34.0±2.2 × 106 versus 34.1±3.4 × 106 cells; P value not significant).
AT1 Receptors on Immune Cells Do Not Regulate Normal Blood Pressure Homeostasis
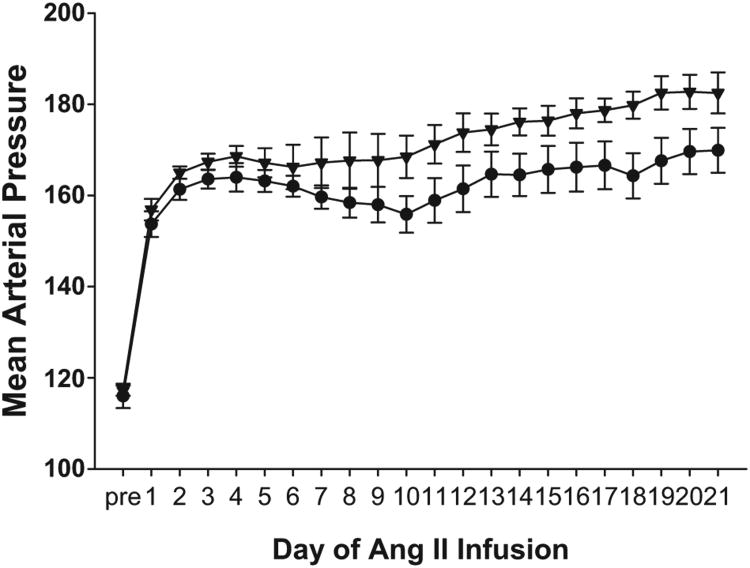
After bone marrow transfer, we allowed 8 weeks for immune reconstitution and then performed unilateral nephrectomy to make the remaining kidney more susceptible to hypertensive injury. After uninephrectomy, baseline blood pressures were measured by radiotelemetry in the experimental groups. As illustrated in Figure 1, baseline mean blood pressures in the BMWT group (116±3 mmHg) were quite similar to those measured previously in nontransplanted WT mice,18 indicating that the bone marrow transfer did not significantly impact baseline blood pressures. Moreover, baseline blood pressures in the BMWT and BMKO groups (116±3 versus 117± 1 mm Hg; P value not significant; Figure 1) were virtually identical, suggesting that AT1 receptors on bone marrow-derived cells do not make a unique contribution to the maintenance of baseline blood pressure.
Figure 1.
Recipients of bone marrow lacking AT1A receptors have an augmented chronic hypertensive response to Ang II. Mean arterial blood pressures measured by radiotelemetry in the experimental groups at baseline (“pre”) and during 3 weeks of Ang II infusion. BMWT, circles. BMKO, triangles. n = 11 per group.
Recipients of AT1A Receptor-Deficient Bone Marrow Have an Augmented Chronic Hypertensive Response to Ang II
After measurement of baseline blood pressures, an osmotic minipump was implanted subcutaneously to chronically infuse Ang II for 4 weeks. After the initiation of Ang II infusion, blood pressures in the BMWT group rose to ≈160 mm Hg and remained elevated throughout the infusion period (Figure 1) in a pattern similar to that seen previously in WT animals infused with Ang II.19,20 These data suggest that the bone marrow transfer procedure did not significantly alter the chronic hypertensive response to Ang II. Surprisingly, during Ang II infusion, the BMKO group had higher blood pressures than the BMWT group (Figure 1), indicating a protective effect of AT1 receptors on immune cells with regard to blood pressure elevation. The statistically significant difference in blood pressure between the groups was particularly evident in the second and third weeks of the Ang II infusion period, during which the increase in blood pressure over baseline was >20% greater in the BMKO group (+58±3 mm Hg) than in the BMWT controls (+47±3 mm Hg; P=0.03).
AT1 Receptor–Deficient Bone Marrow Chimeras Have Exaggerated Proteinuria in Ang II–Induced Hypertension
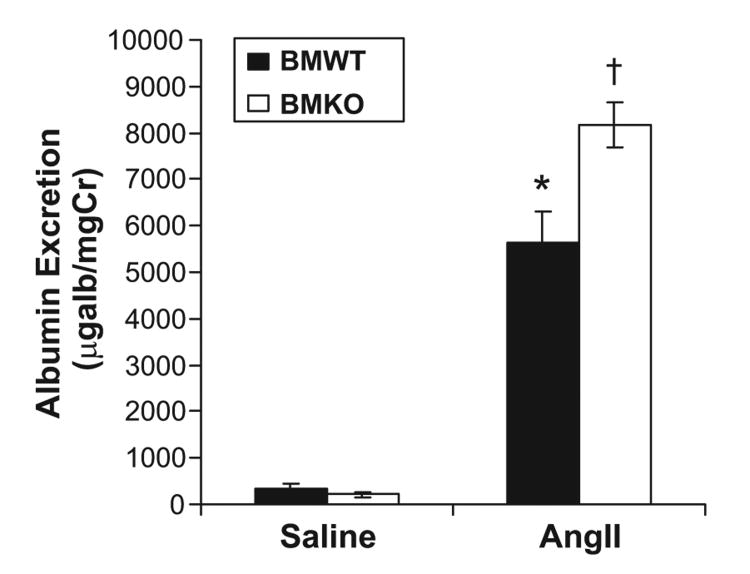
To determine whether the higher blood pressures in the Ang II–infused BMKO (BMKO-Ang) group were associated with exacerbated proteinuria, we measured urinary albumin excretion in the experimental groups after 4 weeks of Ang II or saline infusion (Figure 2). Albuminuria in the saline-infused BMWT (BMWT-saline) group was mild (351 ± 115 μg/mg of creatinine), on par with that seen previously in nontransplanted, uninephrectomized 129/SvEv mice,25 and not different from that in the saline-infused BMKO (BMKO-saline) group (207±44 μg/mg of creatinine). Ang II infusion was associated with more than a 15-fold increase in urinary albumin excretion in the BMWT group (5619±653 μg/mg of creatinine; P<0.0002 versus BMWT-saline) and in the BMKO group (8177±495 μg/mg of creatinine; P<0.0001 versus BMKO-saline). Moreover, the BMKO-Ang mice had ≈50% more urinary albumin excretion than the Ang II-infused BMWT (BMWT-Ang) group (P=0.003).
Figure 2.
Deficiency of AT1A receptors on bone marrow cells leads to increased albuminuria after angiotensin II infusion. Urinary albumin excretion (micrograms per milligram of creatinine) in the experimental groups after 25 days of Ang II or saline infusion. *P<0.0002 vs BMWT-saline. †P<0.0001 vs BMKO-saline, P=0.003 vs BMWT-Ang.
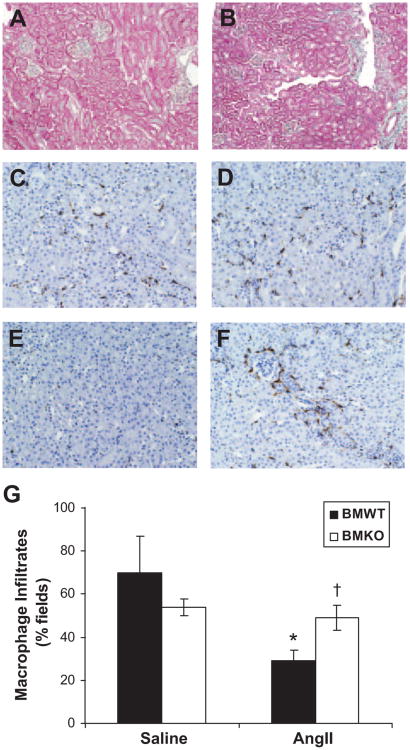
To assess whether the increased albuminuria in the BMKO-Ang group was associated with more severe kidney damage, the kidneys were scored for histological injury after 4 weeks of saline or Ang II infusion (Table). On a semiquantitative scale that includes glomerulosclerosis, interstitial disease, fibrosis, and vascular injury, the 2 saline-infused groups had minimal kidney damage (Figure 3A and Table). Compared with saline infusion, Ang II caused a marked increase in the severity of kidney injury in the BMWT (0.8±0.8 versus 8.3±0.8 au; P<0.0001; Figure 3B) and in the BMKO groups (1.0±0.7 versus 7.0±0.6 au; P=0.0006).
Figure 3.
AT1 receptor-deficient bone marrow chimeras have enhanced renal accumulation of macrophages in Ang II–dependent hypertension. A, Representative kidney section from BMWT-saline group showing minimal injury (×10). B, Representative kidney section from BMWT-Ang group showing mild glomerulosclerosis, mononuclear cell infiltrates, and rare fibrosis. C through F, Representative kidney sections from BMWT-saline (C), BMKO-saline (D), BMWT-Ang (E), and BMKO-Ang (F) groups stained brown for macrophages (×20). G, Proportion of high-powered (×40) fields containing ≥5 F4/80+ macrophages. *P<0.007 vs BMWT-saline. †P<0.03 vs BMWT-Ang.
Table. Recipients of WT and AT1A Receptor–Deficient Bone Marrow Have Similar Levels of Kidney Injury Induced by Angiotensin II.
| Kidney Injury Scores, au | Glomerular Injury | Tubulointerstitial Disease | Vascular Damage | Total Injury |
|---|---|---|---|---|
| BMWT-saline | 0.2±0.2 | 0.6±0.6 | 0.0±0.0 | 0.8±0.8 |
| BMKO-saline | 0.5±0.3 | 0.5±0.5 | 0.0±0.0 | 1.0±0.7 |
| BMWT-Ang | 3.0±0.5‡ | 5.1 ±0.3¶ | 0.1 ±0.1 | 8.3±0.8* |
| BMKO-Ang | 2.2±0.4§ | 4.8±0.3‖ | 0.00±0.00 | 7.0±0.6† |
n≥14 in BMWT-Ang and BMKO-Ang groups. n=4 to 5 in BMWT-saline and BMKO-saline groups.
P<0.0001 vs BMWT-saline.
P=0.0006 vs BMKO-saline.
P<0.006 vs BMWT-saline.
P<0.03 vs BMKO-saline.
P<0.0001 vs BMWT-saline.
P<0.0001 vs BMKO-saline.
Moreover, mild fibrosis was evident in the sections from nearly all of the Ang II–infused BMWT and BMKO animals (13 of 16 [81.3%] and 13 of 14 [92.9%]; P value not significant by χ2) but in none of the saline-infused BMWT (0 of 5; P<0.003 versus BMWT-Ang by Fisher exact test) or BMKO sections (0 of 4; P<0.002 versus BMKO-Ang). However, by this metric, there was no difference in the degree of kidney injury between the BMWT-Ang and BMKO-Ang groups (P value not significant).
AT1 Receptor Deficiency on Immune Cells Leads to Enhanced Accumulation of Mononuclear Cells in the Kidney During Ang II–Dependent Hypertension
Infiltration of inflammatory cells into the kidney has been associated with higher blood pressures during RAS activation.8 Therefore, to determine whether differences in immune cell infiltration might explain the augmented hypertensive response of the BMKO group to Ang II, the kidneys were stained for the immune cell markers F4/80 for macrophages and CD3 for T lymphocytes. Despite the apparent lack of kidney injury, macrophages were present throughout the renal interstitium in the saline-infused BMWT and BMKO groups (70±17 versus 54±4%; P value not significant; Figure 3C, 3D, and 3G), but the extent of macrophage infiltration was not different between the groups. With Ang II infusion, the degree of macrophage infiltration was significantly diminished in the BMWT group (29±5%; P<0.007 versus BMWT-saline; Figure 3E and 3G). On the other hand, Ang II infusion had no effect on the severity of renal macrophage accumulation in the BMKO-Ang group (49±6%; Figure 3F and 3G) as compared with the saline-infused BMKO group. Accordingly, the extent of macrophage infiltration was significantly greater in the BMKO-Ang group compared with the BMWT-Ang controls (49±6% versus 29±5%; P<0.03; Figure 3G).
Next, to measure the accumulation of T lymphocytes in the kidney with saline or Ang II infusion, we stained the kidneys for CD3. Because nearly all of the T cells were clustered around vessels in the kidney, we used a published scoring system to characterize the density of perivascular T-cell infiltrates in the 2 groups.23 In all 4 of the experimental groups, ≥80% of the renal vessels were surrounded by ≥5 T cells (Figure S2A). However, as illustrated in Figure S2B and S2C, the proportion of dense perivascular clusters containing >20 T cells was enhanced in the kidneys from the BMKO-Ang group (75.5%) compared with BMWT-Ang controls (50%; P<0.001 by χ2 analysis; Figure S2D). Thus, AT1 receptor deficiency on bone marrow–derived cells during Ang II infusion leads to enhanced accumulation of macrophages and T lymphocytes in the kidney.
AT1 Receptors on Bone Marrow–Derived Cells Regulate Renal Expression of MCP-1
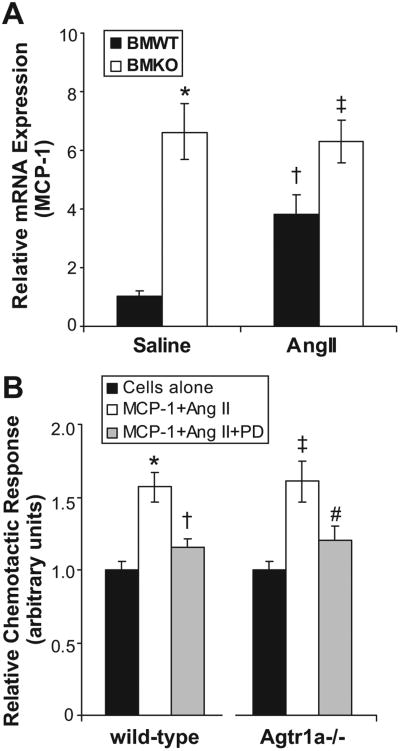
Ang II has been reported to stimulate renal expression of MCP-1 and the T-cell chemokine CCL5.26,27 Therefore, to determine whether altered chemokine generation might be responsible for differences in immune cell infiltration between our experimental groups, we measured renal mRNA expression of MCP-1 and CCL5 in the groups after saline or Ang II infusion. In BMWT kidneys, Ang II induced significantly more MCP-1 expression than saline infusion (1.04±0.18 versus 3.83±0.63 au; P=0.02), consistent with previous studies (Figure 4A).28,29 In addition, however, the BMKO animals had enhanced renal expression of MCP-1 compared with their BMWT counterparts after either saline (P=0.05) or Ang II infusion (P=0.02). Accordingly, the augmented renal expression of MCP-1 in BMKO-Ang kidneys compared with BMWT-Ang controls may have contributed to the persistent accumulation of macrophages in the kidneys from the BMKO-Ang group. By contrast, in our experiments, Ang II did not induce renal expression of the T-cell chemokine CCL5 more than saline control, and there were no differences between the BMWT and BMKO kidneys in CCL5 expression (Figure S3).
Figure 4.
Determinants of macrophage migration in the experimental groups. A, Renal MCP-1 expression measured by realtime PCR in the experimental groups after saline or Ang II infusion. *P=0.05 vs BMWT-saline. †P=0.02 vs BMWT-saline. ‡P=0.02 vs BMWT-Ang. B, Freshly isolated peritoneal WT or Agtr1a−/− macrophages were cultured in the upper chamber of a chemotaxis well with vehicle (black) or MCP-1 plus Ang II added to the lower chamber in the absence (white) or presence (gray) of AT2 receptor antagonist PD123319. MCP-1 with Ang II induced significant chemotaxis that was nearly abrogated by PD123319 compound in both groups. N=6 per group. *P=0.001 vs WT cells alone. †P<0.004 vs WT MCP-1/Ang II. ‡P<0.002 vs Agtr1a−/− cells alone. P value not significant vs WT MCP-1/Ang II. #P=0.04 vs Agtr1a−/− MCP-1/Ang II.
AT 1 Receptor–Deficient Macrophages Have Normal Migratory Capacity
Other groups have reported altered motility of AT1A receptor-deficient macrophages in response to Ang II.13 Moreover, we noted that WT macrophages exposed to Ang II for 4 hours developed dendritic leading edges that were less prominent on AT1A receptor–deficient (knockout [KO×]) macrophages (Figure S4). Therefore, to determine whether this difference in morphology was associated with altered migratory responses, we carried out in vitro cell migration studies using WT and KO macrophages. We were not able to demonstrate a migratory response of the macrophages to Ang II alone (data not shown). Next, because MCP-1 is an important macrophage chemokine that was upregulated in the BMKO kidneys, we incubated WT and KO macrophages with MCP-1 (1 μg/mL) together with Ang II (1 μmol/L). In this setting, WT and KO macrophages demonstrated a robust and equivalent chemotactic response (Figure 4B). Because the AT2 receptor has been shown to have functional effects in mononuclear cells,30 we repeated the chemotaxis experiment exposing WT and KO macrophages to MCP-1 and Ang II in the presence of AT2 receptor antagonist PD 123319 (10 μmol/L). PD 123319 significantly inhibited transwell migration of both the WT and the KO macrophages (Figure 4B). However, the degree of inhibition was similar between the WT and KO cells. Thus, the enhanced accumulation of macrophages in the kidneys of the BMKO-Ang animals cannot be ascribed to altered motility of these cells in response to Ang II or dysregulated AT2 receptor activation in the BMKO macrophages.
AT1 Receptors on Bone Marrow–Derived Cells Influence Renal Cytokine Generation in Ang II–Induced Hypertension
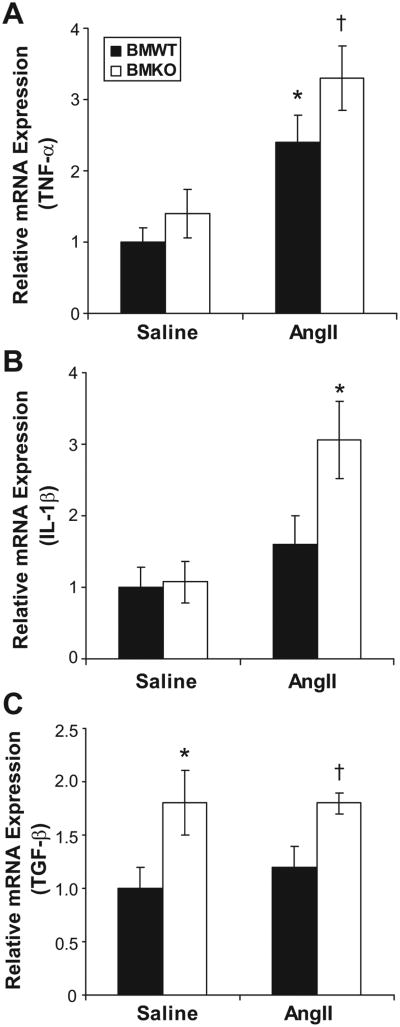
Inflammatory cells infiltrating the kidney can directly secrete cytokines and also regulate secretion of cytokines from renal parenchymal cells through paracrine mechanisms. These cytokines, in turn, can have important effects on blood pressure regulation.31,32 Therefore, we used real-time PCR after 4 weeks of Ang II or saline infusion to examine renal expression of several cytokines that have been shown to affect blood pressure or kidney injury including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6. As shown in Figure 5A, compared with saline infusion, Ang II caused induction of renal TNF-α expression in both the BMWT (P<0.07) and BMKO (P<0.03) groups. However, whereas TNF-α expression was numerically higher in both of the BMKO groups compared with their BMWT counterparts, these differences did not approach statistical significance. IL-1β expression was nearly equivalent in the kidneys from the saline infused groups (1.00±0.29 versus 1.08±0.29 au; P value not significant; Figure 5B). However, Ang II infusion caused significant induction of IL-1β only in the BMKO group (3.06±0.54 au; P=0.02 versus BMKO-saline) such that renal IL-1β expression was 85% greater in the BMKO-Ang group than in the BMWT-Ang mice (1.62±0.39 au; P=0.05; Figure 5B). Thus, activation of AT1 receptors on immune cells by exogenous Ang II appears to constrain IL-1β expression in the kidney during hypertension. Although we could not consistently detect IL-6 in the kidneys from the saline-infused groups, renal IL-6 expression was ≈ 100% greater in the BMKO-Ang group than in the BMWT-Ang controls (1.00±0.25 versus 1.97±0.39 au; P=0.06). Thus, the accumulation of mononuclear cells in the kidneys from the BMKO-Ang group leads to exaggerated generation of vasoactive cytokines that can enhance the chronic hypertensive response to Ang II.
Figure 5.
AT1 receptors on immune cells influence renal mRNA expression for inflammatory mediators. Relative expression measured by real-time PCR for (A) TNF-α. *P<0.07 vs BMWT-saline. †P<0.03 vs BMKO-saline. B, IL-1β. *P=0.02 vs BMKO-saline, P=0.05 vs BMWT-Ang. C, TGF-β. *P=0.05 vs BMWT-saline; †P=0.02 vs BMWT-Ang. mRNA expression normalized to BMWT-saline group in each panel.
Macrophages and some regulatory T-cell populations produce TGF-β, an important mediator of fibrosis and tissue remodeling that has been linked to AT1 receptor signaling.33,34 As shown in Figure 5C, with saline or Ang II infusion, the BMKO groups had substantially enhanced renal expression of TGF-β compared with their BMWT counterparts (P=0.05), suggesting a role for AT1 receptors on immune cells in regulating renal TGF-β expression in response to endogenous Ang II. However, compared with saline, exogenous Ang II did not induce renal TGF-β expression in this experiment (Figure 5C). Another T-cell population reportedly influenced by AT1 receptor activation is the type 1 T-helper cell subpopulation, marked by IFN-γ production.35 However, we were not able to consistently detect IFN-γ in the kidneys from the saline-infused groups, and there were no differences in renal IFN-γ expression between the BMWT-Ang and BMKO-Ang groups (1.00±0.34 versus 1.07±0.49 au; P value not significant). Thus, the induction of renal IFN-γ expression by Ang II noted in other studies does not require AT1 receptor activation on immune cells in this model.
Other mediators regulated by Ang II that could potentially influence blood pressure elevation in this model include vasodilatory prostaglandins. For example, Ang II has been shown to trigger arachidonic acid release from bone marrow cells via AT1 and AT2 receptor–mediated pathways.36 We, therefore, quantitated urinary excretion of prostaglandin E2 and prostacyclin by ELISA in the Ang II-infused BMWT and BMKO groups (n=14 per group). We found that urinary prostaglandin E2 (2663±297 versus 2988±404 pg per 24 hours; P value not significant) and prostacyclin (10 321 ±1177 versus 11 643±2097 pg per 24 hours; P value not significant) were similar in the BMWT and BMKO groups, suggesting that differential generation of vasodilatory prostaglandins did not contribute to the enhanced blood pressure elevation in the Ang II-infused BMKO group.
Discussion
Activation of AT1 receptors promotes blood pressure elevation and hypertensive end-organ damage.2,3,5,7,19 Induction of inflammatory signaling cascades by Ang II can clearly potentiate hypertensive renal injury25,37 and can even exacerbate blood pressure elevation,8 pointing to a possible role for AT1 receptors on immune cells in Ang II–dependent hypertension. Therefore, to directly examine the actions of AT1 receptors on bone marrow–derived cells in the pathogenesis of hypertension, we generated chimeras lacking AT1 receptors solely on immune cells (BMKO) and WT controls (BMWT) and then chronically infused these mice with Ang II. To concomitantly study effects on blood pressure elevation and on kidney injury, we used the 129/SvEv mouse strain that is permissive to hypertensive kidney injury and proteinuria.25,38 To further accentuate the degree of hypertension and kidney injury, mice underwent unilateral nephrectomy before induction of hypertension. On the basis of previous studies showing the importance of immune cells in Ang II–dependent hypertension,9,37 we anticipated that hypertension would be ameliorated in the BMKO group because of the abrogation of AT1 receptor–mediated effects in immune cells.
In this model, baseline blood pressures before Ang II infusion were similar to those we have reported previously in unmanipulated animals,18 suggesting that neither the uninephrectomy nor the bone marrow transfer procedure had a significant impact on baseline blood pressures. Moreover, the similar baseline blood pressures in the experimental groups indicate that AT1 receptors on immune cells do not regulate normal blood pressure homeostasis. These findings are consistent with reports that immune-deficient animals lacking lymphocytes have similar blood pressures to WT controls.9 Our results further indicate that AT1 receptors on lymphocytes do not measurably influence the determination of normal blood pressure.
The response of our BMWT group to chronic Ang II infusion was quite similar to that seen previously in mice not subjected to bone marrow transfer,19,20 suggesting that the bone marrow transplantation procedure did not impact the pattern of blood pressure elevation. By contrast, we found that the BMKO animals had an enhanced chronic hypertensive response to Ang II. These data suggest that AT1 receptors on bone marrow–derived cells actually play a protective role by limiting Ang II–induced blood pressure elevation. In light of previous studies showing that activation of inflammatory responses by Ang II can increase blood pressure,8 our data would indicate that this proinflammatory effect may involve complex interactions between nonhematopoietic cell lineages and the immune system rather than through simple activation of AT1 receptors on immune cells.
The protective actions of AT1 receptors on bone marrow– derived cells in our study contrast with the results from the Rag1-deficient model in which transfer of AT1 receptor– deficient lymphocytes before Ang II infusion yielded a blunted hypertensive response compared with transfer of WT lymphocytes.9 However, the 2 models are quite different in that the Rag1-deficient animal develops in the absence of functional lymphocytes, whereas in our model the experimental animal is immune competent during development. Moreover, in the Rag1 model, only lymphocytes were transferred, whereas in our model, all of the bone marrow–derived cell lineages may be impacted, particularly macrophages. Finally, the timing of immune cell transfer differs between the models. In the Rag1 model, lymphocytes were transferred 3 weeks before Ang II infusion, whereas in our model, bone marrow cells were transferred 10 weeks before Ang II infusion to allow for full bone marrow engraftment. The timing of immune cell transfer could have prominent effects on the circulation of immune cells through the kidney, and the impressive macrophage infiltration into the kidneys of our saline-infused BMWT and BMKO animals demonstrates that Ang II infusion is not required to drive renal mononuclear cell accumulation after adoptive transfer. We, therefore, speculate that actions of AT1 receptors on bone marrow–derived cells may have divergent effects on blood pressure elevation, depending on the repertoire of immune cells resident in the kidney when exogenous Ang II is administered.
More generally, the amplified response to RAS activation seen in our BMKO group is consistent with several other models using AT1 receptor–deficient bone marrow (KO) chimeras. For example, in the ureteral obstruction model, the KO chimeras had worse renal fibrosis and disease.13 Similarly, in the Tsukuba mouse, which exhibits a low level of chronic RAS activation, the KO chimera had worsened atherosclerosis.14 In 2 other important bone marrow transfer studies, one for kidney injury in Goodpasture syndrome12 and the other for atherosclerosis in the setting of low-density lipoprotein receptor deficiency,39 deletion of AT1 receptors solely from bone marrow-derived cells did not impact disease progression. However, these models are each quite different from our current model. Goodpasture syndrome has an antibody-mediated pathogenesis, and previous studies suggest that B cells, which produce antibodies, are not critical to the development of Ang II–dependent hypertension.9 The low-density lipoprotein receptor–deficiency model capitalizes on the susceptibility of the C57BL/6 strain to vascular pathology, to which the 129/SvEv mice used in our studies are relatively resistant.40
In the current studies, the BMKO animals also had higher levels of albuminuria than controls after 4 weeks of Ang II infusion. However, the degree of kidney injury using our semiquantitative scoring system was similar in the BMWT and BMKO groups. Because the blood pressure was >160 mmHg in both groups during the second and third weeks of Ang II infusion, the similar degree of kidney injury supports the previously established notion that kidney injury correlates with a certain threshold level of blood pressure elevation that exceeds the ability of the kidney to protect itself from hemodynamic injury via autoregulatory mechanisms.41,42 We acknowledge that alternative models using lower doses of Ang II or lower intrinsic levels of RAS activation over a longer duration as in the Tsukuba mouse model14 may be more effective in detecting subtle effects of immune cells on blood pressure or injury by avoiding the dramatic hemodynamic effects of high-dose Ang II. Indeed, macrophage functions have now been shown to influence blood pressure in the setting of salt-induced volume expansion alone.43 Nonetheless, in view of the similar levels of glomerulosclerosis in our Ang II-infused BMWT and BMKO groups, we speculate that the enhanced urinary albumin excretion in the BMKO group may be attributable to a direct hemodynamic effect of the higher BMKO blood pressures on glomerular filtration of albumin.
As in previous studies, Ang II infusion in our experiments was associated with a dramatic infiltration of macrophages and T lymphocytes into the kidney.25,29,44,45 In many kidney diseases, the density of mononuclear cell accumulation correlates with the degree of renal dysfunction and is predictive of disease progression.46 Surprisingly, however, we find that actions of AT1 receptors on macrophages appear to paradoxically limit their accumulation in the kidney during Ang II infusion. Ang II is known to stimulate chemokines in the kidney,27,28,47 which, in turn, could influence mononuclear cell infiltration. We, therefore, measured chemokine levels in the kidney. We found that Ang II caused significant induction of MCP-1 expression in BMWT kidneys, consistent with previous reports.28,29 Moreover, renal expression of MCP-1 was significantly enhanced in the BMKO groups compared with the BMWT controls after saline or Ang II infusion. We, therefore, posit that exaggerated renal MCP-1 expression in the BMKO-Ang group led to the persistent accumulation of macrophages in the kidney in the setting of hypertension.
We had initially posited that the enhanced renal accumulation of immune cells in the BMKO-Ang group might relate to effects of AT1 receptor activation on the motility of mononuclear cells. However, when we directly examined the chemotaxis of WT and AT1A receptor-deficient macrophages, we found their patterns of migration to be similar. Moreover, we cannot implicate differential activation of the AT2 receptor in macrophages in the BMWT and BMKO groups to explain the persistence of macrophages in the kidney of the BMKO-Ang mice, because an AT2 receptor antagonist inhibits chemotaxis of the WT and AT1A receptor–deficient macrophages equivalently.
Because damage to the renal vasculature and tubular epithelium was not evident in the experimental groups, the higher blood pressures in the BMKO-Ang group cannot be attributed to accentuated loss of vascular or epithelial architecture in the BMKO kidneys versus controls. We, therefore, examined whether the enhanced accumulation of mononuclear cells in the BMKO kidneys led to exaggerated local generation of cytokines that have the capacity to influence blood pressure or kidney function. Because IL1-β and IL-6 are known to have effects that can modulate blood pressure, we propose that the increased renal levels of these mediators in the BMKO-Ang mice augmented the hypertensive response of the BMKO animals to Ang II. For example, IL-1α and IL1-β stimulate vascular reactivity,48–50 but also trigger the generation of IL-6.51 Because mice genetically deficient for IL-6 have a severely blunted hypertensive response to Ang II,32 this cytokine clearly potentiates Ang II–dependent hypertension, possibly through effects on the epithelial sodium channel.52,53 These vasoactive cytokines, therefore, have the capacity to exaggerate blood pressure elevation through effects on renal blood flow and/or epithelial sodium transport.
Several studies have shown that Ang II stimulates renal expression of TGF-β in hypertension both through AT1 receptor activation54 and through stimulation of the immune system.25 In our experiments, consistent with previous studies,25,33 Ang II caused significant but similar renal fibrosis in both the BMWT and BMKO groups, despite enhanced TGF-β expression in the BMKO-Ang kidneys compared with the BMWT-Ang controls. In the BMKO-Ang group, the upregulation of TGF-β expression may have been related to the augmented renal accumulation of macrophages,29 which act as potent producers of TGF-β. The similar renal fibrosis in the Ang II–infused BMWT and BMKO groups despite the discrepancy between these groups in TGF-β expression highlights the complexity of the fibrotic process in the setting of hypertension. Given the higher blood pressures and augmented TGF-β expression in the BMKO-Ang group compared with the BMWT-Ang controls, it is also tempting to implicate this cytokine in contributing to blood pressure elevation through a mechanism separate from fibrosis. In this regard, some studies indicate that TGF-β may directly stimulate vascular contractility55 and vascular smooth muscle cell proliferation during hypertension,56 and blockade of TGF-β has been reported to lower blood pressure.57 Indeed, the induction of MCP-1 leading to persistent renal macrophage infiltration and TGF-β expression after Ang II infusion has been proposed as a mechanism of salt-sensitive hypertension.29
Perspectives
Activation of the RAS leads to hypertension and progressive kidney injury. The importance of AT1 receptor stimulation to the pathogenesis of hypertension is highlighted by the efficacy of ARBs in lowering blood pressure in broad populations of patients with essential hypertension.5 Although treatment with ARBs slows the progression of kidney disease associated with hypertension, ARBs do not prevent this complication altogether.6,7 Therefore, to develop targeted therapies to further limit end-organ damage in hypertension, determining which tissue pools of AT1 receptors mediate blood pressure elevation and hypertensive kidney injury will be critical. In the present studies, we have found, surprisingly, that actions of AT1 receptors on bone marrow–derived cells limit the extent of Ang II-induced blood pressure elevation and albuminuria by regulating the accumulation of inflammatory cells in the kidney and the renal generation of vasoactive cytokines. Accordingly, further examination of IL-1– and IL-6–mediated inflammatory cascades should offer important insights into the pathogenesis of hypertension that can be exploited to develop novel therapeutic interventions. Moreover, therapeutic strategies that block AT1 receptors in the kidney without preventing activation of the local RAS in the immune system may yield even greater benefits in hypertension than generalized Ang receptor blockade.
Supplementary Material
Acknowledgments
We acknowledge the outstanding administrative support from Brooke Flythe.
Sources of Funding: This work was supported by funding from the Veterans' Administration Medical Research Service.
Footnotes
Disclosures: None.
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://hyper.ahajournals.org/cgi/content/full/55/1/99
Contributor Information
Steven D. Crowley, Division of Nephrology, Department of Medicine Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C.
Young-Soo Song, Division of Nephrology, Department of Medicine Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C..
Gregory Sprung, Division of Nephrology, Department of Medicine Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C..
Robert Griffiths, Division of Nephrology, Department of Medicine, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C..
Matthew Sparks, Division of Nephrology, Department of Medicine, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C.
Ming Yan, Division of Nephrology, Department of Medicine, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C..
James L. Burchette, Division of Nephrology, Department of Pathology, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C.
David N. Howell, Division of Nephrology, Department of Pathology, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C.
Eugene E. Lin, Division of Nephrology, Department of Medicine, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C.
Benson Okeiyi, Division of Nephrology, Department of Medicine, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C..
Johannes Stegbauer, Division of Nephrology, Department of Medicine, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C..
Yanqiang Yang, Division of Nephrology, Department of Medicine, Duke University Medical Center and Durham Veterans' Affairs Medical Center, Durham, N.C..
Pierre-Louis Tharaux, Paris Cardiovascular Research Center, Institut National de la Sante et de la Recherche Medicale, Universite′ Paris Descarte, Paris, France.
Phillip Ruiz, Department of Pathology, University of Miami, Miami, Fla.
References
- 1.Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Buhler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- 2.Gavras H, Lever AF, Brown JJ, Macadam RF, Robertson JI. Acute renal failure, tubular necrosis, and myocardial infarction induced in the rabbit by intravenous angiotensin II. Lancet. 1971;2:19–22. doi: 10.1016/s0140-6736(71)90008-0. [DOI] [PubMed] [Google Scholar]
- 3.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley SD, Tharaux PL, Audoly LP, Coffman TM. Exploring type I angiotensin (AT1) receptor functions through gene targeting. Acta Physiol Scand. 2004;181:561–570. doi: 10.1111/j.1365-201X.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 5.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction in Hypertension Study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 8.Muller DN, Dechend R, Mervaala EMA, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-{kappa}B inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez De Lema G, De Wit C, Cohen CD, Nieto E, Molina A, Banas B, Luckow B, Vicente AB, Mampaso F, Schlondorff D. Angiotensin inhibition reduces glomerular damage and renal chemokine expression in MRL/lpr mice. J Pharmacol Exp Ther. 2003;307:275–281. doi: 10.1124/jpet.103.053678. [DOI] [PubMed] [Google Scholar]
- 11.Nataraj C, Oliverio MI, Mannon RB, Mannon PJ, Audoly LP, Amuchastegui CS, Ruiz P, Smithies O, Coffman TM. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest. 1999;104:1693–1701. doi: 10.1172/JCI7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hisada Y, Sugaya T, Tanaka S, Suzuki Y, Ra C, Kimura K, Fukamizu A. An essential role of angiotensin II receptor type 1a in recipient kidney, not in transplanted peripheral blood leukocytes, in progressive immune-mediated renal injury. Lab Invest. 2001;81:1243–1251. doi: 10.1038/labinvest.3780338. [DOI] [PubMed] [Google Scholar]
- 13.Nishida M, Fujinaka H, Matsusaka T, Price J, Kon V, Fogo AB, Davidson JM, Linton MF, Fazio S, Homma T, Yoshida H, Ichikawa I. Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest. 2002;110:1859–1868. doi: 10.1172/JCI200215045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato H, Ishida J, Nagano K, Honjo K, Sugaya T, Takeda N, Sugiyama F, Yagami K, Fujita T, Nangaku M, Fukamizu A. Deterioration of atherosclerosis in mice lacking angiotensin II type 1A receptor in bone marrow-derived cells. Lab Invest. 2008;88:731–739. doi: 10.1038/labinvest.2008.42. [DOI] [PubMed] [Google Scholar]
- 15.Ito MR, Ono M, Itoh J, Nose M. Bone marrow cell transfer of autoimmune diseases in a MRL strain of mice with a deficit in functional Fas ligand: dissociation of arteritis from glomerulonephritis. Pathol Int. 2003;53:518–524. doi: 10.1046/j.1440-1827.2003.01516.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen BJ, Cui X, Sempowski GD, Domen J, Chao NJ. Hematopoietic stem cell dose correlates with the speed of immune reconstitution after stem cell transplantation. Blood. 2004;103:4344–4352. doi: 10.1182/blood-2003-07-2534. [DOI] [PubMed] [Google Scholar]
- 17.Steel CD, Stephens AL, Hahto SM, Singletary SJ, Ciavarra RP. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (N Y) 2008;37:26–32. doi: 10.1038/laban0108-26. [DOI] [PubMed] [Google Scholar]
- 18.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43:364–369. doi: 10.1161/01.HYP.0000112225.27560.24. [DOI] [PubMed] [Google Scholar]
- 21.Spurney RF, Ruiz P, Pisetsky DS, Coffman TM. Enhanced renal leukotriene production in murine lupus: role of lipoxygenase metabolites. Kidney Int. 1991;39:95–102. doi: 10.1038/ki.1991.12. [DOI] [PubMed] [Google Scholar]
- 22.Spurney RF, Ibrahim S, Butterly D, Klotman PE, Sanfilippo F, Coffman TM. Leukotrienes in renal transplant rejection in rats: distinct roles for leukotriene B4 and peptidoleukotrienes in the pathogenesis of allograft injury. J Immunol. 1994;152:867–876. [PubMed] [Google Scholar]
- 23.Dragun D, Tullius SG, Park JK, Maasch C, Lukitsch I, Lippoldt A, Gross V, Luft FC, Haller H. ICAM-1 antisense oligodesoxynucleotides prevent reperfusion injury and enhance immediate graft function in renal transplantation. Kidney Int. 1998;54:590–602. doi: 10.1046/j.1523-1755.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- 24.McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D'Agostino RB, O'Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1–M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–F524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz-Ortega M, Lorenzo O, Egido J. Angiotensin III increases MCP-1 and activates NF-kappaB and AP-1 in cultured mesangial and mononuclear cells. Kidney Int. 2000;57:2285–2298. doi: 10.1046/j.1523-1755.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolf G, Ziyadeh FN, Thaiss F, Tomaszewski J, Caron RJ, Wenzel U, Zahner G, Helmchen U, Stahl RA. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells: role of the angiotensin type 2 receptor. J Clin Invest. 1997;100:1047–1058. doi: 10.1172/JCI119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K. Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest. 1999;103:627–635. doi: 10.1172/JCI2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sriramula S, Haque M, Majid DSA, Francis J. Involvement of tumor necrosis factor-{alpha} in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 33.Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- 34.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, Ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension. 2003;42:31–38. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- 36.Richmond RS, Tallant EA, Gallagher PE, Ferrario CM, Strawn WB. Angiotensin II stimulates arachidonic acid release from bone marrow stromal cells 10.3317/jraas. 2004.037. J Renin Angiotensin Aldosterone Syst. 2004;5:176–182. doi: 10.3317/jraas.2004.037. [DOI] [PubMed] [Google Scholar]
- 37.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishola DA, Jr, van der Giezen DM, Hahnel B, Goldschmeding R, Kriz W, Koomans HA, Joles JA. In mice, proteinuria and renal inflammatory responses to albumin overload are strain-dependent. Nephrol Dial Transplant. 2006;21:591–597. doi: 10.1093/ndt/gfi303. [DOI] [PubMed] [Google Scholar]
- 39.Lu H, Rateri DL, Feldman DL, Jr RJ, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le TH, Fogo AB, Salzler HR, Vinogradova T, Oliverio MI, Marchuk DA, Coffman TM. Modifier locus on mouse chromosome 3 for renal vascular pathology in AT1A receptor-deficiency. Hypertension. 2004;43:445–451. doi: 10.1161/01.HYP.0000112423.28987.00. [DOI] [PubMed] [Google Scholar]
- 41.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol. 1993;265:F391–F398. doi: 10.1152/ajprenal.1993.265.3.F391. [DOI] [PubMed] [Google Scholar]
- 42.Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure-dependent. Hypertension. 2003;41:201–206. doi: 10.1161/01.hyp.0000049881.25304.73. [DOI] [PubMed] [Google Scholar]
- 43.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 45.Mervaala EM, Muller DN, Park JK, Schmidt F, Lohn M, Breu V, Dragun D, Ganten D, Haller H, Luft FC. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. 1999;33:389–395. doi: 10.1161/01.hyp.33.1.389. [DOI] [PubMed] [Google Scholar]
- 46.Atkins RC. Macrophages in renal injury. Am J Kidney Dis. 1998;31:xlv–xlvii. doi: 10.1016/s0272-6386(14)70003-4. [DOI] [PubMed] [Google Scholar]
- 47.Luft FC, Mervaala E, Muller DN, Gross V, Schmidt F, Park JK, Schmitz C, Lippoldt A, Breu V, Dechend R, Dragun D, Schneider W, Ganten D, Haller H. Hypertension-induced end-organ damage: a new transgenic approach to an old problem. Hypertension. 1999;33:212–218. doi: 10.1161/01.hyp.33.1.212. [DOI] [PubMed] [Google Scholar]
- 48.Dorrance AM. Interleukin 1-beta (IL-1beta) enhances contractile responses in endothelium-denuded aorta from hypertensive, but not normotensive, rats. Vascul Pharmacol. 2007;47:160–165. doi: 10.1016/j.vph.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM. Interleukin-1beta, but not interleukin-6, enhances renal and systemic endothelin production in vivo. Am J Physiol Renal Physiol. 2008;295:F446–F453. doi: 10.1152/ajprenal.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper AL, Beasley D. Hypoxia stimulates proliferation and interleukin-1alpha production in human vascular smooth muscle cells. Am J Physiol. 1999;277:H1326–H1337. doi: 10.1152/ajpheart.1999.277.4.H1326. [DOI] [PubMed] [Google Scholar]
- 51.Sironi M, Breviario F, Proserpio P, Biondi A, Vecchi A, Van Damme J, Dejana E, Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989;142:549–553. [PubMed] [Google Scholar]
- 52.Li W, Xu YJ, Zhang ZX. Detection of the mRNA level of the subunits of amiloride-sensitive Na+ channel in human bronchial epithelium cells from patients with chronic obstructive pulmonary disease [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2004;27:533–536. [PubMed] [Google Scholar]
- 53.Li K, Zhu H, Guo D, Hering-Smith KS, Chao J, Dong Y. Interleukin-6 stimulates epithelial sodium channels via proteolysis in the mouse cortical collecting duct cells. Hypertension. 2008;52:e35. doi: 10.1152/ajpregu.00207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S, Ohta K, Hamaguchi A, Omura T, Yukimura T, Miura K, Inada Y, Wada T, Ishimura Y, Chatani F, Iwao H. Contribution of renal angiotensin II type I receptor to gene expressions in hypertension-induced renal injury. Kidney Int. 1994;46:1346–1358. doi: 10.1038/ki.1994.404. [DOI] [PubMed] [Google Scholar]
- 55.Zhu Z, Tepel M, Neusser M, Zidek W. Transforming growth factor beta 1 modulates angiotensin II-induced calcium influx in vascular smooth muscle. Eur J Clin Invest. 1995;25:317–321. doi: 10.1111/j.1365-2362.1995.tb01708.x. [DOI] [PubMed] [Google Scholar]
- 56.Fukuda N, Hu WY, Kubo A, Endoh M, Kishioka H, Satoh C, Soma M, Izumi Y, Kanmatsuse K. Abnormal regulation of transforming growth factor-beta receptors on vascular smooth muscle cells from spontaneously hypertensive rats by angiotensin II. Hypertension. 1998;31:672–677. doi: 10.1161/01.hyp.31.2.672. [DOI] [PubMed] [Google Scholar]
- 57.Lavoie P, Robitaille G, Agharazii M, Ledbetter S, Lebel M, Lariviere R. Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J Hypertens. 2005;23:1895–1903. doi: 10.1097/01.hjh.0000182521.44440.c5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.