Introduction
A recent national survey shows that 73% of Canadians are taking at least one natural health product (NHP), while more than one-third report taking 3 or more NHPs simultaneously.1-3 Of particular concern, patients with chronic medical conditions are more likely to take NHPs.4-6 These patients are also most likely to be prescribed conventional medications, and therefore the risk of interactions and patient harm is even greater.4-6 For example, 58% of patients taking narrow therapeutic index cardiovascular medications reported concurrent NHP use.6
The Study Of Natural health product Adverse Reactions (SONAR) is a multicentre study assessing a community pharmacy‒based active surveillance system to identify adverse events following NHP use, with a particular focus on NHP–prescription drug interactions. The study was developed in partnership with Health Canada to train participating pharmacists to ask individuals collecting prescription medications about 1) concurrent NHP/drug use in the previous month and 2) experiences of adverse events. If an adverse event was identified and if the patient provided written consent, a research pharmacist (CN) followed up with a detailed phone interview. This study was approved by the Human Research Ethics Board at the University of Alberta.
A patient identified in our study presented with increased bruising following the concurrent intake of clopidogrel, flaxseed oil and an additional long-chain omega-3 fatty acid supplement.
Case description
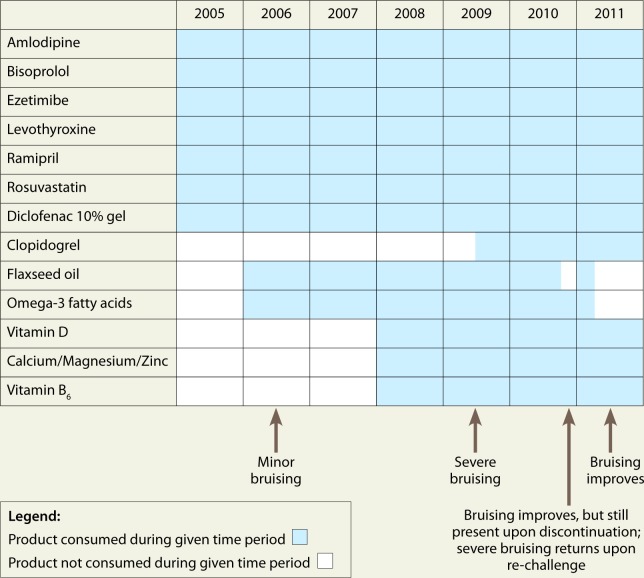
A 68-year-old woman presented to her community pharmacy on February 1, 2011, with concerns of increased spontaneous bruising on multiple areas of her body since 2007, increasingly since she was started on clopidogrel in June 2009. She reported the bruises as varying in size, with some appearing as large as a baseball, and no recollection of injury or direct causes for the bruising. Her medical conditions included hypertension, hypercholesterolemia, placement of a coronary stent in 2000, glucose intolerance, metabolic syndrome, fatty liver, decreased kidney function, hypothyroidism and vitamin D deficiency. Her medications included amlodipine (5 mg), bisoprolol (10 mg), ezetimibe (10 mg), levothyroxine (50 mcg), ramipril (20 mg) and rosuvastatin (10 mg), all of which were taken for 9 years prior to the addition of clopidogrel (75 mg). In addition to these medications, the patient reported taking numerous NHPs, including omega-3 fatty acids (500 mg, providing 200 mg of docosahexaenoic acid [DHA] and 300 mg of eicosapentaenoic acid [EPA]), flaxseed oil (1000 mg), vitamin B6 (100 mg), vitamin D3 (2000 IU) and calcium (666 mg)/magnesium (334 mg)/zinc (40 mg). After an extensive medication history, taken as part of the SONAR study protocol, the patient revealed that she had noticed an increase in bruising after initiating the omega-3 fatty acids and flaxseed oil in 2006; however, it was not until the addition of clopidogrel that the symptoms became enough of a concern to seek advice.
Prior to seeking help from a health professional, the patient tried discontinuing the flaxseed oil for a 2-week period in December 2010. She reported a decrease, but not a disappearance, in spontaneous bruising, and the bruising appeared again after rechallenge. On February 14, 2011, she discontinued both the omega-3 supplement and the flaxseed oil and noticed considerable improvement in the frequency and quantity of bruising. Figure 1 provides a detailed timeline of when all products were taken and the subsequent development of the adverse event.
Figure 1.
Timeline of adverse event occurrence relative to prescription drug and natural health product intake
Discussion
Omega-3 fatty acids are commonly taken by patients with cardiovascular disease.7 Numerous studies have been designed to investigate the benefit of omega-3 fatty acids on the outcomes and mortality associated with this population.7 A systematic review by León et al.7 found that although fish oil supplementation (which provides long-chain omega-3 fatty acids) did not show any benefit on arrhythmic events or all-cause mortality, a significant 20% reduction in deaths from cardiac disease was observed. Despite this benefit, it is important to be aware of possible risks and interactions associated with the use of these products.
This particular case describes a potential clinically relevant interaction between clopidogrel and 2 natural health products containing omega-3 fatty acids. The omega-3 fatty acids product contains the long-chain polyunsaturated fatty acids, EPA and DHA, whereas the flaxseed oil product is a rich source of alpha-linolenic acid (ALA), a plant source of omega-3 fatty acids. ALA is converted to only small amounts of DHA and EPA in the body because this conversion is inefficient in humans and only occurs at a rate of approximately 5%.8
Omega-3 fatty acids, particularly EPA and DHA, produce a reduction in thrombosis via a decrease in the production of thromboxane A2 and prostacyclin I2.9 Cohen et al.10 found an overall increase in bleeding time in patients taking escalating doses (1–8 grams daily) of omega-3 fatty acids, both alone and in combination with antiplatelet agents such as ASA (≤325 mg daily) and clopidogrel (75 mg daily). Omega-3 fatty acids were provided to patients in this study in the form of Lovaza.10 A 1 g capsule of Lovaza contains approximately 465 mg of EPA and 375 mg of DHA from pharmaceutical-grade fish oil sources.10 The mechanism of antiplatelet activity found in this study is described as an increase in the negative platelet surface charge, which reduces the response of platelets.10 Additionally, Gajos et al.11 reported significant potentiation of platelet response in patients who recently underwent percutaneous coronary intervention when omega-3 fatty acids (1 g daily) were added to standard dual antiplatelet therapy with clopidogrel (75 mg daily) and ASA (75 mg daily). Omega-3 fatty acids were provided to patients in this study in the form of Omacor.11 A 1 g capsule of Omacor contains approximately 460 mg of EPA and 380 mg of DHA.11 Further study demonstrated larger pores in the fibrin network, increased clot susceptibility to lysis and decreased thrombin formation when patients undergoing percutaneous coronary intervention were given the same combination as in the study above.12 Alternatively, Watson et al.13 retrospectively investigated reports of bleeding complications in patients treated with ASA (mean ± SD dose 161 ± 115 mg), clopidogrel (mean dose 75 mg) and high-dose fish oil (mean ± SD dose 3 ± 1.25 g) and found no significant increase in the risk of bleeding compared with those taking aspirin and clopidogrel alone.
Although reported less frequently, flaxseed has been found to exhibit some inhibition of platelet aggregation.14 It can be reasonably hypothesized that its effects on platelet aggregation would be similar to the omega-3 fatty acids reported.15 Nevertheless, a 3-month trial in healthy humans taking flaxseed demonstrated no changes in platelet aggregation.16
This case presents a positive rechallenge of the size and frequency of bruising in a patient taking flaxseed in combination with omega-3 fatty acids and clopidogrel. Although the bruising did not disappear, a marked improvement was experienced upon discontinuation of the flaxseed oil alone.
This adverse event could have been avoided or minimized if the patient had discussed her intention to use NHPs concurrently with prescription medications with her health care providers. Responsibility for such discussion is shared. Pharmacists and physicians should routinely inquire about all therapies (prescription, over-the-counter medications and NHPs) during history-taking, while patients should be encouraged to discuss their health care decisions with their health care teams. Unfortunately, it is estimated that only one-third of patients report NHP use to their family physicians, while only 24% of pharmacists regularly ask their patients about concurrent NHP–prescription drug use.17-19 Significant spontaneous external bruising warrants a more detailed history about signs and symptoms indicating possible internal bleeding, which did not occur because this patient confirmed she did not discuss polypharmacy with her health care providers (nor was she asked). This case highlights the important issue of discussing all therapies with all health care providers and the ongoing issue of preventing, identifying and/or reporting adverse events due to NHP-drug interactions.
Conclusion
Although several mechanisms explaining how omega-3 fatty acids can alter platelet aggregation and clot properties have been proposed, few published case reports demonstrate clinical adverse events associated with this effect.9-12,14 In fact, some studies even conclude a lack of risk.13,16 The present case demonstrates that clinically significant increased bleeding may occur with concurrent use of these natural health products. Caution is warranted when patients are taking flaxseed and other omega-3 fatty acids alone and especially in combination with other antiplatelet drugs such as clopidogrel. It is not uncommon for patients to omit discussion about NHP use with their physician or pharmacist.20,21 Proactive screening and discussions around concurrent NHP-drug use are imperative to avoid preventable adverse events. ■
References
- 1. Natural Health Products Directorate—Health Canada Natural health product tracking survey—2010 final report. Ipsos-Reid; 2011. January Available: http://epe.lac-bac.gc.ca/100/200/301/pwgsc-tpsgc/por-ef/health/2011/135-09/report.pdf (accessed Feb. 23, 2012).
- 2. Kelly JP, Kaufman DW, Kelley K, et al. Recent trends in use of herbal and other natural products. Arch Intern Med 2005;165:281-6 [DOI] [PubMed] [Google Scholar]
- 3. Berger E. Berger Population Health Monitor: Pharmacy medication and consumer health products report. Survey 2. Toronto (ON): The Hay Health Care Consulting Group; 2002 [Google Scholar]
- 4. Roy-Byrne PP, Bystritsky A, Russo J, et al. Use of herbal medicine in primary care patients with mood and anxiety disorders. Psychosomatics 2005;46:117-22 [DOI] [PubMed] [Google Scholar]
- 5. Quandt SA, Chen H, Grzywacz JG, et al. Use of complementary and alternative medicine by persons with arthritis: results of the National Health Interview Survey. Arthritis Rheum 2005;53:748-55 [DOI] [PubMed] [Google Scholar]
- 6. Wood MJ, Stewart RL, Merry H, et al. Use of complementary and alternative medical therapies in patients with cardiovascular disease. Am Heart J 2003;145:806-12 [DOI] [PubMed] [Google Scholar]
- 7. León H, Shibata MC, Sivakumaran S, et al. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ 2009;338:a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson BM, Ma DW. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis 2009;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calder PC. n-3 fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004;107:1-11 [DOI] [PubMed] [Google Scholar]
- 10. Cohen MG, Rossi JS, Garbarino J, et al. Insights into the inhibition of platelet activation by omega-3 polyunsaturated fatty acids: beyond aspirin and clopidogrel. Thromb Res 2011;128:335-40 [DOI] [PubMed] [Google Scholar]
- 11. Gajos G, Zalewski J, Rostoff P, et al. Reduced thrombin formation and altered fibrin clot properties induced by polyunsaturated omega-3 fatty acids on top of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention (OMEGA-PCI Clot). Arterioscler Thromb Vasc Biol 2011;31:1696-702 [DOI] [PubMed] [Google Scholar]
- 12. Gajos G, Rostoff P, Undas A, Piwowarska W. Effects of polyunsaturated omega-3 fatty acids on responsiveness to dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: the OMEGA-PCI study. J Am Coll Cardiol 2010;55:1671-8 [DOI] [PubMed] [Google Scholar]
- 13. Watson PD, Joy PS, Nkonde C, Hessen SE, Karalis DG. Comparison of bleeding complications with omega-3 fatty acids + aspirin + clopidogrel—versus—aspirin + clopidogrel in patients with cardiovascular disease. Am J Cardiol 2009;104:1052-4 [DOI] [PubMed] [Google Scholar]
- 14. Cunnane SC, Ganguli S, Menard C, et al. High α-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr 1993;69:443-53 [DOI] [PubMed] [Google Scholar]
- 15. Leyva DR, Zahradka P, Ramjiawan B, et al. The effect of dietary flaxseed on improving symptoms of cardiovascular disease in patients with peripheral artery disease: rationale and design of the FLAX-PAD randomized controlled trial. Contemp Clin Trials 2011;32:724-30 [DOI] [PubMed] [Google Scholar]
- 16. Austria JA, Richard MN, Chahine MN, et al. Bioavailability of alpha-linolenic acid in subjects after ingestion of three different forms of flaxseed. J Am Coll Nutr 2008; 27:214-21 [DOI] [PubMed] [Google Scholar]
- 17. Kennedy J. Herb and supplement use in the US adult population. Clin Ther 2005;27:1847-58 [DOI] [PubMed] [Google Scholar]
- 18. Busse JW, Heaton G, Wu P, et al. Disclosure of natural health product use to primary care physicians: a cross-sectional survey of naturopathic clinic attendees. Mayo Clin Proc 2005;80:616-23 [DOI] [PubMed] [Google Scholar]
- 19. Tiralongo E, Braun L, Wilkinson JM, et al. Exploring the integration of complementary medicines into Australian pharmacy practice with a focus on different practice settings and background knowledge. J Complement Integr Med 2010;7(1):Article 37. [Google Scholar]
- 20. Barnes J, Mills SY, Abbot NC, et al. Different standards for reporting ADRs to herbal medicines and conventional OTC medicines: face-to-face interviews with 515 users of herbal medicines. Br J Clin Pharmacol 1998;45:496-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vickers KA, Jolly KB, Greenfield SM. Herbal medicine: women’s views, knowledge and interaction with doctors: a qualitative study. BMC Altern Med 2006;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]