Abstract
The objective of this study was to define the regional differences in rat vaginal smooth muscle contractility and morphology. We evaluated circumferential segments from the proximal, middle, and distal rat vagina (n = 21) in vitro. Contractile responses to carbachol, phenylephrine, potassium chloride, and electrical field stimulation (EFS) were measured. Immunohistochemical analyses were also performed. The dose–response curves for carbachol- and phenylephrine-dependent contractions were different in the distal (P = .05, P = .04) compared to the proximal/middle regions. Adjusted for region-dependent changes in contractility, the distal vagina generated lower force in response to carbachol and higher force in response to phenylephrine. There was less force with increasing EFS frequency in the distal (P = .03), compared to the proximal/middle regions. Cholinergic versus adrenergic nerves were more frequent in the proximal region (P = .03). In summary, the results indicate that functional and morphological differences in smooth muscle and nerve fibers of the distal versus proximal/middle regions of the vagina exist.
Keywords: adrenergic, cholinergic, electrical field stimulation, rat vagina, vaginal smooth muscle
Introduction
Pelvic organ support is maintained by the levator ani muscles, the vagina, and the connective tissue attachments between the vagina and the pelvic sidewall. Smooth muscle fibers from the vaginal wall connect to muscle fibers of the levator ani muscles.1
The muscularis of the vagina consists of an inner circular and outer longitudinal sheet of smooth muscle. Tonic contraction at rest maintains vaginal tone and relaxation allows for vaginal distension during sexual arousal and childbirth. Damage to the vagina can result in the loss of vaginal support and prolapse of the pelvic organs. However, the various factors leading to the progression of prolapse are still not well understood.
Studies have shown that the smooth muscle content in the anterior and posterior vagina is significantly decreased in women with prolapse.2–5 Morphometric analysis has also shown smaller and fewer nerve bundles in the posterior vaginal wall of women with prolapse.3 Few studies have made attempts to correlate these biochemical changes with functional data. It is generally believed that the proximal and distal vagina have different embryologic origins. The upper vagina arises from the Müllerian ducts, while the lower vagina is derived from the urogenital sinus.6,7 This difference in origin invokes the idea that regional differences may exist in the morphology and contractility of vaginal smooth muscle. Regional differences have been demonstrated in both the rabbit and the rat.8–10 Histologically, a greater percentage of smooth muscle is found in the proximal vagina, compared to the distal region.8,9 Variation in contractile patterns has been observed with a phasic contractile response predominantly observed in the proximal vagina and a tonic response seen in the distal vagina.8,9
Vaginal smooth muscle contractility is governed by a complex web of receptor and nerve-mediated interactions, including sympathetic, parasympathetic, and nonadrenergic noncholinergic (NANC) pathways. In muscle strip studies, direct membrane depolarization can be assessed through potassium chloride (KCl) stimulation. Nerve-mediated interactions can be studied through electrical field stimulation (EFS), and receptor-mediated interactions can be assessed through stimulation with various agonists and antagonists.
α1 and α2 adrenoreceptors appear to be the main mediators of smooth muscle contraction in the distal rat vagina,11 while M2 and M3 muscarinic receptors (MRs) appear to be the main mediators in the proximal region.10 Most studies have utilized longitudinal strips of vaginal smooth muscle, whereas we believe that circumferential segments better reflect the mechanics of the vaginal canal during childbirth, sexual intercourse, and prolapse progression. Smooth muscle bundles in the vaginal wall are supplied by nerve fibers expressing protein gene product (PGP 9.5), vesicular acetylcholine transporter (VAChT), tyrosine hydroxylase (TH), as well as various other nerve fibers, and the distal vagina is particularly well-innervated.12 The PGP 9.5 is a cytoplasmic protein expressed in all peripheral nerves, VAChT mediates acetylcholine storage by synaptic nerves, and TH catalyzes the conversion of the amino acid l-tyrosine to dihydroxyphenylalanine. The PGP 9.5 is therefore used as a specific marker for total peripheral innervation, while VAChT and TH are used as specific markers of cholinergic and adrenergic nerve fibers, respectively. Few studies have examined the nerve-mediated interactions in vaginal smooth muscle through the use of EFS. More data are needed to fully elucidate the details of vaginal smooth muscle contractility.
In the development of graft materials for use during reconstructive pelvic surgery, the role of smooth muscle in vaginal support has largely been ignored in spite of its critical role in the maintenance of sexual function, preservation of vaginal tone, and compliance. We hypothesize that the prolapsed vagina has reduced smooth muscle contractility; thus, restoring smooth muscle function is an important factor to consider in the development of a graft material. We therefore aimed to define the regional differences in contractility of circumferential segments of the vagina in an uninjured state as a basis for recognizing compromised smooth muscle function in future studies. We augmented our functional analysis of the 3 vaginal regions with histochemical techniques to evaluate the morphological differences and immunohistochemical staining to quantify the nerve density.
Methods
Tissue Preparation
The Institutional Animal Care and Use Committee at the University of Pittsburgh approved the use of animals for this study (#0911806). The animals (purchased from Hilltop Lab Animals, Inc, Scottdale, Pennsylvania) were housed in a temperature- and light-controlled room with free access to food and water. Virgin female Long Evans rats (n = 26), aged 12 weeks and of body weight between 208 and 260 g were used. Immediately following sacrifice, the vagina of each rat was excised intact and the paravaginal tissues were meticulously removed from the vagina using dissecting scissors. During preparation, the vaginal tissue was bathed in oxygenated Krebs solution (95% O2-5% CO2). The vagina was then divided by thirds into circumferential segments proximal, middle, and distal. The mean lengths were the same for each segment (mean ± standard deviation [SD]) proximal 7.12 ± 0.79 mm, middle 7.29 ± 0.66 mm, and distal 7.06 ± 1.0 mm (P = .32). Within 30 minutes of excision from the animal, the vagina was set up for a functional assay.
Functional Assays
The functional assays were performed on fresh circumferential segments of vaginal tissue from each rat. After the vagina had been opened longitudinally and the urethra was excised, the circumferential vaginal segments (proximal, middle, and distal) were carefully suspended between 2 wires using stationary clips and then immersed in an organ bath (20 mL) containing oxygenated Krebs solution (95% O2-5% CO2) at 37°C. Contractile responses were monitored with a pressure transducer (Transbridge 4M, World Precision Instruments, Sarasota, Florida) and recorded using Chart (version 5) software on a PowerLab system (sampling at 40 Hz, AD Instruments, Colorado Springs, Colorado). Each segment was adjusted to a tension of 0.5 g and then allowed to equilibrate for at least 60 minutes. Constant tension was applied in order to elongate the specimens to varying lengths to account for changes in size. The equilibrium period following the preload allows the muscles to relax, which causes the length of most fibers to approach Lo or the optimal length for force generation. Final resting tension was 0.1 to 0.2 g.
To evaluate muscle-mediated contractions, contractile response to increasing concentrations of KCl (10, 20, 30, 40, 60, 80, and 120 mmol/L) was measured (n = 7). These concentrations were chosen based on the results from dose–response curves in preliminary experiments, in which we found the greatest change in force between 10 and 40 mmol/L KCl, with a plateau generally reached by 120 mmol/L KCl. Figure 1 depicts a representation of force generated to demonstrate how peak force was measured. Once the response to a dose of KCl plateaued, the tissue was washed with Krebs solution 3 times (10 minutes for each wash), prior to application of the next KCl dose. Contractile responses were normalized to tissue dry weight and volume, and half maximal effective concentration (EC50) values (the concentration required to produce 50% of the maximal contractile response) were calculated. The tissue exposed to KCl was subsequently used to evaluate nerve-mediated contractile response. Electrical field stimulation at frequencies of 1 to 64 Hz, intensity of 20 V, and duration of 2 seconds (n = 6) was applied using a S88, Grass Telefactor stimulator. The tissue samples were washed with Krebs solution 2 times (5 minutes for each wash) between the trials. Contractile responses were again recorded and normalized to tissue dry weight and volume.
Figure 1.

Representation of peak force generated, which is the maximum resulting force obtained between the introduction of a stimulant (40 mmol/L potassium chloride [KCl]) and the removal of the stimulant via a wash cycle (Krebs). Subtraction of the baseline from the peak value yields the net force generated.
For analysis of receptor-mediated contractions, the vaginal tissue (n = 14) was prepared in the same manner as in the KCl/EFS trials. Prior to stimulation with each agonist, the tissue was exposed to 120 mmol/L KCl that was used in normalizing the data to account for variability in muscle contractility. Potassium chloride allows us to evaluate the muscle-mediated contractions through direct membrane depolarization. We divided the force generated by each agonist by the force generated by stimulation with 120 mmol/L KCl in order to take into account the contribution of muscle-mediated contractions and to isolate the effect of receptor-mediated contractions. After the response to the 120 mmol/L dose of KCl plateaued, the tissue was washed with Krebs solution 3 times (10 minutes for each wash). Carbachol (n = 7), a MR agonist, or phenlyephrine (n = 7), an α1-adrenoceptor (AR) agonist, was then added to the organ bath in increasing concentrations (10−8-10−4 mol/L), noncumulatively. After the response to a concentration plateaued, the tissue was washed with Krebs solution 3 times (10 minutes for each wash), prior to the application of the next dose. Contractile responses were normalized for variability in muscle mass (by tissue dry weight and volume) and variability in muscle contractility (120 mmol/L KCl).
Histochemical and Immunohistochemical Assays
Histochemical and immunohistochemical assays were also preformed on fresh circumferential segments (proximal, middle, and distal) obtained from virgin rats (n = 5). Each segment was fixed in 4% paraformaldehyde overnight and then exposed to graded concentrations of sucrose (cryoprotectant) before embedding into optimal cooling temperature medium (Tissue-Tek, Miles Inc, Elkhart, Indiana). For histochemical analysis of gross morphology, and to measure vaginal perimeter, 10 to 20 μm serial cryostat sections of the vaginal wall were obtained. The sections were then processed for hematoxylin and eosin staining according to the methods of the Center for Biological Imaging (University of Pittsburgh). For immunohistochemistry, the 10 μm sections were air-dried for 1 hour, permeabilized in 0.2% Triton X-100 for 10 minutes, and then incubated for 1.5 hours in 2% bovine serum albumin (BSA). Using the indirect probing method, the sections were first incubated for 1 hour at room temperature, with primary antibody diluted in PBDT (1× PBS, 0.2% BSA, 2% Donkey Serum (Jackson Immuno Research Laboratories, Inc, West Grove, Pennsylvania; no. 017-000-00), and 0.03% Triton) against PGP 9.5 (1:10 000; rabbit polyclonal antibody: Accurate Chemical, Westbury, New York, YBG78630507), TH (1:1000; rabbit polyclonal antibody; Sigma-Aldrich T8700, St. Louis, Missouri), and VAChT (1:5000 rabbit monoclonal antibody; Sigma-Aldrich V5387). Following primary incubation, the sections were rinsed with PBS and incubated for 1 hour at room temperature with Cy3-conjugated f(ab) donkey antirabbit immunoglobulin G (1:100; Jackson Laboratories no. 711 165 152) diluted in PBDT. The sections were again washed in buffer and mounted in Fluoro-Gel medium (EMS, Hatfield, Pennsylvania; 17985-10). In control experiments, no immunofluorescence staining was observed when the primary antiserum was omitted.
Morphometric Analysis
All images were captured using a Nikon microscope synced to a Nikon color digital camera, before being imported into Elements software (NIS-Elements AR 3.2) for quantification. To measure the length of the vaginal perimeter in the 3 regions of the vagina, ×4 images of hematoxylin and eosin-stained cross-sections of the vaginal wall were prepared and manual tracings of the outlines of the muscularis layer were performed. For comparative analysis of vaginal thickness, measurements were obtained at all 4 quadrants of the wall, and an average value of the 4 locations was obtained for each section. All measurements were done by blind estimation in duplicate, resulting in a repeat variability ratio of 0.01. To evaluate fibers immunoreactive for PGP 9.5, TH, and VAChT in the 3 regions of the vagina, images were randomly captured at ×10 magnification, in all 4 quadrants of the vagina. For analysis, all images were acquired and the same threshold was applied. Pixels of binary images whose intensity did not exceed the threshold value were automatically removed and considered negative. The number of profiles per unit area of the vaginal wall for the 4 locations was measured (fractional area) and averaged for each section. Nerves in all layers of the vaginal wall were quantified, but to prevent bias, attempts were made to exclude vascular innervation. For normalization, the fractional area of VAChT and TH immunoreactive nerves were expressed as a percentage of total nerves (PGP 9.5 immunoreactive nerves).
Statistical Methods
Functional Assays
Statistical analysis was performed using SPSS statistical software (version 17.0; SPSS Inc, Chicago, Illinois). Linear regression was used to analyze dose–response curves. Terms were added to approximate the shape of each curve, which varied by stimulus. Peak force and EC50 values were compared using 1-way analysis of variance (ANOVA). Significance was set at P < .05.
Histochemical and Immunohistochemical Assays
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, Inc, La Jolla, California). The mean value (±SD) of a parameter, for each of the 3 vaginal segments was calculated and the data for each region of the vagina were pooled. A paired t test was used to compare the percentage of each nerve type within a segment. The difference in nerve type, vaginal wall thickness, and perimeter of the segments were compared using 1-way ANOVA, with Bonferroni posttests. Significance was set at P < .05.
Results
Functional Assays
Muscle- and Nerve-Mediated Contractions
Dose–response curves for KCl stimulation were constructed, after normalizing the force generated by each segment by its volume (data not shown) and dry weight (Figure 2A). Given the delicate nature of the individual segments and the difficulty in handling them during volume measurements prior to the functional assays, dry weight, which was obtained after completion of the experiment, was thought to be a more accurate measurement for use in normalization of the data. Therefore, force normalized to dry weight was used during the statistical analysis. After the normalization, the mean peak force generated per unit dry weight in response to KCl was similar in the proximal, middle, and distal regions: proximal 1217.0 ± 532.9 mN/g; middle 1537.3 ± 1043.3 mN/g; distal 1338.8 ± 591.5 (P = .73). The EC50 values for the proximal, middle, and distal regions were also similar (P = .22, Figure 2B).
Figure 2.

A and B, Dose–response curves for potassium chloride (KCl) stimulation, normalized to dry weight (n = 7). The maximum force generated in response to KCl was similar in the proximal, middle, and distal regions (P = .73). The average half maximal effective concentration (EC50) values were also similar in all the regions (P = .22). Error bars indicate standard error of the mean.
In the EFS trials, when normalized to dry weight, the peak force generated by segments from the distal region (690.5 ± 363.9 mN/g) was lower than the peak force generated by segments from the proximal (1131.9 ± 962.7 mN/g) and middle (1001.4 ± 563.8 mN/g) regions; however, this difference was not statistically significant (P = .53). In comparing the response curves, no difference was observed between the segments from the proximal and the middle regions (P = .81), but less force was generated with increasing frequency in segments from the distal region, compared to the proximal (P = .03) and middle (P = .02) regions (Figure 3).
Figure 3.

Frquency–response curves for electrical field stimulation (EFS), normalized to dry weight (n = 6). Error bars indicate standard error of the mean. When comparing the reponse curves, no difference was observed between the proximal and the middle regions (P = .81), but less force was generated with increasing frequency in the distal region, in comparison to the proximal (P = .03) and middle (P = .02) regions.
Receptor-Mediated Contractions (MR and α1-AR)
No difference in force per unit dry weight with increasing concentration of carbachol was seen between the segments from the proximal and middle regions (P = .86). However, there was a tendency of a difference between the proximal and distal regions (P = .07), and the response curve was significantly different between the middle and distal regions (P= .05). In response to phenylephrine, again, no difference was seen between the segments from the proximal and middle regions (P = .55), but there was a significant difference between the proximal and distal regions (P = .04), as well as the middle and distal regions (P = .007).
Peak force was examined after adjusting for response to 120 mmol/L KCl (Figure 4). No difference in mean peak force was observed between the vaginal regions after stimulation with carbachol (units mN/mN): proximal 2.44 ± 1.03, middle 2.41 ± 1.94, and distal 1.61 ± 0.79 (P = .44). Similarly, no significant difference in mean peak force was seen in response to phenylephrine (units mN/mN): proximal 1.11 ± 0.74, middle 1.37 ± 0.43, and distal 1.51 ± 0.64 (P = .48). However, the distal region of the vagina had a trend toward a lower overall force in response to carbachol (Figure 4A), and a higher force in response to phenylephrine, in comparison to the proximal and the middle regions (Figure 4B).
Figure 4.
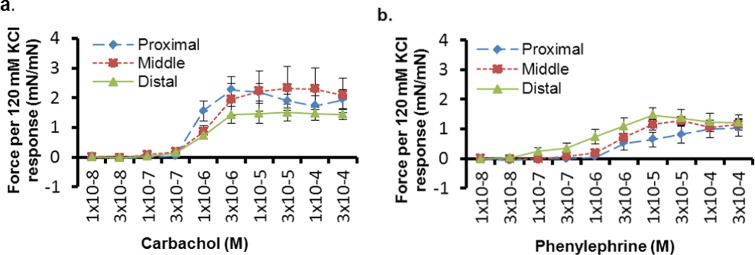
A and B, Dose–response curves normalized to potassium chloride (KCl) response for carbachol (A) and phenylephrine (B). There was no difference in peak force between the vaginal regions (P > .05). However, the distal region had a trend toward a lower overall force in response to carbachol (10−8-10−4 mol/L, n = 7), and a higher force in response to phenylephrine (10−8-10−4 mol/L, n = 7), in comparison to the proximal and middle regions. Error bars indicate standard error of the mean.
Histochemical and Immunohistochemical Assays
Gross Tissue Morphology (Morphometric Analysis)
Hematoxylin and eosin staining of segments from the proximal, middle, and distal regions showed (Figure 5) very dense (compact) smooth muscle bundles in the proximal cross sections. The distal region contained more loosely dispersed muscle bundles, and exhibited a distinct sphincter-like structure. Other observable differences included the increased presence of circumferentially aligned muscle bundles in the distal region, which were almost absent in the middle and sparse in the proximal region. Measurement of the perimeter of the vagina showed no significant differences (P = .12) between the proximal (1.59 ± 0.06 cm), middle (1.77 ± 0.06 cm), and the distal (1.64 ± 0.22 cm) regions, but the wall thickness was significantly different (P = .03) between the proximal and the distal regions (units cm): proximal 0.036 and distal 0.043 (Figure 6B).
Figure 5.

Hematoxylin and eosin-stained partial cross sections of the vaginal wall. The images portray gross morphological differences between the epithelium (E), subepithelium (S), and muscularis (M) layers of the (A) proximal (B) middle, and (C) distal regions of the vagina. The smooth muscle bundles (arrows) were very dense (compact) in the proximal region. The distal region had more loosely dispersed muscle fibers and exhibited a distinct sphincter-like structure (line). Bar = 100 μm.
Figure 6.

Panel (A) shows the full cross sections of the proximal, middle, and distal regions, while panel (B) includes the corresponding mean values of the perimeter and thickness measurements for each region. There were no differences in the average perimeter (P = .12), but the distal region had a larger wall thickness compared to the proximal region (P = .03), as well as an observable fusion to the urethra. Error bars indicate standard deviation. Bar = 100 μm.
Nerve Density (Morphometric Analysis)
Immunolabeling of nerves with PGP 9.5 revealed a homogenous distribution of peripheral nerves throughout the vagina (Figure 7). In the distal region, an increased number of varicose terminals were observed in the epithelium and subepithelium layers. Highly innervated circumferentially aligned smooth muscle was also seen in these segments and was fused with the urethral sphincter (Figure 7C). More selective probing for cholinergic and adrenergic nerve fibers revealed distinct patterns in the distribution of each nerve type. Adrenergic nerves were predominantly muscle and epithelium bound, while cholinergic nerves populated the muscularis and subepithelial layers, and had a greater degree of association with blood vessels (Figure 8). Quantitative analysis showed a similar percentage of both cholinergic and adrenergic nerves in the middle and distal regions, but the proximal region had a higher (P = .03) percentage of cholinergic nerves (65.9 ± 16.1), compared to adrenergic nerves (33.8 ± 12.2, Figure 9).
Figure 7.

Grayscale images of partial cross sections of the (A) proximal, (B) middle, and (C) distal regions of the vaginal wall. The images highlight nerves immunolabeled with PGP 9.5 (white), in the epithelium (E), subepithelium (S), and muscularis (M) layers. Note the increased number of varicose terminals (arrows) in the epithelium and subepithelium of the distal vagina, as well as the highly innervated circumferentially aligned smooth muscle (line) fusing with the urethral sphincter (U). Bar = 100 μm.
Figure 8.
Black and white images of corresponding partial cross sections of vesicular acetylcholine transporter (VAChT; cholinergic) and tyrosine hydroxylase (TH; adrenergic) immunolabeling of the proximal, middle, and distal regions of the vaginal wall. The nerves immunoreactive for VAChT and TH were distinct, resulting in variable staining patterns. Note the increased density of cholinergic fibers along the length of the vagina. Bar = 100 μm.
Figure 9.

The graph illustrates the density of cholinergic (vesicular acetylcholine transporter [VAChT]) and adrenergic (tyrosine hydroxylase [TH]) nerves in the proximal, middle, and distal regions of the vagina. Mean values of nerve type in each region are shown as a percentage of total nerve density (protein gene product [PGP] 9.5). There were significant differences in the average distribution of cholinergic versus adrenergic nerves present in the proximal segments (P = .03), when compared using a paired t test, but no significant differences were observed in nerve type in the middle and the distal regions. Error bars indicate standard deviation.
Discussion
This study was designed to define regional differences in the contractility and morphology of circumferential vaginal segments in an uninjured state, as a basis for recognizing compromised smooth muscle function in future studies. We believe that this work is important given the oversight of vaginal smooth muscle function that has occurred during the development of graft materials for reconstructive pelvic surgery. Vaginal smooth muscle is essential for the maintenance of sexual function, preservation of vaginal tone, and overall compliance of the vagina. We hypothesize that the prolapsed vagina has reduced smooth muscle contractility; thus, restoring smooth muscle function is an important factor to consider in the development of graft materials. The most significant findings of this study were the differences seen in the response curves of the distal region, compared to the other vaginal regions, in response to carbachol, phenylephrine, and EFS. The distal region also appeared to have an increase in circumferentially aligned muscle bundles on morphometric analysis and had a greater wall thickness compared to the proximal vagina. A greater distribution of cholinergic compared to adrenergic nerves was also found in the proximal region. These findings confirm that regional differences do in fact exist between various regions of the vagina.
Prior studies have demonstrated that vaginal smooth muscle contractility is mediated by adrenergic and cholinergic nerves as well as NANC modulators.10–12 Our finding that smooth muscle contractility in the proximal and middle regions of the vagina is preferentially induced by cholinergic stimulation, which is consistent with the results of Basha et al10 who showed a greater maximal contractile response to carbachol in the upper two-thirds of the vagina, compared to the lower one-third. Indeed, our immunohistochemical results corroborated this finding by demonstrating a greater distribution of cholinergic, compared to adrenergic, nerves in the proximal region. Our functional studies also suggest that the distal region of the vagina is more likely to respond to adrenergic stimulation. This is consistent with the finding by Onol et al11 that α1 and α2 adrenoreceptors are the main mediators of contraction in the distal rat vagina. We were unable to confirm this finding in our immunohistochemical analysis, as there was no significant difference between the proportion of cholinergic and the adrenergic nerves in the distal region. This may be attributed to small sampling and future studies are warranted.
In response to EFS, we found that less force was generated with increasing frequency in the distal region, compared to the proximal and middle regions. In prior EFS studies, Giraldi et al12 were able to induce frequency-dependent contractions in the distal vagina at 1 to 50 Hz, but reported no or weak responses using circular proximal strips. We were able to demonstrate a contractile response to 1 to 64 Hz in all vaginal regions (proximal, middle, and distal). Contractions in the proximal tissue appeared to be frequency dependent and an increase in force was observed with increasing frequency starting at 2 Hz. In the middle and the distal regions, the force generated with increasing frequency remained relatively constant after 2 Hz. A decrease in the force generated was observed in all 3 regions from 1 to 2 Hz. This may be explained by a potential corelease of inhibitory transmitters from nerve terminals during 2 Hz stimulation, which could have reduced the contractility of vaginal smooth muscle, although further studies are needed to clarify this point.
Our histochemical studies demonstrated gross morphological differences in the various vaginal regions. Most notable were the changes observed in smooth muscle density along the length of the vagina, suggesting that the proximal, middle, and distal vaginal regions play distinct roles, perhaps during sexual intercourse and parturition. A sphincter-like structure was observed in the distal vagina which was previously described by Giraldi et al.12 We suspect that this structure plays a role in tonic contraction and aids the bulbocavernosus muscle in the maintenance of genital hiatus support. These circumferentially oriented fibers may also be more susceptible to injury during parturition, which could provide an explanation for the greater propensity for tears to originate in the distal vagina, as well as the loss of vaginal tone associated with this region.
It has been reported that the distal vagina is the most richly innervated region.12 Our immunohistochemical results, however, demonstrated a homogenous distribution of nerves. In fact, the results of our EFS contractility assays showed weaker nerve-mediated contractile responses in the distal vagina. Looking further at classification of nerve distribution, we saw an increased density of cholinergic nerves in the proximal vagina, which correlated well with our functional analysis showing greater than 50% decline in the maximum force generated in response to phenylephrine compared to carbachol in this region. We did not find a significant difference in the distribution of cholinergic versus adrenergic nerves with immunohistochemical analysis in the middle or distal regions, and are unable to correlate this finding with our functional assays. We suspect that this variability is a combination of small sampling, sensitivity of measure, as well as NANC mediators playing a role in the middle and distal regions. Additional studies specifically focusing on this relationship are needed to clearly delineate the structure to function relationship. Our work will provide a basis for future analysis.
It is possible that some of the disparities between this study and other studies result from variations in the methods of normalization that were utilized. In their smooth muscle strip studies looking at KCl stimulation, Basha et al9 chose to normalize force generated to cross-sectional area. We chose to normalize to dry weight obtained after completion of the functional assays, which is a technique that has been used in prior studies.13,14 We were concerned about tissue handling prior to the assays and felt that our volume measurements were not as accurate given the small size and delicate nature of the individual segments. Because the mean lengths for our vaginal segments were not significantly different, tissue weight may serve as an index of cross-sectional area. Similar to Basha et al’s10 study on MR expression, we did normalize our receptor-mediated contractions to response to KCl.
Our study is unique in that we examined 3 distinct regions (proximal, middle, and distal) of circumferentially divided vaginal tissue in a rat model. Onol et al11 examined circularly cut tissues, but divided the vaginal tissue into proximal and distal halves. Basha et al10 divided the vagina longitudinally into a proximal segment consisting of the upper two-thirds and a distal segment consisting of the lower one-third. We used circumferential vaginal segments to better approximate the mechanical changes that the vagina undergoes during childbirth and sexual intercourse, and likely in the progression of prolapse. Our decision to examine the proximal and middle region of the vagina separately was driven by the clinical observation that these regions are often dissimilar in patients with prolapse. Some patients have a well-supported apex (proximal region), but have significant prolapse at the midpoint of their anterior and/or posterior vaginal walls. In the current study, we were not able to detect a difference in contractility between the proximal and the middle segments in response to KCl, carbachol, and phenylephrine. However, in the EFS studies, the proximal region exhibited a frequency-dependent response, whereas the middle region appeared to be frequency independent. Further investigation is needed to explain this finding. Given the above-cited distinctions with prior studies, it was important for us to define regional differences in vaginal smooth muscle contractility using a 3-region circumferentially divided model with uninjured tissue. We will use these findings to examine injured vaginal tissue in a prolapse model in future studies.
The findings in the current study may be limited by the relatively small sample size. This likely explains the inability to detect the differences in mean peak force in response to various stimuli, whereas differences in the response curves were demonstrated. An additional limitation is that NANC modulators, such as vasoactive intestinal polypeptide and nitric oxide, are known to innervate the vagina,12 and were not examined in the current study. Also, during EFS, contractile response only was evaluated. It is our plan in future studies to precontract the vaginal tissue and to measure the resulting relaxation response to EFS. Finally, our immunohistochemical assays did not include staining for smooth muscle α actin, which can be used to document the smooth muscle content in each segment. We did, however, stain for PGP 9.5 to look for peripheral nerve distribution, given that the focus of this study was on muscle contractility.
This study suggests that circumferential contractile responses in the proximal and the middle regions of the vagina are preferentially induced by cholinergic stimulation, whereas the distal region is more likely to respond to adrenergic stimulation. Although overall contractility was similar among the different vaginal regions as shown by KCl-induced contractile responses, nerve-mediated contractile responses were stronger in the proximal and middle vagina than in the distal region. We suspect that these differences will persist in prolapsed vaginal tissue and plan to test this hypothesis in future studies. The differences in contractility may serve to aid surrounding structures in vaginal support such as the uterosacral ligaments in the proximal region, the arcus tendineus fascia pelvis in the mid vagina, and the perineal body in the distal region. It is important to understand the contractility of each region so that the graft materials can be designed to preserve remaining native contractile function and prevent further progression of prolapse. Our study suggests that in the development of vaginal graft materials, it is imperative to take differences in contractile function and underlying morphology between the distal one-third and the other segments of the vagina into account. Additional research is needed to understand how to apply these regional differences to graft development.
Acknowledgments
We would like to thank Leslie Meyn, MS, for her assistance with the statistical analysis.
Footnotes
Authors’ Note: The contractility assay findings were delivered as an oral presentation at the 31st Annual Meeting of the American Urogynecologic Society in Long Beach, CA, October 2010.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the National Institutes of Health (grant number NIHR01 HD045590).
References
- 1.DeLancey JO, Starr RA. Histology of the connection between the vagina and levator ani muscles. Implications for urinary tract function. J Reprod Med. 1990;35(8):765–771. [PubMed] [Google Scholar]
- 2.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(1):56–63. [DOI] [PubMed] [Google Scholar]
- 3.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric properties of the posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(6):1501–1508. [DOI] [PubMed] [Google Scholar]
- 4.Badiou W, Granier G, Bousquet PJ, Monrozies X, Mares P, de Tayrac R. Comparative histological analysis of anterior vaginal wall in women with pelvic organ prolapse or control subjects. A pilot study. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):723–729. [DOI] [PubMed] [Google Scholar]
- 5.Takacs P, Gualtieri M, Nassiri M, Candiotti K, Fornoni A, Medina CA. Caldesmon expression is decreased in women with anterior vaginal wall prolapse: a pilot study. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(8):985–990. [DOI] [PubMed] [Google Scholar]
- 6.Breech LL, Laufer MR. Müllerian anomalies. Obstet Gynecol Clin North Am. 2009;36(1):47–68. [DOI] [PubMed] [Google Scholar]
- 7.Massé J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol. 2009;53(2-3):411–424. [DOI] [PubMed] [Google Scholar]
- 8.Oh SJ, Hong SK, Kim SW, Paick JS. Histological and functional aspects of different regions of the rabbit vagina. Int J Impot Res. 2003;15(2):142–150. [DOI] [PubMed] [Google Scholar]
- 9.Basha M, Chang S, Smolock EM, Moreland RS, Wein AJ, Chacko S. Regional differences in myosin heavy chain isoform expression and maximal shortening velocity of the rat vaginal wall smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1076–R1084. [DOI] [PubMed] [Google Scholar]
- 10.Basha M, Labelle EF, Northington GM, Wang T, Wein AJ, Chacko S. Functional significance of muscarinic receptor expression within the proximal and distal rat vagina. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1486–R1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onol FF, Ercan F, Tarcan T. The effect of ovariectomy on rat vaginal tissue contractility and histomorphology. J Sex Med. 2006;3(2):233–241. [DOI] [PubMed] [Google Scholar]
- 12.Giraldi A, Alm P, Werkström V, Myllymaki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002;14(4):271–282. [DOI] [PubMed] [Google Scholar]
- 13.Trujillo M, Candenas L, Cintado CG, et al. Hormonal regulation of the contractile response induced by okadaic acid in the rat uterus. J Pharmacol Exp Ther. 2001;296(3):841–848. [PubMed] [Google Scholar]
- 14.Li Y, Je HD, Malek S, Morgan KG. ERK 1/2-mediated phosphorylation of myometrial caldesmon during pregnancy and labor. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R192–R199. [DOI] [PubMed] [Google Scholar]



