Abstract
Objective:
We tested the hypothesis that fetal pulmonary arterial circulation reacts to changes in fetal oxygenation status at near-term gestation.
Study Design:
A total of 20 rhesus macaques underwent fetal Doppler ultrasonography at near-term gestation. Right pulmonary artery (RPA), umbilical artery (UA), ductus arteriosus (DA), and ductus venosus (DV) blood velocity waveforms were obtained, and pulsatility index (PI) values were calculated. Fetal right and left ventricular cardiac outputs were determined. Ultrasonographic data were collected during 3 maternal oxygenation states: room air (baseline), hyperoxemia, and hypoxemia.
Results:
Fetal RPA PI values increased (P < .05) during maternal hypoxemia and decreased (P < .05) during maternal hyperoxemia, compared with baseline. Maternal hyperoxemia increased (P < .05) DA PI values from baseline. Fetal cardiac outputs, UA, and DV PI values were not affected.
Conclusions:
Our results demonstrate that at near-term gestation, fetal pulmonary arterial circulation is a dynamic vascular bed that reflects acute and short-term changes in fetal oxygenation.
Keywords: Doppler echocardiography, fetal physiology, fetal pulmonary physiology, hypoxemia, hyperoxemia, lung, heart
Introduction
Hypoxia is a common but unwelcomed challenge the fetus may experience during pregnancy. Significant fetal hypoxia is estimated to affect about 0.6% to 0.8% of pregnancies. Hypoxia triggers fetal defense mechanisms, including transient bradycardia and peripheral vasoconstriction.1,2 The peripheral vasoconstriction and subsequent increase in peripheral vascular resistance aim to redistribute fetal cardiac output toward the critical vascular beds, such as the fetal brain. Noninvasive antepartum testing to assess for fetal hypoxia includes fetal heart rate tracing, biophysical profile, and fetal hemodynamic evaluation by Doppler ultrasonography.3 Invasive testing has also been suggested by some studies, such as amniotic fluid erythropoietin concentration in certain high-risk pregnancies.4–6
Recent experimental and clinical studies have demonstrated that fetal pulmonary arterial circulation becomes responsive to fetal oxygen status toward the term of pregnancy.7–11 In sheep fetuses, studies have shown that acute fetal hypoxemia increases pulmonary arterial vascular impedance.10 In human fetuses, it has been demonstrated that an increase in fetal oxygenation leads to decreased pulmonary vascular impedance and increased pulmonary volume blood flow.7 This acquired ability of the pulmonary arteries to vasoconstrict or vasodilate during the last trimester has been attributed to the development of smooth muscle in small pulmonary arteries.11–15 Endothelium-derived nitric oxide (NO) from the pulmonary vasculature contributes to the control of vascular tone in the different fetal oxygen states.12,16 Hyperoxemia increases NO production leading to vasodilatation of the pulmonary arteries, whereas the effect of hypoxemia is opposite.12,17,18
In the present study, using noninvasive Doppler ultrasonography in a nonhuman primate model at near-term gestation, we tested the hypothesis that fetal pulmonary arterial circulation is a dynamic vascular bed that reacts to changes in fetal oxygenation status. Specifically, we wanted to investigate the effects of fetal hypoxemia and hyperoxemia on pulmonary arterial vascular impedance, fetal cardiac output, and placental vascular impedance.
Materials and Methods
A total of 20 rhesus macaques (Macaca mulatta) with singleton pregnancies underwent fetal Doppler ultrasonography at 150 to 157 days of gestation (term 165 days). The primate participants were housed in the Oregon National Primate Research Center. The animal care program is compliant with federal and local regulations regarding care and participation in research experiments. The center is accredited by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. The Oregon Health and Science University Animal Care and Use Committee approved all experiments reported in this article.
Time of conception was recorded for each animal, and the gestational age was confirmed with fetal ultrasound biometry at 90 to 100 days of gestation. For ultrasound examination, the animals were sedated with 10 mg/kg intramuscular injection of ketamine HCl (Ketaved, Bioniche Teoranta, Inverin, Co Galway, Ireland). Once appropriately sedated, each animal was positioned in the supine position and intubated with an endotracheal tube. The first Doppler ultrasonographic examination was performed while the participants breathed room air. After the baseline measurements were obtained, maternal oxygenation was manipulated. Maternal hyperoxemia was achieved by the administration of 100% oxygen via a nonrebreathing anesthesia circuit. For maternal hypoxemia, 12% oxygen was administered via the same circuit. The baseline measurements (room air) were always obtained first, the order of the other 2 oxygenation states alternated with each participant.
Maternal heart rate and oxyhemoglobin saturation were continuously monitored during the ultrasonographic examinations. For hypoxemia phase measurements, maternal oxyhemoglobin saturation was allowed to reach 75% for 5 minutes prior to starting the examination and this oxyhemoglobin saturation level was maintained until the data collection was completed. For hyperoxemia phase measurements, 100% oxygen was administered for a period of 5 minutes before starting the data collection. Participants were continued on the 100% oxygen, while the ultrasonographic data were obtained. All the examinations were completed within 60 minutes. The animals were then returned to their home cages and allowed to recover from anesthesia. All the ultrasonographic examinations were performed by a single investigator (J.R.).
Image-directed pulsed and color Doppler equipment (GE Voluson 730 Expert, Kretztechnik, Zipf, Austria) was used for ultrasonography. The lowest high-pass filter level (100 Hz) was used and an angle of less than or equal to 15° between the vascular flow and Doppler beam was accepted for analysis.
Fetal right pulmonary artery (RPA), ductus arteriosus (DA), and umbilical artery (UA) blood velocity waveforms were obtained to calculate their pulsatility index (PI = [peak systolic velocity − end diastolic velocity]/time-averaged maximum velocity over the cardiac cycle) values. The RPA blood velocity waveforms were obtained from the proximal part of the artery, immediately after the bifurcation of the main pulmonary artery. Fetal heart rate (FHR) was measured from the UA blood velocity waveforms. From the fetal venous circulation, ductus venosus (DV) blood velocity waveforms were recorded and the PI value for veins (PIV) was calculated. Three consecutive cardiac cycles were analyzed and their mean values were used for further analysis.
For fetal cardiac output calculations, pulmonary (PV) and aortic valve (AoV) diameters were measured from frozen real-time images during systole using the leading edge-to-leading edge method.19 Mean values of 3 separate measurements were used to calculate the cross-sectional area (CSA = [valve diameter/2]2 × π) of each valve. Cross-sectional area calculations were based on the assumption that the cross sections of the valves were circular. From the blood velocity waveforms across the PV and AoV, time–velocity integrals (TVI) were obtained by planimetry of the area underneath the Doppler spectrum. Volumetric blood flows across the PV (right ventricular cardiac output [RVCO]) and AoV (left ventricular cardiac output [LVCO]) were calculated using the formula (Q = CSA × VTI × FHR).19 All ultrasonographic recordings were obtained during fetal apnea and absence of fetal movements.
Statistical analyses were performed using Prism 5 software for Mac OS X. One-way comparisons between baseline, hyperoxemia, and hypoxemia measurements were made with 1-way repeated measures analysis of variance (ANOVA) and Bonferroni posthoc analysis. Statistical significance was established at a P < .05. All values are given as mean (standard deviation).
Results
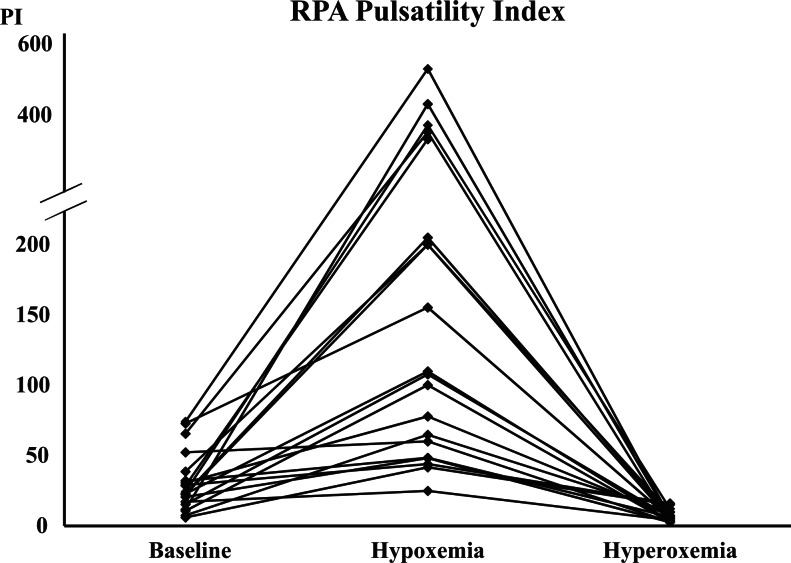
Maternal hypoxemia significantly increased fetal RPA PI values. There was over a 5-fold increase in RPA PI mean values between baseline and maternal hypoxemia phases (Table 1, Figures 1, and 2). During maternal hyperoxemia, RPA PI values decreased significantly, with over a 4-fold decrease in RPA PI mean values between these 2 phases (Table 1, Figures 1 and 2). The mean (standard deviation [SD]) time interval between the beginning of the hypoxemia or the hyperoxemia phase and the RPA PI measurement was 14 (3) minutes. Maternal hypoxemia did not affect the DA PI values. During hyperoxemia phase, however, the DA PI values increased significantly (Table 1, Figure 3). Maternal hypoxemia or hyperoxemia did not affect UA PI or DV PIV values (Table 1). FHR did not change significantly during the experiment. Fetal LVCO and RVCO were not affected by maternal hypoxemia. In addition, during maternal hyperoxemia, both ventricular cardiac outputs remained comparable with baseline values (Table 1).
Table 1.
Doppler Ultrasound Parameters at Baseline, Hypoxemia (12% Oxygen Administration), and Hyperoxemia (100% Oxygen Administration) for n = 20
| Variable | Baseline Mean (SD) | Hypoxemia Mean (SD) | Hyperoxemia Mean (SD) |
|---|---|---|---|
| FHR (bpm) | 168 (24.8) | 157 (21.0) | 151 (18.5) |
| RPA PI | 30.3 (20.6) | 166.4 (133.8)a | 6.9 (4.1)a |
| DA PI | 2.4 (0.3) | 2.3 (0.2) | 2.8 (0.5)a |
| DV PI | 0.3 (0.1) | 0.3 (0.1) | 0.3 (0.1) |
| UA PI | 1.1 (0.1) | 1.1 (0.2) | 1.1 (0.1) |
| RVCO (mL/min) | 149.1 (37.4) | 145.5 (30.1) | 137.1 (30.4) |
| LVCO (mL/min) | 93.9 (21.7) | 87.4 (20.6) | 82.8 (22.8) |
Abbreviations: FHR, fetal heart rate; PI, pulsatility index; RPA, right pulmonary artery; DA, ductus arteriosus; DV, ductus venosus; UA PI; umbilical artery; RVCO, right ventricular cardiac output; LVCO, left ventricular cardiac output.
a P < .05 compared with baseline.
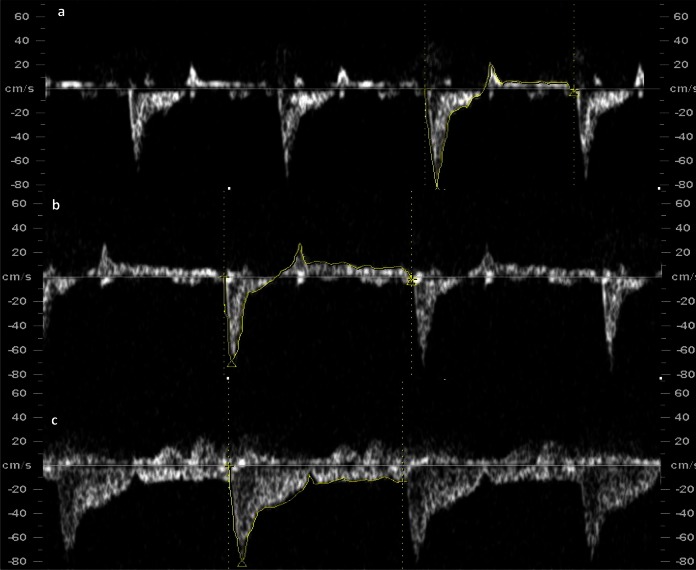
Figure 1.
Right pulmonary artery blood velocity waveform tracing at A, baseline [pulsatility index (PI) = 10.7], B, hypoxemia (PI = 100.1), and C, hyperoxemia (PI = 2.66).
Figure 2.
Right pulmonary artery (RPA) pulsatility index (PI) values at baseline, during hypoxemia, and hyperoxemia.
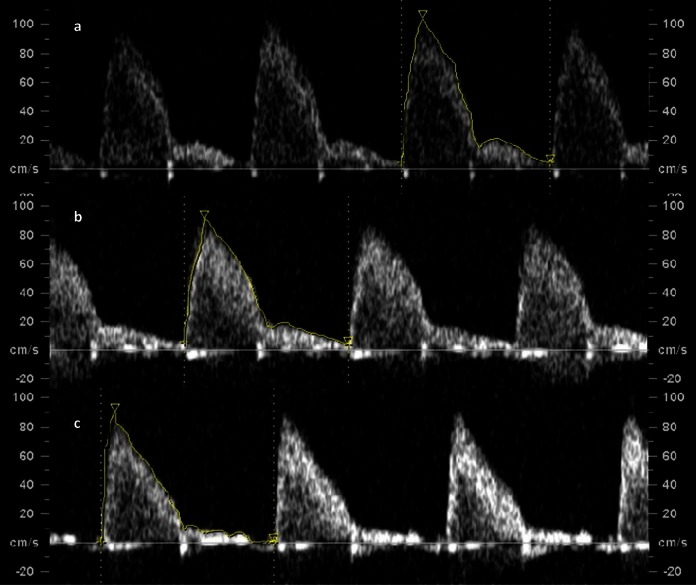
Figure 3.
Ductus arteriosus blood velocity waveform tracing at A, baseline (pulsatility index [PI] =2.4), B, hypoxemia (PI = 2.4), and C, hyperoxemia (PI = 3.1).
Comment
We found that maternal hypoxemia significantly increased fetal pulmonary arterial vascular impedance, and the effect of maternal hyperoxemia was opposite. Our results demonstrate that fetal pulmonary arterial circulation is a dynamic vascular bed that reacts to acute changes in fetal oxygenation, at least at near-term gestation. On the other hand, placental arterial vascular impedance was not affected by short-term changes in fetal oxygen status. In addition, fetal cardiac outputs and the pulsatility of DV blood velocity waveform remained unchanged during the study.
Vasodilatation decreases pressure wave reflection and vascular impedance, and the effect of vasoconstriction is opposite.20 The decrease in vascular impedance during hyperoxemia represents the downstream vasodilatation of the pulmonary arterial vascular bed. The increase in pulmonary arterial vascular impedance reflects vasoconstriction of the pulmonary arterial circulation. This vasoreactivity of the pulmonary arterial circulation is dependent on gestational age. In human pregnancies, it has been shown that pulmonary arterial circulation does not react to maternal hyperoxemia during the second trimester of pregnancy.7 However, between 31 and 36 weeks of gestation, pulmonary arterial vascular impedance significantly decreases during maternal hyperoxemia.7 Experiments on sheep fetuses have revealed similar results.9 In fact, it has been demonstrated that the sensitivity of the pulmonary arterial circulation to changes in fetal oxygenation increases significantly during the last trimester of pregnancy.11 The acquired ability of the pulmonary arterial circulation to regulate its vascular resistance has been attributed to the development of muscular layer in small pulmonary arteries during the third trimester of pregnancy.11–15 Endothelium-derived NO from the pulmonary arteries is thought to be the most important mediator responsible for controlling the vascular tone in the pulmonary arterial circulation.12,16 Oxygen is a critical substrate for NO synthesis, and the increase in fetal oxygenation during hyperoxemia activates NO synthesis and causes pulmonary arterial vasodilatation.21 In addition, increased pulmonary arterial volume blood flow increases shear stress of the vessel wall, thus potentiating NO production by increasing NO synthase expression.21,22 The effect of fetal hypoxemia on NO synthesis is opposite.17 The decrease in NO synthesis leads to increased pulmonary arterial vascular tone and pulmonary vascular resistance.11,23,24 In addition, hypoxemia is known to inhibit prostacyclin production, another potent mediator of pulmonary arterial vasodilatation present in the pulmonary vessels of the fetus and newborn.12,25
During maternal hyperoxemia, fetal DA PI values increased significantly, while maternal hypoxemia did not affect DA PI values. The increase in fetal oxygenation leads to pulmonary arterial vasodilatation, increased pulmonary arterial volume blood flow, and decreased pulmonary arterial vascular resistance. The decrease in pulmonary arterial vascular resistance, especially during diastole, causes the increase in DA PI values during maternal hyperoxemia. In normal circumstances, fetal systemic arterial vascular resistance is lower than the pulmonary arterial vascular resistance, thus allowing unidirectional shunting across the DA toward the descending aorta.7 When pulmonary arterial vascular resistance decreases, the proportion of DA volume blood flow of the RVCO decreases, thus decreasing forward blood flow across DA, especially during diastole.7 If the pulmonary arterial vascular resistance decreases below the systemic vascular resistance, the DA blood velocity waveform pattern may have a retrograde diastolic blood flow component. On the other hand, fetal hypoxemia did not affect DA PI values. Fetal hypoxemia causes a significant reduction in pulmonary volume blood flow and an increase in pulmonary vascular resistance.11 However, even in normal physiologic conditions fetal pulmonary arterial bed is under acquired vasoconstriction at near-term gestation.7 Thus, it seems that during fetal hypoxemia the volume blood flow shift from the pulmonary circulation to DA was not large enough to affect DA PI values.
UA PI values were not affected by the manipulation of fetal oxygenation. Our finding is in agreement with experimental sheep studies that have shown that UA vascular impedance does not change during fetal hypoxemia.26 Experimental studies on sheep fetuses have shown that placental embolization, and thus decreased number of small tertiary villi arterioles, increases UA vascular impedance.27 Altogether, our study confirms that at least short-term fetal hypoxemia does not alter UA vascular impedance.
Fetal hypoxemia causes a significant increase in the DV diameter and increases the pulsatility of DV blood velocity waveform pattern, mainly by decreasing forward blood flow velocity during atrial contraction.28,29 In addition, it has been proposed that fetal hypoxemia augments atrial contraction.28 In the present study, we did not find any significant changes in the pulsatility of the DV blood velocity waveform during maternal hyperoxemia or hypoxemia. The most likely explanation for these different results is that the fetal hypoxemic period in the present study was shorter than that in previous experiments.
Fetal right and left ventricular cardiac outputs remained unchanged during maternal hyperoxemia and hypoxemia. A study in human fetuses demonstrated that during maternal hyperoxemia fetal cardiac outputs do not change, however the distribution of right ventricular cardiac output is significantly affected.7 Pulmonary arterial volume blood flow increases significantly, while the volume of blood flow across the DA decreases.7 Our results in the present study support these findings. Previous experimental studies have shown that during hypoxemia, fetal cardiac output, especially the right ventricular output, increases.10 In the present study, we applied maternal hypoxemia only for a short time period, which could explain the unchanged cardiac output.
The blood velocity waveform pattern of the branch pulmonary artery is unique in the fetal circulation. The waveform profile is characterized by a rapid early systolic acceleration to its peak velocity, followed by a rapid decline and variable amount of diastolic blood flow. The noninvasively derived Doppler ultrasonographic blood velocity waveform tracing is similar to that obtained from invasive blood flow monitoring in fetal sheep.11 In the present study, we found that fetal hypoxemia mainly affects the diastolic blood flow component of the RPA. The vasoconstriction of the pulmonary arterial bed increases retrograde diastolic blood flow. On the other hand, the pulmonary arterial vasodilatation during hyperoxygenation is associated with antegrade diastolic blood flow pattern. Our findings demonstrate that at near-term gestation, fetal pulmonary arterial vascular impedance reflects changes in fetal oxygenation, even acute and short-term alterations.
The present study has certain limitations. We did not directly measure fetal arterial blood gas values during the different oxygenation states. However, previous studies have shown that maternal hyperoxygenation with 50% oxygen significantly increases fetal partial oxygen tension in human pregnancies.30 In addition, experimental studies on fetal sheep have shown that by decreasing maternal inspiratory oxygen content, fetal partial oxygen tension decreases.10,11 Based on these results, our experimental model is sufficient to modify fetal partial oxygen tension. Intraobserver variability in the measurements of different blood velocity waveform indices could introduce a potential error. Previous studies have shown an excellent repeatability of these measurements, and the intraobserver variability for RPA PI values is less than 4%.7 In addition, noninvasive Doppler-derived volume blood flow calculations correlate well with volume blood flow measurements by invasive techniques.31
In conclusion, the results of our experimental study demonstrate that at near-term gestation, fetal pulmonary arterial circulation is a dynamic vascular bed that reflects acute and short-term changes in fetal oxygenation. Fetal hypoxemia increases pulmonary arterial vascular impedance, and the effect of fetal hyperoxemia is opposite. Monitoring of fetal pulmonary arterial vascular impedance could provide clinically useful information about fetal oxygenation status at near-term gestation.
Acknowledgment
This study was supported by NIH grant HL087710. Additionally the publication was made possible by Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Road map for Medical Research.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by NIH grant HL087710.
References
- 1. Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120(6):817–824 [DOI] [PubMed] [Google Scholar]
- 2. Hanson MA. Do we now understand the control of the fetal circulation? Eur J Obstet Gynecol Reprod Biol. 1997;75(1):55–61 [DOI] [PubMed] [Google Scholar]
- 3. Signore C, Freeman RK, Spong CY. Antenatal testing-a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brace RA, Cheung CY, Davis LE, Gagnon R, Harding R, Widness JA. Sources of amniotic fluid erythropoietin during normoxia and hypoxia in fetal sheep. Am J Obstet Gynecol. 2006;195(1):246–254 [DOI] [PubMed] [Google Scholar]
- 5. Loukovaara M, Teramo K, Alfthan H, Hamalainen E, Stefanovic V, Andersson S. Amniotic fluid S100B protein and erythropoietin in pregnancies at risk for fetal hypoxia. Eur J Obstet Gynecol Reprod Biol. 2009;142(2):115–118 [DOI] [PubMed] [Google Scholar]
- 6. Buescher U, Hertwig K, Wolf C, Dudenhausen JW. Erythropoietin in amniotic fluid as a marker of chronic fetal hypoxia. Int J Gynaecol Obstet. 1998;60(3):257–263 [DOI] [PubMed] [Google Scholar]
- 7. Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S, Huhta JC. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: a randomized study. Circulation. 1998;97(3):257–262 [DOI] [PubMed] [Google Scholar]
- 8. Morin FC, Egan EA, Ferguson W, Lundgren CE. Development of pulmonary vascular response to oxygen. Am J Physiol. 1988;254(3 pt 2):H542–H546 [DOI] [PubMed] [Google Scholar]
- 9. Morin FC, Egan EA. Pulmonary hemodynamics in fetal lambs during development at normal and increased oxygen tension. J Appl Physiol. 1992;73(1):213–218 [DOI] [PubMed] [Google Scholar]
- 10. Makikallio K, Erkinaro T, Niemi N, et al. Fetal oxygenation and Doppler ultrasonography of cardiovascular hemodynamics in a chronic near-term sheep model. Am J Obstet Gynecol. 2006;194(2):542–550 [DOI] [PubMed] [Google Scholar]
- 11. Lewis AB, Heymann MA, Rudolph AM. Gestational changes in pulmonary vascular responses in fetal lambs in utero. Circ Res. 1976;39(4):536–541 [DOI] [PubMed] [Google Scholar]
- 12. Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90(4):1291–1335 [DOI] [PubMed] [Google Scholar]
- 13. Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol. 1995;57:115–134 [DOI] [PubMed] [Google Scholar]
- 14. Kiserud T. Physiology of the fetal circulation. Semin Fetal Neonatal Med. 2005;10(6):493–503 [DOI] [PubMed] [Google Scholar]
- 15. Levin DL, Rudolph AM, Heymann MA, Phibbs RH. Morphological development of the pulmonary vascular bed in fetal lambs. Circulation. 1976;53(1):144–151 [DOI] [PubMed] [Google Scholar]
- 16. Mital S, Konduri GG. Vascular potassium channels mediate oxygen-induced pulmonary vasodilation in fetal lambs. Biol Neonate. 2000;77(1):58–68 [DOI] [PubMed] [Google Scholar]
- 17. Liao JK, Zulueta JJ, Yu FS, Peng HB, Cote CG, Hassoun PM. Regulation of bovine endothelial constitutive nitric oxide synthase by oxygen. J Clin Invest. 1995;96(6):2661–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. North AJ, Lau KS, Brannon TS, et al. Oxygen upregulates nitric oxide synthase gene expression in ovine fetal pulmonary artery endothelial cells. Am J Physiol. 1996;270(4 pt 1):L643- L649. [DOI] [PubMed] [Google Scholar]
- 19. Rasanen J, Wood DC, Weiner S, Ludomirski A, Huhta JC. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation. 1996;94(5):1068–1073 [DOI] [PubMed] [Google Scholar]
- 20. Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–2869 [DOI] [PubMed] [Google Scholar]
- 21. Black SM, Johengen MJ, Ma ZD, Bristow J, Soifer SJ. Ventilation and oxygenation induce endothelial nitric oxide synthase gene expression in the lungs of fetal lambs. J Clin Invest. 1997;100(6):1448–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uematsu M, Ohara Y, Navas JP, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269(6 pt 1):C1371–C1378 [DOI] [PubMed] [Google Scholar]
- 23. Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol. 1997;272(5 pt 1):L1005–L1012 [DOI] [PubMed] [Google Scholar]
- 24. Steudel W, Ichinose F, Huang PL, et al. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81(1):34–41 [DOI] [PubMed] [Google Scholar]
- 25. Ibe BO, Raj JU. Endogenous arachidonic acid metabolism by calcium ionophore stimulated ferret lungs. Effect of age, hypoxia. Lab Invest. 1992;66(3):370–377 [PubMed] [Google Scholar]
- 26. Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Acute hypoxemia does not affect the umbilical artery flow velocity waveform in fetal sheep. Obstet Gynecol. 1990;75(4):590–593 [PubMed] [Google Scholar]
- 27. Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol. 1989;161(4):1055–1060 [DOI] [PubMed] [Google Scholar]
- 28. Tchirikov M, Eisermann K, Rybakowski C, Schroder HJ. Doppler ultrasound evaluation of ductus venosus blood flow during acute hypoxemia in fetal lambs. Ultrasound Obstet Gynecol. 1998;11(6):426–431 [DOI] [PubMed] [Google Scholar]
- 29. Kiserud T, Ozaki T, Nishina H, Rodeck C, Hanson MA. Effect of NO, phenylephrine, and hypoxemia on ductus venosus diameter in fetal sheep. Am J Physiol Heart Circ Physiol. 2000;279(3):H1166–H1171 [DOI] [PubMed] [Google Scholar]
- 30. Nicolaides KH, Campbell S, Bradley RJ, Bilardo CM, Soothill PW, Gibb D. Maternal oxygen therapy for intrauterine growth retardation. Lancet. 1987;329(8539):942–945 [DOI] [PubMed] [Google Scholar]
- 31. Schmidt KG, Di Tommaso M, Silverman NH, Rudolph AM. Doppler echocardiographic assessment of fetal descending aortic and umbilical blood flows. Validation studies in fetal lambs. Circulation. 1991;83(5):1731–1737 [DOI] [PubMed] [Google Scholar]