Abstract
Sialic acid immunoglobulin–like lectin (Siglec)-6 is a transmembrane receptor that binds sialyl-TN glycans and leptin. Among eutherian mammals, only human placentas express Siglec-6. Previous work has implicated Siglec-6 in preeclampsia (PE). Preeclampsia, a leading cause of maternal and perinatal morbidity and mortality, is characterized by placental abnormalities. This study provides a comprehensive analysis of Siglec-6 protein expression during human pregnancy by disease state (PE), biologic compartment (basal plate, chorionic villi, or maternal plasma), gestational age (24-41 weeks), and labor status. Siglec-6 protein was increased in both the basal plate and chorionic villi of preterm PE placentas (P < .05). However, expression did not differ at term by disease state, compartment, or labor status. Siglec-6 was not detectable in maternal serum. Overexpression of Siglec-6 protein in preterm PE placentas may contribute to or represent a response to PE pathogenesis and suggests that preterm PE pathogenesis is distinct from term PE.
Keywords: preeclampsia, placenta, siglec, leptin
Introduction
Sialic acid immunoglobulin–like lectin (Siglec)-6 is a transmembrane receptor whose function is currently unknown. It has been shown, using in vitro techniques, that Siglec-6 binds sialyl-Tn glycans and leptin.1 Leptin is a placenta and adipocyte-produced peptide hormone involved in a variety of processes including energy homeostasis and immunity. Siglec-6 is in the rapidly evolving family of Siglecs (CD33 related) and its gene is found only in primates. Siglec-6 displays a restricted tissue expression pattern. It is expressed in the B-cells of all primates evaluated. However, among primates, it is uniquely expressed in human placenta, potentially due to unique elements in the human Siglec-6 promoter sequence.2 The possibility of a soluble form of Siglec-6 existing in maternal blood is supported by Northern blot analyses that have demonstrated multiple different placental messenger RNA (mRNA) transcripts.1,3 One alternatively spliced form of Siglec-6 mRNA is predicted to encode a soluble variant, although a soluble protein has not been demonstrated to date.3 We and others have previously determined by gene expression profiling that Siglec-6 is overexpressed in placentas from pregnancies complicated by preeclampsia (PE) compared to the controls.4–7
Preeclampsia is a disorder of pregnancy characterized by hypertension and proteinuria in the second half of pregnancy. Preeclampsia is almost exclusively limited to human pregnancy, with only rare case reports that PE may occur spontaneously in nonhuman primates.8–10 Approximately 5% of human pregnancies are complicated by PE, resulting in significant maternal and perinatal morbidity and mortality. Although the pathogenesis remains to be fully understood, the placenta is thought to play a central role in the etiology of PE. Aberrant placentation in early gestation has been proposed as the inciting event in PE pathogenesis.11 Defects have been noted in both the basal plate and chorionic villi of placentas from PE pregnancies. In the basal plate, or maternal/fetal interface, invasion of fetal cytotrophoblasts (CTBs) into the maternal decidua and uterine myometrium is shallow and uterine spiral arteries are inadequately remodeled.12 Defects in the chorionic villi include altered nutrient transport, increased shedding of syncytiotrophoblast (STB) debris, and altered villous morphology.13–15 Siglec-6 mRNA is overexpressed in the basal plate4,5 and chorionic villi5–7 of preterm PE placentas. Consistent with the mRNA findings, immunoreactivity of Siglec-6 is increased in preterm PE compared to preterm labor placentas,4 but a quantitative analysis of Siglec-6 protein in PE has not been conducted previously. Siglec-6 protein in the placenta has been immunolocalized exclusively to trophoblasts, both STB and CTB. Fetal stroma and maternal decidual cells do not express Siglec-6.1,2,4 These observations raise the question of whether Siglec-6 may be part of pathologic processes in PE and if Siglec-6 may serve as a predictive or diagnostic marker of PE. Of note, the Siglec-6 ligand, leptin, has also been shown by many investigators to be overexpressed in PE.4–7,16–18
The current studies aimed to quantify the expression of Siglec-6 in placentas from viable pregnancies (24-41 weeks of gestation) by disease state (PE), biologic compartment (basal plate, chorionic villi, or maternal plasma), gestational age, and labor status. The first goal of these studies was to determine whether Siglec-6 protein expression in PE is quantifiably different from controls. Differences in expression by compartment (basal plate vs chorionic villi) will provide clues to a possible role for Siglec-6 in PE placental abnormalities. Samples from preterm (24-36 completed weeks of gestation) and term (37-41 completed weeks of gestation) deliveries were evaluated separately because there are several lines of evidence that suggest preterm PE may have a different pathogenesis than term PE.15,19,20 Additionally, maternal plasma samples from PE and normal pregnancies were evaluated to determine whether a soluble form of Siglec-6 is detectable. Cultured trophoblast cell lines overexpressing Siglec-6 were also evaluated for expression of a soluble form of Siglec-6. The ability to detect Siglec-6 in maternal blood is a necessary first step in evaluating Siglec-6 as a predictive or diagnostic marker of PE. Finally, given that labor has been implicated in regulating Siglec-6 expression,2 the effects of labor on Siglec-6 expression were evaluated to complete this broad analysis of Siglec-6 expression in human pregnancy.
Methods
Tissue and Plasma Collection
Informed consent was obtained from each parturient tissue donor under protocols approved by the University of California San Francisco Committee on Human Research and University of Colorado Multiple Institutional Review Board. Exclusion criteria included pregnancies complicated by multiple gestations, premature rupture of the membranes, clinical signs of infection, diabetes or other autoimmune diseases, and known genetic or fetal anomalies. Gestational age was determined using standard dating criteria.21 Inclusion in the PE groups was determined according to the 2002 American College of Obstetrics and Gynecology diagnostic criteria (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or higher after 20 weeks of gestation in a previously normotensive woman and proteinuria ≥300 mg protein in a 24-hour urine collection).22 Nonlabored placentas were collected from term pregnancies delivered by a scheduled cesarean section. Maternal blood, collected between time of hospital admission and delivery, was processed to isolate plasma within 1 hour of collection and stored at −80°C, as previously described.23 Plasma evaluated in these studies was collected from participants in the preterm labored and preterm PE groups. Immediately after delivery, the basal plate was dissected from the placenta, rinsed in cold phosphate-buffered saline (PBS, RICCA Chemical Company, Arlington, Texas) and cut into ∼3 mm3 pieces, which were snap frozen in liquid nitrogen and stored at −80°C. Samples of the underlying chorionic villi were then collected. Villous tissue was rinsed with cold PBS, snap frozen in liquid nitrogen, and stored at −80°C. All samples were processed and frozen within 1 hour of delivery. Characteristics of participants from which placentas and plasma were collected are presented in Table 1. Samples were analyzed by preterm delivery (24-36 completed weeks of gestation) and term delivery (37-41 completed weeks of gestation). All of the preterm samples, both cases and controls, were from labored deliveries. For term control versus PE comparisons, all samples were from labored deliveries.
Table 1.
Participant Characteristics
| Maternal Age, Years ± SD | Gestational Age, Weeks ± SD | Gravity ± SD | Parity ± SD | |
|---|---|---|---|---|
| Placental samples | ||||
| Preterm labored control, n = 10 | 29.3 ± 8.0 | 30.6 ± 4.5 | 2.3 ± 1.4 | 0.4 ± 0.5 |
| Preterm labored PE, n = 11a | 27.8 ± 8.4 | 29.5 ± 4.8 | 2.3 ± 1.2 | 0.4 ± 0.5 |
| NS | NS | NS | NS | |
| Maternal plasma | ||||
| Preterm labored control, n = 5 | 27.8 ± 3.4 | 29.4 ± 4.9 | 2.5 ± 2.5 | 1.0 ± 1.4 |
| Preterm labored PE, n = 6b | 31.0 ± 7.6 | 28.8 ± 5.0 | 3.0 ± 1.8 | 1.5 ± 1.0 |
| NS | NS | NS | NS | |
| Placental samples | ||||
| Term control, n = 5 | 34.8 ± 5.6 | 38.6 ± 0.9 | 2.8 ± 1.3 | 1.2 ± 1.3 |
| Term PE, n = 5c | 29.8 ± 6.9 | 37.6 ± 0.7 | 2.8 ± 2.0 | 0.8 ± 1.3 |
| NS | NS | NS | NS | |
| Placental samples | ||||
| Term no labor, n = 5 | 32.0 ± 5.0 | 39.0 ± 0.0 | 2.6 ± 1.1 | 1.0 ± 0.7 |
| Term with labor, n = 5 | 28.4 ± 6.3 | 40.6 ± 1.0 | 2.8 ± 1.6 | 1.6 ± 1.5 |
| NS | P < .01 | NS | NS |
Abbreviations: NS, nonsignificant difference; PE, preeclampsia; SD, standard deviation; HELLP, hemolysis, elevated liver enzymes, low platelets; IUGR, intrauterine growth restriction.
a One participant had HELLP, 2 had IUGR, and 1 had HELLP with IUGR.
b One participant had IUGR and 1 had HELLP with IUGR.
c One participant had HELLP.
Culture of Cell Lines for Controls
The BeWO human choriocarcinoma trophoblast cells (American Type Culture Collection, Manassas, Virginia) were cultured in growth medium (F12 K medium [Cellgro, Manassas, Virginia] supplemented with 10% fetal bovine serum [Hyclone, Rockford, Illinois], 100 U/mL penicillin, and 100 μg/mL streptomycin [Invitrogen, Grand Island, New York]). HTR-8/SVneo immortalized first trimester human trophoblast cells (derived by Charles Graham24) were cultured in growth medium (RPMI 1640 medium supplemented with 5% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin). Cells were maintained in monolayer cultures on plastic at 37°C in 5% CO2, 95% air, and 100% humidity.
Generation of Siglec-6 Overexpressing Cells for Positive Controls
To generate Siglec-6 overexpressing cells, BeWO cells that lack Siglec-6 expression were transfected with empty PCDNA3.1+ vector (Invitrogen) or PCDNA3.1+ vector containing full-length Siglec-6. Full-length Siglec-6 complementary DNA ([cDNA] NM_001245.5) was transferred from a p-Babe plasmid1 into the PCDNA3.1+ vector using EcoRI and NotI restriction sites. Restriction digestion and sequencing confirmed the presence or absence of Siglec-6 in PCDNA3.1+.
HTR-8/SVneo cells, which also lack Siglec-6 expression, were transfected with pCDH-CMV-MCS-EF1-Puro vector (System Biosciences, Mountain View, California) or pCDH-CMV-MCS-EF1-Puro vector containing full-length Siglec-6. Full-length Siglec-6 cDNA was transferred from PCDNA3.1+ into pCDH-CMV-MCS-EF1-Puro using EcoRI and NotI restriction sites. The HTR-8/SVneo cells were originally generated by immortalization using a neomycin selection vector, thereby necessitating a puromycin selection vector to generate cells with stable expression of Siglec-6.
Transfections were performed according to manufacturer’s protocols with lipofectamine (Invitrogen) and 2 μg of DNA. Briefly, cells were grown to 80% confluence and then cultured in OptiMEM containing lipofectamine and DNA plus serum-free, antibiotic-free F12K (BeWO) or RPMI 1640 (HTR-8/SVneo) medium for 12 hours. Cells were allowed to recover for 72 hours before passaging into growth medium with selection antibiotics. The BeWO cells were selected with 200 μg/mL G418 (Invitrogen) and HTR-8/SVneo cells were selected with 1 μg/mL puromycin (Roche, San Francisco, CA). These concentrations were sufficient to kill untransfected cells within 72 hours as determined in kill curve assays.
Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR) for Siglec-6 Expression
Total RNA was isolated from BeWO and HTR-8/SVneo cells using Trizol (Invitrogen) and then cDNA generated with ImProm-II reagents (Promega, Madison, Wisconsin) according to manufacturers’ protocols. Templates were amplified with Assay on Demand Kits (Applied Biosystems, Carlsbad, California) as described previously,25 and samples were run in triplicate. Relative quantification was determined with the standard curve method (Applied Biosystems User Bulletin #2). 18S ribosomal RNA was used for amplification normalization. Conditioned medium was collected from cells grown to 60% to 80% confluence in either serum-free or growth medium for 24 hours and snap frozen in liquid nitrogen before storage at −20°C.
Immunoblotting for Siglec-6 Expression in Tissue
Snap frozen placental basal plate and chorionic villous tissues were homogenized in 1% sodium dodecyl sulfate. Samples were then sonicated for 5 seconds and centrifuged for 10 minutes at 13,000 g to remove insoluble material. Protein concentrations were determined with Biorad DC protein concentration assay per manufacture’s protocol. Placental protein lysates (60 μg) were separated on 7.5% polyacrylamide gels (Biorad) under reducing conditions and transferred to polyvinylidene fluoride membranes (Biorad, Hercules, California). The membranes were incubated in 5% nonfat dry milk (NFDM) in TBS-T (10 mmol/L Tris base, 150 mmol/L NaCl, and 0.05% Tween 20, pH 8.4) for an hour to block nonspecific binding. The blot was then washed 3 times for 10 minutes each in TBS-T (standard washing). The membrane was next incubated in the presence of an anti-Siglec-6 antibody (1:100, R&D Bioscience, Minneapolis, Minnesota) diluted in 5% bovine serum albumin (BSA) in TBS-T overnight at 4°C. After standard washing, the membrane was incubated in the presence of horseradish peroxidase (HRP) conjugated anti-sheep immunoglobulin Trublot antibody (1:1000, eBioscience, San Diego, California) diluted in 5% NFDM in TBST-T for an hour at room temperature. Standard washing was followed by a 5-minute wash in TBS without Tween. Visualization of the immunoreactive bands was achieved using chemiluminescence (Western Lightening Plus-ECL, Perkin Elmer, Waltham, Massachusetts) and exposure to XR film (Kodak, Rochester, New York). Actin immunoblotting was carried out to evaluate protein loading on immunoblots of placental lysates. The membranes were first incubated in stripping reagent for 10 minutes at room temperature (Reblot Plus Mild, Millipore, Billerica, Massachusetts). Membranes were then reblocked using 5% BSA in TBS-T for 30 minutes followed by incubation in the presence of anti-actin antibody (1:10 000, Sigma, St. Louis, Missouri) for 30 minutes at room temperature. Following standard washing, the membrane was incubated for 30 minutes in the presence of HRP-conjugated anti-chicken immunoglobulin (1:100 000, Sigma). Washing and visualization were as for Siglec-6. Imaging of films and band densitometry were performed using a Biorad Chemi Doc XRS and QuantityOne software. Tissue Siglec-6 expression was normalized to actin to control for protein loading. Data are reported as fold expression from the lowest expressing nonzero sample. In preterm PE versus preterm labor placental tissue studies, samples were run on 2 gels, each gel containing both preterm labor and preeclamptic samples of similar gestational age. Relative expression was normalized to a 25-week gestation control sample run on each gel.
Immunoblotting for Siglec-6 in Plasma and Conditioned Medium
Plasma samples (3 μL) and conditioned medium samples (38 μL) were separated on 4% to 20% and 7.5% polyacrylamide Criterion gels, respectively (Biorad). Positive controls of term placental basal plate and negative controls of conditioned medium from cells not transfected with Siglec-6 were run on each gel. Immnunoblotting and densitometry were carried out as described above for Siglec-6, excluding actin detection and normalization.
Statistics
Student t test was carried out to determine statistically significant differences in averages between groups for Siglec-6 expression and participant characteristics. Linear regression was used to evaluate the expression of Siglec-6 in preterm placental samples by gestational age. Prism software version 5.0 was used to conduct statistical analyses. P < .05 was considered significant.
Results
Siglec-6 Expression in Preterm PE
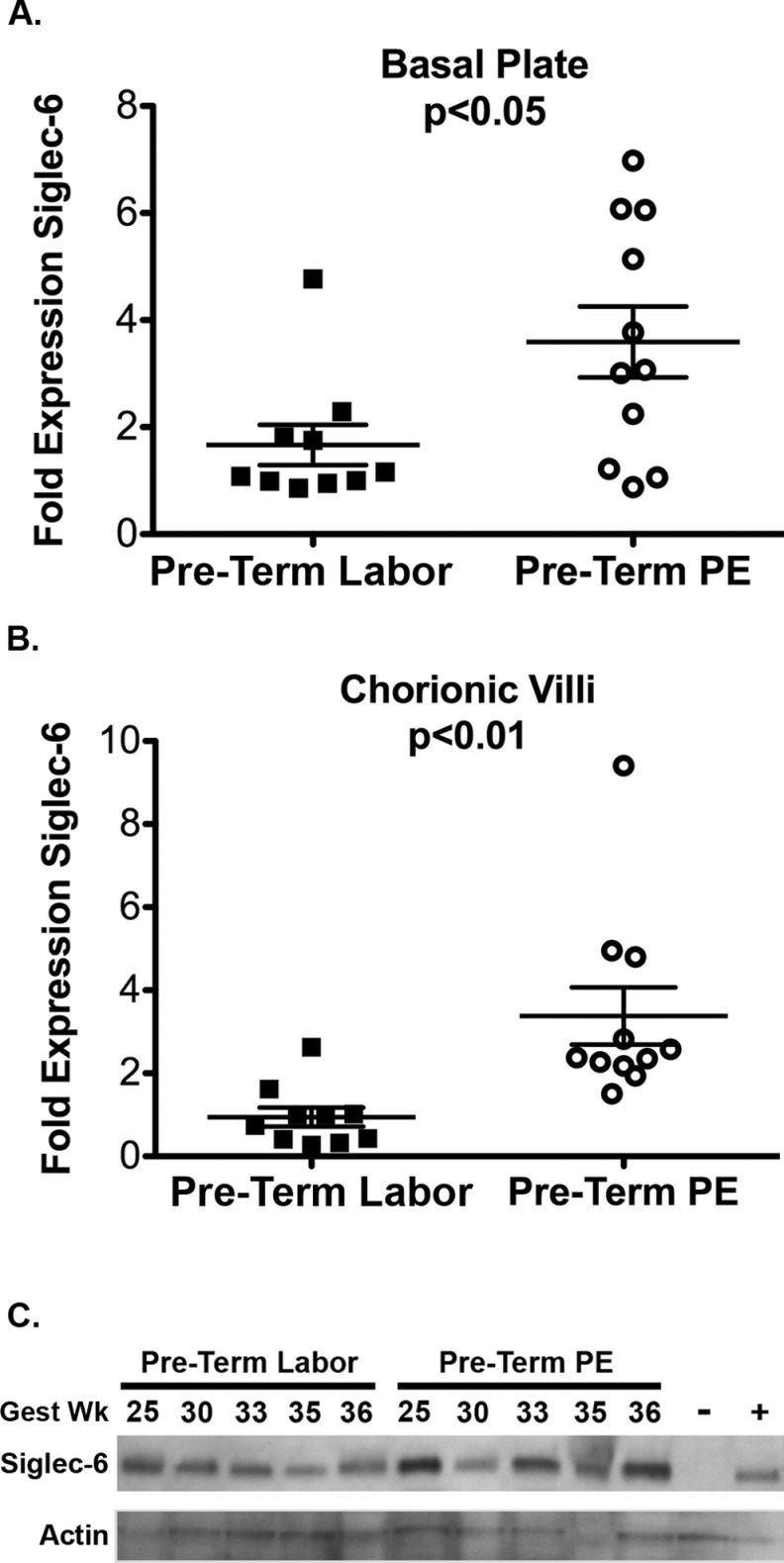
In the preterm period, Siglec-6 expression was higher in PE placentas (n = 11) compared to controls (n = 10), as determined by immunoblotting. In both the basal plate and chorionic villi, Siglec-6 expression was approximately double in the PE samples (Figure 1A and B). These data suggest a misregulation of Siglec-6 expression in both compartments of preterm PE placentas. With regard to the individual samples, 2 fell more than 2 standard deviations outside the mean. Among the preterm labor basal plate samples (Figure 1A, closed squares), there was nothing clinically remarkable about the participant with high basal plate Siglec-6 expression. Among the preterm PE chorionic villi samples (Figure 1B, open circles), the participant with high Siglec-6 expression was remarkable only in her young age (19 years old), which is a risk factor for PE. However, there is a similar young woman (18 years old) in the control group who does not have high Siglec-6 expression, so young age is unlikely to explain the high Siglec-6 expression in this preterm PE sample. Thus, there are no clinically apparent reasons to explain these outliers. The statistically significant increase in Siglec-6 expression in preterm PE basal plate and chorionic villi persists with removal of the outliers (average ± standard error of the mean; basal plate: 1.3 ± 0.2 vs 3.6 ± 0.7, P < .01; chorionic villi: 1.4 ± 0.4 vs 2.8 ± 0.4, P < .01).
Figure 1.
Siglec-6 expression is increased in preterm PE placentas compared to controls. Siglec-6 expression levels were determined from immunoblots of placental tissue protein lysates (gestational age range 24-36 weeks, all from labored deliveries). Siglec-6 expression was normalized to actin expression to control for protein loading. Levels are reported as fold expression relative to a 25-week gestation sample run on all blots. Siglec-6 expression is increased in the basal plate (A) and chorionic villi (B) of preterm PE placentas (n = 11, open circles) compared to preterm labored placentas (n = 10, closed squares). (C) Sample immunoblots of Siglec-6 and actin from preterm labor and preterm PE chorionic villi are shown. Gestational age of each sample in weeks (gest wk) is indicated. The BeWO cells transfected with empty vector (negative control, −) or Siglec-6 expressing vector (positive control, +) are shown. PE indicates preeclampsia; Siglec-6, sialic acid immunoglobulin–like lectin-6.
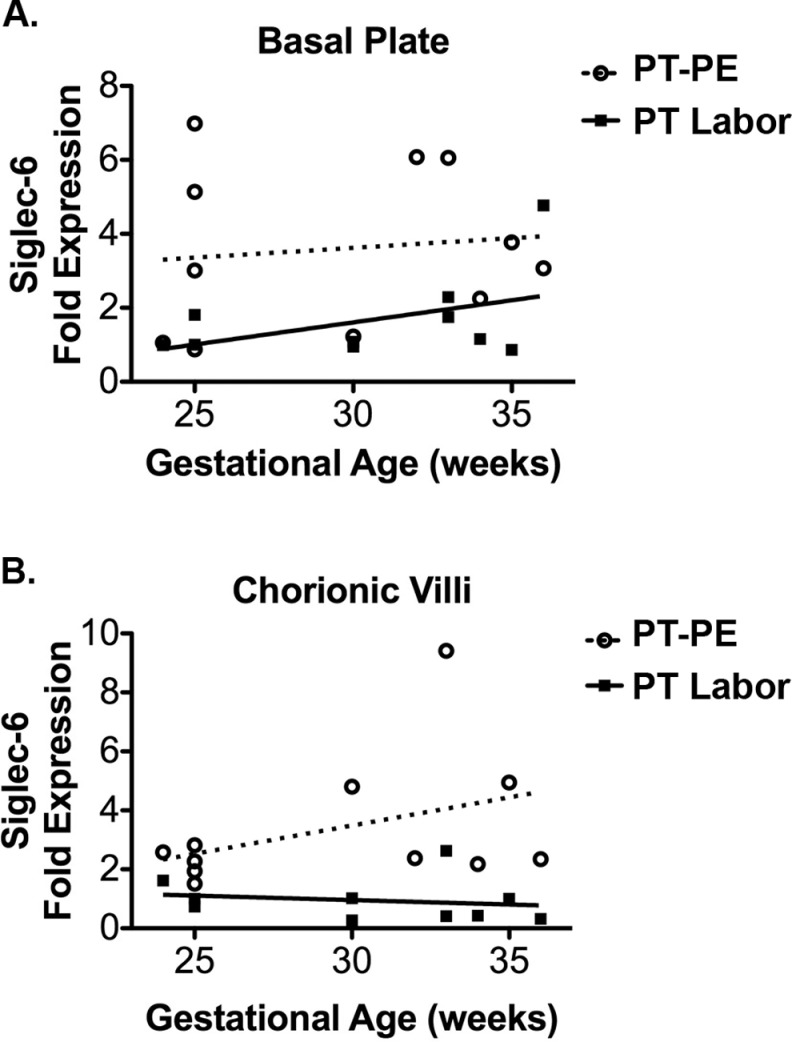
Siglec-6 Expression Across Preterm Gestation
Linear regression was used to determine whether Siglec-6 expression varied by gestational week in the preterm period. In the basal plate, Siglec-6 expression did not vary by gestational age among controls or PE samples (Figure 2A). Similarly, in the chorionic villi there was no relationship between Siglec-6 expression and gestational age in either control or PE samples (Figure 2B). Evaluation of Siglec-6 expression by gestational week from term pregnancies also showed no relationship (basal plate: term controls, R 2 = .10, P = .61 and term PE, R 2 = .48, P = .20. chorionic villi: term controls R 2 = 0.01, P = .86 and term PE, R 2 = .11, P = .59).
Figure 2.
Siglec-6 expression does not change across preterm gestation in either control or PE groups. Siglec-6 expression levels were determined from immunoblots of placental tissue protein lysates (gestational age range 24-36 weeks, all from labored deliveries). Siglec-6 expression was normalized to actin expression to control for protein loading. Levels are reported as fold expression relative to a 25-week gestation sample run on all blots. Linear regression of Siglec-6 expression by gestational age showed no trend in either the basal plate (A) or chorionic villi (B) of preterm labor (n = 10, closed squares, solid line) or preterm PE samples (n = 11, open circles, dashed line). Basal plate: preterm labor, R 2 = .20, P = .19, and preterm PE, R 2 = .01, P = .74. Chorionic villi: preterm labor, R 2 = .03, P = .61, and preterm PE, R 2 = .16, P = .23. PE indicates preeclampsia; Siglec-6, sialic acid immunoglobulin–like lectin.
Soluble Siglec-6 Expression
Plasma samples from preterm labored (n = 5) and PE (n = 6) patients were used to look for evidence of a soluble form of Siglec-6 as a potential biomarker of PE. The antibody used detects an N-terminal peptide of Siglec-6 and would be able to recognize either full length or an extracellular portion of Siglec-6. However, a soluble form of Siglec-6 was not detected in maternal plasma samples from either control or PE groups (data not shown). As an alternative method to look for evidence of soluble form of Siglec-6, cultured trophoblast cells (BeWO and HTR-8/SVneo cells) stably overexpressing Siglec-6 were created. Immunoblot analysis of the culture medium from Siglec-6 overexpressing cells also did not provide evidence of a soluble form of Siglec-6 (data not shown). These data do not support that a soluble form of Siglec-6 exists that is detectable using immunoblotting methods.
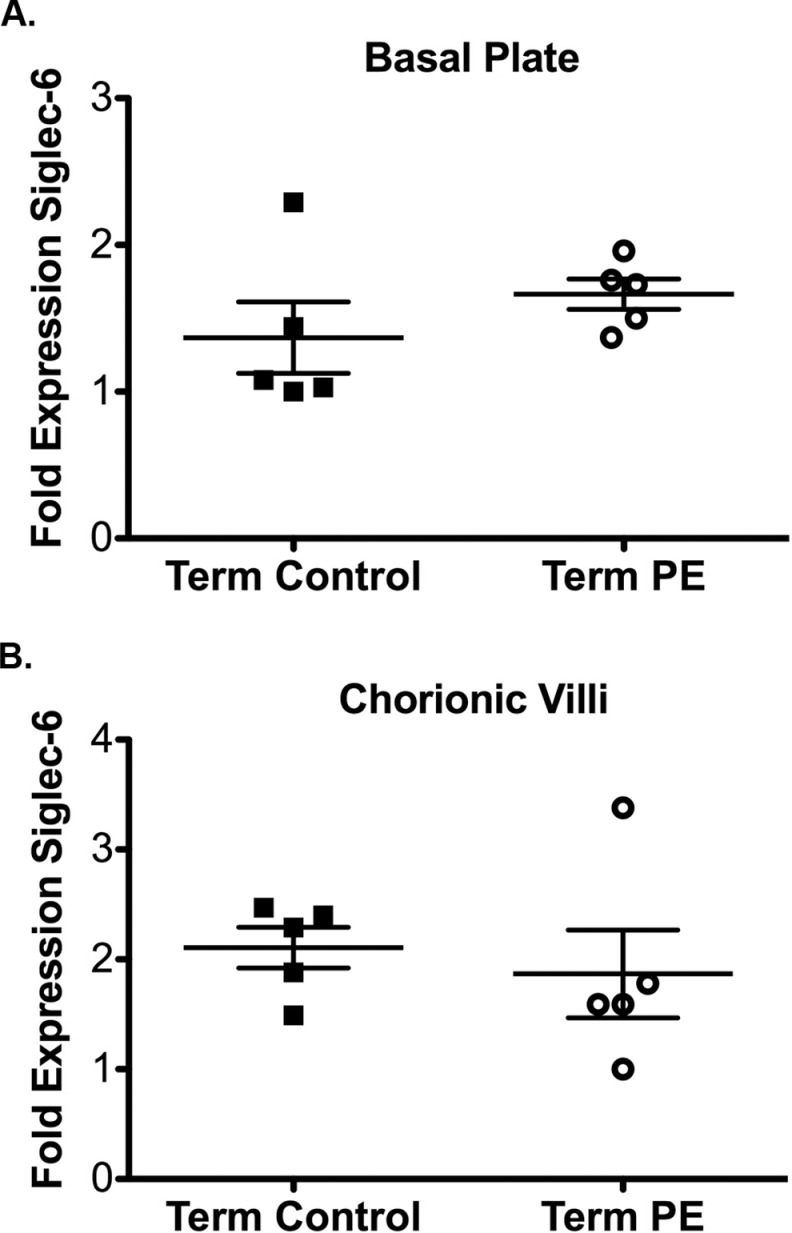
Siglec-6 Expression in Term PE
Siglec-6 expression was also evaluated in placental tissues from term control (n = 5) and term PE (n = 5) samples; all samples were from labored deliveries. In contrast to the preterm samples, there were no differences in Siglec-6 expression between term control and PE groups in either the basal plate or chorionic villi (Figure 3A and B). These data show that overexpression of Siglec-6 in PE is unique to preterm PE.
Figure 3.
Siglec-6 expression is unchanged in term PE placentas compared to the controls. Siglec-6 expression levels were determined from immunoblots of placental tissue protein lysates (gestational age range 37-41 weeks, all from labored deliveries). Siglec-6 expression was normalized to actin expression to control for protein loading. Fold expression is reported relative to the sample with the lowest expression. Siglec-6 expression is unchanged in the basal plate (A) and chorionic villi (B) of term PE (n = 5, open circles) and control placentas (n = 5 closed squares). PE indicates preeclampsia; Siglec-6, sialic acid immunoglobulin–like lectin-6.
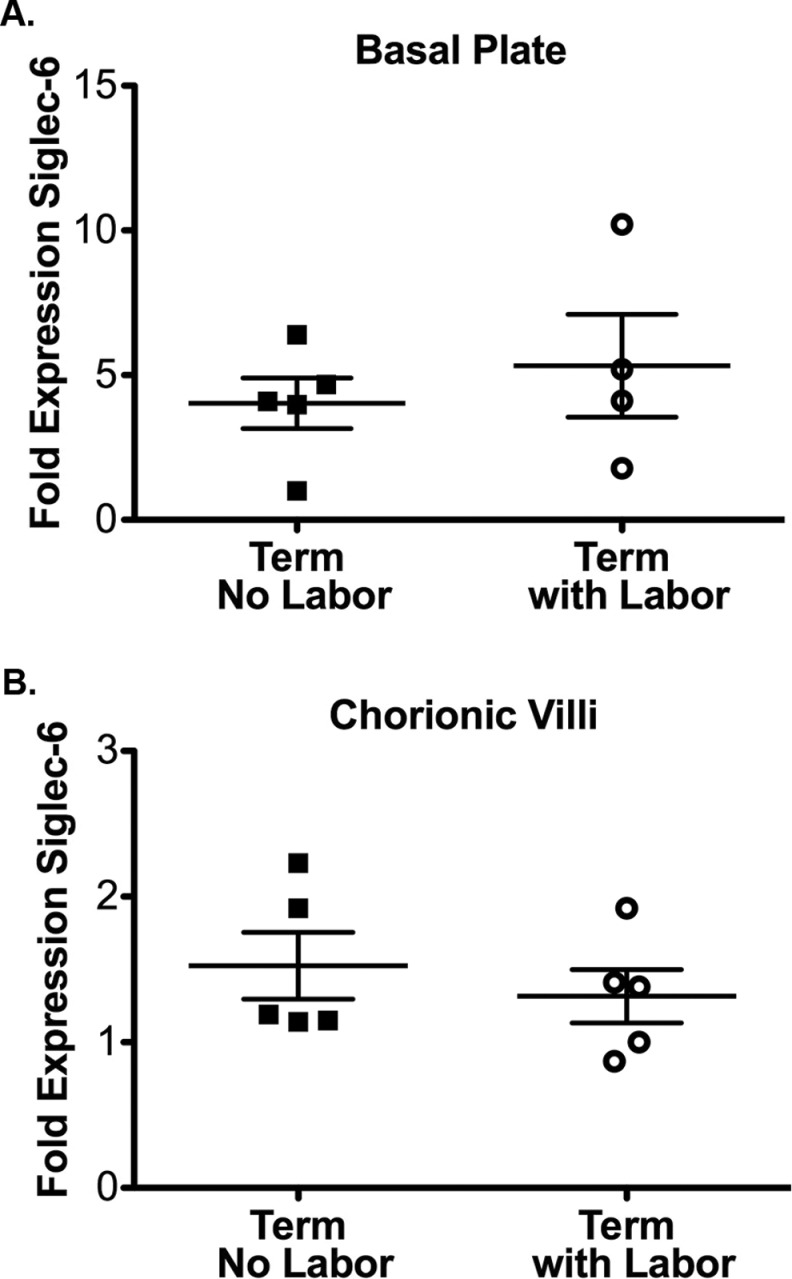
Effect of Labor on Siglec-6 Expression
A previous study had shown, by immunohistochemistry, that Siglec-6 immunoreactivity is generally increased in labored placentas at term, compared to nonlabored controls.2 We evaluated the effect of labor on Siglec-6 expression in our samples using immunoblot analysis. For these studies, immunoblots of term, control samples from labored (n = 5), and nonlabored (elective cesarean section, n = 5) deliveries were evaluated. Surprisingly, labor did not have an effect on Siglec-6 protein expression in either the basal plate or the chorionic villi of term control placentas (Figure 4A and B). The labor and nonlabor groups were different with respect to the participant characteristic of gestational age. In the nonlabored group, all the samples were from 39 weeks of gestation; this is expected due to term cesarean sections being typically scheduled at 39 weeks. In the labored group, the samples were from 37 to 41 weeks of gestation pregnancies. We were unable to obtain sufficient numbers of nonlabored preterm samples to determine whether Siglec-6 is altered by labor in the preterm period. In pregnancies that deliver preterm and fulfill our inclusion criteria, there are very few instances where a cesarean section would be performed without an antecedent labor.
Figure 4.
Siglec-6 expression is unchanged by labor at term. Siglec-6 expression levels were determined from immunoblots of placental tissue protein lysates (gestational age range 37-41 weeks). Siglec-6 expression was normalized to actin expression to control for protein loading. Fold expression is reported relative to the sample with the lowest expression. Siglec-6 expression is unchanged in the basal plate (A) and chorionic villi (B) of term labored placentas (n = 4-5, open circles, lysate from 1 basal plate labored sample was of insufficient quality for immunoblot analysis) compared to term unlabored controls (n = 5, closed squares).
Discussion
This work provides a comprehensive analysis of Siglec-6 protein expression in placentas from viable human pregnancies by disease state (PE), biologic compartment (basal plate, chorionic villi, and maternal plasma), gestational age (24-41 weeks gestation), and labor status. These studies have shown that Siglec-6 protein is overexpressed in preterm PE compared to preterm labored controls. In preterm PE, overexpression of Siglec-6 was present in both the basal plate and chorionic villi. Misregulation of Siglec-6 in both compartments may be indicative of a role for Siglec-6 in defects of trophoblast differentiation. Overexpression could also be a response to the altered placental environment in PE. Thus, the overexpression of Siglec-6 in PE placentas could either be a marker of PE or part of the pathologic processes in PE.
The inability to detect Siglec-6 in maternal plasma in preterm PE by immunoblotting does not support its utility as a marker of disease. However, other detection techniques may prove more fruitful. Finally, there was no trend by gestational age in Siglec-6 protein expression in either controls or PE samples. These findings are in contrast to previous immunohistochemistry studies that demonstrated increased Siglec-6 immunoreactivity with increasing gestational age during the preterm period.4 Because immunoblotting is more quantitative than immunohistochemistry, these results suggest that Siglec-6 expression in the preterm period is unaltered by gestational age. Alternatively, the antibodies used in the separate methods may be recognizing different Siglec-6 forms.
Unlike for preterm PE, there was no difference in Siglec-6 protein expression at term between PE and controls. The overexpression of Siglec-6 in preterm but not term PE may reflect the premature aging phenotype of PE placentas. The premature aging phenotype stems from the findings that many of the morphologic features of PE placentas are consistent with placentas of an older gestation. In PE, Siglec-6 expression may turn on early as part of the premature aging phenotype but by term, expression is normally high and thus expression in PE is indistinct from the controls. Alternatively, this difference between preterm and term PE may reflect that they have different underlying pathologic origins.
This work showed no difference in Siglec-6 expression between labored and nonlabored placentas at term, by immunoblotting. Work by others using immunohistochemistry has shown that Siglec-6 immunoreactivity was increased in the chorionic villi of term placentas from labored vaginal deliveries compared to scheduled cesarean sections with little or no labor.2 Our studies do not corroborate these earlier findings as we found similar levels of Siglec-6 expression in all term control placentas evaluated in both the basal plate and chorionic villi. However, our study evaluated fewer samples than the previous study (n = 9 vs n = 29) and may, therefore, be underpowered to detect small differences. However, several important differences between studies may also explain the different results. First, previous studies used immunohistochemistry while immunoblotting was employed in this study. Second, the antibodies used were different and may be recognizing different isoforms of Siglec-6, although both are directed against N-terminal antigens and there is no evidence of protein isoforms. Third, samples for previous studies were collected at sea level (San Diego, California), whereas our term samples were collected at altitude (approximately 5400 feet above sea level, Aurora, Colorado). If hypoxia is involved in the regulation of Siglec-6 expression, the chronic hypoxia of altitude could be masking the effects of the more acute hypoxia associated with labor. Thus, population differences may account for the differences between studies. Although our work does not support a role for Siglec-6 regulation in labor, this question remains open. Siglec-6 expression is increased in preterm PE and may be important in either the pathogenesis of or response to this disease. Understanding how Siglec-6 may play a role in preterm PE as well as whether hypoxia regulates Siglec-6 expression will be important questions to answer going forward.
Acknowledgments
We would like to thank Anita Kramer and Anne Mailhot for assistance with sample collection and preparation. We are grateful to Dr Ajit Varki for provision of PCDNA3.1+ vector containing full-length Siglec-6. We would like to thank Barb Hughes and Anna Thomas for administrative support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health Grants TL1 RR025778 (K.K.R.), K12 HD001271 (V.D.W.), March of Dimes Basil O’Conner Award (V.D.W.), and RO1 HD060723 (V.D.W.).
References
- 1. Patel N, Brinkman-Van der Linden EC, Altmann SW, et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999;274(32):22729–22738. [DOI] [PubMed] [Google Scholar]
- 2. Brinkman-Van der Linden EC, Hurtado-Ziola N, Hayakawa T, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17(9):922–931. [DOI] [PubMed] [Google Scholar]
- 3. Takei Y, Sasaki S, Fujiwara T, Takahashi E, Muto T, Nakamura Y. Molecular cloning of a novel gene similar to myeloid antigen CD33 and its specific expression in placenta. Cytogenet Cell Genet. 1997;78(3-4):295–300. [DOI] [PubMed] [Google Scholar]
- 4. Winn VD, Gormley M, Paquet AC, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150(1):452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA. Differential placental gene expression in preeclampsia. Am J Obstet Gynecol. 2008;199(5):566 e561–e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sitras V, Paulssen RH, Gronaas H, et al. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30(5):424–433. [DOI] [PubMed] [Google Scholar]
- 7. Tsai S, Hardison NE, James AH, et al. Transcriptional profiling of human placentas from pregnancies complicated by preeclampsia reveals disregulation of sialic acid acetylesterase and immune signalling pathways. Placenta. 2011;32(2):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thornton JG, Onwude JL. Convulsions in pregnancy in related gorillas. Am J Obstet Gynecol. 1992;167(1):240–241. [DOI] [PubMed] [Google Scholar]
- 9. Benirschke K, Kaufmann P. Pathology of the Human Placenta. 4th ed New York, NY: Springer; 2000. [Google Scholar]
- 10. Baird JN., Jr Eclampsia in a lowland gorilla. Am J Obstet Gynecol. 1981;141(3):345–346. [DOI] [PubMed] [Google Scholar]
- 11. Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357(9249):53–56. [DOI] [PubMed] [Google Scholar]
- 12. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 13. Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105(6):632–640. [DOI] [PubMed] [Google Scholar]
- 14. Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572(pt 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113(5):580–589. [DOI] [PubMed] [Google Scholar]
- 16. Meller M, Qiu C, Kuske BT, Abetew DF, Muy-Rivera M, Williams MA. Adipocytokine expression in placentas from pre-eclamptic and chronic hypertensive patients. Gynecol Endocrinol. 2006;22(5):267–273. [DOI] [PubMed] [Google Scholar]
- 17. Li RH, Poon SC, Yu MY, Wong YF. Expression of placental leptin and leptin receptors in preeclampsia. Int J Gynecol Pathol. 2004;23(4):378–385. [DOI] [PubMed] [Google Scholar]
- 18. Laivuori H, Gallaher MJ, Collura L, et al. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol Hum Reprod. 2006;12(9):551–556. [DOI] [PubMed] [Google Scholar]
- 19. von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–148. [DOI] [PubMed] [Google Scholar]
- 20. van der Merwe JL, Hall DR, Wright C, Schubert P, Grove D. Are early and late preeclampsia distinct subclasses of the disease—what does the placenta reveal? Hypertens Pregnancy. 2010;29(4):457–467. [DOI] [PubMed] [Google Scholar]
- 21. Practice Bulletin #107. Induction of labor. American College of Obstetricians and Gynecologists. 2009. [Google Scholar]
- 22. American College of Obstetrics and Gynecology A. ACOG Practice bulletin: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99(1):159–167. [DOI] [PubMed] [Google Scholar]
- 23. Lynch AM, Murphy JR, Byers T, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198(4):e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham CH, Hawley TS, Hawley RG, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206(2):204–211. [DOI] [PubMed] [Google Scholar]
- 25. Winn VD, Haimov-Kochman R, Paquet AC, et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148(3):1059–1079. [DOI] [PubMed] [Google Scholar]