Abstract
We hypothesized that chronic hypoxia disrupts mitochondrial function via oxidative stress in fetal organs. Pregnant guinea pig sows were exposed to either normoxia or hypoxia (10.5% O2, 14 days) in the presence or absence of the antioxidant, N-acetylcysteine (NAC). Near-term anesthetized fetuses were delivered via hysterotomy, and fetal livers, hearts, lungs, and forebrains harvested. We quantified the effects of chronic hypoxia on cytochrome oxidase (CCO) activity and 2 factors known to regulate CCO activity: malondialdehyde (MDA) and CCO subunit 4 (COX4). Hypoxia increased the MDA levels in fetal liver, heart, and lung with a corresponding reduction in CCO activity, prevented by prenatal NAC. The COX4 expression paralleled CCO activity in fetal liver and lung, but was unaltered in fetal hearts due to hypoxia. Hypoxia reduced the brain COX4 expression despite having no effect on CCO activity. This study identifies the mitochondrion as an important target site in tissue-specific oxidative stress for the induction of fetal hypoxic injury.
Keywords: fetal, hypoxia, mitochondria, guinea pig, cytochrome c oxidase
Introduction
Chronic intrauterine hypoxia can result from placental dysfunction, maternal complications, and living at high altitude.1 Under conditions of reduced oxygenation, fetal development is compromised and results in intrauterine growth restriction (IUGR),2,3 preterm birth, and respiratory distress syndrome.4 In more severe cases, IUGR can lead to still birth and perinatal mortality.5,6 In the long term, growth restricted offspring has an increased risk of cardiovascular disease, metabolic syndromes, and pulmonary disease.7 Thus chronic intrauterine hypoxia is a leading cause of fetal morbidity and mortality whose consequences may impact the offspring through programming mechanisms, yet the underlying cellular mechanisms altered by fetal hypoxia remain largely unexplored.
Hypoxia has been demonstrated to cause oxidative stress in adult and fetal tissues.8–11 Cellular damage by oxidative stress occurs as a result of excessive generation of reactive oxygen species (ROS) beyond the antioxidative capacity of the tissue.12 Reactive oxygen species can be generated by a variety of sources including the mitochondrial electron transport chain (ETC), xanthine oxidase, nicotinamide adenine dinucleotide phosphate oxidase, and other metabolic pathways such as β-oxidation.13,14 Under conditions of reduced oxygen levels, when the ETC is compromised, the mitochondrion is a primary contributor of excessive electrons resulting in the generation of superoxide anions (O2 −) and oxidative stress. In the fetus, this can lead to adverse cellular effects including oxidation of lipids in the cellular membranes and mitochondrial dysfunction.
We have recently reported that chronic intrauterine hypoxia increases oxidative and nitrosative stress in fetal heart ventricles as evidenced by increased malondialdehyde (MDA), peroxynitrite, and 3-nitrotyrosine expression.15,16 Additionally, studies have shown a link between chronic intrauterine hypoxia and oxidative stress in other fetal organs such as the brain.17
The mitochondria have been shown to be both generators of O2 − and targets of oxidative stress.18–21 We propose that oxidative stress under conditions of chronic hypoxia could impact mitochondrial function and induce cellular injury. We tested the role of oxidative stress on fetal mitochondrial ETC function by administering the antioxidant, N-acetylcysteine (NAC), to normoxic and hypoxic (HPX) pregnant sows and measuring cytochrome oxidase (CCO) activity of near-term fetal organs. This enzyme is the major site of cellular O2 consumption and is the terminal complex in the mitochondrial ETC which catalyzes the reduction of O2 to H2O. The CCO is vulnerable to oxidative stress since it is inhibited by several oxidative species including MDA, a by-product of lipid peroxidation.22,23 The CCO activity and assembly are regulated by expression of several catalytic and regulatory subunits such as COX4. This is a nuclear-encoded protein shown to be widely expressed and regulated under conditions of hypoxia.24 We hypothesized that COX4 expression may also regulate CCO activity in the context of fetal hypoxemia. A reduction in CCO activity would be expected to result in diminished adenosine triphosphate (ATP) synthesis and reduced energy production capacity in the affected tissues. This study will investigate the effects of hypoxia on mitochondrial CCO activity in fetal guinea pig liver, heart, lung, and brain, and the roles of MDA levels and COX4 expression in regulating CCO activity.
Methods
Animal Model
Female Duncan-Hartley guinea pigs (term = 65 days) were purchased from a commercial breeder (Elm Hill Breeding Labs, Chelmsford, Massachusetts) and time-mated. Pregnant guinea pig sows were placed in a plexiglass chamber containing 10.5% O2 for 14 days (hypoxic [HPX]) and normoxic controls were housed under normal room air (normoxic [NMX]; 21% O2).17 N-Acetylcysteine, at a dose of ∼580 mg NAC/kg per day, was administered in the drinking water to both hypoxic (HPX + NAC) and normoxic (NMX + NAC) animals for 10 days prior to tissue collection. The dose regimen selected was based on previous studies shown to inhibit oxidative responses in rats25 and guinea pigs.26 At near term (62-day gestation), pregnant sows were anesthetized with ketamine (80 mg/kg intraperitoneally [i.p.]) and xylazine (1 mg/kg i.p.), and given lidocaine, subcutaneously, along the midline of the abdomen. An abdominal incision was made and fetuses removed via hysterotomy. Fetal body weight and organ weights of liver, heart, brain, and placenta were measured at the time of excision and normalized to their respective fetal body weight (relative organ weight). Fetal liver (right lobe), heart (left ventricle), lung (right lobes), and brain (forebrain) were collected and snap frozen in liquid N2 and stored at −80°C until analyzed. The methods employed were approved by University of Maryland Animal Care Committee and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Malondialdehyde Assay
Malondialdehyde, a product of lipid peroxidation, was measured by a thiobarbituric acid reactive substances (TBARS) assay as an index of oxidative stress. The MDA levels of frozen tissue homogenates (fetal liver, heart, lung, and brain) were quantified by TBARS assay per the manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, Michigan). Briefly ∼30 mg of frozen tissues of each group were ground into powder using a mortar and pestle, lysed in radioimmunoprecipitation assay buffer (0.5 mol/L Tris-HCl, pH 7.4, 1.5 mol/L NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mmol/L EDTA), and spun at 1600g to remove cellular debris. The supernatant was incubated with the color reagent for 1 hour in boiling H2O (100°C). The MDA–TBA adduct of tissue homogenates formed at high temperatures is measured colorimetrically at 535 nm. Protein concentration was determined for each sample using the Bradford method27 and quantification of MDA expressed as nmols of MDA/mg protein.
Mitochondrial Isolation and Cytochrome c Oxidase Assay
Enriched tissue mitochondrial fractions were generated in a similar manner as described by Gostimskaya and Galkin.28 Briefly, powdered frozen fetal guinea pig liver, heart, lung, and brain (30-50 mg) were homogenized in ice-cold mitochondrial isolation buffer (250 mmol/L sucrose, 5 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1 mmol/L EDTA, pH 7.5) in a Next Advance Bullet Blender for 5 minutes at power setting 9, and centrifuged in 4°C at 500g to remove cellular debris, the supernatant is then recentrifuged at high speed (12.5 × 1000g) to generate an enriched mitochondrial fraction. Total protein of each sample was determined using the Bradford method.27
The CCO is the terminal complex of the ETC and the main site of O2 consumption. The CCO activity was quantified by monitoring the oxidation rate of reduced cytochrome c after the addition of 0.5 µg isolated mitochondria solubilized with 0.05 mmol/L n-dodecyl-beta-d-maltoside at 550 nm in assay buffer (100 mmol/L K+PO4 −, pH 7.4, and 0.22 mmol/L reduced cytochrome c) in a 96-well plate reader and expressed as the units of CCO (µmoles of oxidized cytochrome c/min per mg of mitochondria).
Mitochondrial Western Blot
The COX4, a subunit of CCO encoded in the nucleus, was analyzed using Western blot analysis. Briefly, 5 µg mitochondrial proteins from fetal liver, heart, lung, and brain were resolved on a 4 to 15% precast gradient sodium dodecyl sulfate–polyacrylamide electrophoresis gel and transferred to an Immun-Blot polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, California). The membranes were then probed with a primary mouse monoclonal antibody (1/5000) that recognizes COX4 (Mitosciences, Eugene, Oregon, MS407) in 5% nonfat dry milk, over night at 4°C. Membranes were then incubated for 1 hour in 5% nonfat dry milk containing horseradish peroxidase-conjugated chicken anti-mouse secondary antibody (1:25 000, Santa Cruz Biotechnology, Santa Cruz, California). Bands were detected and visualized using ECL (Western Lightning Plus, Perkin Elmer, Waltham, Massachusetts) and quantified using densitometry in ImageJ (ImageJ, US National Institutes of Health, Bethesda, Maryland, http://imagej.nih.gov/ij/). The intensity of COX4 was normalized to total mitochondrial protein as measured by Coomassie stain of the PVDF membrane and expressed as a ratio of COX4/Coomassie.
Statistics
Data are expressed as mean ± standard error of the mean. A 2-way analysis of variance with hypoxia and drug treatment as independent variables was performed to determine statistical significance between means. Student-Newman-Keuls post hoc test was used to analyze the statistical significance between appropriate groups (NMX vs HPX, NMX vs NMX + NAC, and HPX vs HPX + NAC). A P value less than .05 is considered statistically significant.
Results
Effects of Hypoxia on Fetal Body Weight and Relative Placental and Brain Weights
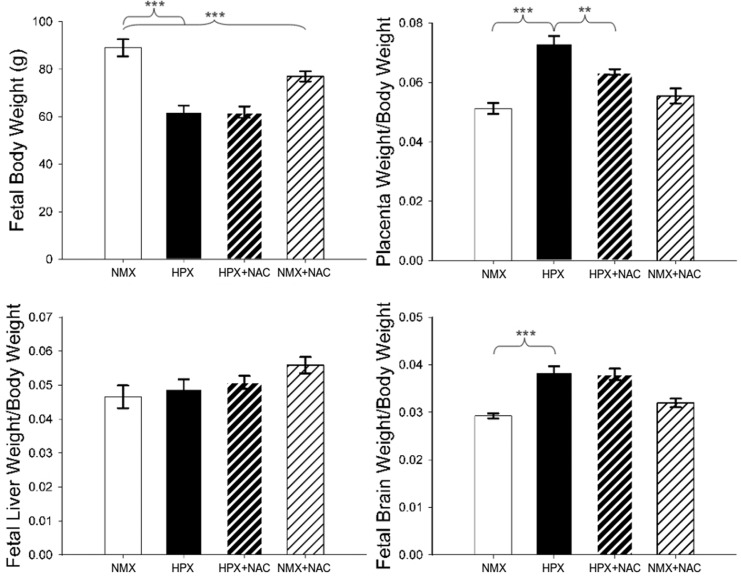
Chronic intrauterine hypoxia reduces fetal body weight by 30.4% (P < .001) compared to NMX controls from 88.97 ± 3.60 to 61.93 ± 2.73 g (Figure 1). N-Acetylcysteine alone (NMX + NAC) reduced the fetal body weight by 13.5% (P < .001) under normoxic conditions from 88.97 ± 3.60 to 76.94 ± 2.16 g (NMX + NAC) but did not the alter fetal body weight in hypoxic conditions. Chronic hypoxia increases the placenta: body weight ratio by 42.5% (P < .001; 0.0512 ± 0.0019 [NMX] vs 0.0730 ± 0.0026 [HPX]). N-Acetylcysteine reduces the hypoxia-induced increase in relative placental weight by 13.0% (P < .01; 0.0730 ± 0.0026 [HPX] vs 0.0635 ± 0.001 [HPX + NAC]). N-Acetylcysteine has no effect on the placenta: fetal body weight ratio in NMX animals. Chronic hypoxia reduces the fetal liver weight by 27.1% (P < .05) compared to NMX controls from 4.20 ± 0.46 to 3.06 ± 0.28 g (HPX) but relative liver weight is unaffected. Hypoxia increases brain: body weight ratio by 31.2% (P < .001) from 0.0292 ± 0.0005 (NMX) to 0.0383 ± 0.0013 (HPX). N-Acetylcysteine administration had no effect on the brain: body weight ratios in either NMX or HPX animals. Hypoxia increases the heart: body weight ratio by 10.7% (P < .05) from 0.0056 ± 0.0002 (NMX) to 0.0062 ± 0.0003 (HPX). Administration of NAC had no effect on the heart: body weight ratios in either NMX or HPX animals.
Figure 1.
Fetal guinea pig body and organ weights. Relative organ weights of placenta, fetal liver, and fetal brain were normalized to their respective fetal body weight (g) and expressed as organ weight/body weight ratios. Fetuses were obtained from animals exposed to normoxia (NMX) or hypoxia (HPX) in the presence or absence of N-acetylcysteine (NAC). Data are mean ± standard error of the mean (SEM), ***P < .001, **P < .01 (NMX n = 8, HPX n = 9, HPX + NAC n = 8, and NMX + NAC n = 10).
Effects of Hypoxia MDA Levels
Malondialdehyde is a product of lipid peroxidation and a marker of oxidative stress. Under normoxic conditions, MDA levels were similar between fetal liver, heart, and lung tissue but were slightly less in the fetal brain (Figure 2). Chronic intrauterine hypoxia increases the MDA levels by 41.7% in fetal liver (1.27 ± 0.13 [NMX] vs 1.8 ± 0.07 [HPX] nmol MDA/mg protein, P < .01), 70.7% in the fetal heart (1.01 ± 0.05 [NMX] vs 1.73 ± 0.17 [HPX], P < .01), and 22.7% in fetal lung (0.66 ± 0.04 [NMX] vs 0.81 ± 0.03 [HPX], P < .05) compared to their respective NMX controls. N-Acetylcysteine significantly reduces the hypoxia-induced increase in MDA levels in the fetal liver, heart, and lungs. The MDA levels in the fetal brain were not significantly altered either by hypoxia (0.64 ± 0.08 [NMX] vs 0.70 ± 0.14 [HPX]) or NAC treatment (0.52 ± 0.04 [NMX + NAC] vs 0.64 ± 0.14 [HPX + NAC]).
Figure 2.
Malondialdehyde (MDA) levels (nmoles/mg protein) of the fetal guinea pig liver, heart, lung, and brain. Fetal tissues were obtained from animals exposed to normoxia (NMX) or hypoxia (HPX) in the presence or absence of N-acetylcysteine (NAC). Data are mean ± standard error of the mean (SEM), **P < .01, *P < .05 (NMX n = 5, HPX n = 4-5, HPX + NAC n = 5-6, NMX + NAC n = 5-6).
Effects of Hypoxia on CCO Activity
The CCO activity is measured as an index of mitochondrial ETC activity.29 Under normoxic conditions, CCO-specific activity units (µmoles of oxidized cytochrome c/min per mg of mitochondria) were highest in the heart (0.78 ± 0.04 units), followed by the lungs (0.28 ± 0.01 units), brain (0.21 ± 0.01 units), and liver (0.15 ± 0.01 units; Figure 3). Chronic hypoxia reduces CCO activity in fetal liver (27.8%, P < .05), heart (29.1%, P < .05), and lungs (19.0%, P < .05) compared to NMX controls but had no effect in the fetal brain. N-Acetylcysteine alone had no effect on CCO activity in NMX controls. However, NAC administration restores the hypoxia-induced decrease in CCO activity in fetal livers, hearts, and lungs to NMX levels but had no effect in the fetal brain.
Figure 3.
Cytochrome oxidase (CCO) activity of fetal guinea pig liver, heart, lung, and brain of animals exposed to normoxia (NMX) or hypoxia (HPX) in the presence or absence of N-acetylcysteine (NAC). Data are mean ± standard error of the mean (SEM) and expressed as CCO-specific activity (1 U = 1 μmol of oxidized cytochrome c/min per mg protein), *P < .05. Fetal liver, lung, and brain NMX n = 8-12, HPX n = 9-12, HPX + NAC n = 8-12, NMX + NAC n = 10-12. Fetal heart (n = 5 for each group).
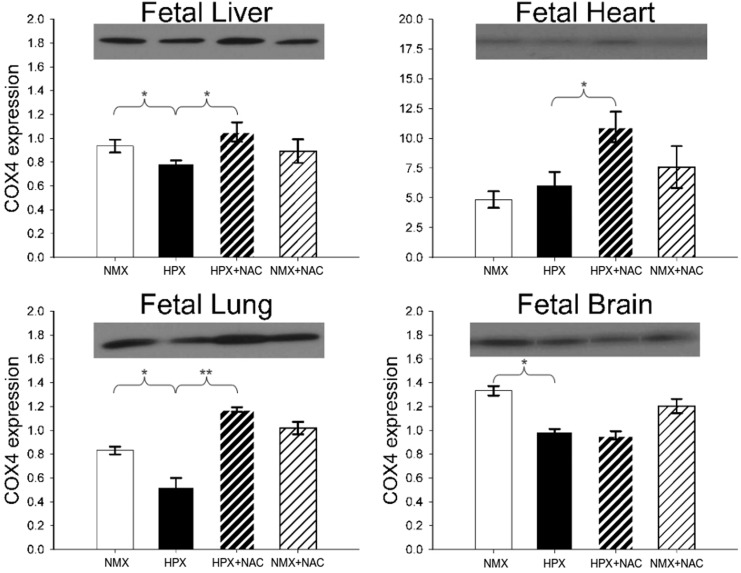
Effects of Hypoxia on COX4 Expression
The CCO subunit 4 is a nuclear-encoded subunit of CCO and has been demonstrated to play an important role in regulating the stability of the mitochondria-encoded catalytic CCO subunit 1. Figure 4 illustrates representative Western immunoblots and the effects of hypoxia and NAC treatment on fetal liver, heart, lung, and brain COX4 protein expression. Chronic intrauterine hypoxia reduces COX4 subunit expression on fetal livers (15%, P < .05), lungs (37%, P < .05), and brains (26%, P < .01) but has no effect in fetal hearts. N-Acetylcysteine prevents the hypoxia-induced decrease in COX4 expression in fetal livers and lungs but has no effect in fetal brains. In contrast, NAC increases COX4 expression in hypoxic fetal hearts but has no effect under conditions of NMX.
Figure 4.
Western blot analysis of cytochrome oxidase subunit 4 (COX4) protein expression of fetal guinea pig liver, heart, lung, and brain of animals exposed to normoxia (NMX) or hypoxia (HPX) in the presence or absence of N-acetylcysteine (NAC). Data are mean ± standard error of the mean (SEM). The COX4 expression is measured as a ratio of COX4 signal/Coomassie stain. *P < .05. Molecular weight (MW) = 16 kDa. NMX n = 3-4, HPX n = 3-4, HPX + NAC n = 3-4, and NMX + NAC n = 3-4.
Discussion
This study indicates that chronic intrauterine hypoxia induces lipid peroxidation and impairs CCO activity in selected fetal organs. We found that chronic hypoxia increased the MDA levels in fetal livers, lungs, and hearts with a parallel reduction in CCO activity. The antioxidant, NAC, reversed the hypoxia-induced responses of both MDA levels and CCO activity. Chronic hypoxia had no effect on either MDA or CCO activity in fetal brains. The COX4 expression was reduced by chronic hypoxia in fetal livers and lungs, which may contribute to the observed decrease in CCO activity. In fetal hearts, however, COX4 expression was not altered by hypoxia and does not appear to contribute to the reduction in CCO activity. Taken together, these results suggest that the decrease in CCO activity by hypoxia in fetal livers, hearts, and lungs is mediated by the inhibitory effects of MDA on CCO activity,22 and the role of COX4 expression in modulating CCO activity differs among the fetal tissues. As expected, hypoxia reduced the fetal body weight, increased relative brain, heart and placental weights, and did not change relative liver weights as previously reported.30 N-Acetylcysteine did not affect any of the hypoxia-induced changes in body or organ weights except for a partial reduction in relative placental growth.
The Effects of Fetal Hypoxia on Mitochondrial Function
A decrease in tissue oxygen levels, as a result of fetal hypoxia can lead to decreased mitochondrial function. Under normoxic conditions, the ETC transfers electrons along complexes I (or II), III, and IV to the final electron acceptor, O2, to generate a pH gradient that drives the production of ATP. The CCO is the terminal complex of the ETC and the major site of cellular O2 reduction to H2O. Its activity is a key regulator of overall mitochondrial oxidative phosphorylation and ATP production.31–35 During hypoxia, the decrease in O2 concentration reduces the electron flow through the mitochondrial ETC resulting in an excess of electrons that interact with oxygen containing moieties or with molecular O2 to generate superoxide anions (O2 −).36–40 As a result, the mitochondria of hypoxic tissues may be the generators of ROS, which can react with biological macromolecules leading to organelle dysfunction and cellular damage.
Peroxidation of cellular lipids produces MDA, a highly reactive lipid peroxide that forms adducts with CCO, antagonizes its activity, and reduces ETC function.22 The elevation of MDA levels in fetal organ generated by hypoxia would be expected to inhibit the activity of CCO and reduce the mitochondrial capacity of ATP synthesis. The restorative effects of NAC on CCO activity indicate an inhibitory effect of oxidative stress on ETC function. This is evident in the fetal liver, lung, and heart but does not occur in the fetal forebrain. The lack of increase in fetal brain MDA was surprising given previous reports of hypoxic stress in the fetal guinea pig brain under similar conditions17 but is consistent with the lack of increase in CCO activity if MDA is an inhibitory factor. The organ-specific variability may be due to the differences in relative tissue hypoxia, MDA formation, or the antioxidant capacity of the fetal organs. Thus, in hypoxic fetal organs, where MDA levels are increased, this may be an important regulatory factor of CCO activity and oxidative phosphorylation.
The Effects of Fetal Hypoxia on COX Expression
The CCO is located in the mitochondrial inner membrane and is a dimer of monomers consisting of 13 subunits.29 The CCO assembly is an important step in regulating catalytic core activity. This regulation is largely dependent on subunit expression and interaction that modulate and stabilize core formation and assembly.41,42 Cytochrome oxidase subunit 4 mediates the progression of CCO assembly.43 Furthermore, COX4 expression is regulated by O2 availability and is degraded by the hypoxia-inducible factor-1α (HIF-1α)-activated mitochondrial protease, Lon, reported to be increased during hypoxia.24,44–46 Thus, a downregulation of COX4 expression and reduction in CCO assembly and activity would be expected to occur under conditions of fetal hypoxia and elevated HIF-1α levels.47
The expression of COX4 in the lung and liver of fetal guinea pig parallels the pattern of CCO activity associated with both hypoxia and hypoxia + NAC. The observation that hypoxia reduces COX4 expression is in agreement with the expected elevation in HIF-1α and Lon levels, presumed to occur during fetal hypoxia. However, the restoration of liver and lung COX4 levels in the presence of HPX + NAC suggests that COX4 is regulated by oxidative stress rather than hypoxia since O2 levels would be expected to remain low in the presence of NAC treatment. This is supported by the sustained brain sparing effect in the presence of HPX + NAC. Previous studies have implicated Lon protease as a factor in degrading oxidized mitochondrial proteins such as COX4.46,48 Thus, NAC may restore COX4 expression by decreasing oxygen radicals in hypoxic fetal guinea pig lungs and livers and preventing oxidation of COX4. However, the effect of NAC differs among fetal organs. In the fetal brain, hypoxia decreased COX4 expression, which was insensitive to NAC. This suggests that the COX4 expression in the fetal brain is regulated independently of oxidative stress. Furthermore, because the reduction in brain COX4 expression did not correspond to a reduction in CCO activity, we hypothesize that COX4 plays a diminished role in regulating brain CCO activity. In fetal hearts, COX4 expression was unaltered by hypoxia, although CCO activity was inhibited. Thus, fetal cardiac CCO activity is likely regulated by MDA in a manner independent of COX4 expression. The differential regulation of COX4 expression by hypoxia and NAC among these fetal organs likely reflects tissue-specific variations in response to hypoxia and generation of oxidative stress. Further, the role of COX4 and its involvement in CCO complex assembly41 may vary between fetal tissues. Finally, our understanding of the regulation of COX4-specific expression in ETC function is derived from a variety of cell types (ie, isolated cancer cells) and conditions (acute in vitro responses) that may be distinct from those of the current study using chronically hypoxic fetal organs. Yet, this study presents a mitochondrial response in the fetus that may elucidate organ-specific challenges to chronic hypoxic stress.
In summary, we studied the effects of chronic hypoxia on tissue-specific regulation of CCO activity by measuring MDA and COX4 expression, 2 factors known to modulate the activity of this enzyme. Our results demonstrate an increase in MDA levels in fetal liver, heart, and lung after chronic intrauterine hypoxia and a concomitant reduction in CCO activity. In the hypoxic fetal brain, a lack of decrease in CCO activity is consistent with a lack of increase in MDA levels. Under conditions of increased MDA, CCO activity was prevented by the antioxidant, NAC. On the other hand, COX4 expression parallels CCO activity in the fetal lung and liver but not in the fetal brain and heart, identifying differences among fetal organs associated with the role of COX4 expression and CCO activity. This study identifies the tissue-specific effects of hypoxia on oxidative stress that lead to a corresponding reduction in mitochondrial function in the fetus. Treatment during gestation may help reverse the effects of intrauterine hypoxia on mitochondrial dysfunction by reducing the ROS-generated inhibitory effects of MDA and other lipid peroxidation products.
Footnotes
Authors’ Note: The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: in part by NIH Grants R01HL 49999 (LPT) and T32-HL072757 (YMA).
References
- 1. Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572(pt 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613–621. [DOI] [PubMed] [Google Scholar]
- 3. Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18(18):2183–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gilbert WM, Danielsen B. Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol. 2003;188(6):1596–1599; discussion 1599-1601. [DOI] [PubMed] [Google Scholar]
- 5. Kramer MS, Séguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14(3):194–210. [DOI] [PubMed] [Google Scholar]
- 6. Julian CG. High altitude during pregnancy. Clin Chest Med. 2011;32(1):21–31, vii. [DOI] [PubMed] [Google Scholar]
- 7. Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guzy RD, Hoyos B, Robin E, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1(6):401–408. [DOI] [PubMed] [Google Scholar]
- 9. Fisher AB. Activation of endothelial NADPH oxidase as the source of a reactive oxygen species in lung ischemia. Chest. 1999;116(1 suppl):25S–26S. [DOI] [PubMed] [Google Scholar]
- 10. Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279(2):L408–L412. [DOI] [PubMed] [Google Scholar]
- 11. Buonocore G, Perrone S, Longini M, Terzuoli L, Bracci R. Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr Res. 2000;47(2):221–224. [DOI] [PubMed] [Google Scholar]
- 12. Raza H, Prabu SK, John A, Avadhani NG. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int J Mol Sci. 2011;12(5):3133–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. [DOI] [PubMed] [Google Scholar]
- 14. Bedard K, Heinz KK. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. [DOI] [PubMed] [Google Scholar]
- 15. Thompson L, Dong Y, Evans L. Chronic hypoxia increases inducible NOS-derived nitric oxide in fetal guinea pig hearts. Pediatr Res. 2009;65(2):188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evans LSC, Liu H, Pinkas GA, Thompson LP. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr Res. 2012;71(1):25–31. [DOI] [PubMed] [Google Scholar]
- 17. Dong Y, Yu Z, Sun Y, et al. Chronic fetal hypoxia produces selective brain injury associated with altered nitric oxide synthases. Am J Obstet Gynecol. 2011;204(3):254.e16–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aon M, Cortassa S, O’Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys Acta. 2010;1797(6-7):865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demin OV, Kholodenko BN, Skulachev VP. A model of O2-generation in the complex III of the electron transport chain. Mol Cell Biochem. 1998;184(1-2):21–33. [PubMed] [Google Scholar]
- 20. Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans. 2003;31(pt 6):1300–1301. [DOI] [PubMed] [Google Scholar]
- 21. Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278(38):36027–36031. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Petersen DR, Schenker S, Henderson GI. Formation of malondialdehyde adducts in livers of rats exposed to ethanol: role in ethanol-mediated inhibition of cytochrome c oxidase. Alcohol Clin Exp Res. 2000;24(4):544–552. [PubMed] [Google Scholar]
- 23. Behn C, Araneda OF, Llanos AJ, Celedón G, González G. Hypoxia-related lipid peroxidation: evidences, implications and approaches. Respir Physiol Neurobiol. 2007;158(2-3):143–150. [DOI] [PubMed] [Google Scholar]
- 24. Fukuda R, Zhang H, Whan KJ, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129(1):111–122. [DOI] [PubMed] [Google Scholar]
- 25. Beloosesky R, Gayle DA, Amidi F, et al. N-acetyl-cysteine suppresses amniotic fluid and placenta inflammatory cytokine responses to lipopolysaccharide in rats. Am J Obstet Gynecol. 2006;194(1):268–273. [DOI] [PubMed] [Google Scholar]
- 26. Wang B-J, Guo Y-L, Chang H-Y, et al. N-acetylcysteine inhibits chromium hypersensitivity in coadjuvant chromium-sensitized albino guinea pigs by suppressing the effects of reactive oxygen species. Exp Dermatol. 2010;19(8):e191–e200. [DOI] [PubMed] [Google Scholar]
- 27. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 28. Gostimskaya I, Galkin A. Preparation of highly coupled rat heart mitochondria. J Vis Exp. 2010;(43):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kadenbach B, Huttemann M, Arnold S, Lee I, Bender E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase1. Free Radic Biol Med. 2000;29(3-4):211–221. [DOI] [PubMed] [Google Scholar]
- 30. Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstet Gynecol. 2008;199(1):78.e1–e6. [DOI] [PubMed] [Google Scholar]
- 31. Pacelli C, Latorre D, Cocco T, et al. Tight control of mitochondrial membrane potential by cytochrome c oxidase. Mitochondrion. 2011;11(2):334–341. [DOI] [PubMed] [Google Scholar]
- 32. Villani G, Attardi G. In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc Natl Acad Sci U S A. 1997;94(4):1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hüttemann M, Helling S, Sanderson TH, et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 2012;1817(4):598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hájek P, Villani G, Attardi G. Rate-limiting step preceding cytochrome c release in cells primed for Fas-mediated apoptosis revealed by analysis of cellular mosaicism of respiratory changes. J Biol Chem. 2001;276(1):606–615. [DOI] [PubMed] [Google Scholar]
- 35. Kunz WS, Kudin A, Vielhaber S, Elger CE, Attardi G, Villani G. Flux control of cytochrome c oxidase in human skeletal muscle. J Biol Chem. 2000;275(36):27741–27745. [DOI] [PubMed] [Google Scholar]
- 36. Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275(33):25130–25138. [DOI] [PubMed] [Google Scholar]
- 37. Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol. 2005;98(1):404–414. [DOI] [PubMed] [Google Scholar]
- 38. Hassanein T, Frederick T. Mitochondrial dysfunction in liver disease and organ transplantation. Mitochondrion. 2004;4(5-6):609–620. [DOI] [PubMed] [Google Scholar]
- 39. Schönfeld P, Schlüter T, Fischer K-D, Reiser G. Non-esterified polyunsaturated fatty acids distinctly modulate the mitochondrial and cellular ROS production in normoxia and hypoxia. J Neurochem. 2011;118(1):69–78. [DOI] [PubMed] [Google Scholar]
- 40. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(pt 2):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fontanesi F, Soto IC, Barrientos A. Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB Life. 2008;60(9):557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fornuskova D, Stiburek L, Wenchich L, Vinsova K, Hansikova H, Zeman J. Novel insights into the assembly and function of human nuclear-encoded cytochrome c oxidase subunits 4, 5a, 6a, 7a and 7b. Biochem J. 2010;428(3):363–374. [DOI] [PubMed] [Google Scholar]
- 43. Stiburek L, Vesela K, Hansikova H, et al. Tissue-specific cytochrome c oxidase assembly defects due to mutations in SCO2 and SURF1. Biochem J. 2005;392(pt 3):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hervouet E, Pecina P, Demont J, et al. Inhibition of cytochrome c oxidase subunit 4 precursor processing by the hypoxia mimic cobalt chloride. Biochem Biophys Res Commun. 2006;344(4):1086–1093. [DOI] [PubMed] [Google Scholar]
- 45. Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405(1):1–9. [DOI] [PubMed] [Google Scholar]
- 46. Pinti M, Gibellini L, De Biasi S, et al. Functional characterization of the promoter of the human Lon protease gene. Mitochondrion. 2011;11(1):200–206. [DOI] [PubMed] [Google Scholar]
- 47. Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14(11):879–897. [DOI] [PubMed] [Google Scholar]
- 48. Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27(2):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]