Abstract
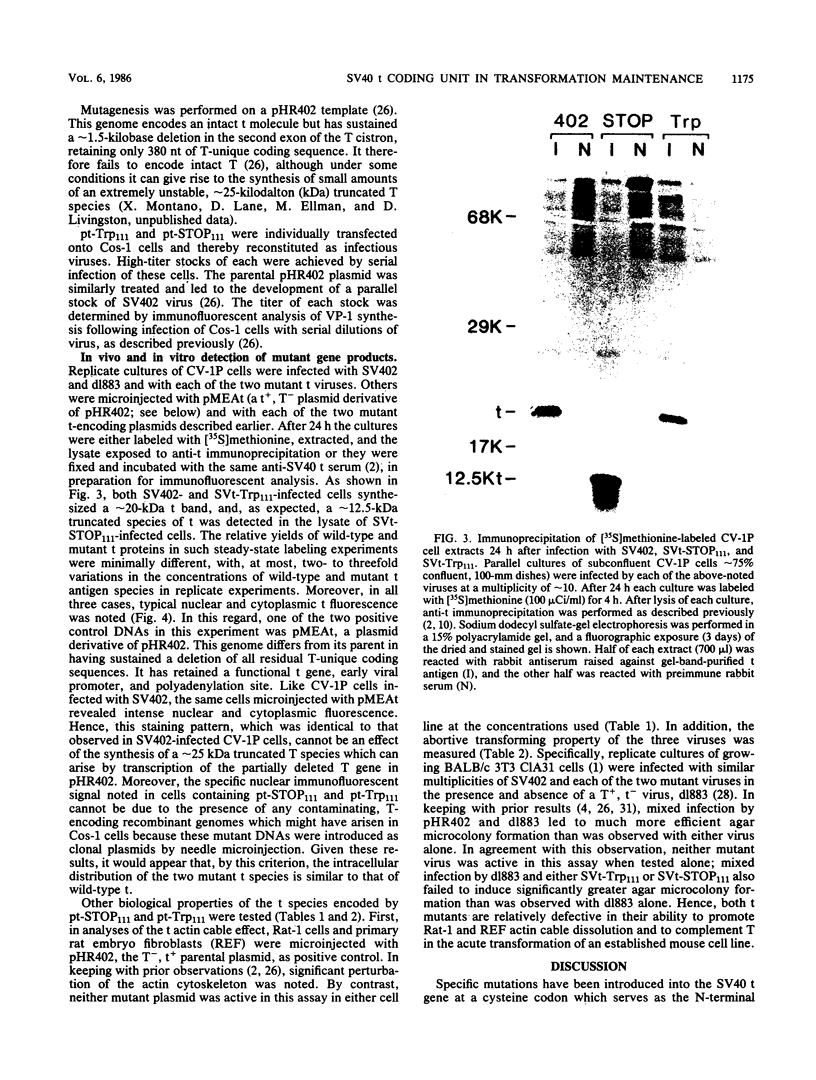
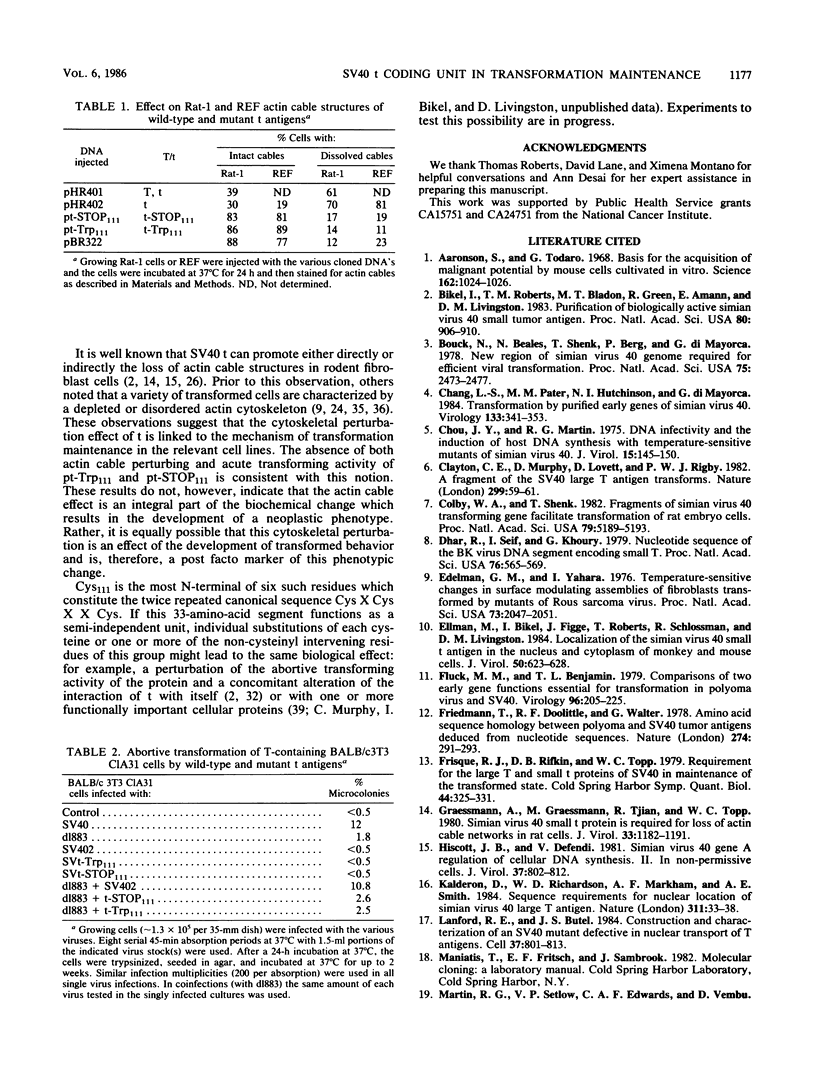
The small t antigen (t) of simian virus 40, a 174-amino-acid-containing protein, when present together with the other early viral protein, large T antigen (T), plays an important role in the maintenance of simian virus 40-induced neoplastic phenotype in certain cells. Indeed, each protein functions in a complementary manner in this process. The t coding unit is composed of two segments, a 5' region of 246 nucleotides which is identical to that of the corresponding 5' region of the T coding unit and a 3' segment of 276 nucleotides which is unique. Two mutant, t-encoding genomes, one bearing a missense and the other a nonsense mutation at the same point in the t-unique coding region were constructed in vitro and found to be defective in their ability to dissolve the actin cytoskeleton of rat fibroblasts and to complement T in the growth of mouse fibroblasts in soft agar. Therefore, the unique segment of the t gene encodes a portion of the t molecule which is essential to its transformation maintenance function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikel I., Roberts T. M., Bladon M. T., Green R., Amann E., Livingston D. M. Purification of biologically active simian virus 40 small tumor antigen. Proc Natl Acad Sci U S A. 1983 Feb;80(4):906–910. doi: 10.1073/pnas.80.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., Beales N., Shenk T., Berg P., di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci U S A. 1978 May;75(5):2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. S., Pater M. M., Hutchinson N. I., di Mayorca G. Transformation by purified early genes of simian virus 40. Virology. 1984 Mar;133(2):341–353. doi: 10.1016/0042-6822(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Murphy D., Lovett M., Rigby P. W. A fragment of the SV40 large T-antigen gene transforms. Nature. 1982 Sep 2;299(5878):59–61. doi: 10.1038/299059a0. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Shenk T. Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5189–5193. doi: 10.1073/pnas.79.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Seif I., Khoury G. Nucleotide sequence of the BK virus DNA segment encoding small t antigen. Proc Natl Acad Sci U S A. 1979 Feb;76(2):565–569. doi: 10.1073/pnas.76.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman M., Bikel I., Figge J., Roberts T., Schlossman R., Livingston D. M. Localization of the simian virus 40 small t antigen in the nucleus and cytoplasm of monkey and mouse cells. J Virol. 1984 May;50(2):623–628. doi: 10.1128/jvi.50.2.623-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M. M., Benjamin T. L. Comparisons of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979 Jul 15;96(1):205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Doolittle R. F., Walter G. Amino acid sequence homology between polyoma and SV40 tumour antigens deduced from nucleotide sequences. Nature. 1978 Jul 20;274(5668):291–293. doi: 10.1038/274291a0. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Topp W. C. Requirement for the large T and small T proteins of SV40 in the maintenance of the transformed state. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):325–331. doi: 10.1101/sqb.1980.044.01.037. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Tjian R., Topp W. C. Simian virus 40 small-t protein is required for loss of actin cable networks in rat cells. J Virol. 1980 Mar;33(3):1182–1191. doi: 10.1128/jvi.33.3.1182-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. II. In nonpermissive cells. J Virol. 1981 Feb;37(2):802–812. doi: 10.1128/jvi.37.2.802-812.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyamura T., Jikuya H., Soeda E., Yoshiike K. Genomic structure of human polyoma virus JC: nucleotide sequence of the region containing replication origin and small-T-antigen gene. J Virol. 1983 Jan;45(1):73–79. doi: 10.1128/jvi.45.1.73-79.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano X., Lane D. P. Monoclonal antibody to simian virus 40 small t. J Virol. 1984 Sep;51(3):760–767. doi: 10.1128/jvi.51.3.760-767.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra B. A., Harvey R., Ely B. K., Markham A. F., Smith A. E. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature. 1983 Aug 4;304(5925):456–459. doi: 10.1038/304456a0. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Figge J., Bladon M. T., Chen L. B., Ellman M., Bikel I., Farrell M., Livingston D. M. Role of small t antigen in the acute transforming activity of SV40. Cell. 1982 Sep;30(2):469–480. doi: 10.1016/0092-8674(82)90244-6. [DOI] [PubMed] [Google Scholar]
- Rundell K., Cox J. Simian virus 40 t antigen affects the sensitivity of cellular DNA synthesis to theophylline. J Virol. 1979 Apr;30(1):394–396. doi: 10.1128/jvi.30.1.394-396.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala M., Atcheson C. L., Kasamatsu H. Stimulation of host centriolar antigen in TC7 cells by simian virus 40: requirement for RNA and protein syntheses and an intact simian virus 40 small-t gene function. J Virol. 1982 Aug;43(2):721–729. doi: 10.1128/jvi.43.2.721-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Sompayrac L., Danna K. J. A simian virus 40 dl884/tsA58 double mutant is temperature sensitive for abortive transformation. J Virol. 1983 May;46(2):620–625. doi: 10.1128/jvi.46.2.620-625.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel C. S., Burgess T. L., Tjian R. Properties of simian virus 40 small t antigen overproduced in bacteria. J Virol. 1981 Feb;37(2):683–697. doi: 10.1128/jvi.37.2.683-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. W., Sanford K. K., Frankel R. Tubulin and actin in paired nonneoplastic and spontaneously transformed neoplastic cell lines in vitro: fluorescent antibody studies. Cell. 1978 Apr;13(4):629–642. doi: 10.1016/0092-8674(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Verderame M., Alcorta D., Egnor M., Smith K., Pollack R. Cytoskeletal F-actin patterns quantitated with fluorescein isothiocyanate-phalloidin in normal and transformed cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6624–6628. doi: 10.1073/pnas.77.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Hearing P., Rundell K. Cellular proteins associated with simian virus 40 early gene products in newly infected cells. J Virol. 1979 Oct;32(1):147–154. doi: 10.1128/jvi.32.1.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]