Abstract
Exercise in the form of daily treadmill training results in significant enhancement of axon regeneration following peripheral nerve injury. Because androgens are also linked to enhanced axon regeneration, we wanted to investigate whether sex differences in the effect of treadmill training might exist. The common fibular nerves of thy-1-YFP-H mice were cut and repaired with a graft of the same nerve from a strain-matched wild type donor mouse. Animals were treated with one of two daily treadmill training paradigms: slow continuous walking for one hour or four higher intensity intervals of two minutes duration separated by five minute rest periods. Training was begun on the third day following nerve injury and continued five days per week for two weeks. Effects on regeneration were evaluated by measuring regenerating axon profile lengths in optical sections through the repair sites and grafts at the end of the training period. No sex differences were found in untrained control mice. Continuous training resulted in significant enhancement of axon regeneration only in males. No effect was found in females or in castrated males. Interval training was effective in enhancing axon regeneration only in females and not in intact males or castrated males. Untrained females treated with the aromatase inhibitor, anastrozole, had significant enhancement of axon regeneration without increasing serum testosterone levels. Two different mechanisms exist to promote axon regeneration in a sex-dependent manner. In males treadmill training utilizes testicular androgens. In females a different cellular mechanism for the effect of treadmill training must exist.
Introduction
Despite the capacity of axons in peripheral nerves to regenerate, functional recovery following peripheral nerve injury is poor (Frostick et al., 1998; Scholz et al., 2009), especially if the injuries involve axotomy. Prominent among the reasons given for the poor functional outcomes following nerve injuries is the slowness of the process of regeneration (Gordon, 2009). Many regenerating neurites from the segment of nerve proximal to the injury site are slow in entering a regeneration pathway distal to the injury. The neurites enter the pathway in a temporally “staggered” manner, and once in the pathway they elongate relatively slowly (Gordon, 2009). Recently, we have shown that modest daily exercise in the form of treadmill training, if applied shortly after peripheral nerve transection and repair, results in a substantial enhancement of axon regeneration. Regenerating axons elongated much further in the first two weeks following nerve injury in trained mice than in untrained controls (Sabatier et al., 2008). This effect was found using two different daily training paradigms: 1) One hour of continuous treadmill walking at a slow speed or 2) four daily repetitions of more intense training for two minute intervals (Sabatier et al., 2008). In addition to increasing axon elongation, during this two week period of training we have previously found that axons of significantly more motoneurons elongated at least four millimeters (English et al., 2009), suggesting that the extent of “staggering” of regeneration is reduced. Using slightly different paradigms, others have reported similar effects of training on axon regeneration (Asensio-Pinilla et al., 2009; Marqueste et al., 2004; Molteni et al., 2004; Seo et al., 2006; van Meeteren et al., 1997).
Androgens are known to have an effect on axon regeneration in peripheral nerves (reviewed by Sharma et al., 2010). Whether the androgen-sensitive motoneurons with axons in the facial nerve are considered (Jones et al., 2000) or less androgen sensitive neurons with axons in the sciatic nerve are involved (Brown et al., 1999), treatments of castrated animals with androgens results in enhanced axon regeneration. Because androgens also regulate the expression of BDNF and its receptor, trkB, in motoneurons, (Osborne et al., 2007; Sharma et al., 2010; Verhovshek et al., 2010) and these molecules have been shown to play a role in axon regeneration (English et al., 2011; Wilhelm et al., 2011) we hypothesized that the effects of treadmill training on axon regeneration in injured peripheral nerves involved androgens. If so, one might expect that male and female mice would respond differently to treadmill training (Aizawa et al., 2010; Aizawa et al., 2008; Hu et al., 1999).
The purpose of this study was to compare the effects of treadmill training on axon regeneration in male and female mice and in mice where the effects of androgens are experimentally manipulated. Our main finding is that slow, continuous treadmill training is effective in enhancing axon regeneration only in intact males, not in females or castrated males. In contrast interval training was found to be effective in promoting axon regeneration only in females and not in intact males or castrated males. Blocking the conversion of androgens to estradiol by treatment with an aromatase inhibitor also resulted in enhanced axon regeneration in untrained females. A preliminary report of these findings has been made (Wilhelm et al., 2010).
Materials and Methods
Animals and surgical methods
All experimental methods were approved by the Institutional Animal Care and Use Committee of Emory University and conformed to the Guidelines for the Use of Animals in Research of the Society for Neuroscience. Axon regeneration was studied in 40 nerves in 36 transgenic mice of the H-strain of the thy-1-YFP mouse (Feng et al., 2000) (Table I). In these mice, a subset of axons in peripheral nerves is marked completely by yellow fluorescent protein (YFP). It is assumed that the marked axons represent a sample of all axons in those nerves (Groves et al., 2005). Founders of the colony from which these mice were taken were obtained from the Jackson Laboratories (Bar Harbor, ME). All mice used were 2–4 months old. The mice were genotyped by viewing a cutaneous nerve on the back of the ear with a fluorescence microscope.
Table I.
Summary of Experimental Groups
| Group | Treatment | No. Nerves Studied |
|---|---|---|
| Males | Untrained | 4 |
| Females | Untrained | 4 |
| Males | Continuous Trained | 4 |
| Females | Continuous Trained | 4 |
| Males | Interval Trained | 4 |
| Females | Interval Trained | 4 |
| Castrated males | Untrained | 4 |
| Castrated males | Continuous Trained | 4 |
| Castrated males | Interval Trained | 4 |
| Females | Anastrozole 1mg/Kg, IP | 4 |
|
| ||
| TOTAL | 40 | |
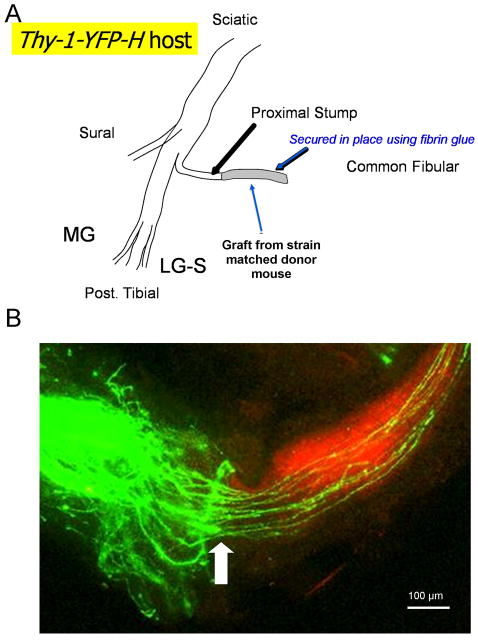
Under isoflurane anesthesia, the common fibular (CF) nerve was isolated and cut just distal to its branch point from the sciatic nerve. Cut nerves were repaired using a short (5–10 mm long) piece of the CF nerve obtained from another, strain-matched anesthetized mouse (Fig. 1A). To repair eight of the nerves studied, this donor mouse was a non-YFP expressing littermate of the thy-1-YFP-H host mouse, but in all other instances the graft was obtained from a different transgenic mouse (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, Jackson Laboratories, stock 007676). This mouse, referred to here as tomato, was developed originally as a Cre-reporter mouse (Muzumdar et al., 2007). The background strain of this mouse is identical to that of the thy-1-YFP-H mice (C57B6). Only mice not expressing Cre were used as graft donors. The tomato mice express strong red fluorescence in all tissues and cell types examined, and they have proven very useful in identification of the extent of the grafts in our experiments (Fig. 1B). The proximal segment of the cut nerve in the host mouse was aligned with the graft on a small rectangle of silastic film (0.007” thickness, part 5–501, Dow Corning, Midland, MI) and secured in place using ca. 6 μL of fibrin glue (de Vries et al., 2002; MacGillivray, 2003). The fibrin glue was manufactured at the time of use from equal parts thrombin and a 1:1 solution of fibrinogen and fibronectin, and was applied to the aligned nerves using a micropipetter.
Figure 1.
A. Diagram of the surgical protocol used. One of the two large terminal branches of the sciatic nerve, the common fibular nerve (shown) of a thy-1-YFP-H mouse, was cut and repaired using a segment of the same nerve harvested from a strain-matched donor mouse. This graft was secured in place using fibrin glue. B. A typical experimental result is shown. A low magnification image taken through the surgical repair site contains the proximal stump of the cut nerve (left) containing green YFP+ axons. In this experiment, the cut common fibular nerve was repaired with a graft from a B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (tomato) mouse. The red fluorescence of the graft tissue (right) is in marked contrast to the green fluorescence found in the host tissue and the regenerating axons in the graft. This contrast aids in precise identification of the interface between the proximal stump and the graft (arrow). In this case, a few YFP+ axons did not invade the graft tissue. They were not measured.
Twelve of the nerves used in this study were from castrated males. Castration was performed at the time of the nerve repair surgery. The testes were identified at the external inguinal rings. The spermatic cords were ligated and the testes removed distal to the ligation.
Treadmill training
Two treadmill training paradigms were used: continuous and interval (Sabatier et al., 2008). For continuous training, the mice were placed on a level, motor driven treadmill at a speed of 10 m/min for one hour daily. For interval training, the treadmill speed was increased to 20 m/min and the mice were run for two minutes, followed by a five minute rest period. This was repeated four times daily. Mice were encouraged to walk at the treadmill speed by gentle coaxing and most required little encouragement. Training was begun on the third day following nerve transection and repair and was conducted five days per week for two weeks. All mice used were acclimated to the treadmill but none had been trained for at least two weeks prior to surgery.
Treatment with aromatase inhibitor
In four intact female thy-1-YFP-H mice, the CF nerve was cut bilaterally and repaired as described above. Beginning at the time of surgery and continuing on a daily basis for the next two weeks, these animals received an intraperitoneal injection of the aromatase inhibitor, anastrozole (1 mg/Kg BW). At this dose, anastrozole is known to produce marked inhibition of the conversion of testosterone or its precursors into estradiol or estradiol precursors and no detectable pharmacologic activity other than aromatase inhibition (Plourde et al., 1994). After two weeks, animals were euthanized and tissue harvested, as described below.
Tissue harvesting and microscopy
Two weeks following nerve repair surgery, the mice were euthanized with an overdose of pentobarbital sodium (150 mg/Kg, IP) and blood was collected from the exposed heart. The collected blood was centrifuged and serum was collected and frozen prior to testosterone analysis. Radio-immunoassay was performed for us by the Yerkes National Primate Research Center of Emory University. Once blood was collected, the mice were perfused transcardially with normal saline solution followed by periodate-lysate-paraformaldehyde fixative solution (McLean and Nakane, 1974). From each mouse, the entire repaired nerve, including the graft and most of the proximal stump, was then harvested, mounted onto a microscope slide, and cover slipped with Vectashield. The edges of these cover slips were sealed with clear nail polish.
The methods for confocal imaging of harvested nerves and measurements of regenerating axons have been described in detail (English et al., 2005; Groves et al., 2005). These methods are summarized here. The mounted nerves were imaged using a laser scanning confocal microscope at low (10x) magnification. Stacks of serial optical sections, each 10 μm thick, were made through the entire thickness of the nerve and multiple contiguous microscope fields were sampled to include the entire repaired nerve. From these stacks, images at the same focal plane were stitched together using Adobe Photoshop. The result of this process was that each repaired nerve could be reconstructed in its entirety as a large stack of aligned optical sections. Profiles of individual YFP+ axons are readily followed through these large stacks from the surgical repair site to the growth cones at their distal tips. The lengths of these profiles were measured using either Image Pro Plus or ImageJ software.
Data Analysis
Ten experimental groups were studied (see Table I). In all animals studied, nerves were cut and repaired. Males and females that were continuously trained or interval trained were compared to males and females that were untrained. Results from all of these groups were compared to untrained, continuously trained, and interval trained castrated male mice. Results from untrained females that were treated with anastrozole were compared to the results from untrained and untreated females. For each nerve studied, a cumulative histogram of the distribution of axon profile lengths measured was constructed, with a bin size of 100 μm. Averages of these histograms were computed for each experimental group. Median axon profile lengths, the axon profile lengths at the 50th percentiles of these distributions, were used to measure the effects of treadmill training in different treatment groups. Significance of differences was first analyzed using a one-way ANOVA. Since the resulting omnibus test was significant (F(9,30)=4.625, p=0.0007), final significance of differences in means was studied using post-hoc paired (Fisher’s Least Significant Difference (LSD)) testing. Probabilities of less than 0.05 were considered statistically significant.
Real time PCR
To investigate sex differences in the effects of the different training paradigms on mRNA expression of BDNF and its receptor, trkB, we used real time PCR analyses. Sequences of the primers used are presented in Table II. Five groups of C57B6 mice each were studied. Two groups of three mice each, one intact males and the other intact females, were trained five days per week for two weeks using the continuous training paradigm described above. Two similar groups of four mice each were trained for two weeks using the interval training paradigm described above. The fifth group of four mice consisted of untrained males and females. This group was considered the controls. At the end of the training period, the mice were euthanized with an overdose of pentobarbital and their lumbar spinal cords were removed and frozen rapidly in liquid nitrogen and stored at −80° C until used.
Table II.
Real-Time PCR primer sequences
| Gene | ID | Primer sequence | Amplicon |
|---|---|---|---|
| BDNF | MGI:88145 | Forward: GGTATCCAAAGGCCAACTGA Reverse: CTTATGAATCGCCAGCCAAT |
183 bp |
| trkB | MGI:97384 | Forward: TTCTGCCTGCTGGTGATGT Reverse: TCCAGTGGGATCTTATGAAACA |
190 bp |
| 18S | Forward: GCAATTATTCCCCATGAACG Reverse: GGCCTCACTAAACCATCCAA |
Frozen spinal cords were hand triturated and RNA was extracted from them using Trizol LS reagent (Invitrogen). The extracted RNA was treated with DNAse I (Invitrogen) and was reverse transcribed using AccuScript™High Fidelity (Stratagene). Real time PCR for BDNF and trkB was performed on this first strand cDNA using a Green I Master in the lightCycler®480 system (Roche) with the following thermal cycle conditions: Initial denaturation at 95°C for 5 minutes; amplification at 95°C for 10 sec, and 40 cycles at 55°C for 10 sec, followed by 72°C for 10 sec. At the end of the program, a melting curve analysis was done and the PCR products were also analyzed using gel electrophoresis to ensure the absence of non-specific PCR product amplification. The 18S ribosomal RNA was used for normalization of the gene expression data. The replication efficiencies of BDNF and trkB and the 18-S ribosomal RNA were verified as similar. Therefore, the comparison CT(ddCT) method was adopted for gene expression analyses (Schmittgen and Livak, 2008).
All data are expressed as fold changes from the values obtained from the control group. Significant changes in expression, relative to untrained controls, were noted if the range of mean fold change ± 95% confidence intervals did not include zero. Significance of differences between the four exercised groups was determined using a two-way (sex and training type) ANOVA.
Results
Sex differences in the effects of treadmill training
Because the androgenic response to exercise in male and female rats is quite different (Aizawa et al., 2010; Aizawa et al., 2008; Hu et al., 1999) we hypothesized that the effects of treadmill training on axon regeneration would be different in males and females. When we reviewed our published data for treadmill training (Sabatier et al., 2008) by sex, we found that it did not contain equal numbers of males and females. Additional experiments were performed such that equal numbers of males and females were studied. Thus the data reported here on the effects of treadmill training in otherwise intact males and females include mostly new observations but also some data already reported.
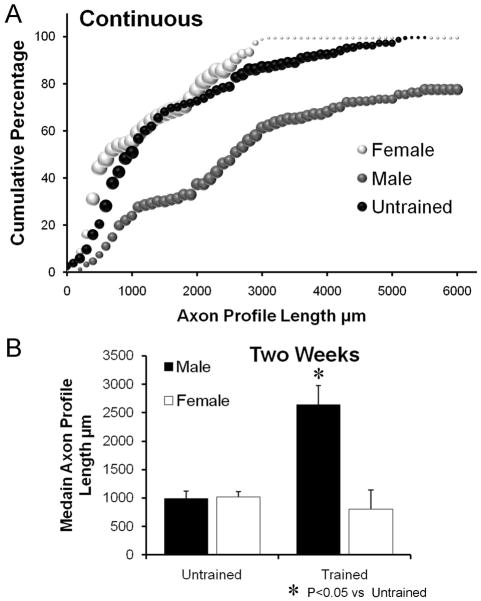
An image taken from a cut and repaired CF nerve is shown in Figure 1B. The lengths of YFP+ axon profiles in these nerves were measured from the proximal surgical repair site to their distal tip and expressed as cumulative histograms (Fig. 2A). Data for untrained males and females are shown as a single histogram because they are virtually identical. Data for continuously trained males and females are shown as separate symbols. Each data point in these graphs, and other cumulative histograms used in this paper, represents the mean percentage of all axon profiles studied in that group that are at least as long as the corresponding axon profile length (N=4 for each group). The size of each symbol is proportional to the standard error of the mean (SEM) of that data point. The effect of training is clear in the males. The distribution of axon profile lengths from continuous trained male mice is shifted to the right of that from untrained controls. For females, no similar shift in the distribution from trained mice was observed. This sex difference is evident if the median axon profile lengths are compared (Fig. 2B). No significant sex difference was found in average median axon profile lengths in untrained mice (LSD, p=0.959). Average median axon profile length is significantly (LSD, p<0.002) greater in the continuous trained males than in untrained controls of either sex or continuous trained females. There is no significant (LSD, p=0.744) difference in average median axon profile length between continuous trained females and untrained males or females. Thus continuous daily treadmill training is effective in enhancing axon regeneration in mice, but only in males.
Figure 2.
A. Distributions of axon profile lengths measured in grafts two weeks following transection and repair of nerves of continuous trained male and female mice and untrained mice of both sexes are shown as cumulative frequency histograms. Each data point in these graphs represents the average of the percentage of axon profiles that are at least as long as the corresponding axon profile length in that group (N=4 for each group). The size of each symbol is proportional to the standard error of the mean (SEM) for that data point. B. Average median (±SEM) lengths of regenerating axons in male and female Untrained and continuous treadmill Trained mice, two weeks after nerve injury.
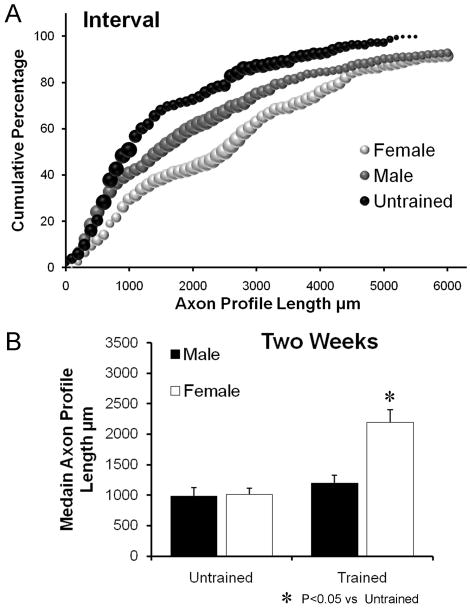
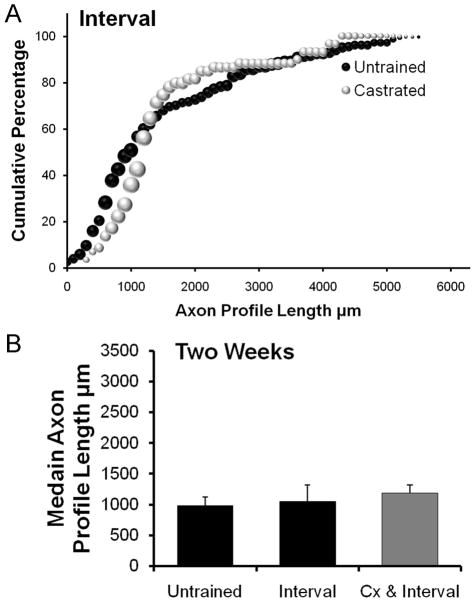
The results of analysis of sex differences in mice receiving daily training using an interval training paradigm are shown in Figure 3. The distributions of axon profile lengths for both males and females are shifted to the right of the respective distributions for untrained males and females (Fig. 3A). However, average median axon profile length is significantly (LSD, p=0.041) greater than in untrained controls only in the interval trained females (Fig. 3B). In interval trained males, no significant (LSD, p=0.397) increase in median axon profile length was found, relative to untrained controls. Thus, daily treadmill training using an interval training strategy is effective in enhancing axon regeneration only in female mice.
Figure 3.
A. Distributions of axon profile lengths measured in grafts two weeks following transection and repair of nerves of interval trained male and female mice and untrained mice of both sexes are shown as cumulative frequency histograms. Each data point in these graphs represents the average of the percentage of axon profiles that are at least as long as the corresponding axon profile length in that group (N=4 for each group). The size of each symbol is proportional to the standard error of the mean (SEM) of that data point. B. Average median (±SEM) lengths of regenerating axons in male and female Untrained and interval treadmill Trained mice, two weeks after nerve injury.
Effects of castration on treadmill training-enhanced axon regeneration
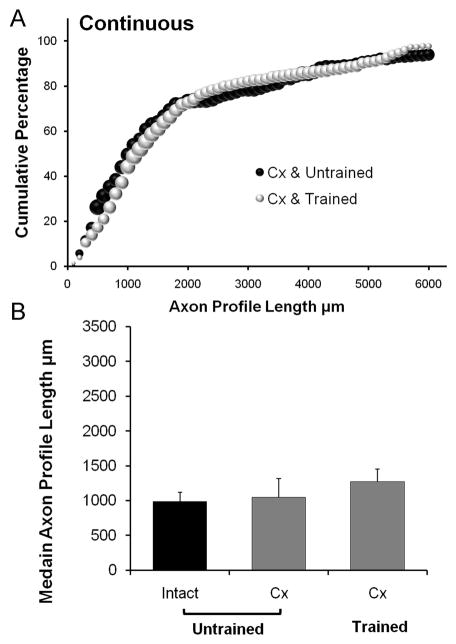
The CF nerves of 12 castrated male mice were cut and repaired, as described above. Four of these mice were then exposed to daily continuous treadmill training for one hour, four were interval trained, and four received no training. The effects of the two training protocols on axon regeneration were studied after two weeks of treatment. Results of these experiments are presented in Figure 4 for continuous training and Figure 5 for interval training. Castration itself did not alter axon regeneration (LSD, p=0.90, data not shown); however no enhancement of axon regeneration was evident in continuous trained castrated males (Fig. 4A). The median axon profile lengths from continuous trained castrated males were not significantly different from the values obtained from untrained males (LSD, p=0.577) (Fig. 4B). Thus, the enhancing effect on axon regeneration found following daily continuous treadmill training is blocked completely in castrated males.
Figure 4.
A. Distributions of axon profile lengths measured in grafts two weeks following nerve transection and repair in castrated untrained (Cx & Untrained) mice and castrated continuous trained mice (Cx & Trained) are shown as cumulative frequency histograms. The format is the same as in Figures 2 and 3. B. Average median (±SEM) lengths of regenerating axons in untrained gonadally intact male (Intact) mice and untrained castrated (Cx) mice or castrated mice trained with a continuous treadmill training paradigm (Trained), each two weeks after nerve injury. N=4 for each category.
Figure 5.
A. Distributions of axon profile lengths measured in grafts two weeks following nerve transection and repair in in castrated untrained (Cx & Untrained) mice and castrated interval trained mice (Cx & Trained) are shown as cumulative frequency histograms. The format is the same as in Figures 2 and 3. B. Average median (±SEM) lengths of regenerating axons in untrained gonadally intact male (Intact) mice and untrained castrated (Cx) mice or castrated mice trained with an interval treadmill training paradigm (Trained), each two weeks after nerve injury. N=4 for each category.
Four cut and repaired CF nerves were studied from castrated male mice that had been interval trained for two weeks. Data from these mice are summarized in Figure 5. Compared to the results from intact male or female mice, no significant difference was found in average median regenerating axon profile lengths (LSD, p=0.696) (Fig. 5B) in castrated male mice exposed to two weeks of interval training.
Effects of aromatase inhibition on axon regeneration
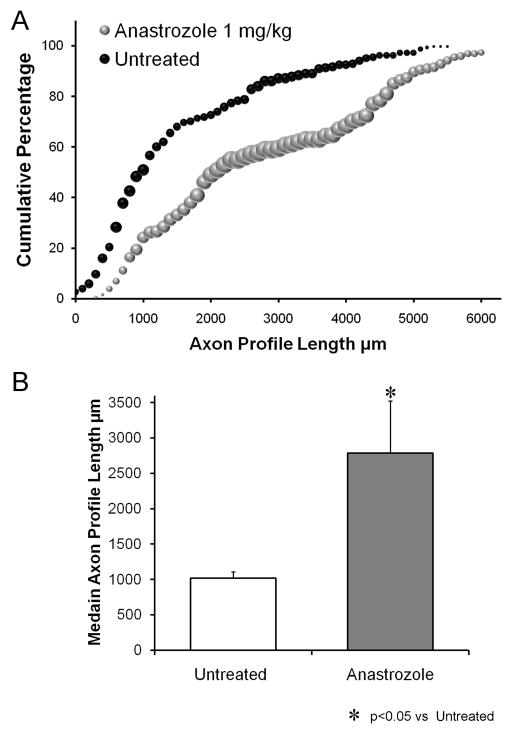
In four female mice, nerve repair surgery was followed by daily systemic treatment for two weeks with the aromatase inhibitor, anastrozole. These mice were not treadmill trained. At the dosage used (1 mg/Kg), anastrozole will block the conversion of testosterone or its precursors into estradiol (Plourde et al., 1994). The distribution of regenerating axon profile lengths in these mice is shown in Figure 6A. Daily anastrozole treatment resulted in a marked shift in the distribution of regenerating axon profile lengths, relative that found in untreated mice (males or females), and a significant (LSD, p<0.004) increase in average median axon profile lengths (Fig. 6B). Thus inhibiting aromatase produced a marked enhancement of axon regeneration in these untrained female mice.
Figure 6.
A. Distributions of axon profile lengths measured in grafts two weeks following transection and repair of the common fibular nerves of untrained and untreated female mice and untrained female mice treated daily with 1 mg/Kg of anastrozole (IP) are shown as cumulative frequency histograms. The format is the same as in Figures 2 and 3. B. Average median (± SEM) lengths of regenerating axons in untrained and untreated female mice and anastrozole treated female mice, each two weeks after nerve injury. N=4 for each category.
Measurements of serum testosterone in untrained and trained mice
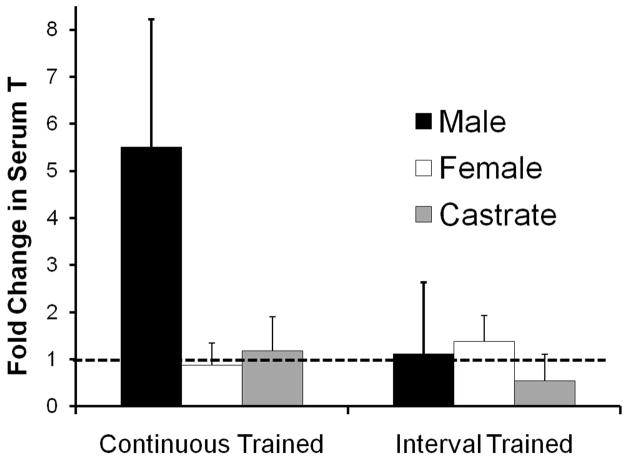
We measured testosterone levels in serum taken from trained and untrained animals at the time of euthanasia. The results of this analysis are summarized in Figure 7. Serum testosterone levels in male and female mice in our sample are similar to those reported for mice of the C57B strain by others (Brouillette et al., 2005). In continuous trained males, these levels are more than five times greater (mean = 5.507, ±2.713 (95% CI)) than serum levels found in untrained males. For all other groups in this series, the mean ratio of trained to untrained controls, less the 95% confidence interval, was less than unity, indicating that no significant change from sex-matched control values was noted. In females trained identically, no significant changes were noted, and no change was found in castrated males trained continuously for two weeks. Interval training for two weeks did not result in a significant increase in serum testosterone levels in either sex. In female mice treated for two weeks with daily injections of the aromatase inhibitor, anastrozole, a small, but significant decrease in serum testosterone, relative to untrained females, was noted (data not shown, mean = 0.799 ± 0.189 (95% CI)). This change was anticipated, as others have shown that similar treatments with similar doses of anastrozole did not result in increases in serum testosterone (Plourde et al., 1994).
Figure 7.
Values for serum testosterone measured in treadmill trained mice. Data are expressed as the mean ratio of serum measurements in trained mice to those of sex-matched untrained controls. Each bar represents the mean (± 95% confidence limits) of four samples. The dashed line at y=1.00 represents the level associated with no change. Continuous trained and Interval trained groups are shown separately.
Real time PCR for BDNF and trkB
To evaluate the effectiveness of our different training paradigms on the expression of BDNF and its receptor, trkB, in males and females, we measured the expression of the mRNA for these two molecules with qRT-PCR. These data are shown in Figure 8. Data are expressed as average fold change (± 95% confidence intervals) in expression relative to controls.
Figure 8.
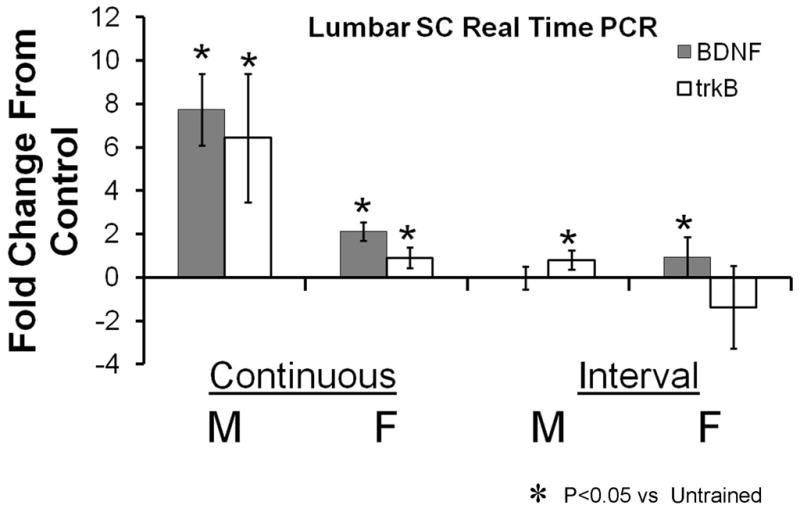
Results of real time PCR analysis for BDNF and trkB expression in the lumbar spinal cord of treadmill trained mice are shown. All data are expressed as mean fold change from the expression found in a group of untrained matched controls. Each bar represents the mean (± 95% confidence intervals) of four samples. The axis line at y=0 represents the level associated with no change. Continuous trained and Interval trained groups are shown separately.
Changes in BDNF mRNA expression were subjected to a two-way analysis of variance, having two levels of training type (continuous and interval) and two levels of sex (male and female). The main effect of training type was significant (F(1, 10) = 87.172, p < .000003), indicating that the mean change in spinal cord BDNF mRNA expression was significantly greater for continuous training than interval training. The main effect of sex was significant (F(1, 10) = 23.517, p < .0007), indicating that the mean change in spinal cord BDNF mRNA expression was significantly different in the males and females. The interaction effect also was significant (F(1, 10) = 46.881, p > .00005), indicating that the effect of sex on BDNF mRNA expression was different in continuous training than in interval training. Post-hoc (Fisher’s LSD) paired testing of the interaction was conducted. As expected, BDNF mRNA expression was significantly greater in continuous trained males than continuous trained females (p<0.00016) and continuous trained males and females both were significantly greater than interval trained males (p<0.01), but not females (p=0.109). No significant sex difference was found for interval trained mice (p=0.159).
Changes in trkB mRNA expression were subjected to a similar two-way analysis of variance. The main effect of training type was significant (F(1, 10) = 20.735, p < .0001), indicating that the mean change in spinal cord trkB mRNA expression was significantly greater for continuous training than interval training. The main effect of sex was significant (F(1, 10) = 19.76, p < .001), indicating that the mean change in spinal cord trkB mRNA expression was significantly higher in the males than females. The interaction effect was not significant (F(1, 10) = 3.698, p > .08), indicating that the effect of sex on trkB mRNA expression was similar (greater in males) in continuous training and interval training.
Discussion
Complete functional recovery following peripheral nerve injury is rare, despite the extensive capacity for axons in peripheral nerves to regenerate (Frostick et al., 1998; Scholz et al., 2009). Poor functional outcomes observed clinically are often attributed to the slowness of the process of regeneration (Gordon et al., 2009). We have shown recently that modest daily exercise in the form of treadmill training results in a significant enhancement of early axon regeneration in mice (English et al., 2009; Sabatier et al., 2008). Two quite different forms of training were applied with comparable outcomes: An hour of daily continuous walking at a slow treadmill speed and repetitions of short intervals conducted at near maximal running speed. Continuous training was chosen because it had been shown to have a beneficial effect on functional recovery following spinal cord injury (Hutchinson et al., 2004). Interval training was studied because it closely resembles the pattern of voluntary exercise noted by others in mice (De Bono et al., 2006). Each paradigm resulted in regeneration of axons to nearly twice the length noted after the first two post-injury weeks in untrained controls.
We have suggested that this enhancement of axon regeneration is produced by a training-induced increase in the neurotrophins brain derived neurotrophic factor (BDNF) and neurotrophin-4/5 (NT-4/5) (English et al., 2011; Wilhelm et al., 2011). Exercise is known to result in increased expression of BDNF in different brain regions (Adlard et al., 2004; Griesbach et al., 2004; Oliff et al., 1998) and in spinal cord motoneurons (Gomez-Pinilla et al., 2001). The expression of BDNF and its receptor, trkB, are regulated by androgens (Osborne et al., 2007; Sharma et al., 2009; Verhovshek et al., 2010). Exogenous androgen treatment is well known to enhance axon regeneration (Brown et al., 1999; Jones et al., 2000) and this enhancement has been linked to BDNF expression (Sharma et al., 2009). Considering this evidence, we wanted to investigate whether sex differences in the efficacy of our two treadmill training paradigms might exist. The main finding of this study is that such sex differences do exist but these differences are not the same for the two training paradigms used. Continuous training at a slow treadmill speed produces enhancement of axon regeneration only in gonadally intact males. More intense interval training for as little as eight minutes daily produced a significant increase in median axon profile length in females.
For continuous training, the mechanism of action seems clear. In intact females and castrated males, no enhancing effects of daily treadmill training at a slow speed for one hour are found. Our continuous training paradigm resulted in elevated serum testosterone in normal males but not females or in castrated mice. The magnitude of the training-induced increase in serum testosterone we observed in continuous trained males is as great or greater than observed after testosterone replacement in castrated rats (Hetzler et al., 2008). Continuous treadmill training resulted in a 6–8 fold increase in BDNF and trkB mRNA expression in the lumbar spinal cords of males and a very much smaller increase in expression of these molecules in females. Thus, we hypothesize that in males, continuous treadmill training enhances the length of regenerating axon profiles by increasing the production of gonadal androgens, which, when combined with activity of motor and sensory neurons, then increases the neuronal expression of BDNF and its receptor, trkB.
The mechanism by which interval training produces a more potent enhancing effect in females than in males is a bit more difficult to explain. The marked effect of interval training in female mice on axon regeneration in peripheral nerves must utilize a cellular mechanism that differs from that of continuous training. No significant increase in serum testosterone was noted in interval trained females or males. In female rats, interval training does not elevate serum testosterone, but it does result in a marked down regulation of skeletal muscle cytochrome C P450 aromatase, an enzyme that converts testosterone or its precursors into estradiol (Aizawa et al., 2008) and a marked increase in expression of skeletal muscle androgen receptors (Aizawa et al., 2010). Changes in both aromatase and androgen receptor expression in muscle with training were not significant in males (Aizawa et al., 2010). The net effect of these sex differences in non-gonadal androgen signaling was that exercise-induced changes in the availability of muscle testosterone were found in males and females, probably by increased gonadal synthesis in males, and decreased metabolism of testosterone to estradiol in females (Aizawa et al., 2008). Whether similar effects in other non-gonadal sources of androgen availability and signaling are found with exercise is not known. When we treated untrained female mice with an aromatase inhibitor, a robust enhancement of axon regeneration resulted without an increase in serum testosterone. Thus, we would speculate that the effectiveness of interval training in females might be the result of an inhibition of aromatase that stimulates a local, androgen-driven effect on the regenerating axons. Neither the source of the localized androgen availability nor the targets of this local androgen effect are yet clear but this speculation can be investigated experimentally.
The tepid increase in expression of BDNF and trkB mRNA in the lumbar spinal cord in both sexes following interval training also might be used to suggest that enhancing axon regeneration following this type of exercise might not include neuronal BDNF or trkB. In contrast to the large sex differences found with continuous training, the effect of interval training on spinal cord BDNF (but not trkB) mRNA expression in females is not significantly different from that found in interval trained males. However, because after either continuous training in males or interval training in females no enhancement of axon regeneration is found in mice conditionally null for the BDNF gene, we suggested that neuronal BDNF is required for the enhancing effects of both training paradigms (Wilhelm et al., 2011). Resolution of this apparent paradox awaits further investigation.
The results presented above are further evidence that exercise in the form of treadmill training has a marked enhancing effect on axon regeneration in cut peripheral nerves. Based on the marked sex differences we discovered, we encourage the application of exercise as a potential treatment for patients recovering from peripheral nerve injuries but in a manner consistent with the sex of the individuals involved.
Acknowledgments
This work was funded by Grants NS057190 (AWE) and K12GM000680 (KW, JCW, MJS) from the USPHS. Thanks are due to the Core facilities of the Yerkes National Primate Research Center of Emory University for conducting the assays of serum testosterone and to Dr. Ken Moberg for his help with the real time PCR analyses. Special thanks are due to Dr. Michael Kutner for his advice on statistical analyses.
Literature Cited
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Iemitsu M, Maeda S, Otsuki T, Sato K, Ushida T, Mesaki N, Akimoto T. Acute exercise activates local bioactive androgen metabolism in skeletal muscle. Steroids. 2010;75:219–223. doi: 10.1016/j.steroids.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Iemitsu M, Otsuki T, Maeda S, Miyauchi T, Mesaki N. Sex differences in steroidogenesis in skeletal muscle following a single bout of exercise in rats. J Appl Physiol. 2008;104:67–74. doi: 10.1152/japplphysiol.00558.2007. [DOI] [PubMed] [Google Scholar]
- Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Brouillette J, Rivard K, Lizotte E, Fiset C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res. 2005;65:148–157. doi: 10.1016/j.cardiores.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Khan T, Jones KJ. Androgen induced acceleration of functional recovery after rat sciatic nerve injury. Restor Neurol Neurosci. 1999;15:289–295. [PubMed] [Google Scholar]
- De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- de Vries J, Menovsky T, van Gulik S, Wesseling P. Histological effects of fibrin glue on nervous tissue: a safety study in rats. Surg Neurol. 2002;57:415–422. doi: 10.1016/s0090-3019(02)00736-x. [DOI] [PubMed] [Google Scholar]
- English AW, Cucoranu D, Mulligan A, Rodriguez JA, Sabatier MJ. Neurotrophin-4/5 is implicated in the enhancement of axon regeneration produced by treadmill training following peripheral nerve injury. Eur J Neurosci. 2011 doi: 10.1111/j.1460-9568.2011.07724.x. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. Neurotrophin 4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005;21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- English AW, Mulligan A, Cucoranu D, Sabatier MJ. Treadmill training enhances axon regeneration in cut peripheral nerves without effecting topographic specificity of reinnervating motoneurons. J Comp Neurol. 2009;517:245–255. doi: 10.1002/cne.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- Gordon T, Chan KM, Sulaiman OA, Udina E, Amirjani N, Brushart TM. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery. 2009;65:A132–144. doi: 10.1227/01.NEU.0000335650.09473.D3. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Groves ML, McKeon R, Werner E, Nagarsheth M, Meador W, English AW. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp Neurol. 2005;195:278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Hetzler LE, Sharma N, Tanzer L, Wurster RD, Leonetti J, Marzo SJ, Jones KJ, Foecking EM. Accelerating functional recovery after rat facial nerve injury: Effects of gonadal steroids and electrical stimulation. Otolaryngol Head Neck Surg. 2008;139:62–67. doi: 10.1016/j.otohns.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Hu Y, Asano K, Mizuno K, Usuki S, Kawakura Y. Serum testosterone responses to continuous and intermittent exercise training in male rats. Int J Sports Med. 1999;20:12–16. doi: 10.1055/s-2007-971084. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Alexander TD, Brown TJ, Tanzer L. Gonadal steroid enhancement of facial nerve regeneration: role of heat shock protein 70. J Neurocytol. 2000;29:341–349. doi: 10.1023/a:1007157105835. [DOI] [PubMed] [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg. 2003;18:480–485. doi: 10.1046/j.0886-0440.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- Marqueste T, Alliez JR, Alluin O, Jammes Y, Decherchi P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J Appl Physiol. 2004;96:1988–1995. doi: 10.1152/japplphysiol.00775.2003. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysate-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007;85:303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Plourde P, Dyroff M, Dukes M. Arimidex: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30:103–111. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- Sabatier M, Redmon N, Schwartz G, English A. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg. 2009;25:339–344. doi: 10.1055/s-0029-1215529. [DOI] [PubMed] [Google Scholar]
- Seo TB, Han IS, Yoon JH, Hong KE, Yoon SJ, Namgung U. Involvement of Cdc2 in axonal regeneration enhanced by exercise training in rats. Med Sci Sports Exerc. 2006;38:1267–1276. doi: 10.1249/01.mss.0000227311.00976.68. [DOI] [PubMed] [Google Scholar]
- Sharma N, Coughlin L, Porter RG, Tanzer L, Wurster RD, Marzo SJ, Jones KJ, Foecking EM. Effects of electrical stimulation and gonadal steroids on rat facial nerve regenerative properties. Restor Neurol Neurosci. 2009;27:633–644. doi: 10.3233/RNN-2009-0489. [DOI] [PubMed] [Google Scholar]
- Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol. 2010;223:183–191. doi: 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- van Meeteren NL, Brakkee JH, Hamers FP, Helders PJ, Gispen WH. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch Phys Med Rehabil. 1997;78:70–77. doi: 10.1016/s0003-9993(97)90013-7. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, Osborne MC, Sengelaub DR. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology. 2010;151:253–261. doi: 10.1210/en.2009-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JC, Cucoranu D, Xu D, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.1411-11.2012. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JC, Wood K, Liu K, English AW. Sex differences in the effect of treadmill training on axon regeneration in mouse peripheral nerves. Soc Neurosci Abstr. 2010:541.523. [Google Scholar]