Abstract
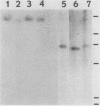
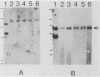
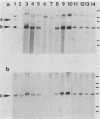
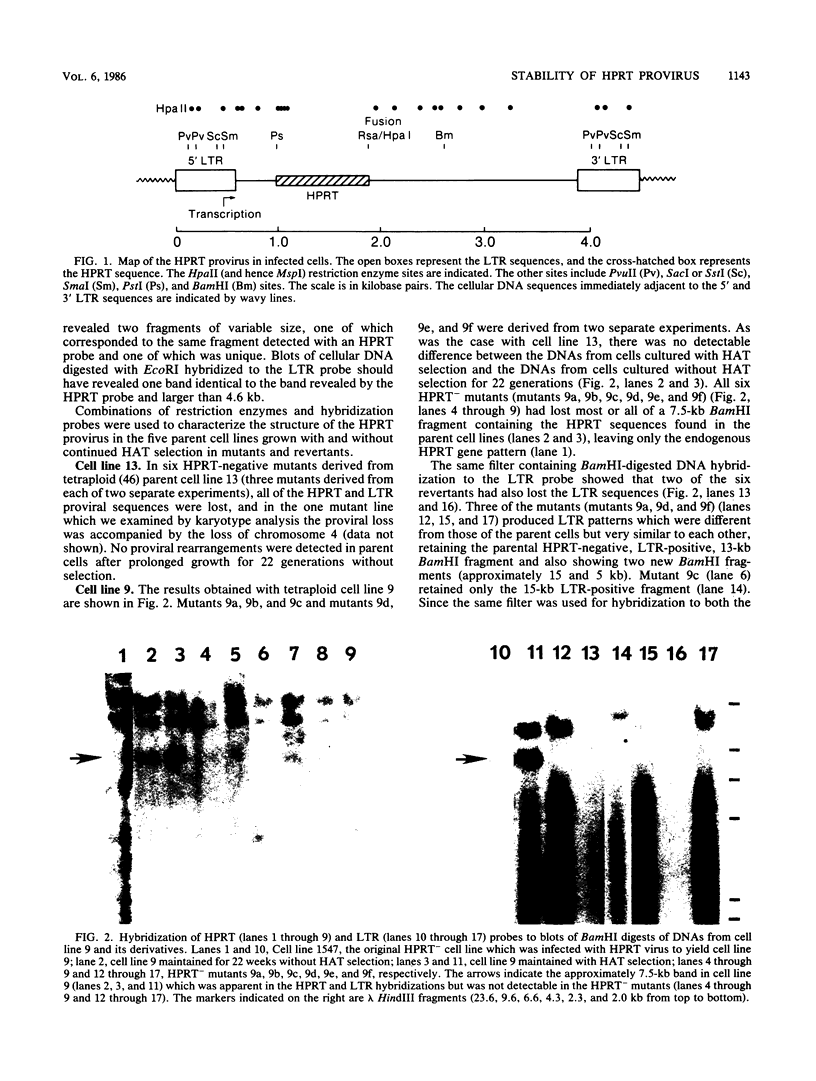
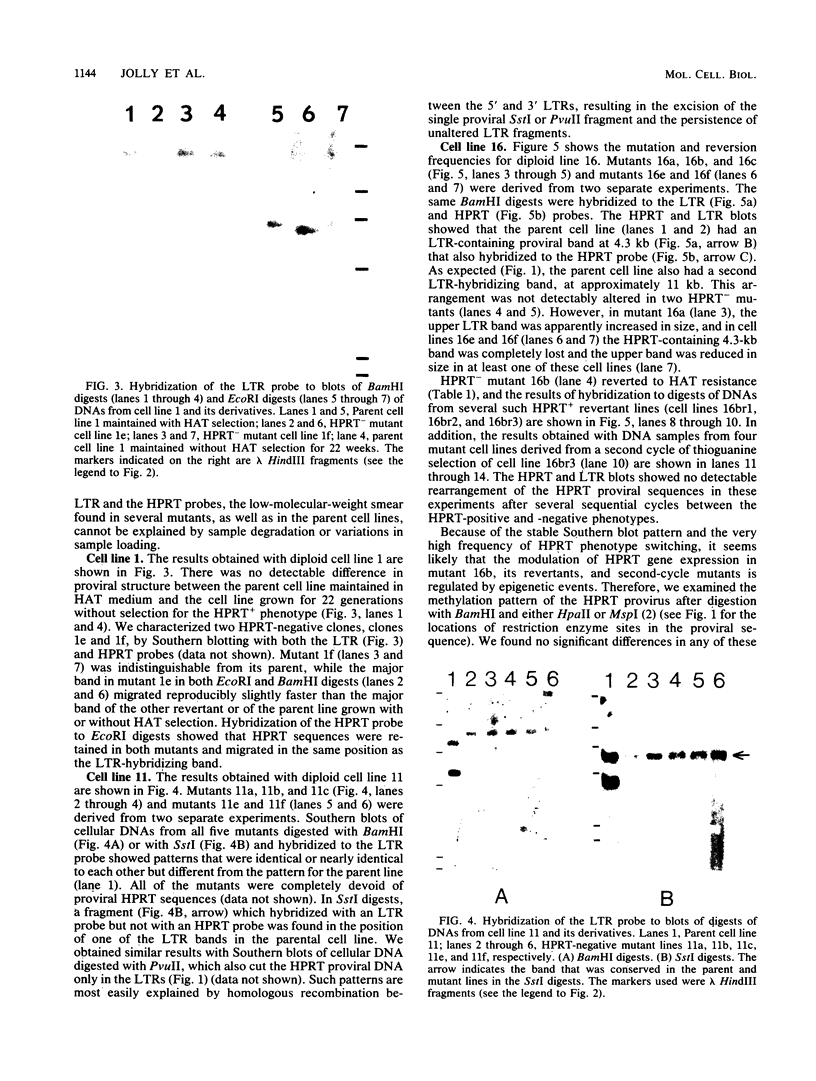
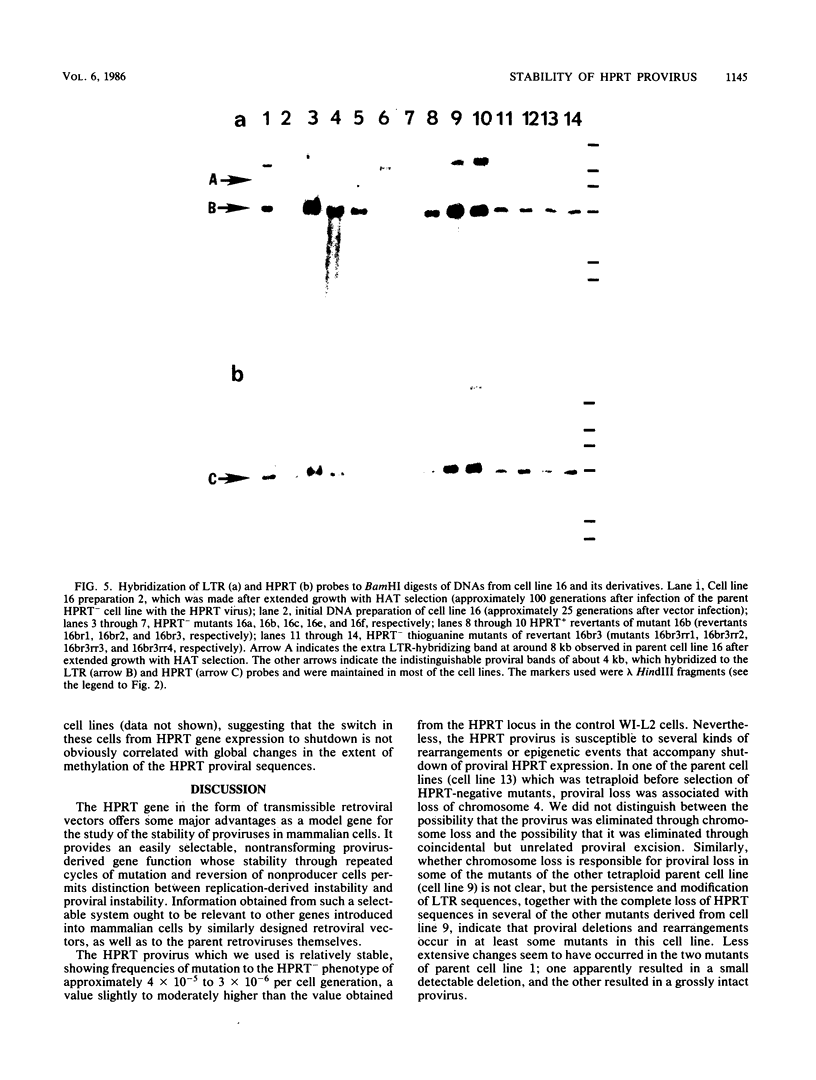
Human lymphoblasts deficient in the enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT) were infected with an amphotropic helper-free retroviral vector expressing human HPRT cDNA. The stability and expression of the HPRT provirus in five cell lines with different proviral integration sites were examined by determining HPRT mutation and reversion frequencies and by blot hybridization studies. Mutation to the HPRT-negative phenotype occurred at frequencies of approximately 4 X 10(-5) to 3 X 10(-6) per generation. Most mutations in each of the five cell lines were associated with partial or complete deletions or rearrangements of the provirus. Several mutants retained a grossly intact HPRT provirus, and in one such mutant HPRT shutdown resulted from a revertible epigenetic mechanism that was not associated with global changes in proviral methylation. Therefore, mutation and shutdown of the HPRT provirus in human lymphoblasts result from mechanisms similar to those reported for several other avian and mammalian replication-competent retroviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bestwick R. K., Machida C. A., Polonoff E., Kabat D. Frequent hereditable shutdown of murine retrovirus gene expression in murine cell lines. Mol Cell Biol. 1984 May;4(5):908–914. doi: 10.1128/mcb.4.5.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Bondurant M., Ramabhadran R., Green M., Wold W. S. "sarc" sequence transcription in Moloney sarcoma virus-transformed nonproducer cell lines. J Virol. 1979 Jan;29(1):76–82. doi: 10.1128/jvi.29.1.76-82.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Kruh G. D. The HPRT locus. Cell. 1979 Jan;16(1):1–9. doi: 10.1016/0092-8674(79)90182-x. [DOI] [PubMed] [Google Scholar]
- Chasin L. A. Non-linkage of induced mutations in Chinese hamster cells. Nat New Biol. 1972 Nov 8;240(97):50–52. doi: 10.1038/newbio240050a0. [DOI] [PubMed] [Google Scholar]
- Chiswell D. J., Enrietto P. J., Evans S., Quade K., Wyke J. A. Molecular mechanisms involved in morphological variation of avian sarcoma virus-infected rat cells. Virology. 1982 Jan 30;116(2):428–440. doi: 10.1016/0042-6822(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Feinstein S. C., Ross S. R., Yamamoto K. R. Chromosomal position effects determine transcriptional potential of integrated mammary tumor virus DNA. J Mol Biol. 1982 Apr 15;156(3):549–565. doi: 10.1016/0022-2836(82)90266-2. [DOI] [PubMed] [Google Scholar]
- Gillespie D. A., Hart K. A., Wyke J. A. Rearrangements of viral and cellular DNA are often associated with expression of Rous sarcoma virus in rat cells. Cell. 1985 May;41(1):279–287. doi: 10.1016/0092-8674(85)90081-9. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jolly D. J., Esty A. C., Bernard H. U., Friedmann T. Isolation of a genomic clone partially encoding human hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5038–5041. doi: 10.1073/pnas.79.16.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Okayama H., Berg P., Esty A. C., Filpula D., Bohlen P., Johnson G. G., Shively J. E., Hunkapillar T., Friedmann T. Isolation and characterization of a full-length expressible cDNA for human hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):477–481. doi: 10.1073/pnas.80.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. L., Bernstein A. Retrovirus transduction: segregation of the viral transforming function and the herpes simplex virus tk gene in infectious Friend spleen focus-forming virus thymidine kinase vectors. Mol Cell Biol. 1983 Dec;3(12):2191–2202. doi: 10.1128/mcb.3.12.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A., Keller G., Phillips R. A., Bernstein A. Retrovirus transfer of a bacterial gene into mouse haematopoietic progenitor cells. Nature. 1983 Oct 6;305(5934):556–558. doi: 10.1038/305556a0. [DOI] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Integration of Moloney leukaemia virus into the germ line of mice: correlation between site of integration and virus activation. Nature. 1980 Oct 2;287(5781):456–458. doi: 10.1038/287456a0. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Shibuya M., Samarut J., Hanafusa H. Revertants and partial transformants of rat fibroblasts infected with Fujinami sarcoma virus. J Virol. 1984 May;50(2):325–334. doi: 10.1128/jvi.50.2.325-334.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Eckner R. J., Jolly D. J., Friedmann T., Verma I. M. Expression of a retrovirus encoding human HPRT in mice. Science. 1984 Aug 10;225(4662):630–632. doi: 10.1126/science.6377498. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Jolly D. J., Friedmann T., Verma I. M. A transmissible retrovirus expressing human hypoxanthine phosphoribosyltransferase (HPRT): gene transfer into cells obtained from humans deficient in HPRT. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4709–4713. doi: 10.1073/pnas.80.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- O'Rear J. J., Temin H. M. Spontaneous changes in nucleotide sequence in proviruses of spleen necrosis virus, an avian retrovirus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1230–1234. doi: 10.1073/pnas.79.4.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A. S., Kirschmeier P. T., Gattoni-Celli S., Weinstein I. B. Design of a retrovirus-derived vector for expression and transduction of exogenous genes in mammalian cells. Mol Cell Biol. 1983 Jun;3(6):1123–1132. doi: 10.1128/mcb.3.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Hughes S. H., Varmus H. E., Bishop J. M. Structure of viral DNA and RNA in mammalian cells infected with avian sarcoma virus. J Mol Biol. 1980 Nov 15;143(4):363–393. doi: 10.1016/0022-2836(80)90218-1. [DOI] [PubMed] [Google Scholar]
- Searle S., Gillespie D. A., Chiswell D. J., Wyke J. A. Analysis of the variations in proviral cytosine methylation that accompany transformation and morphological reversion in a line of Rous sarcoma virus-infected Rat-1 cells. Nucleic Acids Res. 1984 Jul 11;12(13):5193–5210. doi: 10.1093/nar/12.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. D., Capecchi N. E., Capecchi M. R. Altered enzymes in drug-resistant variants of mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3145–3149. doi: 10.1073/pnas.70.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. Formation of infectious progeny virus after insertion of herpes simplex thymidine kinase gene into DNA of an avian retrovirus. Cell. 1981 Oct;26(1 Pt 1):67–77. doi: 10.1016/0092-8674(81)90034-9. [DOI] [PubMed] [Google Scholar]
- Shin S. I. Nature of mutations conferring resistance to 8-azaguanine in mouse cell lines. J Cell Sci. 1974 Mar;14(2):235–251. doi: 10.1242/jcs.14.2.235. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Zakut R., Canaani E. Frequent generation of nonrescuable reorganized Moloney murine sarcoma viral genomes. Proc Natl Acad Sci U S A. 1984 Jan;81(2):294–298. doi: 10.1073/pnas.81.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Stuhlmann H., Jähner D., Wagner H., Werner E., Jaenisch R. Retrovirus genomes methylated by mammalian but not bacterial methylase are non-infectious. Nature. 1983 Jul 21;304(5923):275–277. doi: 10.1038/304275a0. [DOI] [PubMed] [Google Scholar]
- Sorge J., Wright D., Erdman V. D., Cutting A. E. Amphotropic retrovirus vector system for human cell gene transfer. Mol Cell Biol. 1984 Sep;4(9):1730–1737. doi: 10.1128/mcb.4.9.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Hoffmann J. W., Goff S. P., Weinberg R. A. Adaptation of a retrovirus as a eucaryotic vector transmitting the herpes simplex virus thymidine kinase gene. Mol Cell Biol. 1982 Apr;2(4):426–436. doi: 10.1128/mcb.2.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Origin of retroviruses from cellular moveable genetic elements. Cell. 1980 Oct;21(3):599–600. doi: 10.1016/0092-8674(80)90420-1. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Wyke J. Revertants of an ASV-transformed rat cell line have lost the complete provius or sustained mutations in src. Virology. 1981 Jan 15;108(1):28–46. doi: 10.1016/0042-6822(81)90525-0. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Temin H. M. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983 Dec;3(12):2241–2249. doi: 10.1128/mcb.3.12.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Gibson M., Spear P. G., Scolnick E. M. Construction and isolation of a transmissible retrovirus containing the src gene of Harvey murine sarcoma virus and the thymidine kinase gene of herpes simplex virus type 1. J Virol. 1981 Sep;39(3):935–944. doi: 10.1128/jvi.39.3.935-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Jolly D. J., Miller A. D., Plent M. M., Esty A. C., Anderson P. J., Chang H. C., Jones O. W., Seegmiller J. E., Friedmann T. Partial phenotypic correction of human Lesch-Nyhan (hypoxanthine-guanine phosphoribosyltransferase-deficient) lymphoblasts with a transmissible retroviral vector. J Biol Chem. 1984 Jun 25;259(12):7842–7849. [PubMed] [Google Scholar]
- Zarling D. A., Temin H. M. High spontaneous mutation rate of an avian sarcoma virus. J Virol. 1975 Jan;17(1):74–84. doi: 10.1128/jvi.17.1.74-84.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]